Biochemical Profiling and Antioxidant Potential of Fruit Tissues: A Comparative Study of Citrus Cultivars Indigenous to Northeast India
Abstract
The different fruit tissues of selected Citrus cultivars of northeast India, namely, Citrus macroptera Montr. (hatkora, HK), Citrus limon (Linn.) Burm. f. (Assam lemon, AL), Citrus maxima Burm. (pummelo, PM), Citrus reticulata Blanco (Khasi mandarin, KM), and Citrus sinensis (L.) Osbeck (Valencia, VC), were subjected to biochemical profiling and quantification of antioxidant potential. Among the cultivars, HK had significantly higher total phenolic content in juice, peel, and pulp, while VC seeds and AL whole fruit had higher total phenolics. Total flavonoid content was higher in HK juice, pulp, and peel. VC exhibited the highest flavonoid content in whole fruit, while AL exhibited the highest in seeds. In the DPPH assay, HK (seeds, whole fruit), PM (pulp, juice), and AL (peel) exhibited the highest antioxidant capacity. The ability to scavenge ABTS radicals exhibited higher capacity in VC (pulp, juice, and whole fruit), HK (seeds), and KM (peel) in contrast to other cultivars. In the ferric reducing ability, HK seeds resulted in the highest, whereas VC juice resulted in the lowest ability. Among the different tissues of the Citrus cultivars, seeds exhibited the highest ferric reducing capacity, subsequently followed by peels and whole fruit, whereas juice exhibited the lowest reducing capacity. Among the different Citrus cultivars, HK showed considerably greater FRAP values than other Citrus cultivars studied.
1. Introduction
Citrus (Citrus L.) belongs to the family Rutaceae and is one of the most important fruit crops of the tropical and subtropical regions of the world and is consumed mostly as fresh produce or juice or as canned goods because of its high nutritional value and special flavor. It can also be used in the pharmaceutical and commercial food industries as medicines, food additives, and spices, respectively.
Citrus fruits possess a variety of shapes and sizes, ranging from oblong to circular. Citrus fruits are exceptionally rich in vitamin C [1] and offer numerous health benefits due to their antioxidant, anti-inflammatory, anticancer, and antibacterial properties [2]. Citrus fruits are valued for their polyphenols, especially flavonoids, which include flavanones like naringin and hesperidin [3]. Secondary metabolites present in the fruits, usually referred to as phytochemicals, have pharmacological effects and are very important for cutting-edge research, and numerous phytochemicals are reported to be present in different parts of the Citrus fruits [4]. These phytochemicals possess different types of bioactive compounds which play an important role in maintaining human health and have anti-inflammatory, antioxidative, and anticancer properties [4]. The phytochemicals such as polyphenols, flavonoids, and vitamins present in the Citrus fruit are major groups of antioxidants. Flavonoids offer strong defense against a variety of chronic illnesses because of their anti-inflammatory and antioxidant properties. Numerous reports on the phenolic makeup of several Citrus species from throughout the globe have been reported [5, 6].
A large gene pool of citrus variability exists in northeastern India, sometimes referred to as the “Citrus Belt of the World,” both in wild and semiwild forms [7]. It has been suggested that this region may be the origin of several Citrus species [8]. The evidence of the presence of diverse and unattended natural populations of Citrus gene pool supports this concept. The region is designated as store house of citrus germplasm due to reports of citrus trees thriving in deep forests that are unaffected by abiotic influences. Apart from the commercial species, certain wild and indigenous species of Citrus like Citrus ichangensis, Citrus indica, Citrus latipes, and Citrus macroptera also occur in northeast India. Citrus macroptera Mont., commonly known as hatkora (HK), is one of the important and popular citrus species of northeast India known for its medicinal as well as therapeutic values since ancient time. Fruits are edible and used in culinary preparations and also in making squashes. Fruits are very hard and look like small ball with storage life of about 1–2 months [7]. Fruit is smaller than grapefruit and contains low juice, bitter in taste. Because of its use in traditional medicines and presence of good amount of antioxidants, during the present time, this fruit is gaining popularity worldwide [9]. It is a good source of major groups of phytochemicals such as alkaloids, tannins, phenols, and F which are directly or indirectly responsible for a wide range of health-promoting effects including reducing risk of chronic and degenerative diseases [10]. However, the information on biochemical profiling and antioxidant potential of different fruit tissues of HK and other indigenous Citrus species of northeast India is scanty. Considering these, the present research was intended to compare the phenolic and flavonoid contents and antioxidant capacities in several fruit tissues like peels, pulp, seeds, juice, and whole fruit (WF) from five different indigenous Citrus cultivars of northeast India. The results of the present study will help the breeders to use the elite indigenous Citrus genetic resources for breeding of superior cultivars and promoting citrus fruit as functional foods.
2. Materials and Methods
2.1. Chemicals
Analytical grade reagents were used for estimation of phenolics, flavonoids, antioxidant activity, and other biochemical parameters procured from Sigma-Aldrich, Hi-Media, and Merck. The analysis was performed using Milli-Q ultrapure distilled water.
