Extraction of Polyphenols and Anthocyanins From Apple Pomace With Natural Deep Eutectic Solvents: Evaluation of Their Antioxidant and Antibacterial Activities
Abstract
The aim of this study was to evaluate the antioxidant and antibacterial properties of apple pomace (AP) extracts using natural deep eutectic solvents (NADES). Six NADES, based on choline chloride (ChCl), were used as environmentally friendly solvents. Four of these were combined with organic acids, while the other two were combined with urea; they were then used to extract bioactive compounds from AP. ChCl:urea mixture proved to be the solvent with the highest total polyphenol content (TPC), with 13.15 ± 4.70 mg gallic acid equivalent/mL. Antioxidant activity and total anthocyanidin content (TAC) were also assessed. ChCl:oxalic acid recorded the highest values with 35.59 ± 9.53 mg extract/mL and 64.81 ± 4.65 malvidin-3-glucose equivalent μg/mL, respectively. Solvent pH plays a crucial role in selective extraction; an acidic pH facilitates selective anthocyanidin extraction, while a basic pH does not. Anthocyanidin extraction correlated with extract antioxidant activity and solvent viscosity. In addition, the antibacterial activity of the extracts against Bacillus cereus, Listeria innocua, and Escherichia coli strains was studied. All extracts showed antibacterial properties against the strains tested. The ChCl:oxalic acid extracts showed particularly low minimum inhibitory concentrations (e.g., 25 mgextract/mL for B. cereus) and EC50 values (e.g., 6.0 ± 0.3 mgextract/mL for B. cereus).
1. Introduction
With a global production of 95.8 million tons in 2022, apple is the fourth most produced and one of the most consumed fruits in the world [1, 2]. According to Kammerer et al., around 25%–30% of apples are processed into value-added products such as jams and pies [3]. Of these processed products, 65% are juices and cider, corresponding from 16.3% to 19.5% of total apple production processed into apple beverages. This estimate corresponds to a range of 15.6–18.7 million tons. However, apple juice and cider production generate a significant amount of apple pomace (AP) as a by-product. Based on 2023 apple production, the amount of AP has been estimated at between 4.7 and 5.6 million tons. At present, AP is still underexploited. In many countries, AP, like many other plant wastes, is disposed of in soil, posing health and environmental problems due to its high moisture content, which encourages bacterial decomposition [4]. In the European Union, biomass waste is mainly converted to methane by anaerobic digestion, but this process does not allow phytochemicals to be recovered. These compounds are interesting molecules to extract because of their diverse biological activities, including antioxidant, antimicrobial, anticancer, and antidiabetic properties [5–8]. AP contains several classes of polyphenols, including dihydrochalcone (phlorizin), flavonol (quercetin and its derivatives), hydroxycinnamic acid (chlorogenic acid), proanthocyanidin (procyanidin B2), and anthocyanin (ideain chloride) [9–11].
Phytochemicals are sensitive compounds, and their extraction and preservation methods can have a significant impact on their biological activities due to the degradation of the molecules [11, 12]. Exposure to air, light, solvents, and temperature variations can adversely affect extract quality. For instance, Ferrentino et al. showed that the total polyphenol content (TPC, indicator of antioxidant activity) of Soxhlet extracts was lower than that of supercritical CO2 (SC-CO2) extracts, measuring 4.13 ± 0.90 mg gallic acid equivalent (GAE)/g of extract and 8.87 ± 0.17 mg GAE/g of extract, respectively [13].
The differences in polyphenol content between the two extraction methods can be attributed to the temperature and exposure to light and air (O2) during the extraction steps. In the case of SC-CO2 extraction, the biomass was processed at lower temperatures in the absence of light and air, compared with Soxhlet extraction, where the temperature was high, and compounds were exposed to air (O2). Lavelli and Corti monitored the antioxidant activity of AP extracts over a 9-month period under varying humidity conditions (2.4%–4.2%). At the start of the study, the total phenolic content was 5176 mg/kg of dry AP, decreasing to 1704 to 4429 mg/mg dry AP at the end of the study, depending on the moisture content [14].
Researchers continue to explore new solvents for the extraction of phytochemicals in order to eliminate the use of volatile organic solvents and preserve polyphenols during extraction processes. Traditional solvents have a number of drawbacks, including high toxicity, non-biodegradability, high cost, and a significant tendency to accumulate in air, water, and soil [15–17]. In recent decades, subcritical and supercritical fluids have been studied for environmentally friendly extractions. The influence of physical parameters such as pressure and temperature has been studied. A well-known process, the DIAMANT process, is used to remove trichloroanisole, the molecule responsible for cork taint in wine [18]. However, the use of subcritical and supercritical fluids requires substantial expenses for the acquisition and maintenance of the devices [19].
