Detection of Dirofilaria repens and Mansonella llewellyni in the United States by Wolbachia Surveillance
Abstract
In mammals, detection of Wolbachia bacteria can be used to diagnose filarial infection, while antibiotic treatment to eliminate Wolbachia can assist in eliminating filarial infections. Because Wolbachia are necessary for survival of several filarioids and closely related to Anaplasma and Ehrlichia, we analyzed Wolbachia DNA amplification by Anaplasma/Ehrlichia qPCR, from 39,526 domestic and wildlife animal blood samples submitted to a diagnostic laboratory between 2017 and 2023. Filarial infection was confirmed by 28S gene amplification, followed by phylogenetic analysis utilizing filarial cytochrome oxidase subunit 1 (cox1), myosin heavy chain (myoHC), and 70 kilodalton heat shock protein (hsp70) gene sequencing. Wolbachia DNA was detected in 57 domestic dogs (Canis familiaris) and three raccoons (Procyon lotor) from 23 states and Puerto Rico. A majority of the Wolbachia sequences from dogs were Dirofilaria immitis-associated (89%, 51/57), whereas DNA from other Wolbachia were associated with insects (9%, 5/57) or Dirofilaria repens (2%, 1/57). D. immitis infection was confirmed by 28S filarial PCR for all samples with D. immitis-associated Wolbachia available for testing (n = 41). D. repens infection was confirmed by 28S and cox1 PCR in the dog infected with D. repens-associated Wolbachia. This dog was originally imported from Slovakia. The Wolbachia DNA amplified from raccoons most closely aligned with Wolbachia from Mansonella ozzardi (98.9%). 28S filarial, cox1, myoHC, and hsp70 sequencing did not align with currently available GenBank sequences but did align with Mansonella. Morphologically, microfilariae from additional raccoons were consistent with Mansonella llewellyni. Molecular surveillance for Wolbachia in wildlife and domestic animals has the potential to identify novel filarial species in the United States, including zoonotic species.
1. Introduction
The domestic dog is host to over 15 filarioid species associated with clinical manifestation of variable severity (Table 1) [3, 5–8]. Manifestations include pruritic lesions and nodules (D. repens [Dirofilaria repens] and O. lupi [Onchocerca lupi]), lymphatic obstruction (Brugia spp.), and cardiopulmonary symptoms (D. immitis [Dirofilaria immitis]) [1, 9]. These can be triggered by the physical obstruction of the adult worm, host immune activation, or even a life-threatening response to antimicrofilarial treatment [9, 10]. The most common canine filarial infection in the United States is D. immitis, the cause of canine heartworm disease.
| Adult filaria niche | Microfilaria niche | Present in the United States | Reported Zoonotic potential | Wolbachia Infection | |
|---|---|---|---|---|---|
| Acanthocheilonema dracunculoides | Skin | Blood | No | — | No |
| Acanthocheilonema reconditum | Skin | Blood | Yes | — | No |
| Brugia ceylonensis | Lymphatic system | Blood | No | — | Suspected |
| Brugia malayi | Lymphatic system | Blood | No | Yes | Yes |
| Brugia pahangi | Lymphatic system | Blood | No | — | Yes |
| Brugia patei | Lymphatic system | Blood | No | — | Suspected |
| Cercopithifilaria bainae | Skin | Blood | Yes | — | No |
| Cercopithifilaria grassii | Skin | Blood | No | — | No |
| Dirofilaria immitis | Pulmonary artery | Blood | Yes | Yes | Yes |
| Dirofilaria repens | Skin | Blood | No | Yes | Yes |
| Onchocerca lupi | Skin | Skin | Yes | Yes | Yes |
| Thelazia californiensis | Eye | Eye | Yes | — | No |
| Thelazia callipaeda | Eye | Eye | No | — | No |
- Note: Information extracted from the literature regarding filarial infection in the domestic dog including the adult and microfilaria niche, if the filarial species is found in the United States, if infection is considered zoonotic, and if Wolbachia is found infecting that filarial species. Multiple publications review canine filarial infection: [1–4].
Due to a lack of screening and the large number of dogs transported around the world, the risk of importing canine filaria species whose known range does not include the United States, such as D. repens or Brugia spp., is concerning considering the potential for zoonosis [11]. Furthermore, the vast majority of canine and feline filaria testing in the United States utilizes assays specific only for D. immitis antigens. Unlike the Modified Knott’s Test (MKT), the D. immitis antigen test (SNAP 4Dx Plus, IDEXX, and WITNESS Canine Heartworm Antigen Test, Zoetis) only detects adult, female D. immitis antigens [12]. Due to this factor, the antigen test will only detect mature infections after approximately 6 months. Sensitivity to other filarioids is very low with occasional reports of cross-reactions to Acanthocheilonema or D. repens. Therefore, methods that are capable of broad detection of multiple filarioid species including those with limited known geographic range should be prioritized for surveillance.
