Antifungal Activity of Powder Blends of Eremomastax speciosa, Moringa oleifera, and Senna alata Against Digestive Candidiasis
Abstract
Fungal infections (candidiasis) are responsible for approximately 5% of the diseases observed worldwide. The treatment of these candidiasis involves the administration of antifungals such as fluconazole most often as monotherapy. The antifungal activities of blends of extracts from the leaf powders of Eremomastax speciosa (ES), Moringa oleifera (MO), and Senna alata (SA), have been scarcely investigated. This study evaluated the in vitro antifungal activity of blends of leaf powder extracts from ES, MO, and SA against yeasts responsible for digestive mycoses. All the extracts are rich in bioactive compounds whose structures include oxygenated and hydroxylated groups and carbonyl and nitrogen groups. The SA extracts had the highest total polyphenol content (25.02 ± 0.08 mg EGA/100 g DM), total flavonoid content (60.48 ± 0.27 mg EQ/100 g DM), and condensed tannin content (5.31 ± 0.25 mg EC/100 g DM). Several blends (M4, M10, etc.) have significant antifungal effects on Candida albicans, Candida krusei, and Candida parapsilosis with minimum inhibitory concentrations (MICs) and minimum fungicidal concentrations (MFCs) of 125 and 250 μg/mL, respectively. All of these compounds have fungicidal effects (MFC/MIC = 2). Quercetin 3-O-(6 ″-acetyl glucoside) appears to be the main bioactive compound responsible for the fungicidal antifungal activities. Only the M10 blend presented a broader spectrum of action than the other blends and fluconazole. The M10 blend had the best antifungal activity and can serve as a basis for in-depth studies to develop a drug based on these three plants in the fight against digestive mycoses associated with the Candida genus.
1. Introduction
Fungal infections are responsible for approximately 5% of the diseases observed worldwide due to the increase in risk factors. Some of these pathologies such as candidiasis, an opportunistic mucocutaneous fungal infection, are caused by saprophytic fungi, mainly Candida albicans (CA) which represent more than 60% of yeasts isolated in humans [1]. We also observed the emergence of non-albicans Candida species which have become pathogenic yeasts in patients infected with HIV. This is the case for Candida africana, Candida krusei (CK), Candida glabrata, Candida tropicalis, and Candida parapsilosis (CP) [2].
Treatment of candidiasis involves the administration of antifungals such as fluconazole or amphotericin B, most often as monotherapy [3]. However, over time, these methods have unfortunately shown their limits. In fact, prolonged use of these drugs leads to resistance of the pathogen to antifungals. The failure of fluconazole treatment, an antifungal agent of the azole family, for the treatment of recurrent oropharyngeal candidiasis has been reported [4]. The widespread and repeated use of azole drugs has led to resistance to antifungal therapies [5]. In addition, the use of antifungal drugs with active ingredients of synthetic origin has led to numerous side effects. All these have favored the development of new techniques such as polytherapy (the use of a blend of at least two molecules for the treatment of a single disease), as well as the search for new molecules of natural origin through the exploitation and valorization of medicinal plants [2]. The use of plants in traditional medicine is common in various populations around the world, due to their high abundance of phytochemical compounds such as phenolic compounds [6]. In Africa, more than 80% of the population uses traditional medicine for treatment [7]. In Cameroon, the flora is composed of a large variety of medicinal plants. Some of these plants are distinguished by their use in the traditional treatment of gastric disorders, their use as vegetables, and their use in the traditional treatment of skin mycoses, such as “red on one side”, Moringa and darter, whose scientific names are Eremomastax speciosa (ES), Moringa oleifera (MO), and Senna alata (SA), respectively.
ES (Acanthaceae) is widely distributed in tropical Africa and is commonly found in Cameroon and Akwa-Ibom in Nigeria due to its medicinal value [8–10]. It is a polymorphic shrub that can reach 2 m high. Its subsessile, erect flowers, and pedicels reach 2 mm [10]. It is used in medicine and as a vegetable [8]. Phytochemical screening of the aqueous extract of ES leaf powder revealed the presence of large families of secondary metabolites such as tannins, alkaloids, flavonoids, anthocyanins, phenols, quinones, sterols, triterpenoids, glycosides, and proteins [11, 12].
MO (Moringaceae) is a fast-growing perennial tree that can reach 7–12 m in height. The purplish-gray trunk is generally straight, but can branch and sometimes reach 3 meters [12]. It is grown and used as a vegetable (leaves, green pods, flowers, roasted seeds), spice (mainly the roots), for cooking, as a cooking and cosmetic oil (seeds), and as a medicinal plant (all parts of the plant, whether transformed into powder or not) [13]. Among the main phytochemical compounds found in the leaves, flowers, seeds, and bark of MO are tannins, saponins, alkaloids, flavonoids, glycosides, steroids, quercetins, terpenoids, gallic acid, catechins, epicatechin, ferulic acid, vanillin, caffeic acid, cinnamic acid, phytosterols, and phenols [14].
SA, also called Cassia alata (Fabaceae), is an ornamental plant native to the Amazon rainforest [15]. It is a low-wooded decorative shrub that is approximately 3 m tall or taller, with pinnately compound leaves consisting of 8–14 pairs of oblong leaves to obovate leaflets (5–16-cm long by 3–8-cm wide) that are rounded at the end [16]. In northern Nigeria and Cameroon, stem, leaf, and root decoctions are used for the treatment of wounds, skin, and respiratory tract infections, burns, diarrhea, and constipation [17]. In Egypt, leaf decoction is used as an intestinal stimulant to stimulate peristaltic contractions and peristaltic strictures and to decrease water absorption from the colon to prevent constipation [18]. The plant has a multitude of bioactive chemical compounds. Some of the constituents are phenolic compounds (rhein, chrysaphanol, kaempferol, aloemodin, and glycosides), anthraquinones (alatinone and alatonal), fatty acids (oleic, palmitic, and linoleic acids), steroids, and terpenoids (sitosterol, stigmasterol, and campesterol).
