Phytochemical Investigation and Biological Activities of Ruta chalepensis Methanolic Extract: Antioxidant, Anti-Inflammatory, Anticollagenase, and Antielastase Properties
Abstract
Ruta chalepensis has long been recognized in traditional medicine for its diverse pharmacological properties. The primary aim of this study was to analyze the phytochemical composition and evaluate the antioxidant, anti-inflammatory, anticollagenase, and antielastase activities of the methanolic extract derived from R. chalepensis. The extract was subjected to phytochemical screening using LC-MS analysis. For evaluation of the antioxidant activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays were used. The anti-inflammatory properties of the extract were investigated using a protein denaturation bioassay. Finally, fluorometric screening kits assessed the anticollagenase and antielastase activities. The phytochemical screening identified 33 compounds, the dominant being 3,3′,4,5,7-pentahydroxyflavone (9.4%), and myricetin (8.9%). The extract showed antioxidant activity, with IC50 values of 30.1 μg/mL for the DPPH assay and 25.4 μg/mL for the ABTS assay. Moreover, the extract displayed significant inhibition of protein denaturation across a range of concentrations. Additionally, the methanolic extract exhibited a dose-dependent inhibitory effect on collagenase and elastase activities. The highest concentration tested, 5%, achieved 41.7% for collagenase inhibition and 62.4% for elastase inhibition. In summary, the methanolic extract of R. chalepensis showcases promising antioxidant and enzyme inhibitory activities, hinting at its potential for a wide array of therapeutic applications.
1. Introduction
Medicinal plants have been a vital component of traditional medicine for ages because of their curative qualities. Numerous traditional uses have been confirmed by modern research, which highlights three main health benefits: antioxidant, anti-inflammatory, and antiaging [1]. These attributes are essential for coping with the complex aging process, marked by decreased physiological functions and an increased vulnerability to disease [2].
Aging is characterized by the progressive reduction of physiological functions and changes in tissue structure, a multifaceted biological phenomenon [2]. Since oxidative stress causes an imbalance between the body’s capacity to neutralize reactive oxygen species (ROS) with antioxidants and the excessive creation of ROS, it is imperative to comprehend ROS’s role in this process [2]. This imbalance causes lipids, proteins, and DNA to get damaged within the cell, hastening aging and raising the risk of diseases such as cancer, heart problems, and neurological disorders [3]. Similarly, chronic inflammation worsens aging by causing tissue damage and promoting disease development. As though acute inflammation serves as a protective response, chronic inflammation—triggered by factors such as genetics, environmental pollutants, and lifestyle choices—can persist and cause harm [4].
Degrading vital skin proteins by enzymes such as collagenases and elastases, mainly collagen and elastin, is one of the most critical consequences of oxidative stress and inflammation [5]. The strength and suppleness of tissues, including skin, blood vessels, and connective tissues, depend on these structural proteins. Wrinkles, decreased skin elasticity, and tissue weakness are prompted by the breakdown of collagen and elastin, which is facilitated by the increased activity of collagenases and elastases as we age [5, 6]. External factors, such as UV radiation, pollution, and poor dietary habits, further accelerate this degradation by promoting oxidative stress and inflammation [7].
Antioxidants and anti-inflammatory agents are essential in mitigating oxidative stress and inflammation, making them crucial for healthy aging [8]. The body’s enzymatic antioxidants—catalase, superoxide dismutase, and glutathione peroxidase—neutralize ROS and reduce oxidative damage [9]. Nonenzymatic antioxidants from external sources, such as vitamins C and E, glutathione, body oils, and flavonoids, also contribute by directly scavenging ROS and enhancing the activity of antioxidant enzymes [9]. Their ability to combat oxidative stress suggests potential solutions for aging [10–12]. Medicinal plants, rich in natural antioxidants such as phenolic acids, flavonoids, tannins, and terpenoids, offer substantial protection against oxidative stress and inflammation [13, 14].
