High Inhibitory Effect of 2-Nitrobenzaldehyde on the Photodegradation of Erythrocytes, the Production of Singlet Oxygen, and Meat Color Change
Abstract
In this study, 2-nitrobenzaldehyde as a protection agent strongly and influentially blocked singlet oxygen generation and consequently meat color change and erythrocyte photodegradation. The preservation rate of the sheep erythrocyte model and the maintenance of the photosensitizer in the presence of sodium azide (NaN3) as a recognized scavenger of singlet oxygen, 2-nitrobenzaldehyde, and 3-nitrobenzaldehyde were decreased in the order of 2-nitrobenzaldehyde > NaN3 > 3-nitrobenzaldehyde. Also, the results from proton nuclear magnetic resonance (1H NMR) declared that the coating of erythrocytes with 2-nitrobenzaldehyde resulted in a reduction of photooxidation by 27.6%. Conversely, the scavenging of singlet oxygen after 5 min of irradiation within the visible spectrum range, when 2-nitrobenzaldehyde was present, was reduced to 41.5%. Similarly, the results of spectroscopic reflectometry indicated that 2-nitrobenzaldehyde has a high deterrent effect on sheep meat photodiscoloration. The results of this study is valuable for the preservation of meat and food products against photoirradiation and are applicable in packaging industry.
1. Introduction
The visual appeal of food plays a crucial role in consumer purchasing decisions, as it strongly influences their perception of quality [1, 2]. An attractive appearance is closely associated with expected quality and serves as a key factor shaping consumers’ willingness to purchase food products [3, 4]. In meat purchasing decisions, visual attributes act as key indicators of the expected quality of the product at the time of sale. These attributes can be divided into intrinsic factors, such as color and fat composition, and extrinsic factors, including price, origin, and quality certifications [5, 6]. Meat products are particularly susceptible to photooxidation, which results from photochemical interactions between light and lipid components. [7]. Furthermore, the interaction of meat with store lighting, along with the persistent exposure of myoglobin and oxymyoglobin to oxygen, leads to the creation of metmyoglobin, a pigment responsible for the brownish-red appearance of the meat [8]. Lipid oxidation can occur via two primary mechanisms: autooxidation and light-induced oxidation. Autooxidation involves the reaction of triplet oxygen with lipids and occurs independently of light exposure. In contrast, light-induced oxidation, also known as photooxidation, is driven by singlet oxygen generated in the presence of a photosensitizer under light exposure [9, 10]. One of the major contributors to photooxidation in food products is the illuminated environment of refrigerated retail display cases, which accelerates color changes in items like ground meats and fresh sausages [11]. Factors such as the type of protein, packaging technique, and the choice of light source (e.g., LED vs. fluorescent lighting) can further influence this color degradation [7]. In its ground state, molecular oxygen contains two unpaired electrons. When it absorbs energy, these electrons pair up, forming singlet oxygen [12]. Singlet oxygen, being highly electrophilic, reacts with lipids, amino acids, nucleic acids, and other biological molecules, causing their degradation [13, 14]. In food systems, singlet oxygen is often generated under light exposure in the presence of photosensitizers like riboflavin and chlorophylls [15, 16]. Its interaction with unsaturated fatty acids leads to the production of lipid hydroperoxides as the initial oxidation products [17]. Moreover, ultraviolet (UV) radiation can exacerbate oxidative damage, including DNA strand breaks, protein oxidation, and the activation of matrix metalloproteinases [18]. The use of antioxidants has become increasingly important for preserving the quality of meat products, particularly in protecting them from photooxidation [19]. Synthetic antioxidants such as tert-butylhydroquinone (TBHQ), butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT) have shown strong singlet oxygen quenching abilities [13, 20]. Additionally, compounds like 2-nitrobenzaldehyde have emerged as valuable photoremovable protecting agents for diverse chemical functionalities [21]. Recent studies have also highlighted the inhibitory effects of 2-nitrobenzaldehyde on the photooxidation of fatty acids when compared to synthetic antioxidants like TBHQ, BHA, and BHT [22]. The objective of this project was to evaluate the protective effects of 2-nitrobenzaldehyde in relation to singlet oxygen scavenging, erythrocyte photodegradation, and meat color change.
