Influence of Cross-Linking by Microbial Transglutaminase on Physicochemical Properties of Eggshell Membrane Collagen
Abstract
Collagen from eggshell membrane (EM) was stabilized via a cross-linking procedure using microbial transglutaminase as an alternative, nontoxic cross-linking reagent for its low stability and low mechanical strength. The physicochemical features of collagen solutions (5 mg/mL), which were cross-linked by different concentrations of MTG (30, 40, 50, 60, and 70 U/g collagen), were examined. The SDS-PAGE, differential scanning calorimetry (DSC), residual amino group content, scanning electron microscopy (SEM), and viscosity of solutions were evaluated. The results revealed that applying higher concentrations of MTG in cross-linked collagen solutions leads to an increase of about 32°C–39°C in denaturation temperatures (Td) and a 75.76% decrease in residual amino group content. The simultaneous effect of different temperatures and concentrations of MTG on viscosity illustrated an improvement of approximately 30 Pa·s in the viscosity of cross-linked collagen at 50°C. The enzymatically cross-linked porous structures displayed finer microstructure with interlinked micron-sized pores, exhibiting a 74% reduction in size compared to the uncross-linked collagen. These results suggest that combining MTG and EM as sources of collagen represents an innovative potential route for the safe cross-linking of collagen-based matrices. This innovation could have practical applications in reducing waste EM, which is a by-product of egg breaking in industrial and domestic settings, and utilize them to produce firmness and stabilize collagen.
1. Introduction
Collagen, a fibrous macromolecule of a glycoprotein origin, is classified as the most abundant structural protein of vertebrates, constituting around 30% of animal protein. Collagen as a biomaterial used in commercial products is principally produced from rats, cows, bovines, and pigs; however, the prevalence of mammalian diseases has raised concerns among consumers about the risk of disease transmission to humans [1]. Therefore, alternative sources have received increasing attention since the potential alternative to mammalian or land animals’ collagen. The results of cytotoxicity, genotoxicity, and biochemical tests have demonstrated that the by-product eggshell membrane (EM) collagen is safely consumed, regarding the risk of autoimmune and allergic reactions [2].
EM, a by-product of egg breaking, is an abundant waste material with more than 9.825 million tons produced worldwide annually that can be reused in multifunctional applications such as electrical devices, biosensors, biomedical engineering, environmental engineering, and energy harvesting [3]. This waste is environmentally friendly and is readily available at a low cost. Moreover, it is nontoxic and composed of various natural proteins. Additionally, it can be modified through processes such as carbonization and dissolving. EM contains collagen, which constitutes 8% of the total weight after going through the extraction process involving acid solvents [4]. Although the quantity of EM collagen is relatively low compared to other food waste–derived collagen sources such as bovine, poultry, sheep, porcine, and marine animals like jellyfish, sandfish, sharks, and teleosts [5, 6], its substantial global annual production highlights the significance of investigating its potential applications. Therefore, it could potentially serve as a substitute for collagen obtained from mammalian sources [4].
Given the low durability of collagen to external factors such as elevated temperature or the presence of enzymes, its structure requires stabilization, which can be achieved through cross-linking techniques [7]. Cross-linking is a crucial process that can transform weak gels into more durable and strong materials by catalyzing covalent or noncovalent bonds by linking polymer chains [8, 9]. Cross-linking is accomplished through the utilization of four distinct categories of agents to enhance collagen-based biomaterials: chemical, physical, photochemical, and enzymatic agents. The most prevalent cross-linking technique employed for collagen treatment is chemical cross-linking, which can be toxic and biologically incompatible, often overshadowing their cross-linking potential [7]. For example, there are concerns regarding glutaraldehyde’s tendency to provoke localized cytotoxicity and induce significant inflammatory responses [10]. Additionally, removing chemical agents requires thorough washing [11]. Chemical agents such as glyceraldehyde [12], genipin [13], and EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide)-NHS(N-hydroxysuccinimide) [14] have been discussed previously. The second cross-linking technique involves physical collagen cross-linking methods, including dehydrothermal drying (DHT) and UV irradiation. These methods typically yield weaker bonds than chemical reagents [7, 8]. Additionally, the combination of prolonged exposure and very high temperatures results in collagen deformation [15]. The third technique involves photochemical reagents, such as riboflavin which have recently garnered significant attention [16]. The final cross-linking method is biological or enzyme-induced, involving agents such as lysyl oxidase and transglutaminase (TG) [7]. Enzymatic cross-linking is recognized as one of the most accepted methods for modifying protein properties, offering an alternative, nontoxic, and efficient approach for altering protein materials [9].
