Real-Time Reverse Transcription Multienzyme Isothermal Rapid Amplification for Rapid Detection of African Horse Sickness Virus
Abstract
African horse sickness (AHS) is an acute infectious disease of equids caused by the AHS virus (AHSV), which can cause up to 90% mortality in naive horses. Reliable and rapid diagnosis is crucial for the surveillance and control of AHSV. As one of the AHSV detection methods recommended by World Organization for Animal Health (WOAH), the RT-qPCR assay has the drawbacks such as complex operation, expensive instruments, and long detecting time, which limit its application in simple laboratories or outdoors. In this study, a real-time reverse transcription multienzyme isothermal rapid amplification (RT-MIRA) assay was established to detect AHSV. Primers and exo-probes were designed, synthesized, and screened based on the conserved regions of the AHSV Seg-7 gene. A series of experiments were conducted to evaluate the performances of the established real-time RT-MIRA for detecting AHSV. The valid testing results showed that this method was highly specific for the detection of AHSV, without exhibiting any cross-reactivity towards other equine viruses or other Orbivirus; its limit of detection (LOD) was 10 copies/μL, which was consistent with that of RT-qPCR, meaning it had good sensitivity for detecting AHSV. Furthermore, the real-time RT-MIRA for AHSV performed good repeatability, and its standard curve exhibited good linearity with a correlation coefficient of R2 = 0.9898, which indicated that the established method could be used for the quantitative detection of ASHV. As no AHS infection cases have been reported in China, 120 simulated clinical samples were tested by the real-time RT-MIRA and RT-qPCR for AHSV, which results showed there was a significant correlation between the two assays, with a κ value of 0.966 and an R2 value of 0.9576. Parallel detection of 396 equine blood samples and 1760 Culicoides by this method and the RT-qPCR showed that all samples were negative for AHSV. Furthermore, the results of the real-time RT-MIRA could be judged by naked eyes under a portable equipment with blue light (480 nm). In conclusion, the real-time RT-MIRA for AHSV was specific and sensitive and had the advantages of convenient operation, visualization, no need for special equipment, and could be a reliable tool for rapid screening and detection of AHSV in field or border ports.
1. Introduction
African horse sickness (AHS) is a noncontagious vector-borne disease caused by the AHS virus (AHSV) [1], which is transmitted between hosts by hematophagous midges of the genus Culicoides [2]. Susceptible hosts to AHSV are horses and other equids (mules, donkeys, and zebras) [3]. AHS is highly lethal in naïve horses with high mortality rates (>90%) [4]. Donkeys are more resistant to the disease, with less severe clinical signs and death expected in around 5%–10% of cases [5]. In general, zebras are resistant to AHSV, exhibit asymptomatic infection, therefore, are considered to be the hosts maintaining for this disease [6]. AHS is generally endemic in countries of the southern Africa and has been recorded previously in Morocco, Spain, Cyprus, Portugal, India, Iran, and Pakistan [7–9]. However, global warming has expanded the range and duration of AHSV vectors activities, which is likely to expand the range of the AHS epidemic [7]. Recent outbreaks of ASH in Thailand and Malaysia in 2020 indicate that AHS may have escaped geographical limitations and entered Southeast Asia [10].
AHSV is a member of the genus Orbivirus in the family Reoviridae [11], which genome consists of 10 double-stranded RNA segments (Seg-1–Seg-10), encoding seven structural proteins (VP1–VP7) and four nonstructural proteins (NS1, NS2, NS3/NS3A, and NS4) [12, 13]. AHSV has a similar morphological structure to the bluetongue virus (BTV). Its outer capsid layer consists of two major structural proteins VP5 and VP2 [14], while the core particle of ASHV are composed of VP1, VP3,VP4, VP6, and VP7 [8, 15]. As the primary determinant of the AHSV serotype, VP2 is the most variable antigen and the main target of virus-neutralizing antibodies [16, 17]. To date, there are nine different serotypes of AHSV (AHSV-1–AHSV-9) identified based on VP2, and the virulence of different types is different [18, 19]. VP3 and VP7, encoded by Seg-3 and Seg-7, are highly conserved group-specific antigenic proteins in AHSV [20]. Furthermore, VP7 is the main target of enzyme-linked immunosorbent assay (ELISA) assay and molecular biology assay for AHSV [21–23].