These secondary metabolites and other biological molecules that characterize the abovementioned three plants possess many biological activities, including antimicrobial activities [16]. Alkaloids are known for their antimalarial, antibacterial, and antiviral properties. Flavonoids and tannins have antiviral, antifungal and antibacterial properties. Some saponins have antibacterial and antifungal properties. Triterpenes are distinguished by their antimicrobial properties [16, 17]. These activities are more significant when the extracts of these plant powders are combined with blends [19]. This is a practice commonly encountered in traditional medicine [20], where powders are frequently used to treat many pathologies (malaria, typhoid fevers, colic, etc.) [21]. This practice suggests that the blend of bioactive compounds from different plants tends to enhance the therapeutic effects of the preparations [22]. However, very few studies have focused on the effects of combinations of extracts from these three plants, ES, MO, and SA, on the growth of the yeasts CA, CK, and CP, which are associated with digestive mycoses. These digestive mycoses constitute one of the entry points for deep visceral candidiasis, especially in at-risk individuals (immunocompromised individuals, HIV-infected individuals, etc.) [3]. Therefore, the objective of this study was to evaluate the in vitro antifungal activity of blends of leaf powder extracts from ES, MO, and SA against pathogenic yeasts of the Candida genus responsible for digestive mycoses (CA, CK, and CP).
2. Materials and Methods
2.1. Sampling and Identification of Plant Matrices
Each leaf (leaflet and petiole) from the different plant species used in this study was collected in September in Dibombari, a district of the City of Douala for SA; in April 2023 in Mendong a district of the City of Yaoundé for ES; and from the AGROTECH agricultural farm, Littoral region (Cameroon) for MO. They were previously identified and authenticated before any powder production at the National Herbarium of Cameroon, where the voucher samples were kept under the references 13684/HNC, 179761/SRF, and 45146/HNC for ES (Figure 1a), MO (Figure 1b), and SA (Figure 1c), respectively. After that, only the leaflets were detached from the petioles for powder production.



2.2. Production of Powders and Extracts
2.2.1. Production of Powders
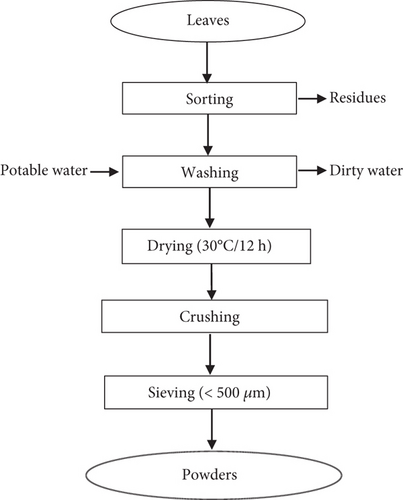
The leaflets were sorted, washed, and dried at 30°C for 12 h using a dehydrator (DORNEX). They were crushed using a blender (Seven 7 Star) and then sieved (< 500 μm) (Figure 2). The powders obtained were stored in opaque light containers, at +4°C throughout the study period.
2.2.2. Extract Production
A mass of 50 g of each powder was macerated in 500 mL of the solvent ethanol:water (70/30). The filtrate obtained was concentrated by evaporation of the solvent at 40°C using a Lauda rotary evaporator, type MC 250. The extract obtained was stored at 4°C in a refrigerator.
2.3. Preparation of Blends Based on the Extracts
Guiadem et al. [24] reported the antifungal activities of plant extracts with an initial concentration range of 0.20 mg/mL (i.e., 20% by mass). To obtain preparations whose volumes corresponded to concentrations differing by 20% by mass, mother solutions with a concentration of 1 mg/100 mL of each powder extract from ES, MO, and SA were prepared. From these three mother solutions, different preparations at 100, 80, 60, 40, and 20% (mass/volume) (Table 1) were obtained. Each of them includes three blends obeying the fundamental law of blends [25] for a total of 15 blends whose M codes (formulation) are presented in Table 1.
| Blend | Formulation code (M) | ES | MO | SA |
|---|---|---|---|---|
| 100 | M1 | 100 | 0 | 0 |
| M2 | 0 | 100 | 0 | |
| M3 | 0 | 0 | 100 | |
| 80 | M4 | 80 | 10 | 10 |
| M5 | 10 | 80 | 10 | |
| M6 | 10 | 10 | 80 | |
| 60 | M7 | 60 | 20 | 20 |
| M8 | 20 | 60 | 20 | |
| M9 | 20 | 20 | 60 | |
| 40 | M10 | 40 | 30 | 30 |
| M11 | 30 | 40 | 30 | |
| M12 | 30 | 30 | 40 | |
| 20 | M13 | 20 | 40 | 40 |
| M14 | 40 | 20 | 40 | |
| M15 | 40 | 40 | 20 | |
- Abbreviations: ES, Eremomastax speciosa; MO, Moringa oleifera; SA, Senna alata.
2.4. Phytochemical Analysis of Plant Extracts
2.4.1. Phytochemical Screening
Standard phytochemical screenings described by Patel et al. [26] and Angelina et al. [27] (Table 2) were used to perform qualitative phytochemical screening of the plant extracts. The various plant extracts were screened for the presence of triterpenes, steroids, phenols, saponins, tannins, flavonoids, and alkaloids.