Both D. immitis and D. repens thrive in areas where domestic dog populations do not receive preventative and high environmental temperature and humidity facilitate vector reproduction and parasite development. Dirofilaria immitis has a worldwide distribution while D. repens is associated with the “Old World” (Europe, Asia, and Africa) (reviewed by [13]). Two reports suggest D. repens is present or has been imported to the “New World” [14, 15]. In Europe, D. repens has spread at a faster rate than D. immitis, reflected in zoonotic and canine transmission [13]. Zoonotic transmission of Dirofilaria species can occur by the bite of an infected mosquito. In a majority of cases, infection is eliminated by the immune system however in atypical cases or immunocompromised individuals, infection may develop and even produce microfilaria. Larval Dirofilaria migrate through the body to a wide variety of body tissues including the skin, eyes, viscera (lungs and mesentery), muscle, lymph nodes, breasts, or male genitalia [2, 13]. In these patients, a dirofilariasis lesion can mimic tuberculosis, fungal infection, or neoplasia, resulting in the need for an excisional biopsy, a cause of costly interventions and emotional distress. Zoonotic D. immitis infection has been described on all continents except Antarctica. Serological surveys suggest D. immitis and D. repens exposure in the human population is similar, 1.5%–9.3%, with higher seroprevalences reported in areas where Dirofilaria are common in canine populations [16–18].
Additional domestic and wild animal species also host filarioids, including the raccoon (Procyon lotor). While the raccoon is host to a wide diversity of filarioids (Brugia beaveri, Dracunculus insignias, Dipetalonema procyonis, Dirofilaria tenuis, and Mansonella llewellyni) little research has addressed the epidemiology, pathology, or zoonotic capacity of these species [19–24]. Mansonella llewellyni is among the most prevalent of these species in the southeastern United States, however reports are restricted to geographically limited studies or case studies [22, 24–29]. Various filaria, including Brugia, Dirofilaria, Mansonella, and Onchocerca spp., are host to endosymbiotic bacteria in the genus Wolbachia [30, 31].
Wolbachia are obligate intracellular bacteria in the family Anaplasmataceae, closely related to Anaplasma and Ehrlichia spp. Elimination of this endosymbiont in infected filarioid species prevents microfilaria production and release and eventually results in premature adult filarioid death [32, 33]. Wolbachia also directly interacts with the mammalian host, as it is believed to drive neutrophil recruitment and activation, as well as, classic activation of macrophages [34, 35]. Given the association of Wolbachia and filaria, Wolbachia is considered a promising target for diagnosis and treatment of various filarioids including Dirofilaria and Mansonella species [36].
This retrospective study assessed Wolbachia detection in animal samples submitted to the North Carolina State University–Vector Borne Disease Diagnostics Laboratory (NCSU–VBDDL) for Anaplasma and Ehrlichia 16S qPCR, because of the close phylogenetic relationship of Anaplasma, Ehrlichia, and Wolbachia, the diagnostic Anaplasma and Ehrlichia 16S qPCR nonspecifically amplifies Wolbachia DNA. The first objective was to assess the mammalian host species and geographic location of Wolbachia infected animals, and to determine the filaria or arthropod species associated with the sequenced Wolbachia. The second objective was to amplify filarioid DNA from Wolbachia positive blood samples and phylogenetically compare non-D. immitis filaria by using three common gene targets: cytochrome oxidase subunit 1 (cox1), myosin heavy chain (myoHC), and 70 kilodalton heat shock protein (hsp70). Based on the detection of Wolbachia and an uncharacterized Mansonella filaria in raccoons in our study, and the lack of deposited Mansonella sequences from the United States in GenBank, the third objective was to further assess the phylogenetic identity and morphology of Mansonella spp. in the raccoons utilizing blood and tissue samples from additional animals.
2. Materials and Methods
2.1. Retrospective Testing
This retrospective study investigated the detection of Wolbachia spp. in animal blood samples submitted to the NCSU–VBDDL for Anaplasma and Ehrlichia qPCR from March 9, 2017 to December 31, 2023. As blood samples were submitted for diagnostic purposes from the animal’s owner, ethical approval for animal use was not applicable. The authors have previously reported the nonspecific amplification of Wolbachia from Ctenocephalides felis utilizing these primers [37]. Wolbachia identity was considered definitive if an NCBI BLAST search returned a single alignment with > 99% homology to an arthropod or filaria associated Wolbachia [38].