2.2. Fruit Materials
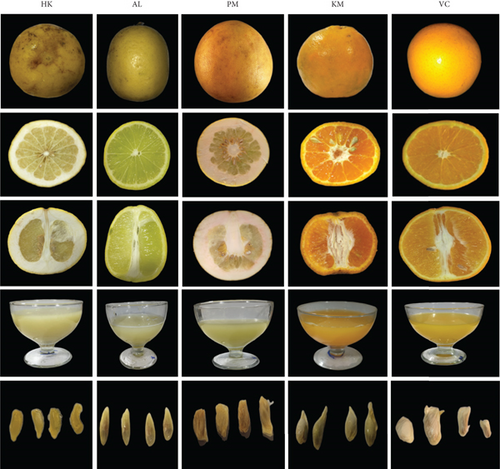
Five indigenous and locally grown Citrus cultivars, namely, HK, Assam lemon (AL), pummelo (PM), Khasi mandarin (KM), and Valencia (VC), belonging to five different citrus species, namely, Citrus macroptera Montr., Citrus limon (Linn.) Burm. f., Citrus reticulata Blanco, Citrus maxima Burm., and Citrus sinensis (L.) Osbeck, were collected from the farmer’s field of Aizawl district, Mizoram, northeast India, during the production year 2022–2023 (Table 1). The fruits were collected at proper stage of marketing based on maturity index. Immediately, it was transported to the postharvest laboratory of Department of Horticulture, Aromatic and Medicinal Plants, Mizoram University, for further analysis (Figure 1). From each cultivar, the fruits were replicated three times and each replication has 10 fruits for analysis of the different parameters. Fruits were separated into different tissues, namely, peel, pulp, juice, seeds, and WF. The tissues from various cultivars were initially dried in the shade at room temperature for approximately 1 month to prevent the loss of bioactive compounds, and afterward, they were ground into a fine powder and stored at −20°C until further examination.
| Scientific name | Cultivars | Abbreviation | TSS (%) | Titratable acidity (%) | Ascorbic acid (mg/100 g) |
|---|---|---|---|---|---|
| Citrus macroptera Montr., f. | Hatkora | HK | 9.2 ± 0.33b | 10.24 ± 1.24a | 61.43 ± 2.66a |
| Citrus limon (Linn.) Burm. | Assam lemon | AL | 5.5 ± 0.48c | 4.08 ± 0.67b | 48.36 ± 3.13b |
| Citrus maxima Burm. | Pummelo | PM | 9.4 ± 0.39b | 1.14 ± 0.17c | 46.70 ± 2.34b |
| Citrus reticulata Blanco | Khasi mandarin | KM | 10.3 ± 0.53a | 0.56 ± 0.08c | 51.02 ± 4.99b |
| Citrus sinensis (L.) Osbeck | Valencia | VC | 10.8 ± 0.38a | 0.69 ± 0.10c | 37.67 ± 1.34c |
| F0.05 | 71.49 | 857.195 | 22.418 | ||
| p0.05 | < 0.001 | < 0.001 | < 0.001 | ||
- Note: Data are expressed as mean ± standard error of triplicate sample. Values in columns followed by different letters (e.g., a, b, and c) are significantly different at p < 0.05. The reported F values and p values are results of one-way ANOVA.
2.3. Determination of Total Soluble Solid (TSS), Titratable Acidity (TA), Ascorbic Acid, and Sugars
To estimate TSS, TA, ascorbic acid, total sugars (TSs), reducing sugars (RSs), and nonreducing sugars (NRSs), juice samples were obtained from 10 different fruits for each replication, and triplicates were employed. For estimation of TSS, Thermocare Erna handheld refractometer (A-Contrast model 0-32) was used and the results were given in °Brix. Estimation of the TA, RS, NRS, and TS of the fruit juice followed the standard technique [11] reported as a percentage. The TA was expressed in citric acid equivalent. To calculate ascorbic acid, sodium salt (2,6-dichlorophenol-indophenol) was used as a redox dye as described by Ranganna [12].
2.4. Extraction of Phenolic Compounds
The phenolic compounds were extracted by following the methods of Ramful et al. [13]. Briefly, 1 g of fruit tissue powder was dissolved in 80% methanol and dimethyl sulfoxide (DMSO) at a 1:1 ratio (v/v). After 12 h of shaking, it was centrifuged at 4°C for 10 min at the speed of 3000 rpm. Methanol (80%) was used to wash the residue twice. The supernatants were pooled and were dissolved up to 50 mL using methanol. Simultaneously, it was preserved at −20°C until subsequent use for analysis.
2.5. Quantification of Total Phenolic and Total Flavonoid Content
The Folin–Ciocalteu (FC) method was used for the estimation of total phenolic content of the fruits according to Xu et al. [14]. Pooled supernatant solution (1 μL) was added to 3-mL Milli-Q water and FC reagent (0.5 mL) and incubated for about 3 min. Further, 2 mL of 20% sodium carbonate (Na2CO3) was mixed and uniformly blended. Absorbance was taken at λ = 650 nm, following incubation for 1 h at room temperature using a Bio-Tek Epoch microplate spectrophotometer (Agilent Technologies, India). The total phenolic content was measured and reported as the mean of triplicate samples in gallic acid equivalent (GAE) per milligram per gram of fresh weight (mg/g FW).
The method defined by Wang et al. [15] was used to quantify the total flavonoid contents. In a Soxhlet extractor, 2.5 g of ground sample was taken and subjected to extraction with methanol (85°C for 12 h). A rotary evaporator was used to desiccate the resulting extract, maintaining a temperature below 4°C. Subsequently, the dried extract was reconstituted using methanol. Further, the extract (1 mL) and 5% sodium nitrate solution (0.3 mL) were added with it and further incubation was done at ambient temperature for about 6 min. Another 6-min incubation was done by adding 10% aluminum nitrate (0.3 mL); subsequently, 1 N sodium hydroxide (4 mL) was added. Finally subjected for 15-min incubation and immediately reading was noted at 510 nm wavelength and the results were expressed in rutin equivalent (RE) as milligram/gram DW.
