Water Physicochemical Properties Influence the Production, Nutritional Composition, and Antioxidant Activity of Gracilaria tenuistipitata From the Northern Bay of Bengal, Bangladesh
Abstract
This study investigates the influence of water physicochemical properties on the growth, production, nutritional composition, and antioxidant properties of Gracilaria tenuistipitata, cultivated at two different sites (Gangamati and Hazipur) along the northern Bay of Bengal, Bangladesh. The water quality parameters were monitored weekly. Results revealed that the Gangamati site is characterized by significantly higher (p < 0.05) salinity (14.75 ± 4.89 ppt), transparency (60.50 ± 9.57 cm), and total dissolved solids (6154.90 ± 1492.50 mg/L) compared to the Hazipur site. The Gangamati site significantly (p < 0.05) outperformed the Hazipur site in terms of daily growth rate and production. Furthermore, proximate composition analysis indicated significantly higher (p < 0.05) protein (25.06 ± 0.90% DW), lipid (1.22 ± 0.05% DW), fiber (7.03 ± 0.76% DW), and carbohydrate (38.41 ± 0.80% DW) contents in seaweed from the Gangamati site. The mineral content of G. tenuistipitata was also influenced by the culture site significantly (p < 0.05). Amino acid analysis demonstrated superior protein quality in Gangamati seaweed, with significantly higher levels of essential (77.286 ± 0.06 mg/g) and nonessential (95.243 ± 0.07 mg/g) amino acids than the Hazipur site. The fatty acid profiles varied significantly between the two sites, with seaweed from the Gangamati site exhibiting higher levels of unsaturated fatty acids (41.27 ± 0.05%) compared to Hazipur, indicating superior nutritional lipid quality indices. Bioactive compounds were significantly higher (p < 0.05) in Gangamati seaweed, along with higher antioxidant activity. Overall, these results indicate that the Gangamati estuary provides a better environment for the cultivation of G. tenuistipitata, resulting in seaweed with superior nutritional and bioactive properties.
1. Introduction
Red seaweeds stand out among marine organisms for their rich biochemical composition and versatile properties, making them valuable for various commercial and ecological applications [1, 2]. Among them, Gracilaria tenuistipitata [3] from the family Gracilariaceae stands out as a significant species, revered for its widespread distribution, rapid growth, and diverse applications across multiple sectors [4]. This species features slender, branching thalli, with colors varying from pinkish–red to dark purplish–red, influenced by environmental and physiological factors. The versatility of G. tenuistipitata stems from its remarkable biochemical composition, which encompasses a rich array of bioactive compounds, essential nutrients, and functional polysaccharides [5]. These include polyphenols, proteins, minerals, amino acids, and fatty acids, each contributing to its diverse range of applications. Gracilaria tenuistipitata represents the untapped potential of marine resources, highlighting its economic, ecological, and cultural importance and the need for responsible stewardship to benefit present and future generations [6].
The cultivation of G. tenuistipitata holds significant importance in the aquaculture industry due to its diverse applications in food, pharmaceuticals, and biotechnology [4]. Water quality parameters encompass a wide range of physical, chemical, and biological factors that directly impact the physiological processes and overall health of aquatic organisms [7]. The growth, production, and nutritional quality of red seaweed, G. tenuistipitata are profoundly influenced by various water quality parameters in its aquatic environment. Nutrient availability performs a vital function in assisting the metabolic processes and biomass accumulation of seaweed. Optimal nutrient levels promote vigorous growth and enhanced production of biomass, ultimately contributing to higher yields of bioactive compounds and essential nutrients [5]. In addition, water temperature significantly impacts the physiological processes and growth rates of seaweed, with specific temperature ranges influencing photosynthetic activity, reproduction, and nutrient uptake. Light intensity and photoperiod are also critical factors regulating photosynthesis and pigment synthesis in seaweeds, thereby influencing their nutritional composition [7]. Furthermore, water physicochemical parameters, such as salinity, dissolved oxygen (DO), and pH, play essential roles in modulating cellular processes, nutrient uptake, and overall metabolic activity in seaweed, ultimately shaping its nutritional quality and functional properties [8].
Understanding the complex interplay between the water physicochemical parameters and their effects on the growth, production, nutrition, and antioxidant activity of seaweed is essential for developing effective cultivation strategies and optimizing yields in seaweed aquaculture systems. Therefore, the current research aimed to investigate the effect of water physicochemical properties on the growth, production, nutrition, and antioxidant activity of G. tenuistipitata. By elucidating the relationships between water physicochemical factors and seaweed physiology, researchers and aquaculturists can enhance the sustainability and efficiency of seaweed cultivation practices and optimize the nutritional properties of G. tenuistipitata for various applications in the food, pharmaceutical, and biotechnology industries.
2. Materials and Methods
2.1. Study Area
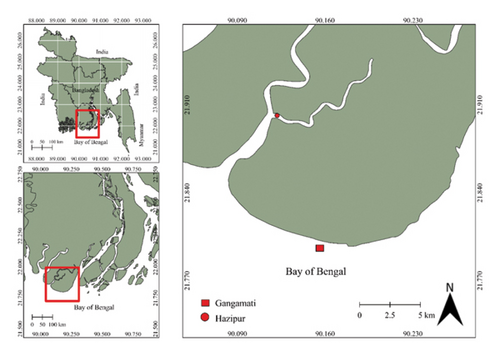
The experiment was performed over 75 days from December 2023 to March 2024 along the Kuakata coast, specifically Gangamati (21.798769N and 90.164684E) and Hazipur (21.900297N and 90.120097E), on the northern Bay of Bengal, Bangladesh (Figure 1). The study areas are notably devoid of coral reefs and seagrass beds, making them distinct in terms of their marine vegetation. The region features a moderate level of water circulation, which influences the estuarine environment. The sediment in the area primarily consists of sandy mud, contributing to the unique physicochemical features of the habitat [4]. These environmental conditions set the stage for the study, providing a specific context that is critical for understanding the ecological interactions and growth patterns observed in marine organisms during the research period.