Dirofilaria immitis antigen test results were obtained if performed by the NCSU–VBDDL (SNAP 4Dx Plus, IDEXX, Maine, USA). For the D. immitis-associated Wolbachia infected dogs, the accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of D. immitis-associated Wolbachia were all calculated in R (version 4.4.1) with the epiR package (version 2.0.80) utilizing SNAP 4Dx Plus results as the gold standard.
Blood stored at − 80°C or extracted DNA from blood was available for 50 animals (47 dogs and three raccoons) that were retrospectively identified as Wolbachia qPCR positive. DNA was extracted utilizing the Qiagen QIAsymphony SP robot with the QIAsymphony DSP DNA Mini Kit (Qiagen, Valencia, CA, USA; catalog no. 937236). Amplification by conventional PCR and sequencing of a portion of the 28S filarioid DNA was utilized to determine the presence and species of infecting filaria [39]. PCR cleanup and sequencing for all reactions was performed by GENEWIZ from Azenta Life Sciences (Azenta, Plainfield, NJ 07080, USA). Sequences were compared to GenBank utilizing NCBI BLAST and those with >99% homology to a single species were considered definitively identified. For D. immitis-associated Wolbachia infected dogs, PPV was calculated utilizing 28S filarioid PCR as the gold standard. This is because Wolbachia PCR negative dogs were not subject to 28S filarioid PCR, therefore only true positive and false positive samples could be identified.
For animals infected with an insect-associated Wolbachia species, or without detectable filarioid DNA, the submitting veterinarian was contacted to obtain medical records. Medical records were reviewed by one author (Edward B. Breitschwerdt) and key clinicopathologic findings were reported in the results. For animal(s) with invasive filarioid species, the owner was contacted to determine the animal’s country of origin and current status.
To further define filarioid species not found in the United States or without a definitive identity, the cox1, myoHC, and hsp70 filaria genes were amplified and sequenced [30, 40, 41]. Phylogenetic trees were constructed to compare sequences to reference sequences acquired from GenBank utilizing the RAxML (version 8.2.12) General Time Reversible model with gamma distribution for rate heterogeneity with 1000 bootstrap replicates [42].
2.2. Prospective Raccoon Sampling
Upon the initial amplification of filarioid sequences from raccoons that did not match publicly available sequences on GenBank, we collected samples from five additional raccoons on January 15, 2024 in Ellerbe, North Carolina with the assistance of a local trapper. Researchers only had contact with raccoons postmortem and animals were not trapped specifically for research purposes, therefore animal use approval was not necessary. Blood was obtained via postmortem cardiac blood draw and preserved in individual EDTA tubes. Four to seven skin snips were collected from each raccoon for incubation in sterile saline to identify adult filarioids.
Two hundred microliters of blood was aliquoted and DNA extracted as previously described utilizing the Qiagen QIAsymphony SP robot. PCR and sequencing of the Wolbachia 16S and 28S filarioid, cox1, myoHC, and hsp70 genes were performed for comparison to filarioid sequences obtained from raccoons identified by the NCSU–VBDDL [30, 39, 40, 43] (Table S1).
For each racoon, five thin blood smears were prepared with 10 µL of blood, air-dried, and stained with either Diff-Quick or Wright-Giemsa. One or more MKT were performed between 126 and 357 days after blood collection for four raccoons with adequate residual blood [44]. All the slides were evaluated by a board-certified pathologist (Cynthia Robveille) in a blinded manner.
To measure microfilaria total length and maximum diameter, 48 microfilariae from four stained blood smear slides from three raccoons and 57 microfilariae from four MKT slides from two raccoons were analyzed. Slides were scanned with the Leica Aperio AT2 by the NCSU College of Veterinary Medicine Histology Laboratory and were analyzed by one author (Cynthia Robveille) using SlideViewer (3DHISTECH Kft). 95% confidence intervals for length and maximum diameter, as well as Wilcoxon rank sum test comparing length and maximum diameter by slide preparation technique (MKT or blood smear), were calculated utilizing the crosstable package (version 0.8.1) in R [45]. Three microfilaria features (head space, nerve ring, and excretory pore) were measured on the MKT slides and reported as a percentage of total body length from the anterior end.
Using the same technique described above, we measured the total length of 15 Mansonella llewellyni microfilariae from a Giemsa-stained thick blood smear slide generated by a previous publication for submission to the Harold W. Manter Laboratory of Parasitology collection [24]. The reference slide (HWML38947) is originally from Georgia, USA.