TG is a natural enzymatic cross-linking agent which catalyzes an acyl-transfer reaction wherein the γ-carboxamide functionalities of glutamine residues within peptides operate as the acyl contributor and, for the most part, the Ԑ-amino groups of lysine residues or other inherent primary amino groups act as the acyl recipient, enabling protein polymerization [17–19]. Microbial transglutaminase (MTG), derived from various species of microorganisms, is a cost-effective, calcium-independent enzyme with a low molecular weight. It is widely utilized in food processing due to its stability across diverse pH and temperature ranges, commercial availability, and ability to enhance the physical properties of protein-based materials, including collagen [20–22]. MTG has various applications in food products, including meat [23], seafood [24], pasta [25], dairy [26], and bakery [22], as well as food packaging [27]. The MTG used in this work was isolated from the culture broth of Streptoverticillium mobaraense (also known as Streptomyces mobaraensis), which has an enzyme activity of 2.95 U/mL [28].
To the best of our knowledge, no studies concerning MTG-based cross-linking of collagen obtained from EM are available in the literature. This study is aimed at assessing the impact of MTG on the physicochemical properties of EM collagen. The electrophoretic characterization of cross-linked collagen samples, differential scanning calorimetry (DSC), residual amino group content (RAC), scanning electron microscopy (SEM), and viscosity of uncross-linked and cross-linked collagen samples were determined. Attempts have been made to elucidate the change in physicochemical properties based on the nature of the cross-linking linkages.
2. Materials and Methods
2.1. Chemicals and Reagents
The raw EM was obtained from a local confectionery store and promptly placed in ice-cold distilled water. All chemicals used were of analytical grade. Pepsin from porcine gastric mucosa with an activity of 500 U/mg proteins was used (Sigma-Aldrich Company (St. Louis, Missouri)). Sodium hydroxide, sodium chloride, Tris (hydroxymethyl) amino methane, and acetic acid were procured from Merck Company (Darmstadt, Germany). Glutaraldehyde (1%, v/v) and formaldehyde (3%, v/v) were obtained from Sigma-Aldrich Company. Additionally, cacodylate buffer (0.1 M) and DMSO (2.5%, v/v) obtained from Fisher Scientific Company. TG (E.C. 2.3.2.13) was derived from the culture broth of S. mobaraense, which was supplied by Ajinomoto Company Ltd. (Tokyo, Japan). Type I collagen was obtained from Sigma-Aldrich Company (St. Louis, Missouri).
2.2. Extraction of EM Collagen
Collagen was extracted from EM according to the methods of Mohammadi et al. [29] with slight modifications. The outer EM was manually removed, rinsed with distilled water, and dried at room temperature. Subsequently, the dried EM was powdered. The EM powder was soaked in 0.7 M NaOH (1:10 (w/v)) with stirring for 72 h at 4°C, with the alkaline solution being changed every 4 h. The alkaline medium (NaOH) facilitates the solubilization of proteins and pigments and prevents the enzymatic degradation of collagen. After rinsing with distilled water until neutral pH was attained, the residues were treated with 0.5 M acetic acid and 120 U/mg pepsin (1:3000) for 48 h with stirring. Pepsin treatment proved effective at cleaving the nonhelical end region (telopeptides) of collagen from EM without damaging the three-dimensional structure of the triple helix of collagen [29]. To eliminate undissolved residues, the solution was filtered through a double layer of cheesecloth. Salt was subsequently added to precipitate the mixture, with the final concentrations of NaCl and Tris (hydroxymethyl) amino methane reaching 2 and 0.05 M (pH 7.0), respectively. The resulting precipitates were separated by centrifugation at 402 RPM and solubilized in 0.5 M acetic acid. Subsequently, the solution was dialyzed against the 0.5 M acetic acid and deionized water using a dialysis bag with a molecular weight cut-off of 30 kDa for 12 h at 4°C. The dialysates were freeze-dried using an Alpha 2–4 LSC freeze dryer (Martin Christ, Osterode am Harz, DE, Germany).