2. Materials and Methods
2.1. Materials
Rose bengal, methylene blue (MB), anthracene, 2-nitrobenzaldehyde, 3-nitrobenzaldehyde, NaN3 and acetonitrile (CH3CN) were procured from Fluka and Merck and used as received without additional purification.
2.2. The Process of Preparing Erythrocytes
Defibrinated sheep blood was obtained from the Kimiakavoshazma Company (https://kimiakavoshazma.com/). Erythrocyte membranes were prepared following a standardized procedure [23] using freshly collected sheep blood treated with heparin. The isolated erythrocytes were suspended in a phosphate buffer at pH 7.4, achieving a protein concentration of roughly 1 mg/mL.
2.3. Preparation of Samples for Photooxygenation of Erythrocytes
Two milliliters of rose bengal (1 × 10−4 M) was added to 100 mL diluted erythrocyte (5 × 10−4 v/v) in a test tube. Individually, 1.6 × 10−6 mol of 2-nitrobenzaldehyde, 3-nitrobenzaldehyde, and NaN3 was introduced into separate test tubes. The test tubes were exposed to light from a sun simulator, consisting of 288 power LED lamps rated at 1 W and 2.3 V (The LEDs emitted visible light in the violet to red region 380–780 nm, overlapping with the absorption maximum of the photosensitizer rose bengal. The measured illuminance was 59660 LUX, corresponding to an estimated irradiance of ~8.74 mW/cm2 at this wavelength range) for duration of 6 h at ambient temperature while maintaining a pressure of 1 atm with air bubbling through the solution. The degradation of erythrocytes was monitored using a Shimadzu 2100 spectrophotometer set to a wavelength of 407 nm.
2.4. Preparation of Samples for 1H NMR Tests
Two milliliters of rose bengal (1 × 10−4 M) was added to fresh erythrocyte in a test tube. 1.6 × 10−6 mol of 2-nitrobenzaldehyde was added to the test tube. The test tubes in the presence and absence of 2-nitrobenzaldehyde were exposed to light from a sun simulator, consisting of 288 power LED lamps rated at 1 W and 2.3 V (59660 LUX, 380–780 nm, 8.74 mW/cm2), at ambient temperature while maintaining a pressure of 1 atm with air bubbling through the solution. After 72 h photooxidation of erythrocyte using hexane, the nonsoluble parts of erythrocyte were extracted. 1H NMR spectra were recorded on a Bruker AMX 300 MHz spectrometer using TMS as an internal standard.
2.5. Preparation of Samples for Photooxygenation of Anthracene
In a standard experimental setup, either 1 × 10−7 mol of 2-nitrobenzaldehyde or NaN3 was added into a 15 mL of CH3CN solution containing anthracene (4 × 10−4 M) and MB (1 × 10−4 M). The samples underwent continuous irradiation using a solar simulator equipped with 288 LED lamps, each rated at 1 W and 2.3 V (59660 LUX, 380–780 nm, 8.74 mW/cm2), for a duration of 5 min at ambient temperature while maintaining a 1 atm air flow in the solution. The resulting products were analyzed using a Shimadzu 2100 spectrophotometer at a wavelength of 375 nm.
2.6. Preparation of Samples for Photodiscoloration of Meat
The lamb meat was purchased from a slaughterhouse in Tehran, Iran. Thin slices of sheep tenderloin were removed from lamb meat and placed into test tubes. The meat test tubes were covered with a cylinder containing 2-nitrobenzaldehyde (1 × 10−4 M) solution. The cylinder containing the meat test tubes was exposed to light from a sun simulator, consisting of 288 LED lamps with a power of 1 W and a voltage of 2.3 V (59660 LUX, 380–780 nm, 8.74 mW/cm2), for a duration of 24 h at room temperature while maintaining an atmospheric pressure of 1 atm with air bubbling through the solution. The discoloration of the meat was assessed by measuring light reflectance at 570 nm, utilizing a Shimadzu 2100 spectrophotometer across the spectral range of 400–1000 nm.