| Chemical families | Characteristics tests | Principles | Positive reactions | Conclusion |
|---|---|---|---|---|
| Alkaloids | Wagner | 1 mL of extract +4 drops I2/KI (1.25 g/2 g in 100 mL) +1 mL of HCl 1% | Precipitation | Alkaloids |
| Steroids and triterpenes | Lieberman-Burchard | 1 mL extract +4 drops acetic anhydride +2 drops of H2SO4 concentrated | Blue–green | Steroids |
| Purplish red or pink | Triterpenes | |||
| Red ring | Unsaturated sterols | |||
| Salkowski | 1 mL extract +2 mL of H2SO4 | Red coloring | Unsaturated sterols | |
| Budjet-kedde | 1 mL+3 drops of picric acid | Orange coloring | Lactonic steroids | |
| Saponins | Foam test | 1 mL extract +10 mL distilled water | Presence of foam 03 cm | Saponins |
| Flavonoïds | Willstatter | 1 mL extract +0.25 mL of HCl concentrated +3 magnesium grain | Red | Flavones |
| Purplish red | Flavanols | |||
| Purplish red | Flavanones | |||
| Tannins and polyphenols | Jellies | 1 mL extract +4 drops of jellies 1% | Precipitation | Polyphenols |
| Salted Jellies | 1 mL extract +4 drops of jellies 1% +04 drops NaCl 10% | Precipitation | Tannins | |
| FeCl3 | 1 mL extract +4 drops of FeCl3 5% | Black blue | Phenolic tannins | |
| Blue–green | Pyrogallic tannins | |||
| Green–black | Catechin tannins | |||
2.4.2. Quantitative Analysis of Some Bioactive Compounds
2.4.2.1. Total Phenolic Content (TPC)
The dosage of total polyphenols was determined spectrophotometrically, according to the colorimetric method using Folin-Ciocalteu reagent [28]. This assay is based on the quantification of the total concentration of hydroxyl groups present in the extract. The protocol used was based on that described by Singleton and Rossi [29] with some modifications. Briefly, 500 μL of each extract was added to glass hemolysis tubes, with a blend of 1 mL of Folin-Ciocalteu reagent diluted 10 times and 800 μL of a 5% sodium carbonate solution 5% (added 4 min later). The tubes were shaken and incubated for 30 min. The absorbance was read at 765 nm. A calibration curve was generated in parallel under the same operating conditions using gallic acid at different concentrations (0–1000 μg/mL).
2.4.2.2. Total Flavonoid Content (TFC)
The determination of flavonoids was carried out by a method based on the formation of a very stable complex between aluminum chloride and oxygen atoms present in Carbons 4 and 5 of the flavonoids [30]. The protocol used was based on that described by Zhishen et al. [31] and Chan et al. [32] with some modifications. In a glass hemolysis tube, 500 μL of extract, standard, or distilled water for the control was added to 200 μL of 5% NaNO2. After 5 min, 200 μL of 10% AlCl3 was added and the medium was vigorously mixed. After 6 min, a 1000-μL volume of 1 M NaOH was added to the medium. The absorbance was read immediately at 510 nm and compared with that of the control. A methanolic solution of quercetin was prepared. Daughter solutions prepared from the mother solution at different concentrations between 0 and 1000 μg/mL will allow the calibration curve to be drawn.
2.4.2.3. Condensed Tannin Content (CTC)
This assay was carried out using the vanillin method combined with hydrochloric acid. This method depends on the reaction of vanillin with the terminal flavonoid group of condensed tannins and the formation of red complexes [33, 34]; this is explained by the ability of tannins to transform into anthocyanidins was red in color after reaction with vanillin [35]. The content of condensed tannins was determined by the vanillin method described by Julkunen-Tiitto [36]. Then, 500 μL of each extract was added to 1500 μL of 4% vanillin/dichloromethane solution and vigorously mixed. Then, 750 μL of concentrated hydrochloric acid (HCl) was added. The obtained blend was left to react at room temperature for 20 min. The absorbance was measured at 550 nm against a blank. Catechin stock solutions at concentrations between 0 and 1000 μg/mL were used to construct a calibration curve.
2.5. Determination of the Chemical Structure of Compounds
Ultra-high-performance liquid chromatography (UHPLC) coupled with mass spectrometry (MS) combines the advantages of these two techniques, namely, high selectivity and separation efficiency, structural information (UHPLC-MS), and further increased selectivity (MS). This analysis method is based on the separation of compounds inside a column in relation to the MS system where they are fragmented by ionization. The different fragments are recorded in the form of a mass spectrum according to their molecular weight. The reconstitution of these fragments allowed us to determine the chemical structure of the compound.
2.6. In Vitro Antifungal Activity Evaluation
The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) were utilized to assess the antifungal activity of leaf powder extracts from ES, MO, and SA against the yeasts CA, CK, and CP. The yeast broth microdilution technique (M27-A2) was employed for this purpose, following the guidelines set by the Clinical and Laboratory Standards Institute (CLSI, formerly known as the National Committee for Clinical and Laboratory Standards, NCCLS) [37].
A series of gradient twofold dilutions of each extract was prepared, along with fungal suspensions in Sabouraud dextrose broth (SDB). Each well received a microvolume of the fungal extract and suspension. After the incubation period, the MICs were determined. These MICs were visually assessed following the corresponding incubation time and were defined as the lowest concentration of the product at which there was no or virtually no growth observed. The test was conducted in triplicate.
For the determination of MFC, aliquots of 5 μL from each well that exhibited no microorganism growth were subcultured in 195 μL of SDB and incubated at 37°C for 48 h. The lowest concentration that did not promote growth after subculture was considered the MFC. Fluconazole (for yeast) served as a positive control. The MFC/MIC ratio was calculated to evaluate the antifungal potency of the extract. If this ratio is less than or equal to 4, the extract is classified as fungicidal; if it is greater than 4, the extract is classified as fungistatic.
2.7. Statistical Analysis
Analyses were carried out in triplicate. Microsoft Excel 2016 software was used for the calculation of means and standard deviations. Statgraphic Centurion 15.2 software (StatPoint Technologies, Inc, Warrenton, Virginia, United States) was used for the analysis of variance (one-way ANOVA) and means were separated using the Duncan multiple range test at p < 0.05.