2.3. Preparation of Enzymatic Cross-Linked Collagen (CC) Matrices
MTG exhibited an enzyme activity of 500 U/g on a dry basis. EM collagen powder was dissolved in 0.1 M acetic acid solution (pH 5.0) to prepare a final concentration of 10 mg/mL. The collagen solution was supplemented with five concentrations of MTG (30, 40, 50, 60, and 70 U/g collagen), designated as UC (uncross-linked collagen), TG30 (30 U/g), TG40 (40 U/g), TG50 (50 U/g), TG60 (60 U/g), and TG70 (70 U/g), and was incubated at 30°C for 30 min. Enzyme-mediated cross-linking solutions were then stirred continuously at room temperature (25°C) for 12 h. The enzymatic reaction was halted by freezing at −40°C, and porous collagen matrices were obtained by lyophilization.
2.4. Identification of Enzymatic CC
2.4.1. Sodium Dodecyl Sulfate Polyacrylamide-Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was conducted according to the procedure of Carvalho et al. with slight modifications [1]. Collagen samples (1 mg/mL) were solubilized in 0.02 M sodium phosphate buffer (pH 7.2) containing 1% (w/v) SDS and 3.5 M urea. The sample blends were gently stirred at 4°C for 12 h to achieve complete dissolution of the proteins. Samples were loaded onto the polyacrylamide gels containing 10% running gel and 4% stacking gel, and electrophoresis was carried out at a constant current of 20 mA per gel. Following electrophoresis, the gel was stained with 0.05% (w/v) coomassie blue R-250 in 15% (v/v) methanol and 5% (v/v) acetic acid and then destained using 30% (v/v) methanol and 10% (v/v) acetic acid, until the bands were prominent. High molecular weight markers, including myosin (200 kDa), macroglobulin (170 kDa), beta-galactosidase (116 kDa), transferrin (76 kDa), and glutamate dehydrogenase (53 kDa), were used to estimate the molecular weight of proteins.
2.4.2. DSC
The thermal denaturation temperature (Td) of UC, TG30, TG40, TG50, TG60, and TG70 collagens was determined using a DSC (PerkinElmer DSC pyris-1, United States). Approximately 5 mg of freeze-dried samples was dissolved in 50 mM acetic acid, sealed in aluminum pans, and loaded into the DSC. A reference was established using an aluminum pan containing 0.5 M acetate buffer solution. The temperature was increased from 10°C to 100°C at a heating rate of 5°C/min. The Td of collagen corresponds to the maximum endothermic point of the curve.
2.4.3. RAC
The RAC was evaluated using the trinitrobenzene sulfonic acid (TNBS) spectrophotometric method reported by Chen et al. with slight modifications [30]. A sample of collagen (0.16 g) was combined with borax buffer (1.2 mL, pH 10.0) and 1.2 mL of a freshly prepared solution of 0.1% (v/v) TNBS in a glass tube. Subsequently, 2.4 mL of 0.1 M HCl was added to the solutions at room temperature (20°C) after a 60-min reaction period in the dark at 50°C. Approximately 30 min after the initial addition of the reagents, the absorbance of the combined solution was quantified at 340 nm via a UV spectrophotometer (Shimadzu-UV200). All samples were evaluated in triplicate.
2.4.4. SEM
The collagen specimens were prepared according to the method described by Schuetz et al. with the key differences being that the percentages of glutaraldehyde and formaldehyde were 1% and 3% (v/v), respectively [31]. Samples were mounted on SEM stubs (carbon adhesive, Ted Pella) and coated with a layer of gold using a sputter coater (VEGA, TESCAN). The samples were then grounded with the conductive paint and prepared for SEM imaging (INCA Penta FET-x3 module by Oxford Instruments, United Kingdom).