Due to its associated high mortality to naïve horse populations and the potential for rapid international spread through Culicoides biting, a sensitive and reliable assay for rapid detection AHS is urgently required to prevent, control, and eliminate this disease. Laboratory diagnostics for AHS include etiological and serological tests. Most of the serological methods detecting AHS are complement fixation (CF), virus neutralization (VN), and ELISA methods, including blocking ELISA, indirect ELISA, and competitive ELISA. Several laboratory etiological methods have been applied to detect AHSV, including virus isolation (VI), immunofluorescence, antigen-detecting ELISA, and molecular biology techniques. Although the VI is the classic gold-standard method, its operation procedure is complex and takes about 2–5 days, not suitable for rapid diagnosis. Relatively, molecular biological detection techniques such as RT-PCR and real-time RT-PCR are more widely used to detect ASHV. Among them, an agarose gel–based RT-PCR developed by Zientara et al. [24] and the real-time RT-PCR methods established by Aguero et al. [25] and Guthrie et al. [26], respectively, are recommended by the World Organization for Animal Health (WOAH). The conventional RT-PCR or real-time RT-qPCR for determination of AHSV type is mostly based on the detection of Seg-2, occasionally aimed at the specific regions of Seg-1 or Seg-3. For rapid DNA/RNA detection, several techniques based on isothermal amplification were developed, such as loop-mediated isothermal amplification (LAMP) [27], rolling circle amplification (RCA) [28], and recombinase polymerase amplification (RPA) [29]. The rapid AHSV RT-LAMP assay based on AHSV Seg-7 has sufficiently high specificity and sensitivity for the detection of AHSV [30].
As a novel isothermal amplification technology, the multienzyme isothermal rapid amplification (MIRA) assay utilizes the synergistic role of multiple enzymes or functional proteins (DNA polymerase, recombinase, helicase, and single-stranded binding protein) to achieve rapid amplification at an isothermal condition. The difference of MIRA with RPA is that it uses a recombinase derived from Streptomyces azure, which improves amplification efficiency and stability and reduces interference [31]. The advantages of reverse transcription (RT)-MIRA/MIRA are rapid reaction and low reaction temperature, which only takes 20–30 min to complete the reaction at 33–42°C, while the RT-LAMP/LAMP reaction needs more than 1 h at 60–65°C. In the meantime, there are several patterns to analyze the RT-MIRA/MIRA amplification products, such as agarose gel or capillary electrophoresis, fluorescence detection, and lateral flow strip. So far, the RT-MIRA/MIRA technique has been used in detection of human, animal, and plant pathogens, such as SARS-CoV-2, hepatitis a virus, classical swine fever virus, pseudorabies virus (PRV), sweet potato feathery mottle virus, and sweet potato chlorotic stuntvirus [32–36]. However, as yet there has not been an RT-MIRA method developed for the detection of AHSV.
In the present study, a real-time RT-MIAR assay based on a highly conserved region within AHSV Seg-7 was established. As a sensitive and reliable method for rapid detection of AHSV, the results of this method in simulated samples, field samples and Culicoides samples were highly consistent with the results of RT-qPCR recommended by WOAH.
2. Material and Methods
2.1. Plasmid and Virus Nucleic Acid
The plasmid pMD18-AHSV-vp7 containing AHSV Seg-7 gene (GenBank: KT030596.1), BTV-1 RNA, epizootic hemorrhagic disease virus (EHDV) RNA, and PRV RNA was preserved at our lab. Equine infectious anemia virus (EIAV) RNA, equine viral arthritis virus (EVAV) RNA, and equine influenza virus (EIV) RNA were purchased from Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
2.2. Designing, Synthesizing, and Screening of Primers for RT-MIRA
For the MIRA/RT-MIRA, the primer length is usually 30–35 bp and the optimal length of the amplicon is 150–500 bp. Five specific primer pairs of RT-MIRA amplification were designed based on the conserved region of the Seg-7 gene (GenBank: KT030596.1) of AHSV (Table 1) by the design software (PrimerPrimer, Version 5.0) and synthesized by Sangon (Shanghai, China). For screening the best RT-MIRA amplification primer pairs, amplification reactions were conducted using the RT-MIRA kits (AMP-Future Biotech, Cat#: WLRB8207KIT) which provide Buffer A (PEG 3wt%, Tris 30 mM), Buffer B (Mg(Ac)2 280 mM) and reaction tubes containing freeze-dried powder consisting of DNA polymerase, recombinase, helicase, single-stranded binding protein, reverse transcriptase, polyA, and dNTP. A RT-MIRA reaction mixture of 29.4 μL Buffer A, 2 μL of each F/R primers (10 μM), 13.1 μL of ddH2O, and 1 μL of the plasmid pMD18-AHSV-vp7 was added to each reaction tube containing freeze-dried powder. After adding 2.5 μL of Buffer B into the reaction mix, the tube was closed, vortexed, centrifuged, and then placed in a thermostatic metal bath at 42°C for 30 min. The results of the amplification reaction were analyzed by a capillary electrophoresis device (Qiagen, QIAxcel Advanced System) to determine the best specific primer pairs for RT-MIRA amplification.