2.2. Seed Collection
The actively growing and young apical fragments of G. tenuistipitata were selected as the seed material for this study. These wild seeds of G. tenuistipitata were sourced from Nuniachara (21.2826N and 91.5751E), Cox’s Bazar, Bangladesh. The collection took place from the shallow tidal zones during low tide. This region is characterized by expansive sand-flat tidal areas. The area experiences low turbulence but is subject to frequent tidal waves reaching up to 2 m in height, creating dynamic yet stable conditions for seaweed growth [9]. The collection of G. tenuistipitata from the seafloor was conducted under regulatory oversight, with all necessary approvals secured from local authorities. The harvested seaweed species were meticulously identified and authenticated against reference samples kept at BFRI herbarium, ensuring the scientific accuracy and reliability of the collected specimens.
2.3. Cultivation of Seaweed
Gracilaria tenuistipitata was cultivated using the square raft technique, which involved constructing rafts from bamboo measuring 1 m by 1 m, and the rafts were supported by ropes and plastic drums that acted as floats. Rafts were equipped with 4 mm coconut husk rope, onto which fragments of growing G. tenuistipitata were attached. To secure the rafts in place, anchors were used, and surface marker floats were attached for easy identification and monitoring. Throughout the cultivation period, no fertilizers, pesticides, or herbicides were applied, ensuring that the seaweed grew in a natural, chemical-free environment. The growth of the seaweed was closely monitored every 15 days, with the biomass carefully weighed using a digital balance to track its development accurately.
2.4. Monitoring of Water Quality
During the experiment, water physicochemical properties were monitored every 7 days interval. The water temperature and pH were measured on-site by a digital pH meter (HI98107, HANNA, Romania). DO levels were assessed on-site using a DO meter (DO-5509, Lutron, Taiwan). Salinity measurements were taken on-site with a Refractometer (HI96822, HANNA, Romania), and transparency was recorded on-site using a multiparameter (HI98194, HANNA multiparameter, Romania). Water samples from each cultivation site were acquired and transported to the laboratory for chemical examination. The chemical properties, including nitrite (HI3873, HANNA, Romania), nitrate (HI3874, HANNA, Romania), and phosphate (HI3833, HANNA, Romania), were analyzed. Each measurement was replicated three times for accuracy, ensuring a comprehensive assessment of the water conditions in each culture system.
2.5. Growth Performance Calculations
2.6. Sample Preparation for Nutritional Analysis
Seaweed samples were carefully dried utilizing a freeze dryer (YJ-10A, Labocon, United Kingdom) operating at −80°C for 48 h. The drying process was deemed complete once a stable dry weight was achieved, indicating that the samples had been sufficiently desiccated. Following the drying phase, the seaweeds were finely crushed into a uniform powder using a blender. The pulverized material was then passed through a 500 μm sieve to ensure consistency in particle size. The seaweed powder was then placed in airtight plastic bags, stored within glass containers, and kept at a controlled temperature of 4°C until subsequent analysis. The picture of the cultured, harvested, and power of G. tenuistipitata is given in Supporting Figure S1.
2.7. Proximate Composition
The proximate compositions of the seaweeds were determined following standardized AOAC methods [12] with slight modifications to suit the specific sample characteristics. The moisture content of the seaweed was assessed according to the AOAC 934.01 method, with minor adjustments. Approximately 2 g of each sample was kept in an oven and dried at 105°C until a persistent weight was reached. The crude protein content was determined by applying the AOAC 981.10 method. The Kjeltec 2300 analyzer was utilized, employing a nitrogen conversion factor of 6.25 to calculate the protein content. The AOAC 991.36 method was employed for crude fat extraction. The Soxtec 2050 system was used, with petroleum ether serving as the solvent for fat extraction. Ash content was measured using the AOAC 930.05 method. The dried samples from the moisture content analysis were subjected to combustion in a muffle furnace at 525°C overnight to achieve complete ashing. The crude fiber was determined through sequential extraction, following the AOAC 962.09 method. This process involved removing soluble components to isolate and quantify the indigestible fiber fraction. Carbohydrate content was calculated by difference, deducting the percentages of moisture, crude protein, crude fat, ash, and crude fiber from the total composition [13].
2.8. Mineral Content
The mineral content of the seaweeds was analyzed utilizing the protocol described by Ullah et al. [14], with slight alterations to enhance accuracy and precision. To begin the analysis, 0.1 g of dried seaweed was placed into a digestion tube, to which 10 mL of concentrated nitric acid (HNO3) was carefully added. The sample was permitted to react and stand for several hours, during which the solution turned colorless, indicating complete digestion of the organic material. Following digestion, the sample was sieved using Whatman filter paper No. 40 to remove any undissolved particles. The resulting filtrate was collected, and 2 mL of this solution was again diluted with distilled water to a final volume of 5 mL, preparing it for mineral analysis. The detection and quantification of specific minerals were carried out using various spectroscopic techniques. A flame emission spectrophotometer (Spectrolab, United Kingdom) was used to measure the amounts of potassium (K) and sodium (Na), equipped with appropriate filters to ensure selective measurement of each ion. The concentrations of calcium (Ca) and magnesium (Mg) were determined using an atomic absorption spectrophotometer (Varian, AAS Spectra 55B, Australia), known for its sensitivity and precision in detecting trace metals. Phosphorus (P) and sulfur (S) were measured using a double-beam UV–VIS spectrophotometer, which provided accurate absorbance readings at specific wavelengths corresponding to these elements. To ensure the reliability and uniformity of the instrumental readings, a quality control solution, prepared from stock solutions of known concentrations, was introduced at the start, midpoint, and end of the analysis sequence.
2.9. Amino Acids
Chromatographic methods as described by Davidson [15] were used for analyzing the amino acid profile of the seaweed. First, 25 mL of 7 N HCl was added to acidify 0.20 g of crushed seaweed, making sure that everything was homogenized completely. The mixture was filtered to get rid of any substances that were not soluble in acid. The acidified samples were filtered utilizing filter paper (Whatman No. 1) after being incubated for 24 h at 120°C. In order to get the samples ready for analysis, the pH was measured with a pH meter (TM 156, HACH, United States of America) and adjusted to a range of 2.9–3.1 using NaOH solutions. Once the pH was attained as intended, the samples were further diluted to a final amount of 250 mL. After being diluted, a 100 mL aliquot was filtered using a 0.45 μm syringe filter and then put into the Sykam amino acid analyzer’s autosampler (S433-D, Sykam, Germany). A capillary column (150 × 4.26 mm) and an auto-injector operating at 43 bars of pressure and 57°C column temperature were used in the study. The mobile phase ran for a total of 60 min at a flow rate of 0.45 mL/min. A dual-channel photometer, that detected absorbance at wavelengths of 440 and 570 nm, was used to identify amino acids. By correlating the sample results with standards for amino acids, the amounts of amino acids found in the samples were ascertained.