Ionic liquids, deep eutectic solvents (DES), and bio-based solvents are being explored as alternatives to conventional solvents such as ethanol and hexane due to their ease of use. Natural deep eutectic solvents (NADES) made from compounds of natural origin are a promising class of solvents. NADES offer advantages such as affordability, renewability, low toxicity, biodegradability, extraction selectivity, and low vapor pressure [15, 16, 19]. However, challenges remain, notably with regard to their higher viscosity than the majority of traditional solvents, and the postextraction separation of phytochemicals [19]. NADES are made up of a hydrogen bond acceptor (HBA) compound and a hydrogen bond donor (HBD) compound, both of biological origin. In particular, the melting point of NADES is lower than that of the HBA and HBD compounds of which they are composed [20]. For example, NADES ChCl:urea (1:2) has a melting point of 12°C, while choline chloride (ChCl) melts at 302°C and urea at around 134°C [21]. Depending on the specific formulation of NADES, its use can enable selective extraction of family compounds and minimizes the need for additional reaction steps such as purification. In previous research, Yu and Bulone successfully extracted and de-glycosylated quercetin derivatives from AP in a one-pot reaction using organic acid-based DES [22]. In this study, ChCl was chosen as the HBA compound due to its well-documented efficiency for polyphenol extraction [23, 24]. Organic acids are among the most polar compounds, followed by amino acids, sugars, and polyalcohols. Existing literature suggests that DES based on organic are generally the most efficient solvent for extracting bioactive compounds [15, 16, 23–25]. Urea-based solvents were employed as reference. These solvents are usually used in scientific research for plant extraction, gas capture, electrodeposition, battery technology, hydrogen production with enzymes, and so on [26, 27]. The aim of the work described in this article was to preserve the bioactivity of molecules extracted from AP via eco-friendly solvents, NADES. Several NADES were tested for the extraction of compounds of interest, in particular for anthocyanins. In this study, the antioxidant (DPPH radical scavenging capacity [DRSC] and TPC) and antibacterial (Escherichia coli, Listeria innocua, and Bacillus cereus) properties were investigated.
2. Material and Methods
2.1. AP
Organic AP (sp. Story) was obtained from La Source du Verger (Tournon, France). The AP was freeze-dried using a Buchi Lyovapor L-200 until a stable weight was achieved. Subsequently, the dried AP was ground into a powder with a diameter of 500 nm using a Pulverisette 19 from Fritsch. The powdered dried AP was then stored at 4°C, protected from light.
2.2. Chemistry Solvents and Reagents in NADES Extraction
Choline chloride (≥ 99%) was purchased from Thermo Fisher Scientific. DL-malic acid (≥ 99%), citric acid monohydrate, DL-lactic acid (∼90%), and Amberlite XAD-16 resin used for NADES extract preparation and separation were procured from Sigma-Aldrich (St. Louis, MO, USA). Oxalic acid dihydrate was obtained from Merck. Phlorizin, isoquercetin, quercetin, procyanidin B2, avicularin, hyperoside, and (−)-epicatechin were acquired from Extrasynthese (Genay, France). Chlorogenic acid and (+)-catechin were provided by Thermo Scientific Chemicals. DPPH was purchased from the Tokyo Chemical Industry (TCI). Acetonitrile (UHPLC-MS grade), ethanol (≥ 96%), and methanol (UHPLC-MS grade) were from Carlo Erba. Bacterial strains (Bacillus cereus (CIP 78.3), Listeria innocua (CIP 80.11T), and Escherichia coli (CIP 104049)) were obtained from the Institut Pasteur (Paris, France). Dehydrated brain–heart infusion (BHI) broth, dehydrated Columbia broth, bacteriological agar, 96-well microplates, and Petri dishes (PDs) were purchased from Dutscher.