3. Results
3.1. Bioactive Potential of Powder Extracts
3.1.1. Bioactive Compounds Present or Absent in Powder Extracts
The presence of major phytochemical groups was detected in the plant extracts studied. The results indicated that all the powder extracts from three plants, namely, ES, MO, and SA, contained alkaloids, flavonoids, polyphenols, saponins, tannins, and triterpenes with a total absence of steroids in all the extracts (Table 3).
| Compounds | ES | MO | SA |
|---|---|---|---|
| Alkaloids | + | + | + |
| Flavonoids | + | + | + |
| Polyphenols | + | + | + |
| Saponins | + | + | + |
| Steroïds | — | — | — |
| Tannins | + | + | + |
| Triterpens | + | + | + |
- Note: (-) absent; (+) present.
- Abbreviation: ES, Eremomastax speciosa; MO, Moringa oleifera; SA, Senna alata.
3.1.2. Contents of Some Bioactive Compounds in Powder Extracts
Several bioactive compounds present in powder extracts and known for their bioactivity (antifungal activity more precisely) [38] were quantitatively evaluated, particularly polyphenols, flavonoids, and condensed tannins. The content of these different bioactive compounds varies significantly from one extract to another (Table 4). The polyphenol contents were 4.08 ± 0.14, 12.96 ± 0.02, and 25.02 ± 0.08 mg EGA/100 g DM; the total flavonoids were 3.95 ± 0.15, 16.03 ± 0.17, and 60.48 ± 0.27 mg EQ/100 g DM; and condensed tannins were 4.54 ± 0.04, 4.40 ± 0.22, and 5.31 ± 0.25 mg EC/100 g DM in powder extracts of ES, MO, and SA, respectively. The SA extracts had the highest contents of polyphenols (25.02 ± 0.08 mg EGA/100 g DM), flavonoids (60.48 ± 0.27 mg EQ/100 g DM), and condensed tannins (5.31 ± 0.25 mg EC/100 g DM). The ES extracts had the lowest contents of polyphenol (4.08 ± 0.14 mg EGA/100g DM), flavonoids (3.95 ± 0.15 mg EQ/100 g DM), and condensed tannins (4.54 ± 0.04 mg EC/100 g DM).
| Extract | Total phenol (mg EGA/100 g DM) | Total flavonoid (mg EQ/100 g DM) | Condensed tannins (mg EC/100 g DM) |
|---|---|---|---|
| Eremomastax speciosa | 4.08 ± 0.14a | 3.95 ± 0.15a | 4.54 ± 0.04a |
| Moringa oleifera | 12.96 ± 0.02b | 16.03 ± 0.17b | 4.40 ± 0.22a |
| Senna alata | 25.02 ± 0.08c | 60.48 ± 0.27c | 5.31 ± 0.25b |
- Note: Means in the same column with different superscript letters are significantly different from each other (p < 0.05).
- Abbreviations: DM, dry matter; EC, equivalent of catechin; EGA, equivalent of gallic acid; EQ, equivalent of quercetin.
3.1.3. Chemical Structures of Bioactive Compounds in Powder Extracts
3.1.3.1. ES
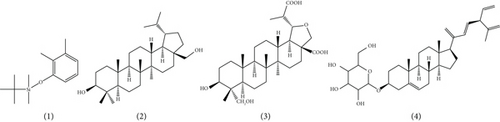
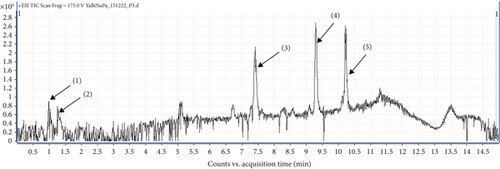
UHPLC-MS/MS analysis of different extracts made it possible to obtain chromatograms, from which the identification of the probable majority of molecules was made possible via MS. These mass spectra are the fingerprints of compounds that can be identified from the data library [39]. The chromatogram (Figure 3) of the hydroethanolic extract of the ES leaf powders presented 04 peaks, whose characteristics and chemical structures are given in Table 5 and Figure 4, respectively. Phenolic compounds, namely 2,3-dimethylphenol-ter-butyldimethylsilylether (1) with a ratio of m/z = 237.1 and a retention time of Tr = 1.26 min; two terpenoids, namely, 20-Hydrobetulin (2) with a ratio of m/z = 445.1 and a retention time of Tr = 9.31 min and 29-carboxy-20-hydro-24-hydroxy-22-oxo-betulinic acid (3) with a ratio of m/z = 507.2 and a retention time of Tr = 10.21 min. Finally, a saponin, namely 18, 22, 24-tri stigmasterol dehydroglucopyranosyl (4), with a ratio of m/z = 569.4 and a retention time of Tr = 11.48 min, was detected.
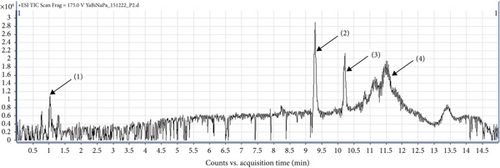
| Pics (signal) | Tr (min) | Fragment m/z | Pseudo molecular ion | Name of chemical compounds | Family |
|---|---|---|---|---|---|
| (1) | 1.26 | 237.1 | [M + H]+ | Tert-butyl(2,3-dimethylphenoxy) dimethylsilane | Phenolic compound |
| (2) | 9.31 | 445.1 | [M + H]+ | 20-Hydrobetuline | Terpenoid |
| (3) | 10.21 | 507.2 | [M + H]+ | Acid-29-carboxy-20-hydro-24-hydroxy-22-oxo-betulinique | Terpenoid |
| (4) | 11.48 | 569.4 | [M + H]+ | 18, 22, 24-Tri dehydroglucopyranosyl de stigmastérol | Saponin |
3.1.3.2. MO
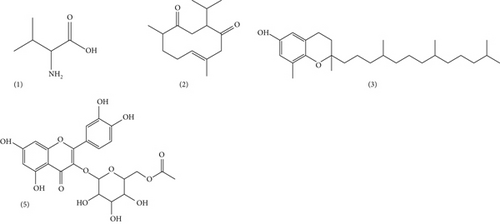
Figure 5 shows the chromatographic profile of hydroethanol extracts from MO powders. The main 05 peaks whose characteristics and chemical structures are given in Table 6 and Figure 6, respectively, are shown. In fact, the results revealed an amino acid, namely, L-valine (1) with a ratio of m/z = 111.01 and a retention time of Tr = 1.05 min; a sesquiterpene (E-3-isopropyl-6,10-dimethylcyclodec-6-ene-1,4-dione), namely, neocurdione (2), with a ratio of m/z = 237.17 and a retention time of Tr = 1.35 min; a phenolic compound, namely, D-delta-tocopherol (3), with a ratio m/z = 403.18 and a retention time of Tr = 8.33 min; and a flavonoid, namely, quercetin 3-O(-6 ″-acetyl glycoside) (5), with a ratio of m/z = 507.21 and a retention time of Tr = 10.20 min. The compound corresponding to peak 4 (m/z = 445.21) could not be identified (4); however, recent studies have reported other compounds that are closely related to this compound, such as kaempferol 3-O-glucoside, with a ratio of m/z = 447 [40].