2.10. Fatty Acids
The procedure described by Eder [16] was followed in the determination of free fatty acids (FFAs) in the seaweed. This process yielded the total lipids from the seaweed, and then, as explained by Hewavitharana et al. [17], fatty acid methyl esters (FAMEs) were synthesized by transesterification utilizing boron trifluoride in methanol. The procedure used a chromatograph (GC-2010 plus Gas, Shimadzu, Japan), which has an SP-2560 capillary column (100 m × 0.25 mm × 0.2 μm film thickness) and an auto-injector (AOC-20i). The carrier gas was helium, which flowed at a rate of 0.8 mL/min. Using a 20: 1 split ratio and 240°C injection temperature, 0.8 μL of the sample was introduced into the gas chromatograph for the FAME assessment. The entire chromatographic run lasted 27.50 min. A Shimadzu GCMS-QP-2020, which worked in the 25°C–50°C temperature range and had a flame ionization detector with a Polyarc reactor, was used for the detection process. By comparing the retention periods and peak regions with predetermined standards, fatty acids were detected and measured, providing precise and trustworthy results in determining the individual components revealed by gas chromatography.
2.11. Nutritional Quality Indices for Dietary Lipids
2.12. Phenolic Contents
With some slight adjustments based on Ullah et al. [19], the Folin–Ciocalteu (FC) technique was utilized for the determination of the phenolic content. To explain it in brief, 0.5 mL of the extract was mixed with 0.1 mL of 1 M FC reagent. Thirty minutes at room temperature (RT) were given to the mixture after it had been mixed with 2.5 mL of concentrated Na2CO3 (7.5%) for 15 min. Utilizing a spectrophotometer (C7200, Peak Instrument, United States of America), the absorbance was determined at 760 nm. The outcomes were given as milligrams (mg GAE/100 g·dm) of gallic acid equivalent per 100 g of dry matter.
The total flavonoid concentration was assessed using the aluminum chloride colorimetric method with slight adjustments as described by Ullah et al. [19]. In brief, 1 mL of the extract was combined with 3 mL of methanol, 0.2 mL of 10% aluminum chloride, and 0.2 mL of 1 M potassium acetate. Following a 30-min RT incubation period, the absorbance at 420 nm was determined. The total amount of flavonoids was reported as milligrams of quercetin equivalent (mg QE/100 g·dm) per 100 g of dry matter. Every measurement was performed three times.
2.13. Antioxidant Activities
2.14. Statistical Analyses
The means and standard deviations of each result are displayed. GraphPad Prism (Version 9) and SPSS (Version 25) were employed for the statistical analysis. To determine if the distribution of the data was normal, the Kolmogorov–Smirnov test was utilized to verify the homogeneity of variance. Data were found to be normally distributed. Comparisons of means between the two sites were conducted using multiple unpaired t-tests to identify any significant differences between the two sites. For statistical tests, a significance level of p < 0.05 was used.
3. Results
3.1. Water Physicochemical Parameters
The data (Table 1) highlight that the Gangamati site exhibits significantly higher (p < 0.05) salinity (14.75 ± 4.89 ppt), water transparency (60.50 ± 9.57 cm), and total dissolved solids (TDS) (6154.90 ± 1492.50 mg/L) compared to the Hazipur site. The water temperature, pH, and DO levels were slightly higher in the Gangamati site, but there was no statistically substantial variance (P > 0.05) between the two sites. On the other hand, nitrite, nitrate, and phosphate levels were relatively higher in the Hazipur site, but no substantial variance (P > 0.05) between the two sites was observed (Table 1).
| Parameters | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| Water temperature (°C) | 23.30 ± 1.36a | 22.50 ± 1.31a |
| Salinity (ppt) | 14.75 ± 4.89a | 6.10 ± 2.17b |
| Water transparency (cm) | 60.50 ± 9.57a | 28.65 ± 10.83b |
| pH | 7.85 ± 0.32a | 7.59 ± 0.28a |
| DO (mg/L) | 8.10 ± 0.51a | 7.72 ± 0.48a |
| TDS (mg/L) | 6154.90 ± 1492.50a | 3326.54 ± 1017.74b |
| Nitrite (NO2) (mg/L) | 0.08 ± 0.05a | 0.11 ± 0.07a |
| Nitrate (NO3) (mg/L) | 0.72 ± 0.53a | 0.86 ± 0.69a |
| Phosphate (PO4) (mg/L) | 0.29 ± 0.18a | 0.37 ± 0.22a |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
3.2. Growth Performance
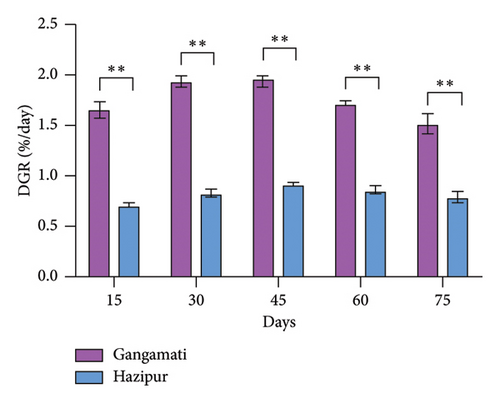
The variability of DGRs for 75 days between the two culture sites is shown in Figure 2. The DGR (%/day) of G. tenuistipitata was substantially higher (p < 0.01) in the Gangamati site than in the Hazipur site. The Gangamati site recorded the highest DGR of 1.97 ± 0.05%/day on the 45th day, while the Hazipur site recorded the lowest DGR of 0.71 ± 0.02%/day on the 15th day (Figure 2). In comparison to the Hazipur site, the Gangamati site produced considerably higher (p < 0.001) G. tenuistipitata (Table 2).
| Production | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| Kg/m2 | 3.09 ± 0.18a | 1.81 ± 0.14b |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
3.3. Proximate Composition
The biochemical composition of G. tenuistipitata cultured in different water quality areas is shown in Table 3. Both samples have a very high moisture content, and the moisture content did not differ significantly between the culture sites. Protein, lipid, fiber, and carbohydrate contents were substantially higher (p < 0.05) in the Gangamati site than in the Hazipur site. Whereas, the ash content was substantially greater (p < 0.01) at the Hazipur site.