| Name | Sequence (5′-3′) | Position | Source |
|---|---|---|---|
| ASHV-S7-1F | CGATAGCAGCAAGAGCCTTGTCCGTTGTAC | 25–54 | Designed in this study |
| ASHV-S7-1R | CCACAGTTGCCAACGCCTGATCATAATCTGGT | 260–291 | Designed in this study |
| ASHV-S7-2F | TCTGGCAGATATTACGTACCGCAAGGTCGAAC | 429–460 | Designed in this study |
| ASHV-S7-2R | GACGACATCTCCAGCCGCAACATTTACTCCAT | 655–686 | Designed in this study |
| ASHV-S7-3F | GTTGATGGAGTAAATGTTGCGGCTGGAGAT | 651–680 | Designed in this study |
| ASHV-S7-3R | GCCAAGTGGGAGAAACATACGCATAACATC | 823–852 | Designed in this study |
| ASHV-S7-4F | CACAAGATGTTATGCGTATGTTTCTCCCACT | 818–848 | Designed in this study |
| ASHV-S7-4R | CGCAACAATTTCATTCCTTTCAGTCGGTGG | 915–944 | Designed in this study |
| ASHV-S7-5F | CCACCGACTGAAAGGAATGAAATTGTTGCG | 915–944 | Designed in this study |
| ASHV-S7-5R | AGCATAAGAACCGACGCGACACTAATGAAA | 1094–1123 | Designed in this study |
| Exo-probe-1 | GCACGCATTACGCGCTGTCATTTTTCAGCAGA (6-FAM-dT) (THF)AA (BHQ1-dT)ATGCAGCCTAT (C3 Spacer) | 851–898 | Designed in this study |
| Exo-probe-2 | GTGTATGCGGCTTTGAGACCAGATTTCAGAA (6-FAM-dT) (THF)AA (BHQ1-dT)GGTGTTGTCGCGCCA (C3 Spacer) | 972–1023 | Designed in this study |
| RNA Template-F | TAA TAC GAC TCA CTA TAG GGT TTT GTC ACC GTT GAT GGA GTA | 641–662 | Designed in this study |
| RNA Template-R | GTA AGT GTA TTC GGT ATT GAC G | 1146–1167 | Designed in this study |
| AHSV-RT-qPCR-F | AGAGCTCTTGTGCTAGCAGCCT | 1038–1059 | [26] |
| AHSV-RT-qPCR-R | GAACCGACGCGACACTAATGA | 1096–1116 | [26] |
| AHSV-RT-qPCR-probe | 6-FAM-TGCACGGTCACCGCT-BHQ-1 | 1080–1094 | [26] |
2.3. Exo-Probe Designing and Screening for Real-Time RT-MIRA
There are four sites of modification on the exo-probe: A dSpacer (tetrahydrofuran, THF) is labeled in the middle position approximately 35 nt from the 5′ end of the exo-probe as the recognition site for exonuclease; the upstream and downstream of the THF site is labeled with a fluorescent group and a quenched group, and a distance of 2–4 nt between the two groups; the 3′ end of the exo-probe is labeled with a modifying group, such as an amino group, phosphate group, or C3 Spacer [31]. Two AHSV-specific exo-probes based on the target regions of the best primer pairs in previous screening were synthesized by Sangon (Shanghai, China) (Table 1).
For screening the optimal group of primers and exo-probe, the real-time RT-MIRA kits (AMP-Future Biotech, Cat#: WLRE8208KIT) were used according to the manufacturer’s instructions with some modifications [35], in which come with Buffer A (PEG 3 wt%, Tris 30 mM), Buffer B (Mg(Ac)2 200 mM), and reaction tubes containing freeze-dried powder consisting of DNA polymerase, recombinase, helicase, single-stranded binding protein, reverse transcriptase, exonuclease, polyA, and dNTP. All subsequent real-time RT-MIRA experiments in this study were conducted using the real-time RT-MIRA kits from Amp-Future Biotechnology Co., Ltd. Each reaction tube with freeze-dried powder was added with the 50 μL reaction mixture of real-time RT-MIRA consisting of 29.4 μL Buffer A, 2.0 μL F/R primer (final concentration 400 nM), 2.5 μL Buffer B, 0.6 μL exo-probe (final concentration 120 nM), 1.0 μL nucleic acid template, and made up to 50 μL with nuclease-free H2O. Buffer B was added in the last step, and the reaction tubes were quickly turned upside down 5–6 times and rapidly centrifuged, then immediately incubated at 42°C for 20 min in ABI QuantStudio 5 qPCR system (ThermoFisher, USA). The fluorescence signals were collected every 30 s with the FAM channel.
2.4. Standard RNA Preparation for AHSV Dection Using Real-Time RT-MIRA and RT-qPCR
To provide a standard AHSV positive RNA template for subsequent assays, a pair of PCR primers was designed (Table 1), named RNA template-F and RNA template-R, whose upstream primer 5′ end contained a T7 promoter. Its amplification range was between the 641–1167 bp of the AHSV Seg-7 gene, which contain the target region of the optimized RT-MIRA primers and the detection region of the RT-qPCR assay for AHSV recommended by WOAH. Plasmid pMD18-AHSV-VP7 containing AHSV Seg-7 gene was used as the template for PCR amplification using the primer pair mentioned above, and then the double-stranded DNA obtained by PCR then served as the DNA template for in vitro transcription reactions with a HiScribe T7 High Yield RNA Synthesis Kit (NEB, Cat#: E2040S,). The product of RNA fragments was purified using a miRNeasy Mini Kit (50; Qiagen, Cat#: 217004). The purified RNA was measured by a spectrophotometer (NanoDrop 2000, ThermoFisher, USA), and diluted to 1 × 107 copies/μL and stored at −80°C.