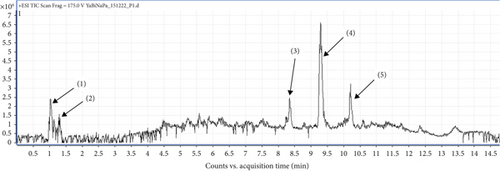
| Pics (signal) | Tr (min) | Fragment m/z | Pseudo molecular ion | Name of chemical compounds | Family |
|---|---|---|---|---|---|
| (1) | 1.05 | 118.08 | [M + H]+ | L- valine | Amino acid |
| (2) | 1.35 | 237.17 | [M + H] + | Neocurdione | Sesquiterpene |
| (3) | 8.33 | 403.18 | [M + H]+ | D-delta-tocophérol | Phenolic compound |
| (4) | 9.31 | 445.21 | / | N. d | / |
| (5) | 10.20 | 507.21 | [M + H]+ | Quercetin 3-O-(6”-acetyl glycoside) | Flavonoïd |
- Abbreviation: N.d, not determined.
3.1.3.3. SA
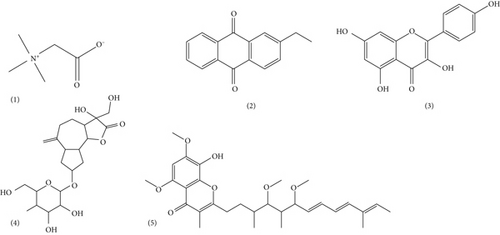
Finally, the chromatogram (Figure 7) of the hydroethanol extract of SA leaves presented five peaks whose characteristics and chemical structures are given in Table 7 and Figure 8, respectively. The chromatographic profile of the SA leaf extract revealed the amino acid betaine (1) with a ratio of m/z = 118.08 and a retention time of Tr = 1.02 min; an anthraquinone, namely, 2-ethylanthraquinone (2) with a ratio of m/z = 237.18 and a retention time of Tr = 1.31 min; a flavonoid, namely kaempferol (3) with a ratio of m/z = 287.04 and a retention time of Tr = 7.45 min; a sesquiterpene lactone, namely, Cyranoside A (4) with a ratio of m/z = 445.19 and a retention time of Tr = 9.29 min; and a phenolic compound, namely, Stigmatellin Y (5) with a ratio of m/z = 507.21 and a retention time of Tr = 10.23 min.
| Pics (signal) | Tr (min) | Fragment m/z | Pseudomolecular ion | Name of chemical compounds | Family |
|---|---|---|---|---|---|
| (1) | 1.02 | 118.08 | [M + H]+ | Betaine | Amino acid |
| (2) | 1.31 | 237.18 | [M + H]+ | 2-Ethylanthraquinone | Anthraquinone |
| (3) | 7.45 | 287.04 | [M + H]+ | Kaempferol | Flavonoïds |
| (4) | 9.29 | 445.19 | [M + H]+ | Cynaroside A | Sesquiterpen lactone |
| (5) | 10.23 | 507.21 | [M + Na]+ | Stigmatellin Y | Phenolic compound |
3.2. In Vitro Antifungal Activity of the Extract Blends
Table 8 presents the antifungal activities (MIC and MFC) of the extracts of the powders of ES, MO, and SA on pathogenic yeasts of the Candida genus, notably CA, CK, and CP. Significant antifungal activity of the extracts from the different preparations was observed.