| Parameters | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| Moisture (% FW) | 90.59 ± 0.52a | 90.05 ± 0.49a |
| Protein (% DW) | 25.06 ± 0.90a | 20.02 ± 0.61b |
| Lipid (% DW) | 1.22 ± 0.05a | 0.60 ± 0.02b |
| Ash (% DW) | 28.28 ± 1.10b | 38.95 ± 1.04a |
| Fiber (% DW) | 7.03 ± 0.76a | 6.82 ± 0.51b |
| Carbohydrate (% DW) | 38.41 ± 0.80a | 33.61 ± 1.07b |
| Total energy (Kcal/100 g) | 264.75 ± 6.83a | 219.84 ± 5.46b |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
3.4. Mineral Content
The mineral content (%) of G. tenuistipitata differed significantly between the different cultivation sites (Table 4). Samples from the Gangamati site had significantly higher (p < 0.05) contents of sodium, potassium, calcium, and magnesium compared to those from the Hazipur site, which exhibited a significantly higher (p < 0.05) phosphorus content. Sulfur levels did not differ significantly between the two sites. The Na/K ratio was significantly lower (p < 0.05) at the Gangamati site (0.29 ± 0.02) compared to the Hazipur site (0.34 ± 0.01).
| Parameters | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| Sodium (%) | 0.78 ± 0.02a | 0.64 ± 0.02b |
| Potassium (%) | 2.69 ± 0.05a | 1.87 ± 0.03b |
| Calcium (%) | 2.48 ± 0.04a | 1.82 ± 0.02b |
| Magnesium (%) | 1.95 ± 0.02a | 1.71 ± 0.01b |
| Phosphorus (%) | 0.26 ± 0.03b | 0.35 ± 0.02a |
| Sulfur (%) | 0.72 ± 0.05a | 0.70 ± 0.03a |
| Na/K | 0.29 ± 0.02b | 0.34 ± 0.01a |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
3.5. Amino Acid Profiles
Table 5 lists a total of 17 amino acids both essential amino acids (EAAs) and non-EAAs (Non-EAAs), along with their ratios, to provide insight into the protein quality of the seaweed from the two culture sites, Gangamati and Hazipur. The amino acids’ profile of G. tenuistipitata from the Gangamati site shows substantially higher (p < 0.05) levels of almost all measured amino acids compared to the Hazipur site, except cystine and histidine, which were substantially higher (p < 0.05) in the Hazipur site. Isoleucine was found only in the Gangamati site. EAA, Non-EAA, TAA, and EAA/Non-EAA ratios were also substantially higher in the Gangamati site compared to the Hazipur site.
| Amino acid (mg/g) | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| Aspartic acid | 21.163 ± 0.07a | 16.232 ± 0.03b |
| Threonine | 10.592 ± 0.02a | 6.656 ± 0.04b |
| Serine | 10.350 ± 0.05a | 7.449 ± 0.01b |
| Glutamic acid | 22.530 ± 0.08a | 16.144 ± 0.03b |
| Glycine | 10.454 ± 0.04a | 8.417 ± 0.05b |
| Alanine | 12.004 ± 0.06a | 11.105 ± 0.02b |
| Cystine | 4.775 ± 0.03b | 4.884 ± 0.01a |
| Valine | 7.599 ± 0.03a | 4.425 ± 0.04b |
| Methionine | 5.273 ± 0.05a | 4.474 ± 0.02b |
| Isoleucine | 5.119 ± 0.01 | — |
| Leucine | 12.273 ± 0.07a | 8.348 ± 0.05b |
| Tyrosine | 6.436 ± 0.02a | 4.384 ± 0.04b |
| Phenylalanine | 9.217 ± 0.04a | 7.352 ± 0.01b |
| Histidine | 5.596 ± 0.02b | 5.683 ± 0.03a |
| Lysine | 7.663 ± 0.05a | 5.050 ± 0.02b |
| Arginine | 13.954 ± 0.06a | 10.536 ± 0.06b |
| Proline | 7.531 ± 0.03a | 5.168 ± 0.04b |
| EAA | 77.286 ± 0.06a | 52.524 ± 0.04b |
| Non-EAA | 95.243 ± 0.07a | 73.783 ± 0.03b |
| TAA | 172.529 ± 0.06a | 126.307 ± 0.04b |
| EAA/Non-EAA | 0.81 ± 0.01a | 0.71 ± 0.01b |
| EAA/TAA | 0.45 ± 0.01a | 0.42 ± 0.01b |
| Non-EAA/TAA | 0.55 ± 0.01a | 0.58 ± 0.01b |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
3.6. Fatty Acid Profiles
The fatty acid composition of G. tenuistipitata cultivated in the Gangamati and Hazipur sites shows significant variation, as detailed in Table 6. Fatty acids which include caprylic acid, capric acid, undecylic acid, tridecanoic acid, myristoleic acid, palmitoleic acid, alpha-linolenic acid, eicosenoic acid, eicosadienoic acid, eicosatrienoic acid, eicosapentaenoic acid, docosadienoic acid, docosahexaenoic acid, and nervonic acid were found in substantially higher concentrations (p < 0.05) in the Gangamati site seaweed than those from Hazipur. Conversely, fatty acids such as lauric acid, myristic acid, pentadecylic acid, palmitic acid, margaric acid, stearic acid, oleic acid, arachidic acid, and behenic acid were substantially higher (p < 0.05) in the Hazipur site samples. Notably, heptadecenoic acid was present only in the Hazipur samples. Certain fatty acids, linoleic acid, arachidonic acid, erucic acid, and lignoceric acid exhibited no substantial difference (p > 0.05) between the two sites.