2.5. Optimization of Temperature in Real-Time RT-MIRA Reaction
To determine the optimal temperature of the real-time MIRA, the standard AHSV RNA was detected using the real-time RT-MIRA kits with the screened optimal primer/probe combination. The reaction temperature of the real-time RT-MIRA was set at 33, 35, 37, 39, and 42°C, respectively. The reaction time was 20 min, and the fluorescence of the FAM channel was collected every 30 s. The optimal temperature of the real-time MIRA was obtained by analysis of the amplification curves of different temperatures.
2.6. Specificity of the Real-Time RT-MIRA Assay
To evaluate the specificity of real-time RT-MIRA assay in the detection of AHSV, the RNA of BTV, EHDV, and other important equine viruses, including PRV, EIAV, EAV, and EIV, were tested by the developed real-time RT-MIRA of AHSV. The positive control was the standard RNA template mentioned above, and the negative control was ddH2O. The reactions were at 35°C for 20 min, and the fluorescence signals were collected every 30 s. Furthermore, a B-BOX Blue light LED epi-illuminator (Smobio, China) was used to detect the real-time RT-MIRA amplification products.
2.7. Sensitivity of the Real-Time RT-MIRA Assay
To determine the sensitivity of the real-time RT-MIRA assay for AHSV, the standard AHSV RNA template was diluted to 1 × 107 copies/μL ~ 1 × 100 copies/μL to detect by this method under optimized conditions. At the meantime, the WOAH-recommended RT-qPCR for AHSV was used to detect these templates in parallel, which were developed by Guthrie et al. [26] with some modifications. The RT-qPCR assay was conducted using the AgPath-ID One-Step RT-PCR Reagents (Applied Biosystems, Cat#: 4387424) in a 25-μL reaction, containing 12.5 μL of 2 × RTPCR buffer, 1.0 μL of 25 × RTPCR enzyme mix, 0.5 μL of each AHSV-RT-qPCR-F/AHSV-RT-qPCR-R primer (final concentration 200 nM), 0.3 μL of AHSV-RT-qPCR—probe (final concentration 200 nM), 1.0 μL of nucleic acid template, and 9.2 μL of nuclease-free H2O. The RT-qPCR was performed on an ABI QuantStudio 5 qPCR System (Applied Biosystems, USA) with the following conditions: 48°C (10 min), 95°C (10 min), and then 40 cycles of 95°C (15 s) and 60°C (45 s), and the flourescence was collected at 60°C. Each concentration of the serial-diluted AHSV RNA templates was tested five times by RT-qPCR and RT-MIRA, respectively. The standard curves of RT-qPCR and RT-MIRA were constructed based on the results (cycle threshold (Ct) values) of the two methods to compare their accuracy.
2.8. Compliance Testing of Simulated Samples
While ASH has never occurred in China, our lab did not have AHSV-positive samples. In order to evaluate the compliance of the established real-time RT-MIRA with RT-qPCR assay for AHSV, 120 simulated samples were prepared by randomly mixing the AHSV standard RNA with AHSV-negative horse or donkey blood samples. These simulated samples were tested in parallel with these two assays. The positive result was determined by the amplification curve and Ct value of the real-time RT-MIRA and RT-qPCR. The efficacy of these two assays were analyzed based on sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) as follows: sensitivity = TP/(TP + FN) × 100%; specificity = TN/(TN + FP) × 100%; PPV = TP/(TP + FP); and NPV = TN/(TN + FN) (TP, true positive; FP, false positive; TN, true negative; FN, false negative). The degree of agreement (κ value) between these two methods was calculated using SPSS software (version 26.0). In addition, the amplification products of the real-time RT-MIRA were observed under 480 nm blue light.
2.9. Testing for Field Samples and Culicoides Samples
To further evaluate the performance of this method, 256 horse blood samples and 140 donkey blood samples were collected from Yunnan province, Guangdong province, and Hebei province in China. The total RNA of samples was extracted by a MagMAX-96 Viral RNA Isolation kit (ThermoFisher, Cat#: AM1836) following the manufacturer’s directions and a KingFisher Flex Purification Instrument (96 Deep Well Processor; ThermoFisher, USA) and tested in parallel with the established real-time RT-MIRA method and the RT-qPCR for AHSV.
From June to September 2023, Culicoides were collected by UV light traps in Shenzhen of Guangdong province, China, while the collection locations were set inside Guanming Farm, Safari Park, Xianhu Botanical Garden, and Mangrove Coastal Ecological Park of Shenzhen City. As described previously with some modifications [37], six battery-powered UV light traps (LTS-M02; Wuhan Lucky Star Medical Treatment Technology Co., Wuhan, China) were set at each collection point. Every two traps are placed 500 m apart and run from 16:00–9:00 h the following day. The insects were collected directly into 70% ethanol and identified by morphology under a stereomicroscope (Leica, S APO), according to the keys and descriptions previously by Liu et al. [38]. During the period from July to September 2023, the frequency of collection was twice per month, and the total collection was six times in 3 months. After the morphological and species identification of culicids, every 20–30 Culicoides of the same species were divided into a batch for RNA extraction. Each batch of culicoides samples was added with 200 μL RNAiso-Plus (Takara, Cat#: 9109) and homogenized by the TissueLyser II homogenizer (Qiagen). The total RNA of samples was extracted following the manufacturer’s directions of RNAiso-Plus and tested in parallel with the established real-time RT-MIRA and the RT-qPCR for AHSV.