| Powder blends (%) | Parameters | Candida albicans (CA) | Candida krusei (CK) | Candida parapsilosis (CP) |
|---|---|---|---|---|
| M1 (100/0/0) | MIC (μg/mL) | 250 | >500 | >500 |
| MFC(μg/mL) | 500 | ND | ND | |
| MFC/MIC | 2 | ND | ND | |
| M2 (0/100/0) | MIC (μg/mL) | 125 | >500 | >500 |
| MFC (μg/mL) | 250 | ND | ND | |
| MFC/MIC | 2 | ND | ND | |
| M3 (0/0/100) | MIC (μg/mL) | >500 | >500 | >500 |
| MFC (μg/mL) | ND | ND | ND | |
| MFC/MIC | ND | ND | ND | |
| M4 (80/10/10) | MIC (μg/mL) | 125 | >500 | 250 |
| MFC (μg/mL) | 250 | ND | 500 | |
| MFC/MIC | 2 | ND | 2 | |
| M5 (10/80/10) | MIC (μg/mL) | >500 | >500 | 250 |
| MFC (μg/mL) | ND | ND | 500 | |
| MFC/MIC | ND | ND | 2 | |
| M6 (10/10/80) | MIC (μg/mL) | 250 | 125 | >500 |
| MFC (μg/mL) | 500 | 250 | ND | |
| MFC/MIC | 2 | 2 | ND | |
| M7 (60/20/20) | MIC (μg/mL) | 250 | >500 | 125 |
| MFC (μg/mL) | 500 | ND | 250 | |
| MFC/MIC | 2 | ND | 2 | |
| M8 (20/60/20) | MIC (μg/mL) | >500 | 250 | 250 |
| MFC (μg/mL) | ND | 500 | 500 | |
| MFC/MIC | ND | 2 | 2 | |
| M9 (20/20/60) | MIC (μg/mL) | 250 | >500 | >500 |
| MFC (μg/mL) | 500 | ND | ND | |
| MFC/MIC | 2 | ND | ND | |
| M10 (40/30/30) | MIC (μg/mL) | >500 | 125 | 125 |
| MFC (μg/mL) | ND | 250 | 250 | |
| MFC/MIC | ND | 2 | 2 | |
| M11 (30/40/30) | MIC (μg/mL) | 250 | >500 | >500 |
| MFC (μg/mL) | 500 | ND | ND | |
| MFC/MIC | 2 | ND | ND | |
| M12 (30/30/40) | MIC (μg/mL) | >500 | >500 | >500 |
| MFC (μg/mL) | ND | ND | ND | |
| MFC/MIC | ND | ND | ND | |
| M13 (20/40/40) | MIC (μg/mL) | >500 | 125 | 250 |
| MFC (μg/mL) | ND | 250 | 500 | |
| MFC/MIC | ND | 2 | 2 | |
| M14 (40/20/40) | MIC (μg/mL) | >500 | >500 | 250 |
| MFC (μg/mL) | ND | ND | 500 | |
| MFC/MIC | ND | ND | 2 | |
| M15 (40/40/20) | MIC (μg/mL) | 250 | >500 | 125 |
| MFC (μg/mL) | 500 | ND | 250 | |
| MFC/MIC | 2 | ND | 2 | |
| Flu | MIC (μg/mL) | 0.5 | 0.25 | 1 |
| MFC (μg/mL) | 2 | 1 | 2 | |
| MFC/MIC | 4 | 4 | 2 | |
- Note: MFC/MIC ≤ 4: fungicidal effect; MFC/MIC > 4: fungistatic effect.
- Abbreviations: Extrac, t; Flu, fluconazol; MFC, minimum fungicidal concentration; MIC, minimum inhibitory concentration.
The 100% preparation, which represents the blends containing mainly the powder extracts of ES (M1), MO (M2), and SA (M3), had significant antifungal effects on only the yeast CA. The MIC and MFC were 250 and 500 μg/mL for M1 and 125 and 250 μg/mL for M2, respectively. No significant antifungal activity was observed against the yeasts CK and CP. The 20%, 40%, 60%, and 80% preparations, which represent the mass variations of the powders in the extracts of the different blends, show antifungal activities with MICs and MFCs of 125 and 250 μg/mL for M4, 250 and 500 μg/mL for M6, M7, M9, M11, and M15 on the yeast CA; 125 and 250 μg/mL for M6, M10, and M13; 250 and 500 μg/mL for M8 on the yeast CK; 125 and 250 μg/mL for M7, M10, and M15; and 250 and 500 μg/mL for M4, M5, M8, M13, and M14 on the yeast CP. The antifungal activity of fluconazole, which represents the reference antifungal agent, was 0.5 and 2 μg/mL for the yeast CA, 0.25 and 1 μg/mL for the yeast CK, and 1 and 2 μg/mL for the yeasts CP.
All blends that exhibited significant antifungal activities possessed fungicidal effects (MFC/MIC = 2) on the yeasts CA, CK, and CP. However, fluconazole exhibited a fungicidal effect on yeast CP (MFC/MIC = 2), CA, and CK (MFC/MIC = 4).
4. Discussion
This study is aimed at evaluating the in vitro antifungal activity of blends of leaf powder extracts from ES, MO, and SA against pathogenic yeasts of the Candida genus responsible for digestive mycoses (CA, CK, and CP). This objective allowed for the preliminary determination of the bioactive potential (presence or absence of bioactive compounds, content of certain bioactive compounds, and their names and chemical structures) of the extracts of the powders studied before any evaluation of antifungal activity. Each powder extract is rich in several bioactive compounds. Those observed in the ES extract are slightly different from those of other authors such as Effiong et al. [8] who highlighted alkaloids, flavonoids, saponins, tannins, and terpenes in the methanolic extract of ES leaves; while Mouokeu et al. [41] highlighted alkaloids, flavonoids, sterols, and triterpenes, but not phenols, saponins, or tannins in the methanol–methyl chloride extract. The results for the bioactive compounds obtained from the MO extract agreed with those of Mahdi et al. [42], who did not note the presence of steroids in the aqueous extract of MO. The bioactive compounds obtained from the SA extract are similar to those reported by several authors. These authors identified the presence of flavonoids, saponins, and tannins and the absence of steroids in the ethanolic extracts of SA leaves [43], unlike Pissang et al. [44], who noted the absence of alkaloids and saponins and the presence of flavonoids and tannins in the hydroethanolic extract of SA leaves. The differences observed between these results are due to numerous factors: the nature of the solvent, the extraction method, the contents of bioactive compounds, the chemical properties of each compound, and even functional groups that characterize the chemical structure of these molecules. This obviously influences the biological activities attributed to each bioactive compound family.
Alkaloids are a group of compounds known for their antimalarial, antimicrobial, and antiviral properties [45]. Flavonoids, which are also polyphenols; have antioxidant, anticancer, antiviral, and antimicrobial properties; and can combat oxidative stress [46, 47]. This large family of phenolic compounds inhibits the function of the cell membrane and fungal adhesion, the formation of biofilms, and the proliferation of biofilm dispersion cells [47]. Saponins have an expectorant effect and are also active against respiratory diseases such as cough and bronchitis. Some saponins have broad-spectrum antibacterial and antifungal properties [48]. Alina et al. [49] reported that saponins may cause severe damage to Candida through cell wall degradation with subsequent disruption of the cytoplasmic membrane and membrane proteins, leading to leakage of cell contents. Like flavonoids, tannins are also a subclass of phenolic compounds that possess similar biological activities. Triterpenes are distinguished by their anti-inflammatory and antimicrobial properties [48]. The multiple biological activities of these compounds, which are primarily chemical compounds, generally vary depending on several factors, some of which have been previously described. One of the most predominant factors is the content of each bioactive compound.