| Fatty Acid (%) | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| C8:0, Caprylic acid | 3.294 ± 0.04a | 2.665 ± 0.02b |
| C10:0, Capric acid | 3.374 ± 0.01a | 2.719 ± 0.02b |
| C11:0, Undecylic acid | 26.341 ± 0.05a | 21.613 ± 0.06b |
| C12:0, Lauric acid | 2.892 ± 0.02b | 2.987 ± 0.01a |
| C13:0, Tridecanoic acid | 1.377 ± 0.01a | 0.993 ± 0.01b |
| C14:0, Myristic acid | 2.506 ± 0.03b | 3.132 ± 0.02a |
| C14:1, Myristoleic acid | 3.663 ± 0.04a | 3.115 ± 0.01b |
| C15:0, Pentadecylic acid | 0.180 ± 0.00b | 0.275 ± 0.01a |
| C16:0, Palmitic acid | 10.69 ± 0.04b | 18.066 ± 0.05a |
| C16:1, Palmitoleic acid | 3.082 ± 0.03a | 2.746 ± 0.01b |
| C17:0, Margaric acid | 0.294 ± 0.01b | 0.427 ± 0.02a |
| C17:1, Heptadecenoic acid | — | 0.243 ± 0.01 |
| C18:0, Stearic acid | 3.623 ± 0.04b | 4.261 ± 0.03a |
| C18:1, Oleic acid | 14.75 ± 0.05b | 15.028 ± 0.04a |
| C18:2, Linoleic acid | 3.978 ± 0.05a | 3.909 ± 0.03a |
| C18:3, Alpha-linolenic acid | 1.599 ± 0.01a | 1.071 ± 0.02b |
| C20:0, Arachidic acid | 0.604 ± 0.01b | 1.081 ± 0.01a |
| C20:1, Eicosenoic acid | 1.980 ± 0.02a | 1.674 ± 0.03b |
| C20:2, Eicosadienoic acid | 0.375 ± 0.00a | 0.309 ± 0.01b |
| C20:3, Eicosatrienoic acid | 1.801 ± 0.03a | 1.714 ± 0.01b |
| C20:4, Arachidonic acid | 0.422 ± 0.02a | 0.396 ± 0.02a |
| C20:5, Eicosapentaenoic acid | 0.927 ± 0.02a | 0.803 ± 0.03b |
| C22:0, Behenic acid | 2.92 ± 0.01b | 3.367 ± 0.02a |
| C22:1, Erucic acid | 1.695 ± 0.03a | 1.640 ± 0.04a |
| C22:2, Docosadienoic acid | 0.602 ± 0.02a | 0.448 ± 0.02b |
| C22:6, Docosahexaenoic acid | 0.509 ± 0.01a | 0.318 ± 0.00b |
| C24:0, Lignoceric acid | 0.633 ± 0.02a | 0.659 ± 0.01a |
| C24:1, Nervonic acid | 5.887 ± 0.04a | 4.338 ± 0.05b |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
3.7. Fatty Acid Ratio
The fatty acid assessment of G. tenuistipitata from the Gangamati and Hazipur sites revealed notable differences in the distribution of saturated fatty acids (SFAs), UFAs, MUFAs, and PUFAs (Table 7). The Gangamati site samples had significantly lower SFA content (58.73 ± 0.06%) compared to Hazipur (62.25 ± 0.08%) but higher levels of UFA (41.27 ± 0.05%), MUFA (31.06 ± 0.05%), and PUFA (10.21 ± 0.03%) than those from Hazipur. The SFA/UFA and SFA/TFA ratios were substantially lower (p < 0.05) in Gangamati compared to Hazipur, indicating a higher proportion of UFAs in the Gangamati samples. Furthermore, the ω − 6/ω − 3 ratio was substantially lower (p < 0.05) in Gangamati compared to Hazipur (Table 7).
| Fatty acids | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| SFA | 58.73 ± 0.06b | 62.25 ± 0.08a |
| UFA | 41.27 ± 0.05a | 37.75 ± 0.04b |
| MUFA | 31.06 ± 0.05a | 28.78 ± 0.03b |
| PUFA | 10.21 ± 0.03a | 8.97 ± 0.03b |
| SFA/UFA | 1.42 ± 0.01b | 1.65 ± 0.01a |
| SFA/TFA | 0.59 ± 0.00b | 0.62 ± 0.01a |
| UFA/TFA | 0.41 ± 0.00a | 0.38 ± 0.01b |
| ω − 6 | 6.20 ± 0.03a | 6.01 ± 0.02b |
| ω − 3 | 3.04 ± 0.02a | 2.19 ± 0.02b |
| ω − 6/ω − 3 | 2.04 ± 0.01b | 2.74 ± 0.01a |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
3.8. Nutritional Lipid Quality Indices
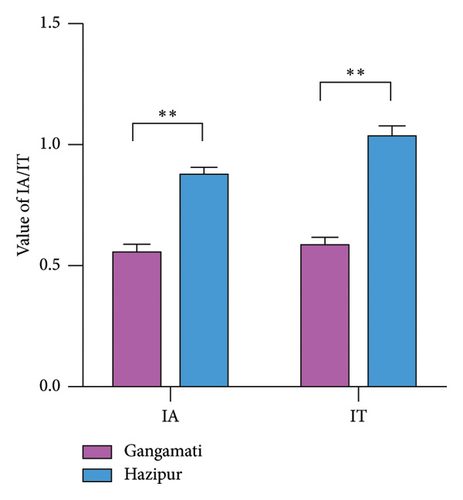
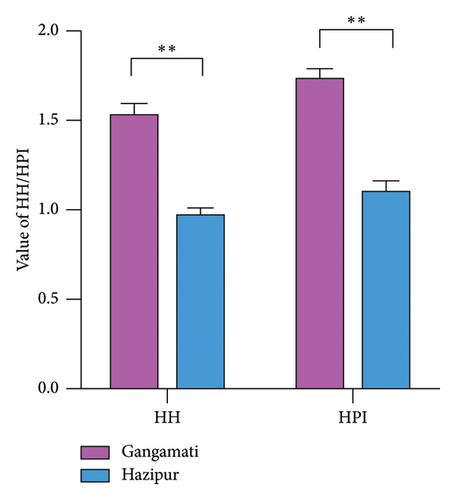
In this study, the IA, IT, HH ratio, and HPI of G. tenuistipitata cultured in different water quality areas differ significantly (p < 0.01). Hazipur site samples have significantly higher values of IA and IT (Figure 3(a)); in contrast, Gangamati site samples have significantly higher HH and HPI values (Figure 3(b)).