2.3. Preparation of NADES
NADES (Table 1) were prepared following a molar ratio and 20% (w/w) of added water, except for ChCl:U. Water content (20%) was reduced to decrease viscosity. The synthesis of NADES was conducted using a heating and stirring method. The compounds were heated to 50°C and stirred continuously until the solution became homogeneous and transparent (approximately 2 h).
| HBA | HBD | Acronym | Molar ratio | pKa of HBDb | HBD structure |
|---|---|---|---|---|---|
| Choline chloride | Citric acid | ChCl:CA | 1:1a |
|
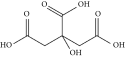 |
| Lactic acid | ChCl:LA | 1:1a | 3.86 | 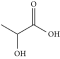 |
|
| Oxalic acid | ChCl:OA | 1:1a |
|
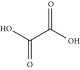 |
|
| Malic acid | ChCl:MA | 1:1a |
|
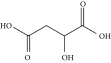 |
|
| Urea | ChCl:U | 1:2 | 0.18 | 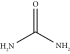 |
|
| ChCl:U:W | 1:2a |
- aAddition of 20% (w/w) of water.
- bData from literature (pKa Values in Water Compilation by R. Williams) [28].
2.4. Characterization of Pure NADES
The viscosity of NADES was measured using a Lovis 2000 M/ME viscometer by Anton Paar (les Ulis, France). The density of NADES was determined using a DM45 Delta Range densitometer by Mettler Toledo (Viroflay, France). The pH of NADES and solutions of diluted NADES (1 gNADES/mLWater) was measured at RT using a pH 211 microprocessor pH meter by Hanna Instruments (Padova, Italy).
2.5. Extraction of Biomolecules
All extractions were conducted according to Moni Bottu et al.’s method with modifications [20]. In round-bottom flasks, 5 g of AP and 50 g of NADES were added and the mixture was heated at 50°C for 45 min. The AP was separated from the liquid extracts by centrifugation (4000 rpm), and the samples were stored at −20°C until further separation. Each extraction was performed in triplicate.
2.6. TPC—Folin–Ciocalteu Assay
Gallic acid served as the reference compound and a calibration curve was prepared using gallic acid solutions ranging from 5 to 250 mg/mL in aqueous solution. Dried extracts were diluted in water to a concentration of 8–20 mg/mL. Then, 250 μL of the sample and gallic acid solutions were mixed with 250 μL of diluted Folin–Ciocalteu reagent (1:1; v/v). After 5 min, 500 μL of Na2CO3 solution (10%; w/v) and 4 mL of distilled water were added and vigorously shaken. The absorbance of the gallic acid and sample solutions was measured at 740 nm using a quartz cuvette (1 cm) and a Cary 50 UV–Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) after 20 min. The TPC was expressed as mg of GAEs per gram of extract. All measurements were performed in triplicate [29].
2.7. DRSC—Antioxidant Activity
The antioxidant activity was assessed using a DPPH stock solution prepared by dissolving 4.5 mg of DPPH in 100 mL of methanol, following the procedure outlined by Alsaud et al. with slight modifications [30]. Trolox was used as the reference compound, and a calibration curve was generated using Trolox solutions ranging from 0 to 0.150 mg/mL in methanolic solution. The extracts were diluted in methanol to yield 13 different concentrations ranging from 0 to 1000 mg/mL to determine the EC50 (half maximal effective concentration or amount of compound/extract necessary to decrease the initial concentration of DPPH to 50% at equilibrium). In each test tube, 200 μL of each solution and 2 mL of DPPH solution were combined. The mixture was vigorously shaken, and the absorbance was measured after 30 min at 515 nm. The antioxidant activity, expressed as the inhibition percentage of the sample, was calculated using the following equation.
2.8. Total Anthocyanin Content (TAC)
The TAC was determined following the methods described by Ribéreau-Gayon and Stonestreet [31]. Briefly, for each sample, 100 μL of extract, 100 μL of a 60% (v/v) ethanol solution containing 0.1% (v/v) hydrochloric acid, and 2 mL of a 2% (v/v) hydrochloric acid solution were added to two separate test tubes. In the first test tube, 400 μL of distilled water was added to the mixture, while in the second test tube, 400 μL of a 15% (w/v) sodium bisulfite solution was added. Both test tubes were vigorously shaken, and after 15 min, the samples were analyzed at 520 nm using a UV–Vis spectrophotometer with a quartz cuvette (1 cm). The TAC was calculated using the following equation.