2.10. Statistical Analysis
The GraphPad Prism (Version 9.0) and SPSS (Version 26.0) were used to statistical analysis, including the probit regression analysis and κ statistics. The statistical analysis’s confidence interval was 95% (p < 0.05).
3. Results
3.1. Results of the Optimal Primers and Exo-Probe
Five ASHV-specific primer pairs for RT-MIRA amplification were screened using pMD18-AHSV-vp7 as a template. The RT-MIRA amplified products were analyzed by a capillary electrophoresis device to determine the best specific primer pairs for RT-MIRA amplification for AHSV. According to the amplification results (Figure 1A), two pairs of specific primers (the pair 4F/4R and the pair 5F/5R) are the optimal primers and could be used in the next experiments.
Two exo-probes were designed based on the target regions (801–927 bp and 898–1123 bp) of the optimal primer pairs (4F/4R and 5F/5R). A real-time RT-MIRA kit was used to screen the optimal group of primers and exo-probe. These findings (Figure 1B,C) indicate that the group of the primer pair 4F/4R and exo-probe 1 had better performance, better amplification efficiency, and better fluorescence amplification curve compared with the group of the primer pairs 5F/5R and exo-probe 2. Therefore, the primer pair 4F/4R and exo-probe1 were the optimal prime and exo-probe combination of the real-time RT-MIRA for AHSV.
3.2. Optimization of Temperature for Real-Time RT-MIRA
For determining the optimal temperature, the real-time RT-MIRA assay’s temperatures were, respectively, set at 33, 35, 37, 39, and 42°C, and the optimal primer-probe combination was used to detect the AHSV-positive RNA template with the real-time RT-MIRA kits. As shown in Figure 1D, although the amplification efficiency was higher at 42 and 39°C, their amplification curves were on an evident upward trend after the plateau. The fluorescence increment was the largest at 35°C, and its amplification curve was a typical S-shaped curve. Therefore, the optimal temperature for real-time RT-MIRA was at 35°C.
3.3. Workflow of the Real-Time RT-MIRA for AHSV
Through the above experiments, a rapid nucleic acid detection method for AHSV based on real-time RT-MIRA was established. Its workflow was as follows: First, a mixture of 29.4 μL Buffer A, 2 μL upstream primer (AHSV-S7-4 F, Table 1; final concentration 400 nM), 2 μL downstream primer (AHSV-S7-4R, Table 1; final concentration 400 nM), 0.6 μL exo-probe (Exo-probe1, Table 1; final concentration 120 nM), and 12.5 μL nuclear-free water was added into each reaction tube containing freeze-dried powder. Then, 1 μL of the nucleic acid samples were added into the reaction tubes, respectively, and the positive and negative controls were added in parallel. 2.5 μL Buffer B was added in the last step, and the reaction tubes were quickly turned upside down 5–6 times and rapidly centrifuged, then immediately incubated at 35°C for 20 min in a fluorescence detection device. The fluorescence signals were collected every 30 s with the FAM channel. In resource-limited diagnostic laboratories or outdoors, the amplification reaction could be performed in a portable warm bath device at 35°C or held by hand for 20 min, and the results were judged under a portable blue light device (480 nm).
3.4. Specificity of the Real-Time RT-MIRA for AHSV
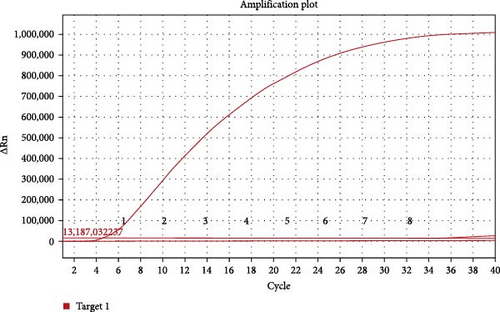
For the specificity analysis, the RNA of other important viruses for equines or other Orbiviruse, including PRV, EIAV, EAV, EIV, BTV, and EHDV, were also tested by the real-time RT-MIRA method. The results showed that only AHSV RNA was positive and the other viruses were negative control were negative (Figure 2B). Besides, under the blue light (480 nm), only the amplification product of AHSV RNA were observed with strong green flourescence (Figure 2A). These results indicate that the developed real-time RT-MIRA method was specific for AHSV detection and had no cross-reactivity with other important equine viruses or other Orbivirus such as BTV and EHDV.