The antiaging benefits of medicinal plants include promoting health, extending cellular longevity, stimulating collagen production for improved skin health, and protecting and repairing DNA. Notable examples include Ginkgo biloba, Panax ginseng, Curcuma longa (turmeric), and Camellia sinensis (green tea), which contain bioactive compounds—flavonoids, ginsenosides, curcumin, and catechins—that provide these protective and rejuvenating effects [15].
As a member of the Rutaceae family, Ruta chalepensis is known for its versatility. In some countries, its leaves are used as a food-decorating herb. The Ruta genus is widely distributed, particularly in Mediterranean climates [16]. The plant thrives in rocky, mountainous areas and is well-suited to dry well-drained soils. Its resilience to harsh conditions and aromatic properties has made it valuable for culinary and medicinal uses in its native regions.
Several Ruta species exhibit various activities, including antioxidant [16–18], anti-inflammatory [18, 19], antibacterial [17], neuroprotective [19], anticancer [17, 20], and antihyperlipidemic properties [21].
In Jordan, Ruta chalepensis is a highly aromatic plant that thrives on the rocky slopes of mountains [17, 22]. Its edible flowers and leaves are rich in flavor and offer unique health benefits. It has been traditionally used to relieve pain, reduce fever, fight inflammation, relax muscles, and treat convulsions [17].
In conclusion, medicinal plants’ antioxidant, anti-inflammatory, and antiaging properties present a compelling natural approach to combating the adverse effects of aging. These plants offer a comprehensive method for enhancing quality of life and extending longevity by reducing oxidative stress, curbing inflammation, and promoting cellular health. This study investigates the phytochemical constituents of the methanolic extract of R. chalepensis and its antioxidant, anti-inflammatory, anticollagenase, and antielastase activities.
2. Materials and Methods
2.1. Plant Material Collection
In the spring of 2023, aerial parts of R. chalepensis were collected from Amman city, Jordan. The plant material was identified and authenticated by Prof. Sawsan Oran. A voucher specimen, numbered RC/2023/20, has been deposited in the Herbarium of the Department of Biological Sciences in Amman, Jordan.
2.2. Preparation of Methanolic Extract
The aerial parts were air-dried in the dark at room temperature for 6 weeks. After drying, they were powdered using an electric blender and extracted with methanolic at a ratio of 1:10 w/w%, following the method described by Abirami et al. [23]. The solvent was subsequently distilled, and the resulting extract was stored at 4°C.
2.3. Phytochemical Analysis
The extract was analyzed using a Shimadzu liquid chromatography system (Tokyo, Japan) equipped with the following modules: CBM-20A Control Bus Module, CTO-30A Column Oven, LC-30AD Liquid Chromatograph, SIL-30AC Auto Sampler, and the LCMS-8030 Triple Quadrupole Mass Spectrometer, which included an electrospray ionization (ESI) source.
The crucial chromatographic separation was performed on a high-quality Primesil RP-C18 column (150 × 4.6 mm, 5 μm). Mobile phase A consisted of water/acetonitrile (80:20% v/v) with 0.01% formic acid (pH 3), while mobile phase B was acetonitrile containing 0.01% formic acid. Both solvents were sourced from Merck.
The samples were meticulously prepared by dissolving the extracted compound in 2 mL of methanol. LC-MS/MS analysis was conducted with a 10 μL injection volume.
The mobile phase’s flow rate was constant at 0.7 mL per minute, and the column temperature was 25°C. The gradient elution program began with 20% mobile phase B, increased to 50% B by 18 min, then ramped to 100% B at 20 min, and held for 1 min. At 22 min, the gradient was reset to 20% B and maintained for 2 min. The total run time was 31 min.
Before injection, all solvents and extracts were filtered through a 0.45 μm PTFE syringe filter. The samples’ mass spectra and retention indices were compared against the NIST 2016 mass spectral library to identify the compounds present in the plant extract.