In terms of the content of certain bioactive compounds, the polyphenol contents obtained from the extracts of ES leaf powders were lower than those reported (297.40 ± 5.71 mg EAG/100 g DM) by Udofia et al. [50] for the ethanolic extracts of Eremomastax polysperma, another species of the same genus. However, the values reported by Kiani et al. [51] for the ethanolic extracts of MO leaves were also greater (16.2 ± 1.33 mg EAG/100 g DM) than those obtained in this study. On the other hand, the polyphenol contents of the SA extracts obtained in this study were greater than those reported (7.84 ± 0.49 mg EAG/100 g DM) by Abubakar et al. [52] for the methanolic extracts of SA leaves. Several factors can explain the differences observed between the polyphenol contents obtained, including the nature of the solvent, the extraction method, and the particle size of the powders. The extraction of total polyphenols is a crucial step for the valorization of bioactive compounds. This depends on the operating conditions and the method used. In recent work, the authors have shown that ethanol in combination with water allows for better extraction of total polyphenols [53]. Indeed, the addition of water to organic solvents, as in this study, increases the solubility of polyphenols by modulating the polarity of the organic solvent. This increase may be due to the weakening of hydrogen bonds in aqueous solutions and the increase in basicity and ionization of polyphenols in such solutions [54]. The solubility of polyphenols also depends on the number of hydroxyl groups, the molecular weight, and the length of the carbon chain of the basic skeleton [53]. In addition, the chemical nature of plant compounds varies from simple to highly polymerized, as plant materials can contain variable amounts of phenolic acids, phenylpropanoids, anthocyanins, and tannins. This diversity is responsible for the great variability of physicochemical properties influencing the extraction of polyphenols.
The content of flavonoids, a phenolic compound, in the extracts of the ES powders was roughly the same as those reported (3.05 ± 0.10 mg EQ/100 g DM) by Udofia et al. [50] for the ethanolic extracts of the ES leaf powders. However, the flavonoid contents of the MO leaf powder extracts were greater than those reported (10.1 ± 0.83 mg EQ/100 g DM) by Kiana et al. [51] for the ethanolic extracts of MO leaves. The flavonoid contents of the methanolic extracts of SA leaves were also observed. The contents are 42.28 ± 0.90 mg EQ/100 g DM [52]. For the MO and SA extracts, the TFCs (16.03 ± 0.17 and 60.48 ± 0.27 mg EQ/100 g DM, respectively) were greater than the total polyphenol contents (12.96 ± 0.02 and 25.02 ± 0.08 mg EGA/100 g DM), respectively. This can be explained by the fact that the assay method used to quantify total polyphenols is based on the reduction of the Folin reagent by the oxidizable groups of phenolic compounds. However, under certain conditions, flavonoids undergo methoxyl substitution and therefore end up in a derived form (methyl derivative) that is not phenolic, which suppresses their reactivity and therefore prevents their reaction with the Folin-Ciocalteu reagent [28]. The method of quantifying total flavonoids is based on the formation of a very stable complex between aluminum chloride and the oxygen atoms present on Carbons 4 and 5 of the flavonoids [30]. In addition to these observations related to the chemistry of these bioactive compounds, as with polyphenols, it is undeniable to mention that the extraction method can also influence the flavonoid contents obtained.
The condensed tannin contents of the studied ES leaf powders were lower than those reported (52.23 ± 1.43 mg EC/100 g DM) by Udofia et al. [50] for ethanolic extracts of Eremomastax polysperma leaves. The same observation was made with the condensed tannin contents of the methanolic extracts of SA reported (59.48 ± 0.50 mg EC/100 g DM) by Abubakar et al. [52]. On the other hand, in the MO extracts, the CTC was greater than that reported (1.45 ± 0.83 mg EC/100g DM) by Kiani et al. [51] for hydroethanolic extracts of SA. The condensed tannin contents of the three extracts were close and low, unlike those of the other phenolic compounds assayed, despite the use of the same solvent. However, the extraction rather offers high contents of total polyphenols and total flavonoids; this shows that the extraction method used, namely, maceration, seems to limit the attainment of high contents of condensed tannins. Indeed, Mahmoudi et al. [54], in a study on the extraction of phenolic compounds from the artichoke flower, showed that maceration was the best extraction technique for total polyphenols and flavonoids while decoction was preferable for the extraction of condensed tannins. Indeed, this method increases the content of condensed tannins due to the heat destruction of polyphenol oxidases (PPOs) which decreases the content of polyphenols, thus causing the rupture of bonds between polyphenols and other substances (proteins, polysaccharides, etc.) [36] making them more accessible in the process.
Regarding the chemical structures of the bioactive compounds contained in the extracts of the leaf powders of ES, MO, and SA, 14 distinct chemical structures were identified. They possess oxygenated, hydroxylated, carbonylated, and nitrogenous functions. Several authors have reported the biological activities of these chemical compounds [54, 55]. The antifungal activities of 20-Hydrobetulin; 29-carboxy-20-hydro-24-hydroxy-22-oxo-betulinic acid; and D-delta-tocopherol, quercetin 3-O-(6 ″-acetyl glucoside), and kaempferol are known via distinct mechanisms of action [54, 56]. The 18, 22, 24-tri dehydroglucopyranosyl moiety of stigmasterol has anti-inflammatory activity [32]. The 2-ethylanthraquinone Stigmatellin Y. has antibacterial activity [57]. However, very few studies have focused on the following compounds and their bioactive potential: tert-butyl(2,3 dimethylphenoxy) dimethylsilane, betaine, neocurdione, and cynaroside A. Taken individually, several of these bioactive compounds whose chemical structures are known have not always given satisfactory results when used alone in the treatment of mycoses; especially digestive mycoses [58]. The combination of these compounds via formulations is increasingly recommended in phytotherapy [59]. This allows the antimicrobial activities of these chemical compounds to be optimized against pathogenic germs. An experimental matrix was generated (Table 8) to evaluate the in vitro antifungal activity of the blends of extracts of the leaf powders of ES, MO, and SA on three yeasts, namely, CA, CK, and CP.