3.9. Phenolic Contents
The bioactive compounds’ analysis of G. tenuistipitata from the Gangamati and Hazipur sites show substantial differences (p < 0.05) in both total phenolic and flavonoid content (Table 8). Gallic acid and quercetin’s linear calibration curves were created and have an r2 value of 0.9976 and 0.9938 respectively. The seaweed samples from the Gangamati site exhibited a higher total phenolic (75.38 ± 1.75 mg GAE/g dry weight) and flavonoid (49.57 ± 0.92 mg QE/g dry weight) content compared to the Hazipur site samples.
| Parameters | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| Total phenolic content (mg GAE/g dry weight) | 75.38 ± 1.75a | 67.04 ± 1.92b |
| Total flavonoid content (mg QE/g dry weight) | 49.57 ± 0.92a | 42.96 ± 0.87b |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
3.10. Antioxidant Activities
The Gangamati site samples exhibited a substantially higher (p < 0.05) ABTS scavenging activity (63.91 ± 0.85%) compared to the Hazipur site (Table 9). Whereas, no substantial difference (p > 0.05) was found in DPPH scavenging activity between the two sites. Trolox’s linear calibration curve was created with an r2 value of 0.9989.
| Parameters | Gangamati (mean ± SD) | Hazipur (mean ± SD) |
|---|---|---|
| ABTS scavenged (%) | 63.91 ± 0.85a | 56.37 ± 0.69b |
| DPPH scavenged (%) | 61.40 ± 0.78a | 60.58 ± 1.24a |
- a-bMeans in a row without a common superscript letter differ substantially (p < 0.05).
4. Discussion
There are very few reports about the effect of water physicochemical properties on the production, nutritional, and antioxidant properties, even though several research studies have been conducted worldwide on the growth, nutrition, and antioxidant properties of various seaweed species. According to Coaten et al. [7] and Ullah et al. [4], seaweed growth, productivity, and nutritional composition are all strongly influenced by ecological conditions. Throughout the study, fluctuations in the physicochemical characteristics of the experimental site were monitored to assess their impact on seaweed production and nutritional quality. According to the current study, a wide range of physicochemical parameters substantially impact the growth of G. tenuistipitata.
The Gangamati site exhibits significantly higher salinity (14.75 ± 4.89 ppt), water transparency (60.50 ± 9.57 cm), and TDS (6154.90 ± 1492.50 mg/L) compared to the Hazipur site. These findings suggest that the Gangamati site is subject to more saline conditions, which could be influenced by factors such as the proximity to coastal areas, tidal influences, or reduced freshwater inflows. The higher salinity levels at the Gangamati site may indicate a more estuarine or brackish water environment, which can have profound effects on the aquatic biota, influencing species composition, synthesis of chlorophyll, and the process of photosynthesis, respiration, growth, reproductive cycles, and overall ecosystem functioning [21–23]. The significantly greater water transparency at the Gangamati site may be attributed to lower suspended particulate matter, which could be a result of the higher salinity causing flocculation and sedimentation of fine particles [22]. Alternatively, it could also indicate less terrestrial runoff or human disturbance in the area compared to Hazipur. Higher transparency is generally associated with reduced turbidity and can affect light penetration, influencing photosynthetic activity and the distribution of aquatic plants and phytoplankton [24, 25]. TDS were also significantly higher at Gangamati, reflecting the elevated concentrations of salts and organic matter dissolved in the water. High TDS levels are often correlated with increased salinity and can be indicative of mineral-rich water inputs, evaporative concentration, or anthropogenic influences such as agricultural runoff or industrial discharges [26].
The slight but nonsignificant increases in water temperature, DO, and pH levels at the Gangamati site compared to Hazipur suggest that these parameters are relatively stable across the two sites. The similarity in temperature between the sites implies that both locations experience similar climatic conditions or that the water bodies have comparable thermal buffering capacities [23]. According to research, the optimum temperature range for seaweed to grow normally is between 22°C and 30°C [4]. In addition, an extensive tolerance to temperature has been reported for Gracilaria sp. [27]. The pH levels at both sites remained within a range that is generally conducive to most seaweed, with no significant difference observed between the Gangamati and Hazipur sites. pH is a crucial determinant of water chemistry, influencing the solubility and toxicity of nutrients and pollutants [7]. The observed pH stability suggests that the buffering capacity of the water bodies is sufficient to maintain a relatively constant pH despite potential inputs of acidic or basic substances. The DO levels, which are critical for the existence of seaweed, were also slightly higher at Gangamati but not significantly different from Hazipur. This could be attributed to similar rates of photosynthesis, respiration, and decomposition, or comparable levels of water mixing and aeration at both sites [28, 29]. The adequate DO levels at both sites indicate that the waters are well-oxygenated, supporting a diverse range of aquatic organisms.
Although nitrite, nitrate, and phosphate levels were relatively higher at the Hazipur site, the differences were not statistically significant. These nutrients are key components of aquatic ecosystems, playing vital roles in the growth and productivity of seaweed. The elevated levels at Hazipur may indicate increased nutrient inputs, possibly from agricultural runoff, wastewater discharge, or natural processes such as nitrogen fixation or organic matter mineralization [30–32]. The insignificant differences in nutrient levels suggest that while there may be some variability in nutrient inputs or processing between the sites, these differences are not large enough to create substantial ecological divergence.
The significant variability in the DGRs of G. tenuistipitata between the Gangamati and Hazipur culture sites over 75 days suggests that water physicochemical parameters play a significant role in the growth performance and production of this seaweed. The higher DGR observed at the Gangamati site indicates that the conditions there are more favorable for the cultivation of G. tenuistipitata, likely due to the greater salinity, water transparency, and TDS, as noted in the environmental parameters. The maximum DGR recorded on the 45th day at Gangamati (1.97 ± 0.05%/day) suggests that the seaweed experienced optimal growth conditions during this period, possibly due to a combination of favorable light availability, nutrient concentration, and stable salinity levels [33, 34]. In contrast, the lower DGR at the Hazipur site, with the minimum recorded on the 15th day (0.71 ± 0.02%/day), could be attributed to less favorable physicochemical factors, such as lower water transparency and different nutrient profiles [33]. These conditions may have limited the photosynthetic efficiency and nutrient uptake of the seaweed, thereby reducing its growth rate. The significantly higher overall production (3.09 ± 0.18 kg/m2) at the Gangamati site further underscores the importance of site selection in seaweed aquaculture, as the environmental conditions directly impact the production of the cultivated seaweed which supports the study of Glenn et al. [35], Mensi et al. [36], and Banik et al. [34].