2.9. Bactericidal/Bacteriostatic Activity
The antibacterial activity of the extracts was assessed against two Gram-positive bacteria, B. cereus (CIP 78.3) and L. innocua (CIP 80.11T), and one Gram-negative bacterium, E. coli (CIP 104049). E. coli and B. cereus were cultured in Columbia broth at 30°C, while L. innocua was cultured in BHI broth at 30°C. Precultures of the bacterial strains were prepared by transferring a bacterial colony of each strain into 0.5 mL of the corresponding broth, followed by overnight incubation at 30°C. After overnight incubation, a cascade dilution was performed until the desired concentration (CFU/mL) was achieved for the antibacterial testing.
2.9.1. Determination of Diameter Inhibition Zone (DIZ)
The antibacterial tests using PDs were conducted following the method outlined by Alsaud et al. [30], with slight modifications. Briefly, cascade dilutions were performed until a concentration of 105 CFU/mL was achieved for PD testing. For each PD, 1 mL of bacterial suspension was added to the appropriate medium PD, and excess liquid was removed. After a brief incubation period, 20 μL of samples (at a concentration of 300 mg/mL, except for oxalic acid at 150 mg/mL) dissolved in phosphate-buffered saline (PBS) and 5 μL of ampicillin (at a concentration of 25 mg/mL) were added. PBS was used as a buffer to maintain a pH of 7.4 and avoid the potential pH effects of NADES on the bacterial strains. In each PD, positive and negative controls (ampicillin and PBS–water, respectively) were included, in addition to the samples. After overnight incubation at 30°C, the DIZ was measured.
2.10. Determination of Minimum Inhibitory Concentration (MIC)
The antibacterial tests were conducted following the method described by Zambrano et al. with slight modification [33]. MICs were determined using a 96-well microplate. The extracts were diluted in PBS solution at 8 different concentrations: 300, 150, 100, 75, 50, 25, 15, and 5 mg/mL. Ampicillin was used as positive control at 25 mg/mL. A cascade dilution was performed until a concentration of 105 CFU/mL was achieved for microplate testing. For the extract test, 25 μL of extract solution was added to 125 μL of appropriate medium and 25 μL of bacterial suspension. Additionally, an extract control was prepared by adding 175 μL of the appropriate medium and 25 μL of the extract solution or ampicillin. The positive control consisted of adding 25 μL of bacterial suspension to 175 μL of the appropriate medium, while the negative control comprised 200 μL of the appropriate medium. All experiments were performed in triplicate. Before incubation, the optical density (OD) was measured at 600 nm, and the microplate was sealed and incubated overnight under appropriate conditions. OD600 was measured after the incubation period. The percentage of inhibition was calculated using the following formula:
2.11. Statistical Analysis
Statistical analyses were performed using the free software R (version 3.4.3, R Core Team [34]). The Shapiro–Wilk test was used to assess normality, while the Fligner–Killeen test was applied to evaluate homoscedasticity [35, 36]. Significant differences between extraction conditions for TAC, TPC, DRSC, and EC50, as well as between pure NADES and NADES with extracts on different bacterial strains, were determined using analysis of variance (ANOVA), followed by a post hoc Tukey test for pairwise comparisons. Statistical significance was set at p < 0.05.
3. Results and Discussion
3.1. Viscosity Effect
Due to the high viscosity of some NADES at room temperature (RT), measurements were carried out at 50°C to ensure consistency, even with the addition of 20% water to reduce viscosity. As shown in Table 2, ChCl:CA had the highest viscosity (162.6 ± 21.2 mPa·s), whereas ChCl:U:W and ChCl:LA exhibited the lowest viscosities (9.32 ± 0.13 mPa·s and 13.64 ± 1.05 mPa·s, respectively). The characterization temperature was set to match the extraction temperature, ensuring a reduction in NADES viscosity. Consistent with the findings of Oomen et al., viscosity was adjusted by adding water. However, under these experimental conditions, the addition of water was not sufficient, and an increase in temperature was necessary [37]. The results obtained in this study are consistent with the literature. Notably, adding 20% water to ChCl:U reduced its viscosity by 91.1%, achieving a final value of 9.32 ± 0.13 mPa·s for ChCl:U:W.
| NADES | Dynamic viscosity (mPa·s)a | Densityb | pH of NADES solutionc |
|---|---|---|---|
| ChCl:CA | 162.6 ± 21.2 | 1.28438 ± 0.00294 | 1.10 |
| ChCl:LA | 13.64 ± 1.05 | 1.13269 ± 0.00832 | 1.70 |
| ChCl:OA | 33.46 ± 0.18 | 1.22439 ± 0.00085 | 0.35 |
| ChCl:MA | 49.14 ± 15.14 | 1.23429 ± 0.00449 | 1.26 |
| ChCl:U | 104.3 ± 7.8 | 1.19219 ± 0.00201 | 8.60 |
| ChCl:U:W | 9.32 ± 0.13 | 1.16191 ± 0.00025 | 9.43 |
| Water | 1.002d | 1.00000d | 6.34 |
- aDynamic viscosity was measured at 50°C.