3. Results
3.1. Diagnostic Screening
In total, 39,526 samples were submitted to the NCSU–VBDDL during study enrollment from at least 45 domestic and wild animal species (Table S2). Eight samples were from an unknown animal species, often with a group or family designation (e.g., unknown primate). Approximately 98% of samples were from domestic dogs (n = 35,179) or cats (n = 3,666). In total, Wolbachia DNA was detected in 60 samples from 23 states and Puerto Rico including raccoons (3/40; 7.50%) and domestic dogs (57/35,179; 0.16%; Figure 1). All raccoon samples tested by the NCSU–VBDDL, infected or uninfected, were from Kentucky.
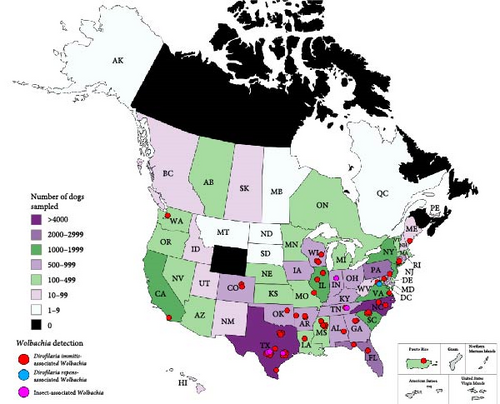
3.2. Domestic Dogs
Based on alignment of the amplified portion of the 16S gene, Dirofilaria immitis-associated Wolbachia was the most frequent Wolbachia in domestic dogs (51/57, 89.5%; GenBank Accession ID CP046578.1, 180/180 bp, 100%). Dirofilaria repens-associated Wolbachia (AJ276500.1, 100%) was detected in a single dog.
Wolbachia associated with insect species were detected in five dogs (5/57, 8.8%) (Table 2). This included Wolbachia pipientis (CP092141.1, 181/181 bp, 100%) in two dogs, C. felis-associated Wolbachia (CP116767.1, 181/181 bp, 100%) in a single dog, and two insect-associated Wolbachia with multiple alignments to Supergroup A Wolbachia (OZ187006.1, 189/190 bp, 99.5% for the 5-year-old dog; OX366347.1, 181/181 bp, 100% for the 10-year-old dog). Their demographic and clinical presentations were broad (Table 2).
| Detected Wolbachia | Age (years) | Breed | Sex | US State | Historical, clinical, and diagnostic findings |
|---|---|---|---|---|---|
| Wolbachia pipientis (n = 2) | 2 | Mixed breed | FC | IN | Weight loss, pancytopenia, and splenomegaly |
| 13 | Labrador retriever | FC | TX | Megaesophagus, aspiration pneumonia, and increased ALP and ALT | |
| Ctenocephalides felis-associated Wolbachia (n = 1) | 1 | German shepherd | FC | TX | Fever and endocarditis (treated with penicillin) |
| Nonspecific insect-associated Wolbachia (n = 2) | 10 | Siberian husky | MC | TX |
|
| 5 | English setter | FI | TX | Sudden onset blindness, reverse sneezing, nasal malignant tumor with pulmonary metastases, and cardiac and pulmonary dirofilariasis | |
- Note: The information was obtained from veterinary clinic records.
- Abbreviations; C, castrated; F, female; I, intact; M, male.
Compared to 4Dx SNAP Plus results generated by the NCSU–VBDDL, a majority of D. immitis-associated Wolbachia 16S qPCR positive dogs were D. immitis antigen positive (37/42, 88%), while five (12%) were antigen negative and nine were not tested (Table 3). Analyzing the 23,085 dogs tested by both 4Dx SNAP Plus (gold standard) and Wolbachia 16S qPCR, detection of D. immitis-associated Wolbachia by 16S qPCR boasted high specificity (100%) and NPV (99%) but limited sensitivity (18%; Table 4). The one D. repens-associated Wolbachia positive and five insect-associated Wolbachia 16S qPCR negative dogs were all 4Dx SNAP Plus D. immitis antigen negative. From medical records, one insect-associated Wolbachia dog tested 4Dx SNAP Plus positive for D. immitis antigens at their home clinic (2 days prior to sample submission) and D. immitis were found during autopsy 23 days later. Evidence of D. immitis infection was not reported in the records for the other insect-associated Wolbachia infected dogs.