2.7. Statistical Analysis
All experiments were conducted in triplicate, and the data were analyzed using SAS software Version 3.9. The results were averaged and statistically evaluated through Duncan’s multiple range test. Graphs were generated using the Python 3.9 statistical package. Data are reported as mean ± standard deviation (SD) with a sample size of n ≥ 2. Statistical significance was determined at p < 0.05. Duncan’s post hoc analysis revealed significant differences between all experimental groups and the control group (without antioxidant) (p < 0.05). Values that were not statistically significant (p > 0.05) were systematically excluded during the analysis.
3. Results and Discussion
3.1. Effect of 2-Nitrobenzaldehyde on Erythrocyte Preservation and Photosensitizer Remaining
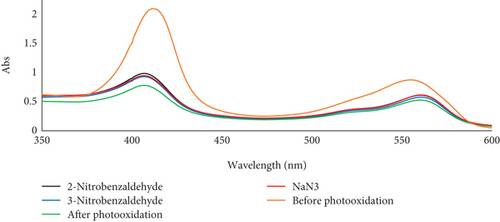
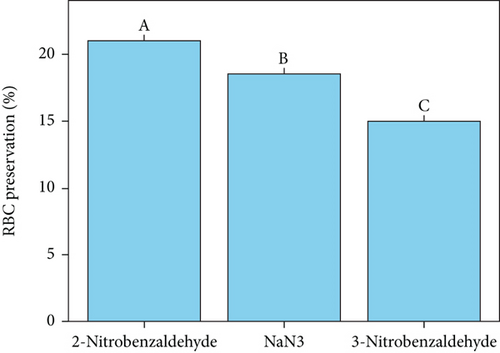
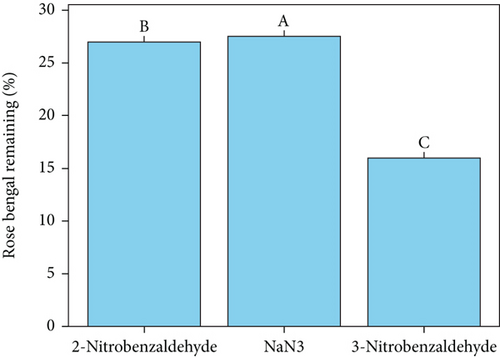
Red blood cells (RBCs) or erythrocytes are often used as a model to investigate lipid oxidation in meat products. This is because residual blood can accelerate the oxidation of phospholipid bilayers through hemolysis and the subsequent release of hemoglobin [24]. The generation of singlet oxygen through photosensitization holds considerable importance in the field of photooxidation of organic substances [25] and food chemistry [12, 14, 17, 26]. The photodegradation of erythrocytes, triggered by singlet oxygen (1O2), was used as a standard model to evaluate the antioxidant effects of 2-nitrobenzaldehyde (Figure 1). The antioxidant results showed that 2-nitrobenzaldehyde, NaN3, and 3-nitrobenzaldehyde were able to prevent the photodegradation of hemoglobin in sheep erythrocyte model by 21%, 18.50%, and 15%, respectively (Figure 1b). It is important to note that the degradation of erythrocyte model was accelerated in the presence of photosensitizer. Therefore, 2-nitrobenzaldehyde showed a significant suppressing effect on singlet oxygen generation and erythrocyte photodegradation compared to the control and other treatments (p < 0.05), as indicated by different uppercase letters. Also, it was declared that inhibition values in the photodegradation of rose bengal as a photosensitizer were obtained as 27%, 27.5%, and 16%, respectively (Figure 1c) which are correlated with results of photo-protecting effect of 2-nitrobenzaldehyde on erythrocyte model. It is crucial to highlight that the photodegradation of hemoglobin ceased in the absence of rose bengal (refer to Table 1, Entry 1), in the absence of oxygen (refer to Table 1, Entry 2), or when the irradiation was stopped (refer to Table 1, Entry 3). Therefore, the presence of a photosensitizer, light, and O2 is vital for the degradation of erythrocytes (refer to Table 1, Entry 4).