2.4.5. Viscometry of UC and Enzymatic CC Solutions
A viscometer (DV-II, Brookfield, Middleboro, Massachusetts) was used to measure the rheological properties of the collagen solutions before and after cross-linking with different concentrations of MTG. For this measurement, the method of Tabarestani et al. was used and all the samples were measured in triplicate [32].
2.5. Statistical Analysis
The data were reported based on three evaluations. The data obtained underwent statistical analysis using a one-way ANOVA, and the probability of statistical significance was set at p < 0.05. All data analyses were performed using the R software package.
3. Results and Discussion
3.1. Electrophoretic Characterization of Collagens
Figure 1 shows SDS-PAGE patterns of the UC, TG30, TG40, TG50, TG60, and TG70 collagens. In these gels, α1, α2, β, and γ chains can be observed. The isolated EM collagen in this pattern showed two α chains with molecular weights of 114 kDa for α1 chain and 98 kDa for the α2 chain. The relative concentration of the α2 chain is lower than that of the α1 chain. It has been previously described that an α3 component may be present [33]. The α3 chain has a similar movement to the α1 chain and cannot be detected separately from the α1 chain under the applied electrophoretic conditions [1]. High molecular weight components, including β chain (dimeric shape) with a molecular weight of around 200 kDa and the γ chain (trimeric shape) with a molecular weight of around 200 kDa, were also observed in the collagen samples. The presence of two different α subunits indicates that the main type of collagen in the EM is Type I collagen [34]. Additionally, the molecular weight of MTG was low (around 37 kDa) with overall dimensions of 65 × 59 × 41 Å as reported by Duarte et al. [35].
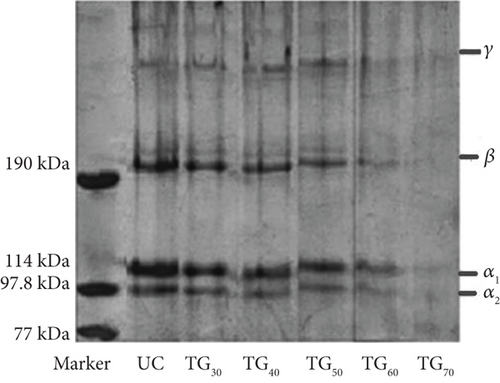
As shown in Figure 1, the CCs were significantly different from the UC. Increasing MTG concentration decreased the intensity of the α and β bands, while the γ band exhibited an initial increase, followed by a subsequent decrease. Additionally, the bands of TG60 disappeared, suggesting extensive cross-linking [36]. This phenomenon might be due to the formation of intermolecular cross-links by MTG, which are insoluble aggregates and are SDS-resistant, preventing complete denaturation and migration [37].
A notable disparity was observed in the subunit molecular masses between UC, TG30, TG40, TG50, and TG60 collagens. Upon treatment with MTG, the intensity of the high molecular weight band increased relative to the untreated samples, demonstrating that TGase effectively induced cross-linking within the collagen [9]. This finding was corroborated by Wu et al. [38]. The electrophoretic patterns of extracted collagen from EM were similar to those of collagens from hake, blue shark, calf [39], marine eel fish [40], sheepshead seabream, and black drum [41].
3.2. DSC Measurement
Figure 2 displays the DSC thermograms of UC and CC solutions. The organization of collagen in a solution depends on the proportion of triple helices to coil structures. When the proportion of molecules in a coiled state reaches 90%, denaturation occurs. Conversely, when at least 70% of molecules maintain their triple helical conformation, the collagen remains undenatured [1]. The Td reflects the transition of collagen from a triple helical conformation to a random coil, identified as the maximum point of the endothermic response in the DSC graph [1]. DSC was conducted to ascertain the Td values of the CCs and explore the impact of MTG on the thermal stability of collagen solutions. It is noteworthy that the optimum reaction temperature of S. mobaraense TG is 55°C [22].