The variances in the biochemical composition of G. tenuistipitata cultivated in the Gangamati and Hazipur sites reflect the varying water environmental conditions and their influence on nutrient accumulation in seaweed. Moisture content is a crucial parameter in the composition of seaweed, influencing its nutritional value, storage stability, and processing characteristics [37, 38]. Seaweeds generally have a high moisture content when freshly harvested [39]. The high moisture content, with no significant differences between the two sites, suggests that water content is a stable trait of G. tenuistipitata. This stability may result from the species’ intrinsic water-retention properties, making it less sensitive to environmental variations [38]. However, the substantial variations in protein, lipid, fiber, carbohydrate, and ash content between the two sites are indicative of how water quality can influence the metabolic processes of seaweed. The significantly higher protein content in the Gangamati site samples compared to those from the Hazipur site suggests that the conditions in Gangamati are more conducive to protein synthesis. Protein accumulation in seaweed is often linked to the availability of nitrogen, a critical nutrient for growth [8]. Although nitrate levels were slightly higher in Hazipur, the overall nutrient profile, including other nitrogen forms, light intensity, and salinity, may have been more conducive to protein synthesis in Gangamati. The higher protein content implies that Gangamati could be a more suitable site for producing seaweed with enhanced nutritional value, particularly for applications where protein content is a key quality criterion [23, 40, 41]. The lipid content, which was also substantially higher in the Gangamati site samples compared to Hazipur, indicates that the conditions in Gangamati may promote lipid biosynthesis or retention. Lipids in seaweed are vital for various metabolic functions and can serve as important bioactive compounds in human nutrition [42]. The higher lipid content in Gangamati seaweed could be related to factors such as higher salinity, which may induce a stress response in seaweed, leading to increased lipid accumulation as a protective mechanism [43]. The carbohydrate content was also substantially higher in Gangamati compared to Hazipur. Carbohydrates are major storage compounds in seaweed and are closely associated with photosynthetic activity and overall energy metabolism [44]. The higher carbohydrate content in Gangamati samples could reflect more favorable photosynthetic conditions, such as better light penetration due to higher water transparency. In addition, the slightly higher fiber content in Gangamati compared to Hazipur suggests that the structural components of the seaweed are more developed in Gangamati, possibly due to a more stable or nutrient-rich environment that supports robust growth [45]. Conversely, the significantly higher ash content in the Hazipur site samples compared to Gangamati suggests a greater accumulation of inorganic minerals and salts in the seaweed from Hazipur. This could be due to the higher levels of dissolved nutrients such as nitrite, nitrate, and phosphate in the water at Hazipur, which, although not significantly different, might still influence the mineral uptake and deposition in seaweed. While a higher ash content might suggest a richer mineral profile, it could also indicate potential environmental stress or the presence of less desirable conditions for optimal growth, leading to the accumulation of excess salts or other inorganic substances [46, 47].
Incorporating seaweeds in diets can help maintain a balanced, healthful diet because of the seaweed’s high nutritional content [14, 41]. Mineral concentrations may differ depending on the seaweed type, location, time of year, habitat, and water physiological characteristics [2]. The mineral composition of G. tenuistipitata is indicative of the influence of water physicochemical factors on the nutrient uptake of seaweed, and the results from this study underscore the variability in mineral content across different cultivation sites [7]. The significantly higher levels of sodium, potassium, calcium, and magnesium in the samples from the Gangamati site compared to those from Hazipur suggest that the water quality and nutrient availability in Gangamati may be more conducive to the absorption of these particular minerals. Such differences could be attributed to varying salinity levels, water transparency, and the overall nutrient profile of the water in these regions [7, 48]. Besides, the significantly higher phosphorus content in the Hazipur samples suggests that the conditions at this site may promote greater uptake of phosphorus, possibly due to differences in the phosphorus availability or its bioavailability in the water [49]. Phosphorus is an essential element for various biochemical processes, including energy transfer and the synthesis of nucleic acids, which could impact the growth of seaweed [50]. Interestingly, sulfur content did not differ substantially between the two sites, indicating that the sulfur availability or uptake mechanisms in G. tenuistipitata are consistent across different environmental conditions. This could suggest a relatively stable sulfur requirement for the species, regardless of the cultivation site. The higher potassium levels in Gangamati also contribute to the lower Na/K ratio observed at this site. This is noteworthy because a lower Na/K ratio is often associated with better nutritional quality, particularly in terms of cardiovascular health benefits [51]. The increased potassium levels may enhance the functional properties of the seaweed, making it more suitable for dietary applications that require high potassium and lower sodium content [52].
The amino acid composition of G. tenuistipitata cultivated in different water quality environments provides valuable insights into the influence of water quality on the nutritional quality of this seaweed. The results demonstrate that the G. tenuistipitata from the Gangamati site had substantially higher levels of almost all measured amino acids than the Hazipur site, indicating that the environmental conditions at Gangamati are more favorable for amino acid synthesis and accumulation. The elevated levels of amino acids such as aspartic acid, arginine, threonine, serine, glutamic acid, and leucine at the Gangamati site suggest that the nutrient availability, water chemistry, or other ecological factors in this estuary may enhance the metabolic processes responsible for amino acid production in seaweed [53]. The absence of isoleucine in the samples of the Hazipur site and its presence in the Gangamati site samples is noteworthy. Isoleucine is an essential branched-chain amino acid important for muscle metabolism and immune function [54, 55]. Its presence exclusively in the Gangamati site samples further underscores the superior protein quality of G. tenuistipitata from this site. The absence in the Hazipur site might indicate either a limitation in its synthesis or a difference in the seaweed’s metabolic response to the environmental conditions there [48]. Interestingly, cystine and histidine were found in significantly higher concentrations in the Hazipur site samples, contrary to the general trend. This suggests that specific water conditions in the Hazipur site might promote the synthesis or retention of these particular amino acids. Histidine, being an important amino acid involved in tissue repair and growth, might be more abundant in the Hazipur site due to different stress conditions or nutrient availability that favor its accumulation [56]. The significantly higher levels of EAA, Non-EAA, and TAA in Gangamati, along with a higher EAA/Non-EAA ratio, highlight the superior protein quality of seaweed from this site. The EAA/Non-EAA ratio is an important indicator of protein quality, as a higher ratio typically reflects a better balance of EAAs, which are crucial for human health and cannot be synthesized by the body [57]. The higher ratio in Gangamati suggests that seaweed from this site may be more nutritionally valuable, particularly in terms of its potential use as a dietary supplement or functional food ingredient [58, 59]. The significant differences in amino acid content between the Gangamati and Hazipur sites emphasize the impact of water quality on its nutritional value. The higher levels of EAAs and overall superior protein quality at Gangamati suggest that this site may be more favorable for cultivating G. tenuistipitata for high-value nutritional products.