| Entry | Reaction condition | Erythrocyte degradation (%) |
|---|---|---|
| 1 | Erythrocyte + air + light | Trace |
| 2 | Erythrocyte + rose bengal + light | Trace |
| 3 | Erythrocyte + rose bengal + air | Trace |
| 4 | Erythrocyte + rose bengal + light + air | 100 |
- aA solution containing erythrocytes (5 × 10−4 v/v) and rose bengal (1 × 10−4 M) was subjected to illumination using a solar simulator, which consisted of 276 power LED lamps rated at 1 W and 2.3 V (59660 LUX, 380–780 nm, 8.74 mW/cm2) and was equipped with a cooling fan. This illumination occurred for a duration of 120 min at a temperature of 25°C while maintaining an atmosphere of 1 atm with air bubbles.
3.2. 1H NMR Evidences on Erythrocyte Preservation in the Presence of 2-Nitrobenzaldehyde
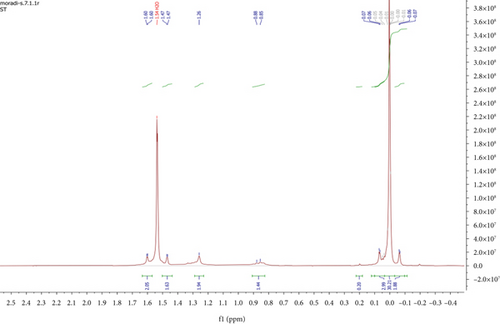
To investigate the effect of 2-nitrobenzaldehyde on the photooxidation of erythrocyte, using hexane, the nonsoluble parts of erythrocyte were extracted and analyzed. 1H NMR spectrum of extracted erythrocyte did not show a signal arising from the 1.70–10.00 ppm (Figure 2a). After adding photosensitizer and light, 1H NMR spectrum underwent more changes between 1H NMR spectrum 1.6 and 3.7 ppm. Figure 2b displays an expanded view of the aliphatic region between 0.5 and 1.5 ppm. The dominant signals observed at approximately 0.9 and 1.3 ppm correspond to the CH3 and CH2 groups in lipids, respectively. When comparing this spectrum with that of isolated erythrocytes treated with a photosensitizer and exposed to light, oxidation reactions were evident. New signals appeared in the 1.8–2.4 ppm range, representing CH2COR, CHCOR, and CH3COR groups. 1H NMR results declared that the rate of erythrocyte photooxidation in the presence of 2-nitrobenzaldehyde 27.6% (SD = 0.45%) was reduced (Figure 2c). These results prove the photo-protecting effect of 2-nitrobenzaldehyde on lipid oxidation and consequently meat spoilage.