2.6. Antioxidant Capacity Assays
To quantify the antioxidant capacity of different fruit tissues of the selected Citrus fruits, different antioxidant assays, namely, 1,1-diphenyl-2-picrylhydrazyl (DPPH), Trolox equivalent antioxidant capacity (TEAC), and ferric ion reducing antioxidant power (FRAP) assays, were used. The DPPH assay was executed according to the radical scavenging activity of DPPH using methods reported by Barreca et al. [16]. After taking 50 μL of the sample, 63 μM DPPH solution was added, and methanol was used to bring the total volume to 4.0 mL. Further, it was incubated in a dark room for 25 min, and reading was taken at 517-nm wavelength. The results were expressed as percentage inhibition of scavenging ability of free radicals.
The TEAC assay relies on the ability to scavenge 2,2 ′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical resulting into a decolorized product. According to the procedure reported by Barreca et al. [16], 5 mL of 7 mM ABTS solution was mixed with 88 μL of potassium per sulfate solution (140 mM) and incubated in dark at room temperature for 14 h. The reading was taken at λ = 734 nm wavelength.
Jang et al. [17] method was followed for estimation of FRAP assay. For this, in 20-μL extract of the fruit, 1.8 mL of Milli-Q water was added, with FRAP reagent (1.8 mL). Subsequently, 30-min incubation was done, and immediately, the absorbance was taken at λ = 593 nm wavelength. For standard calibration curve, iron(II) sulfate heptahydrate (0–5 mM) was used and the reducing ability was expressed as millimolar. The estimation was done thrice and expressed as mean value. The DPPH, TEAC, and FRAP were expressed as Trolox equivalent millimole/gram.
2.7. Statistical Analysis
Analysis of variance (ANOVA) was performed by OPSTAT software, followed by Duncan’s multiple range test (DMRT) at 95% confidence level. Each and every data are shown as the average ± standard error of triplicates. PAST 4.0 software was used to create Pearson’s correlation for various biochemical characters (phenolics, flavonoids, and antioxidants) (DPPH, ABTS, and FRAP) across five distinct fruit parts (peel, pulp, juice, seed, and WF).
3. Results and Discussion
3.1. TSS, TA, and Ascorbic Acid Content
Table 1 depicts the TSS and TA values of the selected Citrus cultivars analyzed. The TSS of fully ripened fruits was in the range of 5.50%–10.80%. VC exhibited the highest TSS (10.8% ± 0.39%), followed by KM (10.3% ± 0.53%) and PM (9.4% ± 0.39%). The lowest TSS was exhibited in AL (5.5% ± 0.48%). The acidity of all the fruits tissues examined ranged 0.56 ± 0.08 to 10.24% ± 1.24%. The juice of HK was the most acidic (10.24% ± 1.24%) while the juice of KM was the least acidic (0.56% ± 0.08%). AL, PM, and VC exhibited 4.08% ± 0.67%, 1.14% ± 0.17%, and 0.69% ± 0.10% of TA, respectively. The ascorbic acid of the different fruits varied between 37.67 and 61.43 mg/100 g. The juice of HK had the highest ascorbic acid (61.43 ± 2.66 mg/100 g). It was followed by juice of KM (51.02 ± 4.99 mg/100 g), AL (48.36 ± 3.13 mg/100 g), and PM (46.70 ± 2.34 mg/100 g), while VC contained the lowest ascorbic acid (37.67 ± 1.34 mg/100 g).
TSS is pivotal for the sweetness and flavor of fruits, enhancing their taste and indicating ripeness, while also influencing attributes such as texture, juiciness, and shelf life. TSS levels are typically higher in WFs compared to their juices due to the presence of pulp, fiber, and other solid components, resulting in a more concentrated solution of sugars, acids, and soluble substances in fruit form [3]. Organic acids are widely recognized as crucial factors influencing fruit quality; these compounds not only shape the fruit’s flavor but also impact its aging process, storage capabilities, and storage potential but its high concentration or low pH value typically signifies a longer storage lifespan [18]. TA, a valuable characteristic in fruits, enhances their sensory appeal and shelf life by providing tartness that enriches flavor and acts as a natural preservative, inhibiting spoilage microorganisms. It is an indicative phenomenon in several fruits that an increase in TSS level often corresponds to decrease the acidity level. The variability in TA within genotypes may be closely linked to a combination of the TSS content and the hereditary characteristics of the plant. In the process of selecting superior genotypes, breeders should place a significant emphasis on the TSS% of the fruit. Jamil et al. [19] also reported variations in TSS among different Citrus species such as oranges, limes, and lemons. Vitamin C acts as a potent antioxidant in fruits, bolstering immune function, shielding cells from oxidative stress, promoting collagen synthesis for healthy skin, and aiding iron absorption, thereby supporting overall vitality and cardiovascular health [3]. Citrus are well known to be a nutrient source of vitamin C in dietary intake [20]. Ramful et al. [13] also noted a noteworthy difference in the ascorbic acid levels among different Citrus species. In line with Martí et al. [21], the concentration of ascorbic acid in citrus fruits is affected by both the particular species and the individual segments of the fruit, which is correlated with the current study. As shown in Table 1, differences in chemical composition can be seen in the varieties studied and can be attributed to genetic and physiological factors. In fact, Lee and Kader [22] reported that the selection of genotype with high amounts of bioactive compounds (as vitamin C) was a more important factor than climatic conditions and cultural practices.