2.4. 1,1-Diphenyl-2-Picrylhydrazyl Radical Scavenging Assay
2.5. ABTS + Scavenging Assay
An innovative method for assessing antioxidant activity was incorporated using the ABTS solution. The process began by mixing 7 mM ABTS solution and 2.4 mM potassium persulfate solution in equal parts (1:1). This mixture was then incubated at room temperature for at least 14 h to allow optimal development of the reactive properties. After incubation, the solution was diluted with methanol until its absorbance reached approximately 1.0 at λ = 734 nm, a standard step in the ABTS + scavenging assay. Samples were then prepared at 10–1000 μg/mL concentrations, with 1 mL of each extract combined with 1 mL of the ABTS solution. The absorbance was determined with a UV/VIS spectrophotometer at 734 nm [24]. A solution of 1 mL ABTS and 1 mL methanol was used for the control. A positive control was provided by AA. All measurements were carried out in triplicate for each extracted sample.
2.6. Anti-Inflammatory Assay
2.6.1. Protein Denaturation Bioassay
The protein denaturation bioassay described by Sailović et al. [25] is a reliable method for assessing the anti-inflammatory activity of plant extracts. Potential therapeutic uses of the extract in inflammatory circumstances can be ascertained by assessing its capacity to suppress protein denaturation. This method involves preparing a reaction with 2 mL of plant extract (from each concentration 31.25–1000 μg/mL), 0.2 mL of egg albumin, and 2.8 mL of phosphate-buffered saline (PBS) at pH 6.4. Then, a mixture was heated to 70°C for five minutes to promote denaturation, and then, it was incubated at 37°C for 15 minutes. It is then cooled to room temperature. Using the spectrophotometer, the absorbance measured at 660 nm allows for the calculation of percent inhibition, providing a quantitative assessment of the extract’s anti-inflammatory potential. The control sample was created using the same method but omitting the plant extract (PBS and egg albumin). The extract’s efficacy is further validated by comparison with positive control, diclofenac sodium (31.25–1000 μg/mL), suggesting it has potential as a therapeutic agent for inflammatory conditions.
2.7. Anticollagenase Activity Determination
A screening kit (Abcam (ab211108), Cambridge, UK) was used to assess the inhibitory effect on the collagenase activity of the R. chalepensis methanolic extract. The analysis involved precisely utilizing various extract concentrations (0.5%, 1%, 2.5%, and 5%). Samples were prepared on a 96-well plate with a clear and flat bottom. In the initial step, collagenase was dissolved in a collagenase assay buffer (CAB). The test samples were created by mixing the extract and testing it with collagenase and the CAB. In the control samples for inhibitor testing, an 80-mM solution of (1,10)-phenanthroline inhibitor was combined with diluted collagenase and CAB buffer. Enzyme control wells were formed by mixing diluted collagenase with CAB, while the CAB buffer was used as a background control. Afterward, the samples were incubated for 15 min at room temperature.
2.8. Antielastase Activity Determination
2.9. Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 10 software (GraphPad Software, San Diego, CA, USA). All data were expressed as the mean ± standard deviation (SD) of three independent experiments. One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test was employed to compare the antioxidant, anti-inflammatory, anticollagenase, and antielastase activities between different concentrations of the Ruta chalepensis methanolic extract and the control group (AA or diclofenac sodium, as appropriate). For comparisons between multiple extract concentrations (10–1000 μg/mL), the IC50 values were calculated using nonlinear regression analysis. p values < 0.05 were considered statistically significant, ensuring high reliability in interpreting the results.