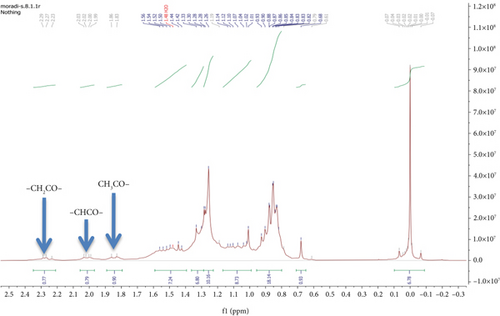
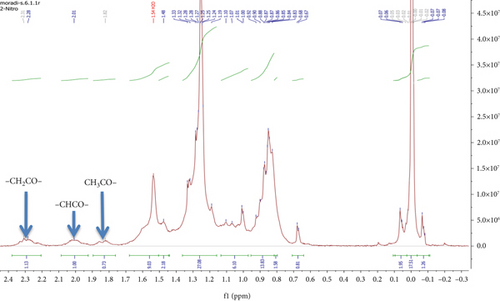
3.3. Anthracene Photooxidation
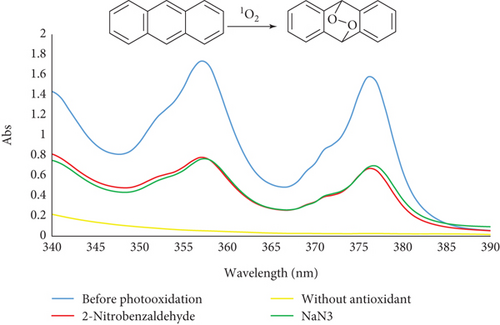
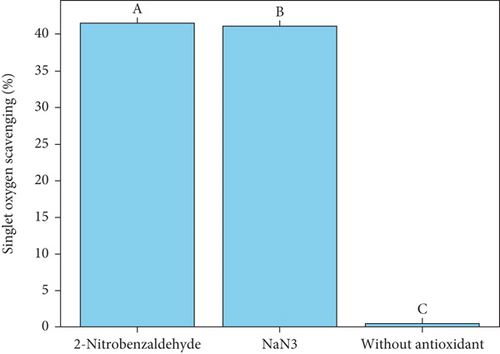
Spectrophotometry offers a more efficient method for identifying singlet oxygen. Typically, a chemical probe is employed to capture singlet oxygen, allowing for detection and quantification through absorbance measurements. One significant reaction of singlet oxygen is the reversible [4 + 2] cycloaddition with conjugated cyclic dienes and polycyclic aromatic hydrocarbons, such as anthracene [13, 27]. The generation of singlet oxygen by MB was confirmed through chemical trapping with anthracene. The UV–Vis spectra of anthracene over time during visible light exposure, with MB acting as the photosensitizer, are shown in Figure 3a. A decrease in the absorption intensity of anthracene (λmax = 375 nm) was observed as irradiation progressed. This reduction is attributed to the formation of anthracene-9,10-endoperoxide (see Figure 3a). The results from the photooxidation of anthracene demonstrated that both NaN3 and 2-nitrobenzaldehyde significantly reduced anthracene oxidation. Notably, 2-nitrobenzaldehyde exhibited a slightly higher inhibition rate (41.5% [SD = 0.1%]) compared to NaN3, a well-known singlet oxygen scavenger, which showed an inhibition of 41% (SD = 0.3%) and also demonstrated significantly greater activity compared to the control and other treatments (p < 0.05), as indicated by different uppercase letters (Figure 3a,b). Moreover, the cessation of oxidation in the absence of light confirms that anthracene oxidation is driven by singlet oxygen under visible light exposure. These results suggest that 2-nitrobenzaldehyde acts as a highly efficient suppressor of singlet oxygen generation.
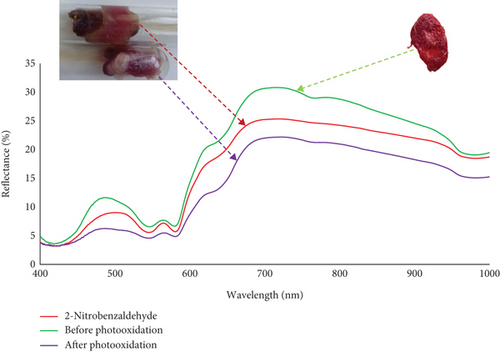
3.4. Meat Reflectometry
Reflectance spectroscopy is a widely used technique for analyzing color changes in meat. The observed color of meat, as well as other materials, results from the interaction of light, visual perception, and the object itself. The reflectometry findings at 570 nm indicated that the covering of fresh meat with 2-nitrobenzaldehyde led to a 69.7% (SD = 1.7%) reduction in discoloration (Figure 4). These findings indicate that the outcomes of meat reflectometry align well with existing evidence regarding the photoprotective properties of 2-nitrobenzaldehyde.