3.2. TSs, RSs, and NRSs
Table 2 depicts the TS, RS, and NRS contents of the fruits of various Citrus cultivars. The levels of TS in the ripe fruits were found to range between 2.04% and 8.77%. PM juice had the highest TS value (8.77% ± 1.05%) followed by VC (8.11% ± 0.16%), KM (6.59% ± 0.11%), and AL (5.10% ± 0.42%). Similarly, the highest RS was found in PM (5.24% ± 0.27%) followed by KM (3.12% ± 0.64%), AL (2.67% ± 0.44%), and VC (1.78% ± 0.15%). The lowest RS among the five species was found in HK (1.11% ± 0.44%). In NRS, VC (6.01% ± 0.03%) exhibited the highest value followed by PM (3.35% ± 1.04%), KM (3.30% ± 0.71%), and AL (2.31% ± 0.50%), whereas HK (0.88% ± 0.09%) exhibited the lowest amount of NRSs.
| Scientific name | Cultivars | Total sugars (%) | Reducing sugars (%) | Nonreducing sugars (%) |
|---|---|---|---|---|
| Citrus macroptera Montr., f. | HK | 2.04 ± 0.35d | 1.11 ± 0.44c | 0.88 ± 0.09c |
| Citrus limon (Linn.) Burm. | AL | 5.10 ± 0.42c | 2.67 ± 0.44b | 2.31 ± 0.50b |
| Citrus maxima Burm. | PM | 8.77 ± 1.05a | 5.24 ± 0.27a | 3.35 ± 1.04b |
| Citrus reticulata Blanco | KM | 6.59 ± 0.11b | 3.12 ± 0.64b | 3.30 ± 0.71b |
| Citrus sinensis (L.) Osbeck | VC | 8.11 ± 0.16a | 1.78 ± 0.15c | 6.01 ± 0.03a |
| F0.05 | 75.068 | 42.053 | 28.699 | |
| p0.05 | < 0.001 | < 0.001 | < 0.001 | |
- Note: Data are expressed as mean ± standard error of triplicate sample. Values in columns followed by different letters (e.g., a, b, and c) are significantly different at p < 0.05. The reported F values and p values are results of one-way ANOVA.
3.3. Total Phenolic and Total Flavonoid Contents
Phenols serve as effective anti-inflammatory agents and antioxidants, safeguarding cells from oxidative harm, enhancing the color, flavor, and aroma of fruits, thereby enriching their sensory appeal and promoting overall health [3]. Phenolic compounds serve as chemopreventive agents that boost defense mechanisms against oxidative stress of the body. Their potent antioxidant characteristics allow them to counteract chemical toxins, unbound radicals, and environmental pollutants (carcinogens), therefore playing a crucial role in preventing damage to genetic material [23]. As detailed in Table 3, the comprehensive assessment of total phenolic content demonstrated significant variability across various anatomical fruit tissues of different Citrus cultivars, exhibiting a broad spectrum in the peel (3.29–5.58 mg/g FW), pulp (2.83–5.01 mg/g FW), juice (0.33–0.63 mg/g FW), seeds (2.08–3.58 mg/g FW), and WF (2.43–3.62 mg/g FW). Peels exhibited the highest total phenolic content, followed by pulp, seeds, and WF, with juice exhibiting the least content. Vallée Marcotte et al. [24] suggested that the low phenolic in juices might be due to the fact that polyphenols can be lost in part during juice processing, mostly due to the oxidative conditions of pulping, pressing, and clarification. In the analysis of the various Citrus cultivars, it was evident that for the peel, pulp, and juice, the HK exhibited notably higher phenolic content among the cultivars studied. Additionally, in the case of seeds, the VC exhibited a greater total phenolic content compared to the other cultivars. Concerning the WF, the AL stood out with significantly greater total phenolic content in contrast to other cultivars. The results of the present investigation are in close conformity with the research of Ramful et al. [13], who reported a wide range of phenolics (188.2–776.7 mg GAE/100 g FW) in the peel of the 21 analyzed Citrus varieties. Li et al. [25] found in their research that grapefruit peels exhibit a greater total phenolic content when compared to other cultivars (mandarin, Yen Ben lemon, Meyer lemon, and orange). Furthermore, the presence of elevated phenolic compounds in Citrus fruit peels demonstrates protection for fruits from UV radiation, pathogens, and potential pests. In line with Barros et al. [26], the higher concentration of phenolics present in peels can be attributed to their location as the fruit’s exterior, making them highly favorable for the synthesis of phenolic compounds. This observation is corroborated by several studies, including those conducted by Xi et al. [27], Barros et al. [26], and Buyukkurt et al. [28], all of which consistently demonstrated the prevalence of higher phenolic levels in Citrus peels in comparison to the pulp.