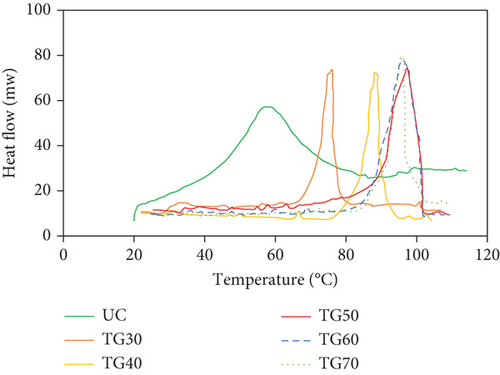
A large endothermic peak was observed at approximately 57°C in the case of UC. This peak can be attributed to a complex thermo-transition process, which includes reducing the triple helix content of collagen and degrading it into smaller polypeptide chains [9]. Additionally, gelatin is derived from the hydrolysis of collagen, which undergoes thermal pretreatment under acidic conditions. It primarily comprises amorphous polypeptide chains along with several smaller peptide fragments [9].
The Td of the collagen solutions was determined to be 57.00, 75.18, 89.23, 96.39, 96.43, and 96.47°C for UC, TG30, TG40, TG50, TG60, and TG70, respectively. Treatment with MTG resulted in an approximate 18°C increase in TG30. Moreover, an increase of about 32°C–39°C in TG40, TG50, TG60, and TG70 was observed for samples exposed to higher concentrations of MTG. The Td values of the CC exhibited a shift toward higher temperatures compared to the UC. Therefore, the thermal stability of collagen increased with enhanced cross-linking, and the temperature required for the denaturation of CC was observed to be higher compared to that of UC. This phenomenon might be attributed to the isopeptide bond catalyzed by MTG, which bundles collagen fibers and molecules together, leading to the creation of a more stable conformation [9]. Additionally, MTG may have provided hydrogen bonding sites for water, facilitating the formation of a stable network structure within the film and inhibiting water evaporation from the dense film matrix due to the reduction of intermolecular space [42]. Our results were consistent with the findings reported by Orban et al., demonstrating that an increase in enzyme concentration resulted in elevated Td [43]. The elevated transition temperature indicates that the collagen is more stable in high-temperature environments. Furthermore, thermal stability played a crucial role in the longevity of collagen-based biomaterials [44]. Our findings are consistent with those of Sallehuddin et al., who found that Td of two combinations of ovine collagen–gelatin–elastin without cross-linking was 64.5–69.12°C and 57.37–61.29°C; however, after cross-linking with genipin, the Td increased to 76.89–81.70°C and 92.50–99.75°C [45]. The thermal stability of collagen-based composite films was improved via cross-linking with TG according to the research by Wu et al. [38]. Additionally, the degradation temperature of collagen fiber cross-linked by 50% casein compound films was higher than that of uncross-linked proteins (306°C and 227°C, respectively) [46]. However, the study conducted by Ricotti et al. revealed that the Td of jellyfish collagen does not change with the degree of cross-linking by genipin, as evidenced by the temperature peak remaining constant at approximately 57°C across all concentrations of genipin [47]. Therefore, despite using the same cross-linking agent in the two mentioned studies, Sallehuddin et al. [45] and Riacci et al. [47], this discrepancy in results suggests that the Td might be affected by factors other than the collagen sources and cross-linking agents.