The fatty acid composition of G. tenuistipitata cultivated at the Gangamati and Hazipur sites reveals significant differences, highlighting the impact of water physicochemical properties on the nutritional quality of this seaweed. The observed variations in fatty acid profiles suggest that each site offers distinct water quality conditions that affect the biosynthesis and accumulation of specific fatty acids in G. tenuistipitata. The higher levels of certain fatty acids in Gangamati samples indicate that its environmental conditions favor the accumulation of medium-chain and PUFAs. These fatty acids are particularly valuable due to their roles in human health, including anti-inflammatory properties and contributions to brain function [41]. The higher levels of these fatty acids in the Gangamati site could be due to factors such as salinity, water transparency, and nutrient availability, which may enhance the metabolic pathways involved in fatty acid biosynthesis [60–62]. Conversely, the higher contents of some other fatty acids in the Hazipur site samples indicate a different metabolic response to the water physicochemical conditions. These fatty acids are typically associated with energy storage and membrane structure. The presence of heptadecenoic acid exclusively in the Hazipur site samples further suggests a site-specific influence, possibly due to variations in water quality or microbial interactions that could affect fatty acid synthesis [61, 63]. The two sites’ dissimilarities in the composition of fatty acids underscore the importance of environmental factors in shaping the biochemical characteristics of seaweed. Factors such as salinity, light intensity, and nutrient concentration are known to influence fatty acid metabolism in marine algae [45, 60, 64].
The Gangamati site, characterized by lower SFA content and higher levels of UFAs, MUFAs, and PUFAs, suggests that the conditions at this site are more conducive to the biosynthesis of health-promoting UFAs. UFAs, especially PUFAs, play a critical role in upholding cardiovascular health, reducing inflammation, and supporting brain function [65, 66]. The lower SFA/UFA ratio in Gangamati samples underscores the potential health benefits of seaweed cultivated at this site, as a higher proportion of unsaturated fats is generally connected with a reduced risk of chronic diseases [59]. The ω − 6/ω − 3 ratio is a crucial parameter in assessing the nutritional quality of dietary fats. A lower ω − 6/ω − 3 ratio, as observed in the Gangamati samples, is generally considered more favorable for health, as it is associated with a reduced risk of inflammation and chronic diseases [67, 68]. The significantly lower ω − 6/ω − 3 ratio in Gangamati suggests that seaweed from this site may offer a better balance of these essential fatty acids, further enhancing its potential as a functional food ingredient.
The analysis of the lipid quality indices reveals significant differences in the G. tenuistipitata cultivated at two distinct estuarine sites. The significantly higher IA and IT values observed in the Hazipur site samples suggest a greater potential risk for cardiovascular diseases associated with the consumption of seaweed from this area [69]. Higher IA values are often associated with an increased likelihood of lipid deposition in arterial walls, leading to atherosclerosis, while higher IT values indicate a greater tendency for thrombosis due to an imbalance in fatty acid composition that favors clot formation [18, 70]. Conversely, the Gangamati site samples exhibit significantly higher HH and HPI values, which are indicative of better health-promoting properties. The elevated HH ratio suggests that the fatty acid profile in these samples is more effective in lowering cholesterol levels and lowering the chance of cardiovascular conditions [71]. Similarly, a higher HPI value reflects a more favorable balance of fatty acids that contribute positively to overall health, including anti-inflammatory and immune-supportive effects [18].
The observed differences in the total phenolic and flavonoid content of G. tenuistipitata from the Gangamati and Hazipur sites suggest that environmental factors significantly influence the accumulation of bioactive compounds in this seaweed. The higher levels of total phenolic and flavonoid content in the Gangamati site seaweed indicate that this site may provide more favorable conditions for the biosynthesis of these compounds, which are well-known for their antioxidant properties [72, 73]. Phenolic compounds and flavonoids play a vital role in the defense mechanisms of plants and algae, protecting them against oxidative stress and other environmental challenges [74]. The elevated levels of these compounds in the Gangamati site could be attributed to variations in water quality, nutrient availability, or other site-specific factors such as light exposure and salinity, which can influence the metabolic pathways involved in the production of secondary metabolites [75].
The higher antioxidant activity, especially ABTS scavenging activity, in G. tenuistipitata from Gangamati suggests that its environmental conditions enhance the production or retention of antioxidant compounds [76]. The ABTS assay is known to be more sensitive to hydrophilic antioxidants, which may indicate that the phenolic or other water-soluble antioxidant compounds are more abundant or more active in the Gangamati samples [77]. This is similar to the higher total phenolic content observed in Gangamati samples, as phenolics are major contributors to antioxidant activity in seaweeds. The lack of substantial difference in DPPH scavenging activity between the two sites may indicate that the lipophilic antioxidant components, or those that react with the DPPH radical, are present at similar levels in both Gangamati and Hazipur samples [78, 79]. These findings highlight the impact of site-specific physicochemical factors on the antioxidant properties of G. tenuistipitata, which could be linked to factors such as water quality, nutrient availability, and stress conditions at each site.
5. Conclusion
Our study is the first to evaluate the influence of water quality parameters on the growth, nutrition, and bioactive properties of G. tenuistipitata. The Gangamati site showed superior growth, production, and nutritional quality, including higher protein, lipids, and favorable amino and fatty acids than the Hazipur site. It also enhanced the bioactive potential and antioxidant activity, making it ideal for seaweed cultivation. Future research should focus on exploring the mechanisms underlying these differences and optimizing cultivation practices to maximize the potential of seaweed as a valuable resource for food, pharmaceuticals, and environmental applications.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Md. Rahamat Ullah: conceptualization, methodology, data curation, formal analysis, writing–original draft, writing, review, and editing. Mohammed Ashraful Haque: project administration, supervision, funding acquisition, writing, review, and editing. Md. Monjurul Hasan: resources, writing, review, and editing. Farhana Yasmin: writing, review, and editing. Aovijite Bosu: writing, review, and editing. Md. Amirul Islam: visualization, investigation, supervision, writing, review, and editing. Mohosena Begum Tanu: investigation, supervision, writing, review, and editing.
Funding
This work was fully supported by the Bangladesh Fisheries Research Institute (BFRI/RSS/02/2023-24).
Supporting Information
Figure S1: (a) Cultured Gracilaria tenuistipitata, (b) harvested Gracilaria tenuistipitata, (c) powder of Gracilaria tenuistipitata from the Gangamati site, and (d) powder of Gracilaria tenuistipitata from the Hazipur site.
Open Research
Data Availability Statement
Data will be made available on request.