| Scientific name | Cultivars | Peel | Pulp | Juice | Seed | Whole fruit |
|---|---|---|---|---|---|---|
| Citrus macroptera Montr., f. | HK | 5.58 ± 1.12a | 5.01 ± 0.77a | 0.63 ± 0.09a | 2.08 ± 0.2a | 2.97 ± 0.10bc |
| Citrus limon (Linn.) Burm. | AL | 4.21 ± 0.28bc | 3.18 ± 0.34bc | 0.33 ± 0.04d | 3.3 ± 0.14a | 3.62 ± 0.40a |
| Citrus maxima Burm. | PM | 4.98 ± 0.53ab | 3.9 ± 0.32b | 0.45 ± 0.04bc | 2.74 ± 0.49a | 2.72 ± 0.11cd |
| Citrus reticulata Blanco | KM | 4.26 ± 0.34bc | 4.73 ± 0.35a | 0.53 ± 0.04b | 3.06 ± 0.12a | 3.34 ± 0.18ab |
| Citrus sinensis (L.) Osbeck | VC | 3.29 ± 0.30c | 2.83 ± 0.25c | 0.37 ± 0.05cd | 3.58 ± 0.34a | 2.43 ± 0.27d |
| F0.05 | 6.176 | 13.609 | 15.866 | 3.169 | 11.902 | |
| p0.05 | 0.009 | < 0.001 | < 0.001 | 0.063 | 0.001 | |
- Note: Data are expressed as mean ± standard error of triplicate sample. Values in columns followed by different letters (e.g., a, b, and c) are significantly different at p < 0.05. The reported F values and p values are results of one-way ANOVA.
Li et al. [25] reported that the high content of phenolic compounds in the peels has been shown to protect the fruits and vegetable against UV light, pathogens, and predators. According to Barros et al. [26], peels contain higher concentrations of these compounds because they are the outer part of the fruit and thus are more predisposed to the synthesis of phenolic compounds. Therefore, Citrus peels as a source of valuable phenolic compounds can be used in food products as active ingredients or even as replacements for synthetic preservatives, such as butylated hydroxyanisole and butylated hydroxytoluene [29], which improves the health-promoting value of the products.
Our results are in the line of conformity with the findings of Mediterranean Citrus cultivars reported by Gorinstein et al. [30] and Abeysinghe et al. [31] where they reported the presence of higher phenolic compounds in fruit peel than other segments. Because of its high phenolic content, the fruits are being used in traditional medicines of Mizo society. Traditionally, HK has a good reputation for remedies for abdominal pains, hypertension, flu, fever, and diarrhea in infants [32].
Flavonoids, classified as a widely distributed category of polyphenolic compounds, are often referred to as “nutraceutical substances” because of their demonstrated anticancer, antimicrobial, and anti-inflammatory properties. Citrus plants contain a wide range of flavonoid constituents, some of which are characteristic of them [33]. In this way, there are several flavanone glycosides specific to Citrus, and some flavanones from orange juices have been reported as markers to differentiate Citrus varieties [20]. The total flavonoid content among various fruit tissues and cultivars in the present study exhibited significant differences. There existed a broad spectrum of variation in flavonoid contents of different fruit tissues, namely, peel (3.02–7.07 mg/g FW), pulp (3.24–6.07 mg/g FW), juice (0.22–0.46 mg/g FW), seeds (18.49–26.22 mg/g FW), and WF (4.74–9.03 mg/g FW) among the five Citrus cultivar studies as depicted in Table 4. Among the various fruit tissues analyzed, the seeds exhibited the highest flavonoid content, followed by WFs, pulps, and peels, while the juice has the lowest. Among the cultivars, the HK juice, pulp, and peel exhibited higher flavonoid content among the cultivars analyzed. The flavonoid content was highest in WF of VC, whereas in the seeds, AL exhibited maximum content than other Citrus cultivars analyzed. Our study is in the line of conformity with the studies of Tripoli et al. [34] where they reported that Citrus peel and seeds are very rich in phenolic compounds, such as phenolic acids and flavonoids. In contrast to our study, it was reported that the peels are richer in flavonoids than the seeds [35]. Since Citrus fruits are consumed after removal of peels and the peel and seeds are not used, it is necessary to estimate these by-products as natural antioxidants in foods [36]. Our findings correspond with earlier investigation by Lalruatsangi et al. [10], who revealed that HK has a higher flavonoid value than other Citrus species, indicating that it is a powerful antioxidant.
| Scientific name | Cultivars | Peel | Pulp | Juice | Seed | Whole fruit |
|---|---|---|---|---|---|---|
| Citrus macroptera Montr., f. | HK | 7.07 ± 0.45a | 6.07 ± 0.29a | 0.46 ± 0.02a | 18.49 ± 1.03d | 5.11 ± 0.40d |
| Citrus limon (Linn.) Burm. | AL | 6.16 ± 0.77b | 4.34 ± 0.54b | 0.36 ± 0.05b | 26.22 ± 0.78a | 8.18 ± 0.40b |
| Citrus maxima Burm. | PM | 4.91 ± 0.34c | 3.92 ± 0.21bc | 0.28 ± 0.02cd | 20.45 ± 0.99c | 4.74 ± 0.50d |
| Citrus reticulata Blanco | KM | 4.57 ± 0.39c | 5.57 ± 0.14a | 0.31 ± 0.03bc | 21.24 ± 0.83c | 5.85 ± 0.33c |
| Citrus sinensis (L.) Osbeck | VC | 3.02 ± 0.13d | 3.24 ± 0.63c | 0.22 ± 0.03d | 24.12 ± 0.94b | 9.03 ± 0.38a |
| F0.05 | 24.155 | 24.456 | 33.526 | 66.675 | 33.358 | |
| p0.05 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
- Note: Data are expressed as mean ± standard error of triplicate sample. Values in columns followed by different letters (e.g., a, b, and c) are significantly different at p < 0.05. The reported F values and p values are results of one-way ANOVA.