3. Results
3.1. Phytochemical Analysis
Table 1 outlines the chemical profile, percentage content, order of elution, and retention times of the methanolic extract of R. chalepensis, whereas the extract’s LC/MS chromatogram is shown in Figure 1. According to this research, the extract has a rich makeup, with 33 components comprising 97.8% of the extract overall. Among the notable compounds, 3,3′,4′,5,7-pentahydroxyflavone was present at a concentration of 9.4%, followed by myricetin at 8.9% and carvacrol at 8.1%. In contrast, gamma-terpinene was the least abundant, representing only 0.1% of the extract.
| Number | Compound | Retention time (min) | Percentage (%) |
|---|---|---|---|
| 1 | Gamma-terpinene | 1.5 | 0.1 |
| 2 | Carvacrol | 2.1 | 8.1 |
| 3 | Limonene | 3.5 | 5.1 |
| 4 | Geraniol | 4.8 | 5.9 |
| 5 | Nonanone | 6 | 2 |
| 6 | Camphor | 7.9 | 6.4 |
| 7 | Piperitone | 8.1 | 1 |
| 8 | Pulegone | 9 | 0.3 |
| 9 | Eucalyptol | 9.8 | 2.4 |
| 10 | Bicyclo[2.2.1]heptan-2-ol | 10.2 | 1.3 |
| 11 | 3-Bornanol | 10.8 | 3.2 |
| 12 | Linalool | 11.2 | 6.9 |
| 13 | 1,4 (8)-p-Menthadiene | 12.1 | 6.2 |
| 14 | Decan-2-one | 13 | 2.2 |
| 15 | 4-Hydroxycinnamic acid | 13.7 | 3.1 |
| 16 | Unknown | 14.1 | 0.2 |
| 17 | 3,4,5-Trihydroxybenzoic acid | 15.2 | 4.1 |
| 18 | 2-Undecanone | 16 | 0.9 |
| 19 | 3,4-Dihydroxycinnamic acid | 16.4 | 1.8 |
| 20 | 2-Dodecanone | 17 | 0.9 |
| 21 | 4-Hydroxy-3-methoxycinnamic acid | 18.1 | 0.8 |
| 22 | 3,7-Dimethyl-1,6-octadien-3-yl acetate | 19.2 | 2.3 |
| 23 | α-Humulene | 21.1 | 0.2 |
| 24 | α-Cadinene | 21.8 | 0.4 |
| 25 | Unknown | 23.1 | 2 |
| 26 | (−)-Viridiflorol | 23.7 | 1.4 |
| 27 | Octadecanoic acid | 24.7 | 2.4 |
| 28 | 3,3′,4′,5,7-Pentahydroxyflavone | 25.8 | 9.4 |
| 29 | 3-Methylquercetin | 26.2 | 1.4 |
| 30 | Myricetin | 27.6 | 8.9 |
| 31 | 3-Caffeoylquinic acid | 28.2 | 0.4 |
| 32 | β-Carotene | 29.6 | 3.1 |
| 33 | Quercetin-3-O-rutinoside | 31.1 | 5.2 |
| Total identified compounds | 97.8% | ||
| Fatty acids | 2.4% | ||
| Flavonoid | 24.9% | ||
| Ketone | 6.0% | ||
| Organic acid | 10.2% | ||
| Monoterpene | 49.2% | ||
| Sesquiterpene | 2.0% | ||
| Tetraterpene | 3.1% | ||
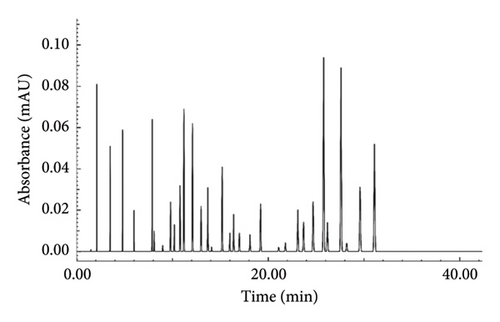
The identified compounds were categorized by chemical structure, with monoterpenes dominating the profile at 49.2%. Flavonoids and organic acids contributed 24.9% and 10.2%, respectively. Sesquiterpenes were the least represented category, making up 2.0% of the extract.