3.5. Sensitivity and Repeatability of the Real-Time RT-MIRA for AHSV
For evaluating the analytical sensitivity of the real-time RT-MIRA, the tenfold serially diluted AHSV RNA templates (1 × 107 to 1 × 100 copies/μL) were tested by the developed method and the WOAH recommended RT-qPCR for AHSV. Individual concentrations of the serial-diluted AHSV RNA were tested in quintuplicate. The sensitivity results of these two assays were shown in Figures 3 and 4. The results demonstrated that the limit of detection (LOD) of the real-time RT-MIRA assay was 10 copies/μL, which was consistent with that of RT-qPCR. Moreover, the real-time RT-MIRA amplification products of 1 × 107 to 1 × 101 copies/μL were observed obvious fluorescence at 480 nm blue light (Figure 3A). Furthermore, the real-time RT-MIRA for AHSV performed good repeatability as shown from the Ct values of five times repeated detection for individual diluted concentrations of the AHSV RNA template (Table 2).

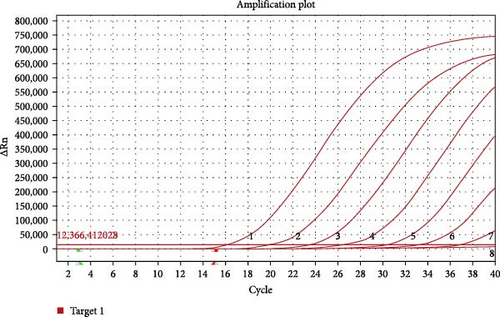
| Initial concentration of quantified AHSV RNA | RT-MIRA | Ct mean ± SD | RT-qPCR | Ct mean ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ct1 | Ct2 | Ct3 | Ct4 | Ct5 | Ct1 | Ct2 | Ct3 | Ct4 | Ct5 | |||
| 1 × 107 | 5.18 | 5.24 | 5.73 | 5.92 | 5.74 | 5.56 ± 0.30 | 15.34 | 15.51 | 15.32 | 15.03 | 15.11 | 15.26 ± 0.17 |
| 1 × 106 | 7.90 | 7.66 | 8.09 | 8.31 | 8.11 | 8.01 ± 0.22 | 19.89 | 19.88 | 19.52 | 19.92 | 19.84 | 19.81 ± 0.15 |
| 1 × 105 | 12.80 | 12.97 | 12.02 | 12.66 | 12.76 | 12.64 ± 0.32 | 23.28 | 23.89 | 23.58 | 23.59 | 23.94 | 23.66 ± 0.24 |
| 1 × 104 | 14.80 | 14.92 | 15.02 | 15.14 | 14.55 | 14.89 ± 0.20 | 26.62 | 26.68 | 27.08 | 27.02 | 26.97 | 26.87 ± 0.19 |
| 1 × 103 | 18.17 | 17.92 | 17.62 | 18.34 | 17.77 | 17.96 ± 0.26 | 29.79 | 30.16 | 30.11 | 30.61 | 30.22 | 30.18 ± 0.26 |
| 1 × 102 | 23.07 | 23.66 | 23.02 | 22.62 | 23.42 | 23.16 ± 0.36 | 33.20 | 34.08 | 33.93 | 33.15 | 34.23 | 33.72 ± 0.45 |
| 1 × 101 | 26.32 | 25.51 | 25.34 | 29.13 | 28.84 | 27.03 ± 1.64 | 37.65 | 36.81 | 36.06 | 35.21 | 35.62 | 36.27 ± 0.87 |
| 1 × 100 | — | — | — | — | — | — | — | — | — | — | — | — |
- Note: “—” represents the unidentified value or invalid calculation result.
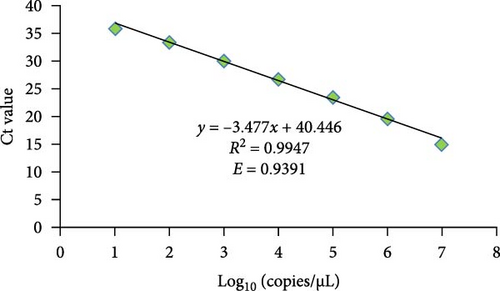
In the real-time RT-MIRA, its standard curve had good linearity (y = −3.5717x + 29.894) with an R2 = 0.9898, the efficiency of the assay was 90.54% obtained by its slope (Figure 3C). The standard curve of RT-qPCR also had good linearity (y = −3.477x + 40.446) with an R2 = 0.9947, the efficiency of the RT-qPCR was 93.91% based on the slope of the standard curve (Figure 4B). Linear regression analysis indicated that the established real-time RT-MIRA could be applied for the quantitative detection of ASHV.