3.4. Antioxidant Capacity
Antioxidant potentials are demonstrated by the capacity to lower the quantity of free radicals produced during oxidation reactions. For evaluation of antioxidant capabilities of different Citrus fruit tissues, namely, peel, pulp, juice, seeds, and WF, three types of radical assays were used, namely, DPPH, TEAC, and FRAP (Table 5).
| Scientific name | Cultivar | Peel | Pulp | Juice | Seed | Whole fruit | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | ||
| Citrus macroptera Montr., f. | HK | 507.94 ± 9.67a | 178.17 ± 14.51b | 116.13 ± 7.05a | 22.37 ± 2.97c | 147.11 ± 8.70b | 95.27 ± 2.35a | 14.37 ± 1.40b | 110.60 ± 6.03a | 71.94 ± 4.02a | 314.61 ± 16.92a | 205.26 ± 7.10a | 132.18 ± 12.37a | 242.37 ± 5.75a | 132.80 ± 4.19c | 97.60 ± 6.87a |
| Citrus limon (Linn.) Burm. | AL | 446.33 ± 6.42b | 168.86 ± 4.15b | 112.43 ± 3.36a | 16.19 ± 1.81d | 98.43 ± 6.36c | 67.60 ± 7.83b | 12.72 ± 2.74b | 77.28 ± 10.66d | 54.26 ± 2.21b | 254.33 ± 8.41b | 181.92 ± 6.02b | 125.45 ± 8.84a | 221.58 ± 1.77b | 119.09 ± 6.21c | 95.26 ± 6.23a |
| Citrus maxima Burm. | PM | 125.26 ± 3.57d | 96.49 ± 9.41d | 91.66 ± 2.22b | 293.41 ± 3.67a | 116.86 ± 16.16c | 56.62 ± 2.46c | 18.31 ± 1.33a | 97.95 ± 2.88bc | 44.62 ± 1.73c | 161.93 ± 3.41c | 107.04 ± 6.88d | 101.53 ± 3.49b | 217.12 ± 2.95b | 102.66 ± 6.68d | 51.62 ± 3.71c |
| Citrus reticulata Blanco | KM | 433.2 ± 10.35c | 224.73 ± 10.05a | 42.77 ± 1.53c | 17.24 ± 2.16d | 139.38 ± 10.27b | 46.07 ± 2.24d | 11.79 ± 2.03bc | 93.74 ± 9.66c | 38.41 ± 5.40d | 120.36 ± 7.70d | 113.74 ± 9.98d | 86.38 ± 5.07c | 139.81 ± 15.52c | 177.10 ± 15.07b | 77.74 ± 14.31b |
| Citrus sinensis (L.) Osbeck | VC | 76.06 ± 1.77e | 142.71 ± 6.16c | 37.06 ± 1.64c | 96.02 ± 2.98b | 246.51 ± 7.17a | 35.61 ± 1.57e | 8.89 ± 0.20c | 121.93 ± 9.44a | 25.28 ± 2.02e | 55.75 ± 1.52e | 162.58 ± 8.83c | 75.74 ± 6.40d | 64.72 ± 4.52d | 198.73 ± 3.68a | 62.28 ± 5.70c |
| F0.05 | 3504.04 | 73.365 | 302.649 | 5431.66 | 92.615 | 98.05 | 11.787 | 12.682 | 80.336 | 374.523 | 87.962 | 28.639 | 267.792 | 71.678 | 18.021 | |
| p0.05 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.001 | 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
- Note: Data are expressed as mean ± standard error of triplicate sample. Values in columns followed by different letters (e.g., a, b, and c) are significantly different at p < 0.05. The reported F values and p values are results of one-way ANOVA.
DPPH is a stable free radical that accepts an electron or hydrogen radical to produce a stable molecule that is diamagnetic. This assay is employed for scavenging free radicals in the presence of an antioxidant to evaluate the ability of DPPH, widely acknowledged as one of the most popular and straightforward colorimetric techniques for evaluating antioxidant capabilities [37]. In our study, DPPH antioxidant capacity varied significantly among the various Citrus cultivars analyzed from 76.06 to 433.20 mM TE/g in peel, 16.19–293.41 mM TE/g in pulp, 8.89–18.31 mM TE/g in juice, 55.75–314.61 mM TE/g in seeds, and 64.72–242.37 mM TE/g in WF (Table 5). For seeds and WF, HK had the highest DPPH values; for pulp and juice, PM had the highest, whereas for peel, AL exhibited the highest DPPH values compared to other cultivars analyzed.