This analysis highlights the diverse chemical composition of R. chalepensis and underscores the importance of bioactive compounds, which hold potential for further research and application.
3.2. Antioxidant Activity
The antioxidant characteristics of the methanolic extract of Ruta chalepensis were assessed using two widely accepted methods: the DPPH and ABTS radical scavenging assays. These assays evaluate the ability of antioxidants to neutralize free radicals by donating hydrogen atoms, which is visually reflected in a color change. In both assays, the IC50 values were calculated to quantify the extract’s antioxidant potential, where a lower IC50 value signifies higher antioxidant activity.
As shown in Table 2, the methanolic extract of R. chalepensis demonstrated an IC50 value of 30.1 ± 0.1 μg/mL for DPPH and 25.4 ± 0.3 μg/mL for ABTS, indicating considerable antioxidant activity. Nevertheless, AA, the positive control (IC50 of 12.5 ± 0.5 μg/mL for DPPH and 17.2 ± 0.2 μg/mL for ABTS), was shown to be more effective in scavenging both radicals than the extract (p < 0.01). These results highlight the significant difference between the antioxidant capacities of the extract and AA, as illustrated by the IC50 values and their statistical significance.
| Sample | DPPH IC50 (μg/mL) Mean ± SD |
ABTS IC50 (μg/mL) Mean ± SD |
|---|---|---|
| Methanolic extract of R. chalepensis | 30.1 ± 0.1 ∗∗ | 25.4 ± 0.3 ∗∗ |
| Positive control (ascorbic acid) | 12.5 ± 0.5 | 17.2 ± 0.2 |
- Note: Values are means ± standard deviation (SD) of triplicates.
- ∗∗Significant differences between the values, with p < 0.01.
3.3. Anti-Inflammatory Assay
3.3.1. Protein Denaturation Bioassay
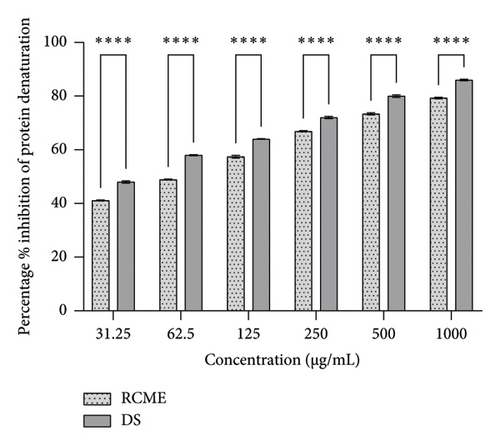
During the inflammatory process, tissue proteins can undergo denaturation, which is crucial for understanding the progression of diseases such as infections, arthritis, type 2 diabetes mellitus, obesity, and cancer [26]. The anti-inflammatory potential of the methanolic extract was evaluated using a protein denaturation bioassay, which focuses on the inhibition of protein denaturation—a key aspect of many inflammatory diseases. Both the extract and the positive control, diclofenac sodium, demonstrated a dose-dependent increase in the percentage of inhibition as concentrations rose.
However, the inhibition percentages for the extract were consistently lower than those of diclofenac sodium at all tested concentrations, as illustrated in Figure 2. Statistical analysis confirmed significant differences in inhibition percentages between the extract and diclofenac sodium at all concentrations (p < 0.05), indicating that diclofenac sodium was a more effective inhibitor of protein denaturation.
3.4. Anticollagenase and Antielastase Activity
This investigation employed a carefully designed method to analyze potential collagenase inhibitors. A self-quenched BODIPY conjugated to type B gelatin served as the fluorogenic substrate for measuring collagenase activity. This substrate gave off a brilliant green fluorescence when collagenase broke it down, making it possible to quantify collagenase activity accurately using fluorescent measures. Figure 3 illustrates the ability of the analyzed extracts to inhibit collagenase’s proteolytic activity.