3.6. Compliance Testing for Simulated Samples
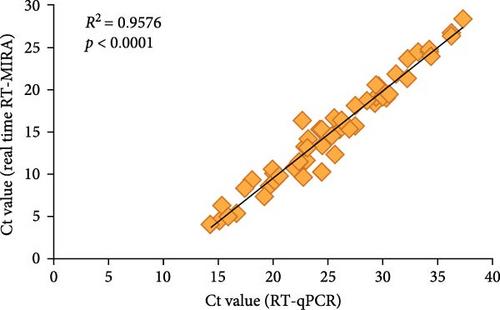
For evaluating the performance of the developed real-time RT-MIRA for AHSV, 120 simulated samples were collected and simultaneously tested by the established method and RT-qPCR. Table 3 showed the detection results of these two methods, which demonstrated that the overall agreement between the real-time RT-MIRA and RT-qPCR detecting the simulated samples was 98.33% (118/120). Compared with the RT-qPCR, the sensitivity, specificity, PPV, and FPV of the established method were 96.30% (52/[52 + 2]), 100% (66/[0 + 66]), 100% (52/[52 + 0]), and 97.06% (66/[2 + 66]), respectively. Furthermore, the statistical analyses indicated that there wes a good correlation between the two assays, with an R2 = 0.9576 (Figure 5) and a κ = 0.966 (p < 0.001). The results of statistical analysis indicated that the established real-time RT-MIRA had a high level of consistency with the RT-qPCR. Beside, the overall agreement between the real-time RT-MIRA visual observation results and RT-qPCR detecting the simulated samples was 95.00% (114/120) and its κ value was 0.898 (p < 0.001), indicating that the real-time RT-MIRA assay could be used for the visual detection for AHSV in outdoor.
| Method | RT-qPCR | κ | p-Value | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | ||||
| Real-time RT-MIRA (real-time fluorescence readout) | Positive | 52 | 0 | 52 | 0.966 | <0.001 |
| Negative | 2 | 66 | 68 | — | — | |
| Total | 54 | 66 | 120 | — | — | |
| Real-time RT-MIRA (blue light instrument) | Positive | 48 | 0 | 48 | 0.898 | <0.001 |
| Negative | 6 | 66 | 72 | — | — | |
| Total | 54 | 66 | 120 | — | — | |
3.7. Testing for Field Samples and Culicoides Samples
There were 396 field samples, including 256 horse blood samples and 140 donkey blood samples collected from Yunnan Province, Guangdong Province, and Hebei Province in China, which were tested in parallel with the established real-time RT-MIRA method and the RT-qPCR for AHSV. The results showed that all field samples were negative for AHSV (Table 4).
| The sources of samples | Horse blood | Donkey blood |
|---|---|---|
| Hebei province | 83 | 65 |
| Yunnan province | 121 | 32 |
| Guangdong province | 52 | 43 |
| Results of real time RT-MIRA | Negative | Negative |
| Results of RT-qPCR | Negative | Negative |
- Abbreviation: RT-MIRA, reverse transcription multienzyme isothermal rapid amplification.
From June to September 2023, more than 1760 culicoides were collected from four sites (Guanming Farm, Safari Park, Xianhu Botanical Garden, and Mangrove Coastal Ecological Park) in Shenzhen of China, and the morphological identification showed that there were seven Culicoides species, including Culicoides homotomus, Culicoides actoni, Culicoides arakawai, Culicoides circumbasalis, Culicoides oxystoma, Culicoides similis, and Culicoides peliliouensis. However, 30 Culicoides were not identified due to incomplete morphology. Total RNA was extracted from 20 to 30 Culicoides of the same species and detected by the developed assay and RT-qPCR. As shown in Table 5, neither RT-MIRA nor RT-qPCR detected AHSV nucleic acid in these Culicoides (Table 5).
| Species | Total number | Collection site | Results of real time RT-MIRA | Results of RT-qPCR |
|---|---|---|---|---|
| Culicoides homotomus | 126 | 1, 2, 3 | Negative | Negative |
| Culicoides actoni | 170 | 2, 3 | Negative | Negative |
| Culicoides arakawai | 482 | 1, 2, 3, 4 | Negative | Negative |
| Culicoides circumbasalis | 282 | 1, 2, 3, 4 | Negative | Negative |
| Culicoides oxystoma | 357 | 1, 2, 3 | Negative | Negative |
| Culicoides similis | 230 | 1, 2, 4 | Negative | Negative |
| Culicoides peliliouensis | 83 | 2, 4 | Negative | Negative |
| Uncertain species | 30 | 1, 2, 3, 4 | Negative | Negative |
- Note: 1: Guanming Farm, 2: Safari Park, 3: Wutong Mountain Forest Park, 4: Mangrove Coastal Ecological Park.
- Abbreviation: RT-MIRA, reverse transcription multienzyme isothermal rapid amplification.
4. Discussion
AHS is a highly lethal viral infectious disease of equines. Once outbreak, it can cause a large number of naïve horses to die, thus, causing economic and animal welfare disasters. AHS is endemic to Southern African. However, due to a combination of globalization and climate change, the worldwide distribution of Culicoide species in the world has expanded rapidly, which may cause the spread of AHS in the world [39–41]. In February 2020, Thailand experienced the first AHS outbreak. Then in September of the same year, Malaysia reported an ASH outbreak to WOAH. The AHS outbreak in Thailand and Malaysia in 2020 was the second occurrence of the disease in Asia after a gap of 60 years, and also the first appearance of the disease in Southeast Asian countries. The outbreaks in these two countries indicate that AHS is breaking out of its original epidemic areas and spreading to Southeast and East Asia. Meanwhile, due to the humid and warm ecological environment in Southeast and East Asia, there are a large number of Culicoide species, which have a suitable ecological chain for AHS transmission. Furthermore, Southeast and East Asia have large numbers of horses naïved to AHSV. Taking China as an example, the number of horses, donkeys, and mules on hand in 2022 was 5.89 million, none of which have a history of AHS vaccination (https://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0D0N&sj=2022). According to statistics, there are 368 species of Culicoides in China [42], and 103 species of Culicoides are found in Yunnan Province adjacent to Thailand, making it the province with the highest number of Culicoides in China. As one important vector of AHSV, C. Imicola is found in Yunnan, Hainan, and Guangdong provinces of China [37, 43, 44]. In this regard, special attention should be paid to the prevalence of AHS in Thailand and Malaysia, and it is necessary to conduct surveys of AHS transmission vectors, monitoring the presence of AHSV in wild equine animals, and Culicoides in China’s border areas. However, conventional detection techniques such as RT-PCR or real-time RT-PCR are not suitable for rapid screening of AHS in the field. Thus, it is urgent to establish a rapid, visualized, and reliable detection assay for AHSV.