| Dirofilaria immitis 4Dx SNAP Plus | |||
|---|---|---|---|
| Positive | Negative | ||
| Dirofilaria immitis-associated Wolbachia (16S qPCR) | Positive | 37 (0.16%) | 5 (0.02%) |
| Negative | 163 (0.71%) | 22,881 (99.11%) | |
| Gold standard | ||
|---|---|---|
SNAP 4Dx Plus Heartworm (95% CI) |
28S filarioid PCR (95% CI) |
|
| Number of samples | 23,086 | 41 |
| Sensitivity | 18 (13–25) | N/A |
| Specificity | 100 (100–100) | N/A |
| Positive predictive value (PPV) | 88 (74–96) | 100 (91–100) |
| Negative predictive value (NPV) | 99 (99–99) | N/A |
Previously extracted DNA for additional analysis was acquired for 47 dogs. 28S filarial PCR amplified D. immitis DNA from all dogs with D. immitis-associated Wolbachia (41/41, 100%). Therefore, when utilizing 28S filarial PCR as the gold standard, detection of D. immitis-associated Wolbachia had a 100% PPV (91%–100%; 95% CI) (Table 4). Dirofilaria repens DNA was amplified from the one dog with D. repens-associated Wolbachia. Despite triplicate PCR, no filarial DNA was amplified from the five dog blood samples containing insect-associated Wolbachia (W. pipientis, C. felis, and nonspecific insect Wolbachia).
The dog infected with D. repens was originally imported to Virginia, USA from Slovakia but was unfortunately lost to follow up. Due to limited sample remaining, only the cox1 filarial gene was amplified. The sequence aligned to D. repens (100%, 655/655 bp; MT345575.1) and was deposited in GenBank (Accession ID PV258722).
3.3. Raccoons
The Wolbachia detected in three raccoons submitted to the NCSU–VBDDL were all most closely aligned with Mansonella ozzardi-associated Wolbachia (AJ279034.1, 179/181 bp, 98.9%). Sequences from 28S filarial PCR aligned 100% with both M. ozzardi (GenBank Accession ID MN432519.1) and Mansonella perstans (MN432520.1).
Prospective sampling identified an additional three (3/5, 60%) Wolbachia spp. qPCR positive raccoons from Ellerbe, NC. No adult filaria were identified from skin snips. All Wolbachia qPCR positive raccoons, but not the Wolbachia spp. negative racoons, were 28S filarial PCR positive with the same Mansonella as raccoons tested by the NCSU–VBDDL. Additional sequencing targeting the cox1 (570 bp), myoHC (517 bp), and hsp70 (468bp) genes was performed for comparison with a wide variety of filarial species (Figure 2). The amplified Mansonella spp. was distinct from human-associated Mansonella (M. ozzardi, M. perstans, and Mansonella streptocerca) and Mansonella previously reported in Nasua nasua (ring-tailed coati) in Brazil [41]. The sequences from raccoon filaria were deposited in GenBank: cox1 (PV258720-1, PV258723-5), myoHC (PV269753-8), and hsp70 (PV299582-6).
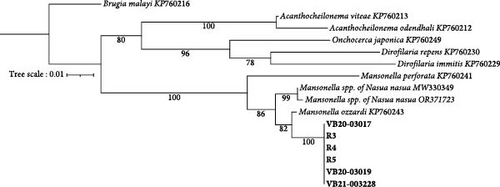
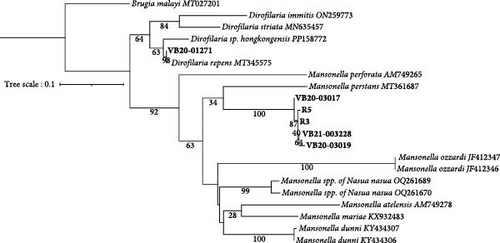
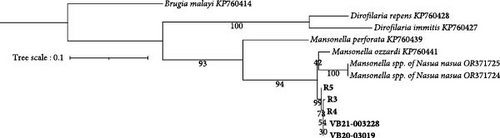
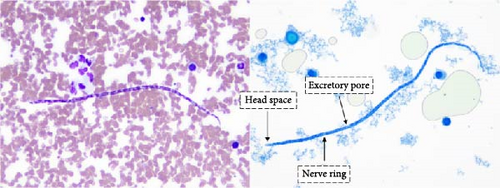
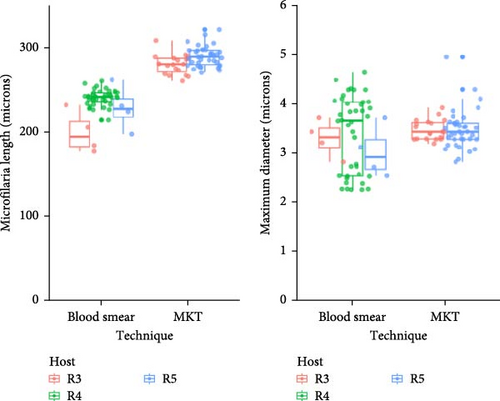
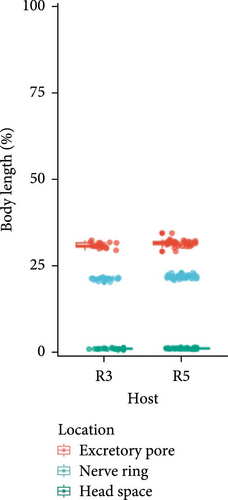
Microfilariae were observed on stained blood smears and MKT in the three PCR positive raccoons, but not in the two PCR negative raccoons (Figure 3A). The length of the microfilariae (Figure 3B) was significantly shorter (p < 0.0001) on the stained thin blood smears (236 ± 5 µm; mean ± 95% CI) compared to the MKT (287 ± 3 µm). Their maximum diameter was not different between the two techniques (3.4 ± 0.3 µm with thin blood smear versus 3.3 ± 0.1 µm with MKT, p = 0.92; Figure 3B and Table S3). On the MKT, microfilariae were not sheathed and had a blunt head with a relatively short head space, a compact column of nuclei beginning and ending with a single row, and a slender attenuated tail devoid of nuclei and sometimes ending in a button–hook curve. The length and morphology of the observed microfilariae were compatible with Mansonella llewellyni based on comparison with the reference slide and previously published literature [46–48] (Table 5). The location of the head space, nerve ring, and excretory pore as a percentage of body length was 1.2%, 21.7%, and 31.4%, respectively (Figure 3C).