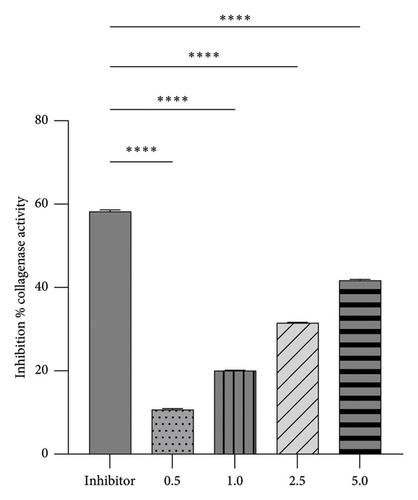
The methanolic extract of R. chalepensis showed a dose-dependent inhibitory effect on collagenase activity, with higher concentrations resulting in more significant inhibition. Notably, the highest concentration tested, 5%, achieved an inhibition of 41.7%. However, this extract was less potent than the known inhibitor 1,10-phenanthroline, which achieved 58.5% inhibition.
The high-throughput in vitro screening test was a crucial part of our research methodology, evaluating the potential of the tested extracts as NE inhibitors. This test involved measuring elastase activity by detecting fluorescent substrate hydrolysis, providing valuable insights into the capabilities of the tested extracts. The percentage of elastase-inhibiting activity in the analyzed extracts is shown in Figure 4.
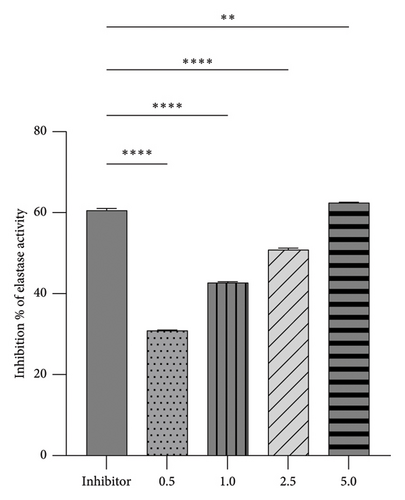
The analyses revealed a range of results. Each concentration of the tested extracts exhibited varying abilities to inhibit elastase, leading to reduced substrate hydrolysis. Control tests with the known inhibitor SPCK were conducted to assess the relative effectiveness of the tested inhibitors. The results indicate that the tested extracts demonstrated a dose-dependent inhibitory effect on elastase activity, with higher concentrations resulting in more significant inhibition. Remarkably, the highest concentration tested, 5%, achieved an inhibition of 62.4%.
These findings highlight the considerable potential of R. chalepensis as an elastase inhibitor. At a 5% concentration, it surpassed the control inhibitor SPCK, indicating a promising future for R. chalepensis as a potential elastase inhibitor.
4. Discussion
Since ancient times, medicinal plants have been utilized in traditional medicine and are valued for their healing abilities [27]. Contemporary scientific research increasingly confirms their traditional applications, especially emphasizing three key health benefits: antioxidant, anti-inflammatory, and antiaging effects.
The use of secondary metabolites in various therapeutic and cosmetic industries is exciting. These metabolites, which possess unique properties, are found in a diverse range of molecules, including acetylated polyacetylene quinones, phenolic lipids, volatile compounds, and polycyclic compounds with three-dimensional structures, such as coumarins, flavonoids, lignans, sesquiterpenes, and spiro compounds. For example, flavonoids are utilized in antiaging creams due to their antioxidant properties [28], while volatile compounds are commonly included in perfumes for their aromatic qualities [29]. The distinctive composition of these metabolites gives plants a wide range of ecological functions, playing a vital role in growth regulation, defense mechanisms, interactions with other organisms and the environment, and protection against biotic and abiotic stress factors [30].