As mentioned earlier, MIRA technology has many superiorities and is quite suitable for field surveys or rapid screening for human or animal epidemic diseases in resource-limited settings. Therefore, in this study, RT-MIRA-specific primers and exo-probe were designed, synthesized, and screened based on the AHSV Seg-7 gene. The optimal specific primers and exo-probe obtained by screening were blasted with the Seg-7 gene sequences of nine serotypes of AHSV, and the results showed that the region was highly conserved (Figure S1), which meant that the specific primers and probe combination were versatile and could be used to detect all nine serotypes of AHSV. In addition, the absence of AHSV in this study and the inability to reflect the RT process by using plasmid as the standard substance, we prepared a section of RNA of the AHSV Seg-7 gene (641–1167 bp) by in T7 vitro transcription, evaluated the established real-time RT-MIRA using this section of RNA as a positive control. The valid test results showed that the established real-time RT-MIRA was highly specific for the detection of AHSV, without exhibiting any cross-reactivity towards other equine viruses or other Orbivirus; the LOD of the established assay was 10 copies/μL, which was consistent with that of RT-qPCR, meaning it had good sensitivity for detecting AHSV. Furthermore, the real-time RT-MIRA for AHSV performed good repeatability, and its standard curve exhibited good linearity with a correlation coefficient of R2 = 0.9898, which indicated that the established method could be used for the quantitative detection of ASHV. As no AHS infection cases have been reported in China, 120 simulated field samples were tested by the real-time RT-MIRA and RT-qPCR for AHSV, which results implied the diagnostic specificity and sensitivity of RT-MIRA were 100% and 96.30%, respectively. Furthermore, there was a significant correlation between the two methods, with a κ value of 0.966 and an R2 value of 0.9576. Additionally, among the 52 positive samples tested by the real-time RT-MIRA, 48 were judged as positive under a blue light instrument.
In the detection of field samples, this study tested 256 horse blood samples and 140 donkey blood samples from Guangdong, Hebei, and Yunnan provinces in China. The results of RT-qPCR and RT-MIRA showed that all samples were negative for AHSV. The survey results of Culicoide midges showed that there is C. Imicola, one of the important vectors for AHSV in Zhongshan City of China [44]. While Shenzhen City, adjacent to Zhongshan City, has the same climate and geographical conditions, and there are lots of horses and wild equine animals imported from overseas, it is necessary to investigate the species of Culicoides in Shenzhen and use the method established in this study to detect whether AHSV is carried in Culicoides. From July to September 2023, 1760 Culicoides were collected from four locations in Shenzhen (Wildlife Park, Xianhu Botanical Garden, Mangrove Coastal Park, and Guangming Farm). There were seven species of Culicoides, but no C. Imicola by morphological identification, which may be related to the investigation location and collection time. Neither RT-MIRA nor RT-qPCR detected AHSV in these Culicoides. The results of the Culicoides survey and detection for ASHV at four sites in Shenzhen for 3 months were not representative. However, in the detection process, the real-time RT-MIRA method showed advantages such as quick, convenience, and visualization, while its detection performance was consistent with RT-qPCR.
5. Conclusion
In this study, a real-time RT-MIRA assay based on ASHV Seg-7 gene was established and evaluated for AHSV detection. Compared with the WOAH recommended RT-qPCR for AHSV, the specificity and sensitivity of this method as good as the RT-qPCR. Moreover, this method has the advantages of fast and easy operation and is suitable for the rapid screening of AHSV. In addition, visualization of the results of real-time RT-MIRA assay makes it possible for an application to monitor the presence of AHSV in wild equine animals and Culicoides in border areas or grassroots farms.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Disclosure
The funders had no role in the design, conduct, and analysis of the study, nor in the writing and submission of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was supported by the National Key Program of Research and Development of China (2021YFD1801203) and the Project of Science and Technology of General Administration of Customs of the People’s Republic of China (2021HK169).
Acknowledgments
This study was supported by the National Key Program of Research and Development of China (2021YFD1801203) and the Project of Science and Technology of General Administration of Customs of the People’s Republic of China (2021HK169).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data supporting the conclusions of this article are provided within the article. Raw data are available from the corresponding author upon request.