The antifungal activities of the blends of extracts of the leaf powders of ES, MO, and SA show particular effects when moving from one blend to another or from one preparation to another. The 100% preparation composed of three blends (M1, M2, and M3) showed that only the extract of SA (M3) had no significant antifungal effect on the three yeast strains studied, unlike the extracts of ES (M1) and MO (M2). This can be explained by the fact that the extracts of SA, unlike the others, contain chemical compounds with nonsignificant antifungal activity. However, it contains kaempferol (Table 5), which belongs to the flavonoid family. The latter must still be combined with sodium butyrate for significant antifungal activity, and its mechanism of action consists of obstructing biofilm formation [60]. Several authors have reported antifungal activities of flavonoids associated with other bioactive molecules [60, 61]. Moreover, the high contents of major bioactive compounds (Table 2) in the powder, notably polyphenols, flavonoids, and condensed tannins, are not sufficient to guarantee any antifungal activity. Prior identification of the chemical structures that characterize these compounds is essential.
The 20%, 40%, 60%, and 80% preparations highlighted the beneficial effects of the blends of extracts from the leaf powders of ES, MO, and SA. The addition of a small mass of MO powder to the other extracts significantly improved the antifungal activity of the preparations. In this regard, for successive increases of 20%, fungicidal effects are observed on CK (M6, M8, M10, and M13) and CP (M4, M5, M7, M8, M10, M13, M14, and M15), and no significant antifungal activity is observed on CA, except for the M11 blend (which has an indifferent effect). This result is explained by increases in the mass of the ES and MO extract powders, which have shown at least fungicidal effects on CA. These increases also imply an increase in the contents of bioactive compounds with fungicidal antifungal activities. The most important compounds are notably D-delta-tocopherol [62] and quercetin 3-O-(6 ″-acetyl glycoside), which act by breaking the cellular membrane of germs [63]. These two bioactive compounds, whose chemical structures have been identified in the extracts of MO leaf powders, are known for their multiple biological activities. In this regard, authors have reported the antifungal (CA, Saccharomyces cerevisiae, and Aspergillus fumigatus) [60, 63], antiviral [5], and antioxidant [56] activities of quercetin. D-delta-tocopherol is better known as an antioxidant [64] and very little known as an antifungal agent [62].
When comparing all these antifungal activities with that of the reference antifungal, fluconazole, the antifungal activities of the extract blends M2, and M4 against the yeast CA; M6, M10, and M13 toward the yeast CK; and M7, M10, and M15 toward the yeast CP were much greater. This difference can be explained by the different types of action mechanisms specific to the different compounds. Indeed, fluconazole is an azole antifungal that acts at the membrane sterol level by blocking the biosynthesis of ergosterol as a result of the inhibition of 14-sterol demethylase, a cytochrome p450 enzyme responsible for the transformation of lanosterol into ergosterol, which is essential for the fungal membrane. They present a broad spectrum of action including yeasts of the Candida genus except Candida glabrata, dimorphic fungi, Cryptococcus neoformans, dermatophytes, and the Aspergillus genus [65]. On the other hand, the bioactive compounds found in the blends act by a combination of action mechanisms ranging from the destruction of the cellular membrane, a change in membrane permeability, a reduction in the expression of virulence factors, and dysfunction of the mitochondria to the obstruction or inhibition of the formation of the yeast biofilm [46, 62].
5. Conclusion
It appears from this study that the extracts of leaf powders of ES, MO, and SA are rich in many families of bioactive compounds (alkaloids, flavonoids, polyphenols, saponins, triterpenes, and anthraquinones) whose structures include oxygenated and hydroxylated groups and carbonyl and nitrogenous groups. This confirms their high bioactive potential. Quercetin 3-O-(6 ″-acetyl glucoside) appears to be the main bioactive compound responsible for the fungicidal antifungal activities of the extracts from MO leaf powders and extract blends. This confirms that it is advantageous to mix bioactive compounds to enhance the functional benefits associated with plant extracts. The M10 blend (composed of 40% by mass of leaf powder in the ES extract, 30% by mass of leaf powder in the MO extract, and 30% by mass of leaf powder in the SA extract) had the best antifungal activity (fungicidal effect) with a broader spectrum. This blend can serve as a basis for in-depth studies to develop a drug based on these three plants in the fight against digestive mycoses associated with the Candida genus.
Nomenclature
-
- ES
-
- Eremomastax speciosa
-
- MO
-
- Moringa oleifera
-
- SA
-
- Senna alata
-
- CA
-
- Candida albicans
-
- CK
-
- Candida krusei
-
- CP
-
- Candida parapsilosis
-
- MIC
-
- minimum inhibitory concentration
-
- MFC
-
- minimum fungicidal concentration
-
- TPC
-
- total polyphenol content
-
- TFC
-
- total flavonoid content
-
- CTC
-
- condensed tannin content
-
- HPLC
-
- high-performance liquid chromatography
-
- MS
-
- mass spectrometry
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
R.J.Z.N.: experimentation, methodology, and data collection; J.A.A.A.: design, methodology, data analysis and processing, writing of the original draft, and correction; W.D.A. and D.S.A.O.: methodology and correction of the original draft; S.B.K., S.C.F., M.I.S., and K.S.S.: contributed to the bibliographic research and to the writing of bibliographic references; E.F.F.: design and methodology; F.A.A.T.: design, methodology, correction of the original draft, and supervision.
Funding
No funding was received for this manuscript.
Acknowledgments
The authors extend their gratitude to all members and leadership of the Laboratory of Process Engineering for the work completed in their facilities and the Faculty of Sciences of the Northeast Federal University of Yakurst for their open collaboration.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