| Techniques (number of microfilariae observed) | Microfilariae length (range) | Microfilariae diameter (range) | Citation |
|---|---|---|---|
| Thin blood smear (n = 48) | 236 (177–262) | 3.4 (2.3–4.6) | This study |
| MKT (n = 57) | 287 (261–321) | 3.5 (2.8–5) | This study |
| 2% formalin | 288 (275–304) | 4 (3–5) | [26] |
| MKT (n = 333) | 270.2 (210–310) | 2.5 | [28] |
| Unreported | 290 ± 5 (unknown) | 2.5 | [48] |
| Knott’s test (n = 60) | 286.5 ± 10.7 (st. dev.) | 2.8 ± 0.2 (st. dev.) | [24] |
- Note: By default, range is reported in parentheses after metrics if available. If not available, the metrics reported by the citation is included and metric indicated in parentheses.
4. Discussion
This investigation supports the interest in targeted Wolbachia qPCR gene amplification as a tool for filaria surveillance in the future. While the majority of sequences were D. immitis-associated Wolbachia, we also reported qPCR amplification of Wolbachia associated with one “Old World” zoonotic filarioid species in the United States (D. repens), and one wildlife filaria species (M. llewellyni) for which DNA sequences were not publicly available.
The establishment of D. repens in North America is a considerable risk for dog and human health, as both the host (dog) and vectors (Anopheles, Aedes, and Culex species) are present. For the present case, there is not sufficient evidence to determine if the D. repens infection was acquired in Europe, prior to importation of the dog to the United States, or in the United States. Reports of autochthonous infections in Florida and Mexico emphasize the potential for D. repens to establish in the Americas [14, 15]. In Europe, D. repens is the primary cause of subcutaneous and ocular dirofilariasis in humans and is among the fastest spreading zoonotic pathogens, with an increase in geographical range and increase in prevalence in established areas [49, 50]. Given that D. immitis antigen assays are not sensitive for detection of D. repens without heat treatment, MKTs or PCR based detection methods in canine and human populations should be implemented in the United States for surveillance purposes, particularly in imported animals [51]. Wolbachia has been repeatedly reported in D. repens-infected dogs in Europe [52, 53].
In this study, diagnostic amplification of Wolbachia DNA also facilitated the detection of M. llewellyni in raccoons. Given the lack of confirmatory DNA sequence or genomic data, the species identification was based on microscopic evaluation of microfilariae in raccoon blood collected prospectively. The previously published morphology (e.g., lack of sheath, blunt head, and curved tail devoid of nuclei) and mean length of M. llewellyni microfilariae were consistent with our findings. It is worth reinforcing that the technique used to visualize the parasites can impact morphometric characterizations, as shown here by a 21.6% increase in microfilaria length on MKT (287 µm) compared to blood smear (236 µm). Despite a high parasitemia in blood, microfilariae were not observed in 1 of 5 blood smears for two raccoons, highlighting the need to perform multiple blood smears or concentration methods (i.e., MKT) to decrease the risk of false-negative results. Given the concentration step, MKT has a greater sensitivity compared to blood smears [54]. In addition, MKT was required for precise morphology assessment.