The findings of this investigation, based on a comprehensive phytochemical analysis using LC-MS, highlight the notable therapeutic potential of the methanolic extract derived from R. chalepensis. This analysis has identified secondary metabolites, including terpenes (monoterpenes, tetraterpene, and sesquiterpene), flavonoids, organic acids, ketones, and fatty acids. These compounds, widely recognized in the scientific literature for their bioactive properties, provide a solid biochemical basis for the observed therapeutic effects of the extract, reinforcing the validity of our findings.
Compared to other studies, our results are particularly noteworthy. Various extracts of R. chalepensis have been analyzed for their phytochemical constituents, yielding intriguing outcomes. According to Alemayehu et al. [31], the methanol extract of R. chalepensis leaves contained alkaloids, flavonoids, anthraquinones, tannins, terpenoids, phenols, and saponins. While the ethyl acetate extract revealed the presence of steroids, terpenoids, and saponins, while the acetone extract was rich in steroids, terpenoids, saponins, and anthraquinones. Additionally, the n-hexane extract contained flavonoids, terpenoids, and anthraquinones.
Further supporting the rich phytochemical profile, the leaves of R. chalepensis L (MK828113) have been shown to house an array of bioactive compounds such as alkaloids, flavonoids, phenols, quinones, and steroids [32]. Moreover, the ethanolic extract of R. chalepensis is particularly abundant in bioactive compounds, with significant levels of quercetin (9.2%), myricetin (8.8%), and camphene (8.0%). These findings, highlighted by Althaher et al. [16], underscore the extract’s rich flavonoid content, further emphasizing its therapeutic promise.
The overlapping presence of essential bioactive compounds such as flavonoids, terpenoids, and steroids across these studies highlights the significant therapeutic potential of R. chalepensis. Each extract exhibits unique and complementary bioactive profiles contributing to its medicinal value.
Ruta chalepensis’s methanolic extract showed antioxidant capabilities, as shown by IC50 values of 25.4 μg/mL in the ABTS test and 30.1 μg/mL in the DPPH test. Because of solid flavonoids and terpenes, these results show a robust scavenging capacity equivalent to well-known antioxidants. This shows that the extract may reduce oxidative stress-related damage in biological systems and efficiently neutralize free radicals.
In comparison, Alemayehu et al. [31] evaluated the DPPH radical scavenging activity of various R. chalepensis leaf extracts. The methanol extract exhibited the highest activity, reaching 93.851% at 1000 mg/mL. The acetone and ethyl acetate extracts also showed considerable activity, peaking at 78.937% and 76.590%, respectively, at the same concentration, while the n-hexane extract had the lowest activity at 64.499%. Controls, such as AA and BHT, demonstrated superior antioxidant capacity, with AA consistently achieving over 97% activity and BHT ranging from 74.454% to 94.687%.
In line with Al-Ghamdi et al. [33], our research has opened up exciting possibilities for future studies. We found that R. chalepensis leaf extracts from Saudi Arabia exhibited significant variation in antioxidant activity depending on the solvent used. The n-butanol extract showed the highest radical scavenging activity at 88.13% with an IC50 value of 0.000299, indicating superior antioxidant capacity. The ethyl acetate extract demonstrated considerable activity at 52.17% with an IC50 of 0.00165. Petroleum ether and chloroform extracts had similar activities (43.53% and 43.10%, respectively), while the ethanol extract showed the lowest activity at 34.83%. These findings indicate the potential for further exploration and optimization of R. chalepensis extracts due to their antioxidant properties.
Additionally, Althaher et al. [16] reported that the DPPH radical scavenging activity of the R. chalepensis ethanolic extract ranged from 2.5 to 100 μg/mL, achieving inhibition between 34.9% and 73.3%. The extract demonstrated a 50% inhibition (IC50) of the DPPH radical at 41.2 ± 0.1 μg/mL. These comparative results highlight the varying antioxidant capacities of R. chalepensis extracts depending on the solvent used, with the methanolic extract consistently showing strong potential for mitigating oxidative stress.