The TEAC assay, also popularly recognized as ABTS assay, is an ideal technique to evaluate the activity of antioxidants that break chains, like scavenging lipid peroxyl radicals and antioxidants that donate hydrogen, such scavenging aqueous phase radicals. The TEAC approach is based on a sample’s ability to neutralize the ABTS radical cation in comparison to Trolox, a standard antioxidant; additionally, it is often employed for investigating the antioxidant capability of the plant [38]. TEAC value varied significantly among the fruit tissues analyzed, namely, in peel (96.49–224.73 mM), pulp (98.43–246.51 mM), juice (77.28–121.93 mM), seeds (107.04–205.26 mM), and WFs (102.66–198.73 mM), in the five Citrus cultivars tested as depicted in Table 5. The results revealed that VC pulp exhibited the highest TEAC value, while AL juice showed the lowest TEAC value. Among the five different Citrus cultivars analyzed, WF and seeds exhibited noticeably higher TEAC values than peels, pulps, and juice among various fruit tissues examined. Compared to other cultivars, pulp, juice, and WF of VC exhibited higher TEAC value whereas, in tissue specific examination, HK seeds had the highest, followed by KM peel as among the examined cultivars. The variation in TEAC among the different cultivars might also be due to the differences in genetic constituents of the cultivars. In addition, environmental factors including soil, climate, and altitude might also influence the phytochemical content and accumulation of bioactive compounds in Citrus fruits which ultimately affect the antioxidant capacity of the cultivars.
In order to determine the antioxidants in foods, FRAP is a straightforward and repeatable technique for determining antioxidant capability [39]. Table 5 depicts the FRAP values among the Citrus cultivars studied, noticeably varied in all the examined fruit tissues, namely, peel (37.06–116.13 mM), pulp (35.61–95.27 mM), juice (25.28–71.94 mM), seeds (75.74–132.18 mM), and WF (51.62–97.60 mM). Among the cultivars, HK seeds exhibited maximum FRAP values, whereas VC juice exhibited minimum FRAP value. The FRAP values of the examined fruit tissues of Citrus cultivars were highest for the seeds, followed by peels and WF tissues. Compared to other cultivars evaluated, all the fruit tissues (peel, pulp, juice, seeds, and WF) of HK exhibited much greater FRAP values.
Antioxidants fight the etiology of major illnesses including cancer, diabetes, and arthritis by scavenging the damaging free radicals in the body. Citrus fruits are an abundant source of antioxidant activity, including vitamin C, hesperidin, and naringin [40]. Moreover, the combined actions of several antioxidant classes, such as F and carotenoids, may potentially be accountable for the effects of antioxidants. According to Reber et al. [41], the individual composition of the extract as well as the antagonistic or synergistic relationships between various bioactive compounds may contribute to the variability in the antioxidant capabilities of different genotypes.
Despite the fact that the DPPH, TEAC, and FRAP assays evaluated the antioxidant capability of plant extracts using various methods, the results of the same fruit tissues obtained are essentially consistent with each other. HK peels exhibited maximum antioxidant activity among the Citrus cultivars examined, followed by AL peels and KM peels. The findings presented herein align with the conclusions drawn by Lalruatsangi et al. [10], who indicated that HK serves as a highly effective source of antioxidants.
Among the different fruit tissues, peel had the highest antioxidant values, succeeded by seeds and WF, whereas juice exhibited the least antioxidant values. Phenolic compounds play a significant role in the antioxidant capacities of date fruits [42, 43]. This parallel ranking of bioactive compounds (phenols, flavonoids) and antioxidant properties observed in the peel, pulp, and juice of 42 Citrus species by Nogata et al. [33] indicates that flavonoids have a significant function in determining antioxidant levels. Our study findings corroborate the results of prior research by Xu et al. [14], who reported that in lemon, in comparison to other parts of the fruits, juice had the lowest activity of FRAP. Likewise, the WFs of the five Citrus cultivars examined in this study achieved a greater ability to neutralize DPPH radicals than their pulp. The findings aligned with the investigations conducted by Barros et al. [26] and Czech et al. [44].
In the current investigation, peels exhibited the highest DPPH antioxidant activity, followed by seeds, pulp, WF, and juice. Citrus seeds are an excellent source of antioxidants since pulp, peel, WF, and juice exhibited lower TEAC and FRAP antioxidant activity than seeds. This difference in antioxidant activity may have been caused by the chemical constituents of each individual phenols. It was reported that grapefruit seeds exhibited lesser antioxidant activity compared to other fruit parts due to the presence of a varied naringin level in the seeds [6]. Thus, the amount and composition of each individual phenols, as well as their intricate interactions, determined the antioxidant capability of various fruits and their tissues. Regarding the examined cultivars, HK and VC showed more antioxidant activity than other cultivars in addition to having higher levels of total phenolic and total flavonoid content.
HK peel has a very pleasant aroma, and because of that, dried peels are used for culinary purposes. Because of its use in traditional medicines and the presence of a good amount of antioxidants, gradually, this fruit is gaining popularity worldwide [9]. Although due to very high acidity, the HK juice is not desired by the people for direct consumption, our results proved that HK peels contain bioactive compounds like phenolics and flavonoids, which contribute to their very high antioxidant activity. These compounds can help to inhibit microbial growth and extend the shelf life of food products. Food technologists should work on the microbial properties of the peels of HK to extend the shelf life of fruits and vegetables. In addition, breeders should emphasize developing some varieties with bigger sized fruits and more yield potentialities that can boost the endangered Citrus species in this remote region of the world. Further studies should be addressed to investigate the genetic or environmental basis leading to such accumulation of phenolic compounds. Also, accessions found to be rich in phenolic could be further used in a crop improvement program to ensure an optimum phytochemical balance for high organoleptic quality and functionality of juices.