Based on a PubMed search for “Mansonella llewellyni Wolbachia” and “Mansonella raccoon Wolbachia” yielding no results and review of the limited publications regarding M. llewellyni [24–29, 48], the authors believe this is the first study highlighting the presence of Wolbachia in M. llewellyni. Wolbachia have been identified in other Mansonella species, including M. ozzardi [31], Mansonella perstans [55], and Mansonella perforate and Mansonella atelensis amazonae [56]. Adult M. llewellyni are located in the raccoons’ subcutaneous connective tissue and intermuscular fascia [27]. No parasites in these locations were found during the autopsy of the three filarial positive PCR raccoons. However, given their small number and size and not using a dissecting microscope, parasites could have been missed [27]. In addition, the presence of microfilariae without the observation of adults has also been reported [24, 27]. In the United States, microfilariae of M. llewellyni have been reported in several raccoons living in Florida [25], Georgia [24], Louisiana [22, 26], Maryland [27], and Tennessee [28] and in a single raccoon in North Carolina [29]. Our study demonstrated for the first time, to the best of our knowledge, M. llewellyni-infected raccoons in Kentucky, and additional cases in North Carolina. The ability of M. llewellyni to cause microscopic lesions was not assessed, as formalin-fixed tissues were not collected. Nevertheless, its pathogenicity in raccoons is likely low as there were no significant gross lesions in the PCR positive raccoons. Given the shared geography of M. llewellyni reservoir hosts (raccoons) and hematophagous arthropod vectors with humans and domestic animals, there is the potential for zoonotic or cross-species transmission but the authors did not locate any reports.
Human Mansonella infection is often asymptomatic but may manifest as acute swelling and itching of the skin, joint pain, or abdominal pain [57]. Successful elimination of M. perstans microfilaria in humans via doxycycline treatment targeting Wolbachia indicates the importance of future investigations involving Mansonella-Wolbachia interactions [36].
The detection of insect-associated Wolbachia species in the absence of detectable filaria DNA was unexpected. While Wolbachia are known to colonize a variety of insects, these Wolbachia strains are not regarded as infectious to mammalian species. None of the dogs had filarial DNA detected by PCR or were D. immitis antigen positive when tested by the NCSU–VBDDL. Interestingly, one dog was infected with D. immitis upon autopsy performed 15 days after blood collection and was D. immitis SNAP 4Dx Plus antigen positive at the home veterinary clinic. Injectable ivermectin and doxycycline was prescribed for treatment. Although unlikely given the slow rate of Wolbachia host switches, the detection of Wolbachia may be an undescribed strain infecting D. immitis or unrelated finding [58]. For all insect-associated Wolbachia dogs, DNA contamination was considered unlikely as these insect and Wolbachia species were not found in the NCSU–VBDDL during the study period, except for limited C. felis samples handled by different personnel. In the absence of detectable filarial infection, the ability of Wolbachia to survive in the mammalian host, invade mammalian cells, or survive in the absence of an arthropod or nematode host is unknown. Additional investigation, including sequential testing to determine if the infection persists, attempted isolation of Wolbachia from hosts with detectable insect-associated Wolbachia and experimental infection will be necessary to understand the potential role of Wolbachia as a mammalian pathogen.
Compared to SNAP 4Dx Plus Heartworm antigen detection (gold standard), detection of D. immitis-associated Wolbachia by amplification with the Anaplasma/Ehrlichia 16S rRNA qPCR boasted high specificity and NPV, but limited sensitivity (18%). While PPV was 88% utilizing SNAP 4Dx Plus Heartworm antigen detection as a gold standard, D. immitis DNA was detected in all samples with D. immitis-associated Wolbachia (100% PPV), suggesting the SNAP 4Dx Plus Heartworm antigen test does not detect all active D. immitis infections. This may be due to the delayed detection of the antigen test (~ 6 months after infection), limited detection of the antigen test (only female D. immitis), or imperfect sensitivity of antigen detection that is improved by heat treatment [59].