On the other hand, a protein denaturation bioassay was used to evaluate the R. chalepensis methanolic extract’s anti-inflammatory potential.
Compared to the positive control (diclofenac sodium), the extract has a smaller but still significant inhibitory effect on protein denaturation. However, the presence of alkaloids and terpenoids, known for their anti-inflammatory effects, supports the potential use of the extract in managing inflammatory conditions [34]. To fully comprehend the extract’s anti-inflammatory properties and efficacy in intricate biological systems, more in vivo research is required.
Similarly, Mokhtar et al. [18] conducted an in vitro study evaluating the anti-inflammatory activity of a polyphenol extract from Ruta graveolens compared to diclofenac. The study assessed the extract’s ability to prevent albumin denaturation and stabilize the membranes of human red blood cells (HRBC) at concentrations of 50, 100, and 200 μg/mL. The findings indicated that the R. graveolens extract demonstrated a dose-dependent inhibition of albumin denaturation (35.77%–50.61%) and a dose-dependent stabilization of HRBC membranes (31.18%–44.12%). These results suggest its potential to prevent protein denaturation, a characteristic feature of inflammation.
Further research has highlighted the anti-inflammatory potential of R. graveolens. Loonat and Amabeoku [35] demonstrated that the R. graveolens extract significantly reduced carrageenan-induced edema, and when combined with indomethacin, the 25 mg/kg extract synergistically reduced edema. Ratheesh and Helen [36] found that the R. graveolens extract at 20 mg/kg exhibited substantial inhibition (90.9%) of carrageenan-induced paw edema in male Wistar rats, surpassing the effectiveness of the standard drug Voveran (72.72%). Moreover, rutin, a primary compound of Ruta, has been reported to possess anti-inflammatory effects by inhibiting lipid peroxidation and mitigating oxidative stress [37, 38].
Although direct comparisons with other studies on R. chalepensis are not available, insights from research on related species like R. graveolens highlight the therapeutic potential of Ruta species in developing effective anti-inflammatory treatments.
Aging is typical for all living things, as the skin acts as a strong barrier between the body and its ever-changing environment [39]. Recent research in phytotherapy has highlighted the potential for managing and preventing skin health issues related to aging and age-related skin diseases. There is growing interest in herbal products for skin protection in many countries, promising remarkable skincare benefits.
Notably, the methanolic extract of Ruta chalepensis has demonstrated antiaging potential in various bioassays for the first time. The extract significantly inhibited collagenase and elastase activities in a dose-dependent manner, achieving 41.7% and 62.4% inhibition at the highest concentration (5%). These enzymes are critical for degrading collagen and elastin, essential structural proteins in connective tissues. Inhibiting these enzymes maintains skin elasticity and prevents wrinkles and sagging [40]. This suggests that the extract could be helpful in dermatological skincare and antiaging formulations. The synergistic actions of many phytochemicals could be the cause of the observed inhibitory efficacy of the extract. Interestingly, there have been no reported data on anticollagenase and antielastase activities for similar or different species of Ruta.
5. Conclusions
Ruta chalepensis methanolic extract reveals its significant bioactive properties, including antioxidant, anti-inflammatory, collagenase, and elastase-inhibitory activities. Although these results are encouraging, the extract’s effectiveness is usually less than well-known controls such as AA and diclofenac sodium. Future research should optimize the concentration of R. chalepensis extract, explore synergistic effects with other bioactive substances, and undertake in vivo investigations to validate its therapeutic potential further. Furthermore, identifying the precise components in the extract that are responsible for these actions may help create more effective derivatives or formulations, increasing Ruta chalepensis’s potential for medicinal use. This plant shows potential as a natural medicinal agent and requires more exploration and development.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
The authors received no specific funding for this work.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The findings of this study are supported by data that can be obtained from the corresponding author upon reasonable request.