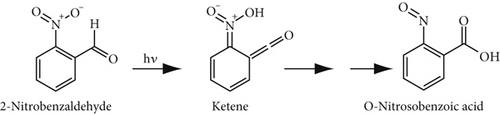
3.5. Mechanistic Insights
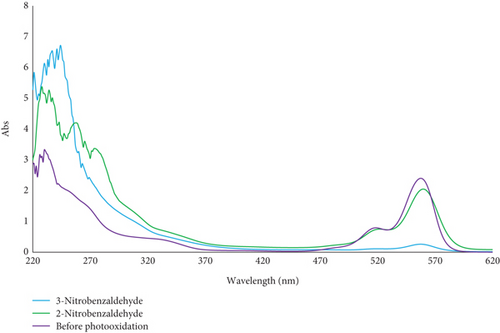
Research has shown that 2-nitrobenzaldehyde functions as a photolabile protecting group with a quantum yield of 0.5, which remains stable in different solvents [28]. Upon photoexcitation, a hydrogen transfer occurs, leading to the formation of a ketene intermediate. This intermediate subsequently undergoes reactions to produce the final nitrosocompound (see Figure 5a). The UV–Vis spectra of 2-nitrobenzaldehyde and 3-nitrobenzaldehyde show strong absorption near 230–290 nm and no significant absorption above 400 nm, while rose bengal absorbs maximally at 562 nm (Figure 5b) during the photooxidation process. Thus, there is minimal spectral overlap, indicating that the photoprotective effect is not due to a simple filter effect. Also, based on our previous study [29], it was confirmed that in the presence of light, oxygen, and non–metallo-photosensitizers, although various types of aldehydes are efficiently converted to carboxylic acid products, the conversion rate of nitrobenzaldehydes is only trace. This finding supports the hypothesis that the photoprotective mechanism of nitrobenzaldehydes involves the generation of nitrosocompounds rather than the oxidation of 2-nitrobenzaldehyde or the physical quenching of the photoexcited photosensitizer or singlet oxygen (via energy or electron transfer). It appears that the inhibitory effect of 2-nitrobenzaldehyde on singlet oxygen production, erythrocyte photooxidation, degradation of photosensitizers, and the meat color change is attributed to its photolability.
4. Conclusion
The color of fresh meat is a critical quality indicator, as consumers often assess its freshness and quality based on appearance. Consequently, identifying an effective and robust photoprotective agent presents a significant challenge in food chemistry. This study demonstrated that 2-nitrobenzaldehyde plays a vital role in inhibiting the production of singlet oxygen, preventing erythrocyte photooxidation, and reducing the degradation of photosensitizers and the stability of meat color. While 2-nitrobenzaldehyde was utilized in this study as a model photoprotective agent, its known toxicity limits its direct use in food-related or biomedical applications. However, its performance in providing effective photostability highlights the potential of nitroaromatic compounds in noncontact roles. Future research will focus on the identification and evaluation of alternative nitroaromatic compounds with improved safety and environmental profiles. Additionally, the development of nanostructured systems, encapsulation techniques, and active packaging applications presents a promising way, allowing these agents to deliver photoprotection while remaining physically isolated from sensitive products. These strategies could broaden the practical relevance of photoprotective systems, especially in packaging technologies where light-induced degradation is a concern. Nonetheless, this necessitates advancements in methods for doping 2-nitrobenzaldehyde into the plastic films used for packaging meat and food products.
Ethics Statement
This article does not contain any studies with human participants or animals conducted by any of the authors.
Disclosure
All the authors gave their consent for the publication of this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Mahdi Hajimohammadi designed the concept and methodology of this research work and wrote, reviewed, and edited the manuscript. Samaneh Moradi analyzed and investigated the experiments.
Funding
We gratefully acknowledge the support from Kharazmi University.
Acknowledgments
We gratefully acknowledge the support from Kharazmi University.
Open Research
Data Availability Statement
The data is available on request from the authors.