Limited research has attempted to utilize Wolbachia detection to diagnose filarial infection and generally report lower Wolbachia detection than filarial detection [60, 61]. Laidoudi et al. also reported the amplification of D. immitis-associated Wolbachia DNA in a majority of hosts with D. immitis DNA (75%, 9/12) as well as hosts without D. immitis DNA detected (29%, 8/29) [62]. In a survey of 307 domestic cats utilizing nested PCR targeting the ftsZ gene, Wolbachia was detected in 34 samples compared to filaria (D. immitis) detection in one sample [63]. The Wolbachia spp. reported in the present study were detected by non-specific amplification of the highly conserved bacterial 16S rRNA gene and had low sensitivity (18%) compared to the SNAP 4Dx Plus Heartworm antigen test. Therefore, qPCR for a highly sensitive and specific Wolbachia gene target, such as ftsZ, may increase diagnostic sensitivity for early or occult infections.
The lack of Wolbachia spp. detection in any additional animal species, specifically the domestic cat, was initially surprising as 3666 cats were tested by the Anaplasma/Ehrlichia qPCR during the study period. The domestic cat is documented to host B. malayi, D. immitis, and D. repens; however, only D. immitis infection is common in areas of the United States. Dirofilaria immitis infection in the cat requires a longer period of maturation and results in fewer adult parasites with a shorter lifespan compared to dogs [64]. Microfilaremia in the cat is typically transient with low microfilaria burdens, reducing the likelihood of detecting Wolbachia spp. by qPCR. Dirofilaria immitis infection is common in wild carnivores (e.g., coyote, fox), however the limited number of samples submitted for diagnostic testing was likely inadequate to detect a filaria infected host [65, 66]. The horse and donkey are host to multiple Onchocerca spp., however the location of microfilariae and adult filarioids in the skin, subcutaneous, or connective tissues prevents detection of Wolbachia DNA within the blood [67, 68].
Limitations of this study include not performing an MKT or PCR screening for filaria in all blood samples subjected to the Anaplasma/Ehrlichia 16S qPCR to assess the efficiency of Wolbachia detection. However, this was not possible due to the large number of samples tested by the VBDDL and practice of freezing samples at − 20°C after submission which is not compatible with the MKT. Furthermore, our inability to locate adult Mansonella from raccoons restricted phenotypic comparisons of adult morphology which is more thoroughly described in the literature [48]. The absence of longitudinal testing of animals prevented our ability to assess the persistence of Wolbachia in filarioid infected and uninfected hosts.
Based upon the findings in this study, there is a need to establish surveillance programs monitoring emerging and atypical filarioid species in humans, wildlife, and domestic animal species. Our findings expanded the spectrum of filarioid parasites colonized by Wolbachia to include M. llewellyni. We also for the first time published color images and genetic sequencing of M. llewellyni, a critical tool for species identification by parasitology and molecular laboratories. Surveillance programs for domestic animals and wildlife would help establish the diversity of filaria infecting the diverse species present in North America, as well as facilitate tracking their zoonotic potential. Our detection of D. repens in an imported dog reinforces the potential for D. repens to be introduced and establish in North America.
Consent
The authors have nothing to report.
Conflicts of Interest
In conjunction with Dr. S. Sontakke and North Carolina State University, Edward B. Breitschwerdt holds US Patent Number 7,115,385 Media and Methods for Cultivation of Microorganisms, which was issued on October 3, 2006. He is a cofounder, shareholder, and Chief Scientific Officer for Galaxy Diagnostics, a company that provides advanced diagnostic testing for the detection of Bartonella spp. infections. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
Charlotte O. Moore: conceptualization, data curation, investigation, formal analysis, methodology, software, writing – original draft and editing, visualization. Cynthia Robveille: conceptualization, investigation, methodology, supervision, writing – original draft, review, and editing. Barbara Qurollo: resources, methodology, writing – review and editing. Edward B. Breitschwerdt: resources, supervision, writing – review and editing.
Funding
This research was supported through donations to the Bartonella/Vector Borne Diseases Research Fund at the North Carolina State University College of Veterinary Medicine.
Acknowledgments
The authors would like to thank Armon Waters and the North Carolina Trappers Association for allowing us to collect samples from trapped raccoons. We would also like to thank Dr. Matthew Breen and Isabella Livingston for assisting in the selection of the 28S filaria primers and providing a D. immitis positive control for the assay. We thank Brittany Thomas and Catherine Byrd at the NCSU–VBDDL for assisting us in acquiring records for the animals included in this study. Finally, we would like to thank Scott L. Gardner, Gabor Racz, and Nicholas Lee at the Harold W. Manter Laboratory of Parasitology for sharing slides from their collection and providing parasitological expertise.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Dryad at https://doi.org/10.5061/dryad.bcc2fqzqp. Sequences are available on GenBank: cox1 (PV258720-5), myoHC (PV269753-8), and hsp70 (PV299582-6).