3.3. RAC
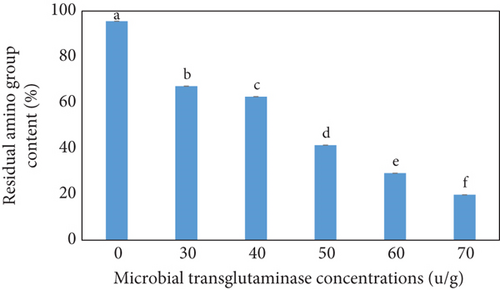
The percentage of RAC of the CC as a function of the MTG concentration is shown in Figure 3. The concentration of RAC decreased slightly from 95.58% for UC to 67.21% and 62.64% for TG30 and TG40, respectively, and then decreased sharply to 41.47%, 29.24%, and 19.82% for TG50, TG60, and TG70, respectively, with a further increase in MTG concentration. In other words, the concentration of residual amino groups exhibited a gradual decline as the MTG concentration increased from 0 to 40 U/g and then rapidly decreased, with a further increase in MTG concentration. According to the statistical analysis results (obtained from Duncan and ANOVA tests), a significant difference among the groups with varying concentrations was observed (p < 0 05), as indicated by the significance letters in Figure 3. Given that the standard deviations were exceedingly small and negligible, the averages were found to be distinctly different. RAC was still present at elevated MTG concentration, which may be related to the unavailability of reactive lysine and glutamine residues for MTG action [36]. Another reason might be that when self-polymerization of MTG occurred, larger molecules were created, which might not be able to access the core of collagen aggregates due to steric hindrance [48].
3.4. SEM
Representative SEM images of collagen matrices before and after cross-linking treatment with different MTG concentrations are illustrated in Figure 4. The original collagen showed highly interconnected pores (Figure 4a). However, the collagen matrices exhibited smaller interconnected pores after cross-linking (Figures 4b, 4c, 4d, 4e, and 4f). Therefore, MTG collagen cross-linking could modify the microstructure of the matrix. Such fine microstructures with interconnected micro-sized fibers creating micron-sized pores were not present in the UC. On average, an increase in MTG concentration resulted in a 76% reduction in pore size. These alterations may be attributed to the development of a more compact and cross-linked protein network [49]. The observed morphological changes are consistent with the SDS-PAGE profile, which indicated that the intensity of the high molecular weight band increased relative to the untreated samples. This outcome is in agreement with the results of Chen et al., who found that the microstructures of individual caseins treated with MTG became more compact [50]. Additionally, several other studies have reported similar results [49, 51].
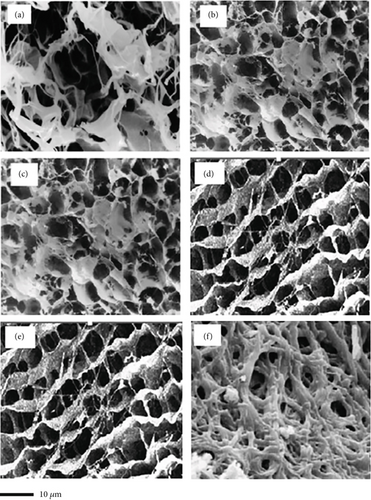
There was no notable difference in the morphology of CCs, especially between TG30 and TG40, as well as between TG50 and TG60. The only difference was attributed to the smoother and less textured matrix microstructure in the TG30 and TG50 compared to TG70. The roughness of the surface of TG70 resulted in an irregular cross section, which may be related to the formation of covalent and noncovalent interactions between protein chains induced by the enzyme [52]. Higher concentrations of MTG had no significant impact on the morphology of collagen matrices. This observation is consistent with recorded data demonstrating that the microstructure of jellyfish collagen samples was not significantly affected at different concentrations of genipin [47].
This reveals that cross-linking with MTG can stabilize the bonding strengths between molecules and fibers, allowing the strengthened microstructure to withstand the freeze-drying process. These diverse microstructures produced through cross-linking with MTG might be related to the increased physicochemical properties of CC matrices, created by the gradual removal of water molecules from the well-structured and reinforced collagen network.
3.5. Viscometry of Collagen Solutions
Figure 5 illustrates the changes in viscosity that occur upon heating, a fundamental physicochemical property of collagen. The high viscosity is attributed to the large percentage of β and γ chains, which collectively result in a higher average molecular mass. Application of elevated temperatures, especially at 45°C and higher, during heat treatment results in the rupture of hydrogen bonds between protein chains and the formation of gelatin polypeptide chains, which contribute to the reduction of intermolecular gaps, subsequently resulting in a decline in viscosity [9, 53].