3.5. Correlation Studies
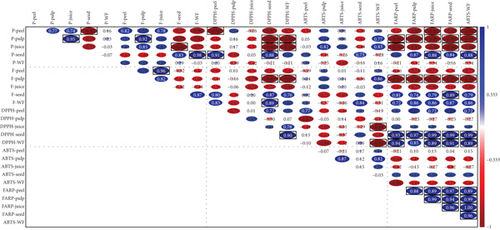
The correlation matrix in Figure 2 represents an extensive pairwise Pearson correlation for various biochemical characters (phenolics, flavonoids, and antioxidants) (DPPH, ABTS, and FRAP) across five distinct fruit parts (peel, pulp, juice, seed, and WF) revealing substantial (p < 0.05) positive and negative relationships between the characters. The matrix represents a positive and negative relationship using blue and red circles (the intensity and size indicate the strength of correlation). The phenolic content (P) in peel is strongly correlated with flavonoids (F) and antioxidant activities. The phenolic content in peel is strongly positively correlated with pulp (r = 0.77), juice (0.74), F-peel (0.82), F-pulp (0.68), and F-juice (0.78), whereas it is strongly negatively correlated with P-seed (−0.95), F-seed (−0.77), and F-WF (0.86). It also exhibited a strong negative correlation with antioxidant activity, such as DPPH (−0.26 to −0.93) and FRAP (−0.65 to −0.9) in the major studied components. It is also observed that a consistent positive correlation exists between total phenols and flavonoids. The existence of a high degree of correlation infers a strong shared biosynthesis pathway where the accumulation of flavonoid significantly contributes to the overall phenolic content in tissues. Similarly, phenol content is higher in WFs due to losses during juicing, particularly through oxidation and enzymatic degradation, as phenols are commonly found in fruit skin, seeds, and pulp. Total phenols show a moderate to strong positive correlation with DPPH radical scavenging activity and ABTS activity. This indicates that phenolics significantly contribute to the ability to scavenge free radicals by these two assays (DPPH and ABTS). Similar correlation of total phenolics negatively correlated with total flavonoids which was also observed by Wairata et al. [45]. FRAP antioxidant activity exhibited a highly strong negative correlation with phenolic (seed) and flavonoid content in peel, pulp, and juice (WF), whereas it showed a strong positive correlation with flavonoid in seed and WF and DPPH in juice, seed, and WF. From the results, we can infer that flavonoids are the primary drivers of FRAP antioxidant activity, as most fruit parts show a strong positive correlation. The presence of multiple hydroxyl groups in flavonoid compounds facilitates efficient electron donation in the FRAP assay. Total phenolics and flavonoids contribute significantly to antioxidant activity by serving as electron donors, free radical scavengers, and chain breakers [46]. The variation of phenolics and flavonoids and antioxidant activities in different parts of pomegranate was also observed by Kennas and Amellal-chibane [47]. Cano et al. [48] reported that the concentration of flavonoids and other bioactive compounds is influenced by different citrus varieties and varies among the tissues. Comparing juices among table grapes, Mohamedshah et al. [49] discovered similar patterns, indicating that a higher percentage of skin and seed phenolics was removed by juicing. Similarly, Siddhartha et al. [3] carried out an assessment and compared the bioactive compounds and antioxidants of the citrus fruits and their juices.
3.6. Limitations of the Study
Since the different Citrus cultivar fruit samples were collected only from a single district, Aizawl, Mizoram, India, this limits the environmental variability and may not represent the full genetic diversity of the cultivars.
4. Conclusion
The different fruit tissues of selected Citrus cultivars of northeast India, namely, Citrus macroptera Montr. (HK), Citrus limon (Linn.) Burm. f. (AL), Citrus maxima Burm. (PM), Citrus reticulata Blanco (KM), and Citrus sinensis (L.) Osbeck (VC), were subjected to biochemical profiling and quantification of antioxidant potential. Among the cultivars, HK had significantly higher total phenolic content in juice, peel, and pulp, while VC seeds and AL WF had higher total phenolics. Total flavonoid content was higher in HK juice, pulp, and peel. VC exhibited the highest F content in WF, while AL exhibited the highest in seeds. HK (seeds, WF), PM (pulp, juice), and AL (peel) exhibited the highest antioxidant capacity in terms of In DPPH. VC (pulp, juice, and WF), HK (seeds), and KM (peel) had higher values for ABTS. For FRAP, HK seeds resulted in the highest, whereas VC juice resulted in the lowest ability. The tissues of the Citrus cultivars show seeds exhibited the highest ferric reducing ability, subsequently followed by peels and WF, whereas juice exhibited the lowest reducing ability. HK showed significantly (< 0.001) greater FRAP reducing ability than other Citrus cultivars studied.
In this study, we have presented the biochemical profiling and antioxidant potential of different fruit parts of Citrus cultivars indigenous to northeast India and we observed clear differences in the phenolics, flavonoids, and antioxidant capacity of different Citrus cultivars. The information in this research offers a clear qualitative and quantitative analysis of the antioxidants and bioactive substances found in the edible portion of native citrus cultivars in northeast India.
Ethics Statement
The authors have nothing to report.
Disclosure
All the authors have given consent to publish the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
T.K.H.: conceptualization, supervision, writing—review and editing. G.M.: methodology, resources, validation, visualization. P.D.: writing original draft, software. K.B.: writing—review and editing, software. P.C.N.: writing—review and editing, software.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
All the data generated or analysed during this study are included in this published article.