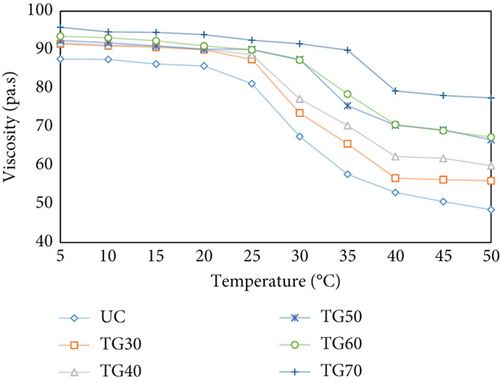
As shown in Figure 5, MTG incorporation into collagen solutions led to a net increase in viscosity. Moreover, as the concentration of MTG increased, the enhancement in viscosity was more pronounced. This can be attributed to enhanced cross-linking of collagen molecules, which promotes the formation of a more stable and interconnected network, creating intermolecular bridges between collagen fibrils; a continuous 3D network forms, sharply increasing viscosity [37]. A comparable investigation demonstrated that increasing the fructose concentration in collagen-based scaffolds cross-linked by fructose enhances viscous behavior [54]. Similarly, Pratiwi et al. reported that increasing the TG concentration enhances thickness, resulting in a more condensed structure due to increased hydrogen bond formation through enzymatic cross-linking [55]. The relative viscosity exhibited a continuous decline with increasing temperature of the collagen solution. For UC, TG30, and TG40, there was a sudden decrease around 30°C; for TG50 and TG60, there was a sudden decrease around 35°C; and for TG70, a sudden decrease occurred around 40°C. In the temperature range of 30°C–50°C, the reduction rate was maintained. The aggregation of collagen fiber bundles and the subsequent release of water resulted in a decrease in viscosity [9]. A similar occurrence was noted in an earlier report at temperatures below 30°C, where a decrease in the yield value of collagen suspensions was attributed to collagen fiber precipitation [56]. An analogous study illustrated that a decline in viscosity began at 30°C, reduced completely at 40°C in the case of black drum, and 39°C for sheepshead; above 40°C, a low viscosity was maintained in both cases [57]. These results indicate that temperature has a more significant influence on viscosity than the enzyme-induced cross-linking effect. This finding aligns with a study that reported that as the temperature increased, the rate of degradation increased, regardless of the presence of TG. This can be explained by the fact that pyrolysis temperature affects the amount of triple helix structure in collagen [9].
4. Conclusion
This study discovered that a large concentration of eggshells is dumped as waste, and its study demonstrated that it is feasible to utilize EM as a significant collagen source. The physicochemical properties of EM collagen solutions cross-linked by different concentrations of MTG were evaluated.
The simultaneous effect of different temperatures and concentrations of MTG on viscosity revealed that an increase in cross-linking concentration resulted in a corresponding increase in viscosity. However, increasing the temperature was observed to accelerate the degradation rate, regardless of the presence or absence of TG. In addition, the thermal stability of CC matrices increased compared to the UC samples. MTG collagen cross-linking could modify the microstructure of the matrix resulting in smaller interconnected pores due to a 76% reduction in pore size. Moreover, the RAC of UC significantly decreased (75.76%) through MTG enzymatic cross-linking. Consequently, this nontoxic enzymatic cross-linking agent was recognized as simple to utilize through in situ cross-linking treatment. MTG collagen cross-linking is an effective technique to provide collagen matrix with strengthening and stabilizing microstructure and it can also improve the physicochemical properties of the collagens. Further studies are required on the physicochemical properties and biological functions of EM collagen as a new resource. Using collagen from different egg batches and investigating the MTG enzymatic activity on EM collagen in the presence of ions such as Co2+, Ba2+, and K+ can be explored in future research. It is noteworthy that the scale and physical conditions of this study could differ from those encountered in industrial settings, which would require different considerations of determination and optimization in production scales. Additionally, the long-term effects of collagen cross-linking should be investigated in future studies.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The research is supported by University of Tehran (10.13039/501100004481) and Shiraz University (10.13039/501100005071).
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