- bDensity was measured at 25°C.
- cMeasurements of solution of diluted NADES (1 gNADES/mLWater) at RT.
- dData from the literature.
The viscosity of ChCl:OA is comparable to that of ChCl:MA (33.49 ± 0.18 mPa·s and 49.14 ± 15.14 mPa·s, respectively). However, it should be noted that ChCl:OA solidifies at RT (< 18°C), whereas ChCl:MA remains liquid. The high viscosity of ChCl:CA is attributed to intermolecular and Van der Waals bonds between NADES molecules and ions [39, 40]. According to Rashid et al., water content significantly influences NADES extraction capacity, with optimal extraction occurring at concentrations below 30%, as higher concentrations lead to a decrease in hydrogen bond availability [16, 37]. Nevertheless, extraction efficiency remains relatively stable when water content is kept below 30%.
3.2. Density Effect
Density measurements were carried out at RT (25°C). As with viscosity, NADES exhibit variable densities, with the highest density observed for ChCl:CA (1.28438 ± 0.00294) and the lowest for ChCl:LA (1.13269 ± 0.00832). Notably, ChCl:OA and ChCl:MA show comparable densities (1.22439 ± 0.00085 and 1.23429 ± 0.00449, respectively). NADES density can be influenced by a variety of factors, including the nature of the HBD and the molar ratio between HBD and HBA [41]. The addition of water can also significantly alter NADES density. In this study, although ChCl:U and ChCl:U:W have the same molar ratio of HBD and HBA, their densities vary, with ChCl:U showing a density of 1.19219 ± 0.00201 and ChCl:U:W a density of 1.16191 ± 0.00025. This difference highlights the impact of compositional variations on NADES density, underscoring the importance of precise control during their synthesis and application.
3.3. pH Effect
The pH measurements were carried out at RT (25°C) to standardize conditions. Attempts to measure the solvent pH directly were unsuccessful, possibly due to factors such as high viscosity, low freezing point, or solvent acidity [39]. NADES solutions were then diluted to a concentration of 1 gNADES/mLWater for pH measurement. The pH values obtained ranged from 0.35 to 9.43. Although these values do not accurately reflect the pH at the time of extraction, they do provide valuable information. pH is temperature-dependent, decreasing as temperature increases (equation (6)) [40]. For instance, pH values reported by Skulcova et al. ranged from 1.2 to 2.74 at 23°C, but decreased to 0.05 to 2.09 when the temperature increased to 60°C.
The pH measurements obtained in this study align well with the existing literature [42]. However, pH can vary depending on factors such as composition and temperature, underscoring the need to control these parameters when using NADES [40]. Table 2 provides values of the viscosity, density, and pH characteristics of the NADES studied. The viscosity was measured under the experimental temperature conditions used due to the high viscosity at 25°C. However, it offers a comprehensive overview of their properties.
3.4. Antioxidant Activity
The results indicate that the TPC values align with the existing literature, confirming the robustness and reliability of our experimental approach (Table 3) [24, 25]. The range of values provided by the literature is between 1 and 9.5 GAE mg/g extract [24]. The observed variation in TPC values, ranging from 2.04 ± 0.19 to 13.15 ± 4.70 GAE mg/g extract, highlights the impact of the NADES composition on phenolic compound extraction. According to Figure 1(a), ChCl:U:W with extracts was significantly higher than all the other TPC values (p < 0.05). In Figure 1(b), pure ChCl:OA was significantly different than pure ChCl:MA, ChCl:U, and ChCl:U:W (p < 0.05). However, pure ChCl:CA and ChCl:LA are not significantly different from ChCl:OA and ChCl:MA, and ChCl:U and ChCl:U:W solvents. Furthermore, significant differences were observed between NADES-containing bioactive compounds and pure NADES, except for ChCl:OA (Figure S1).
| Sample | DRSC EC50 (mgSample/mL) | TPC (GAE mg/g of extract) | ||
|---|---|---|---|---|
| With extract | Pure | With extract | Pure | |
| ChCl:CA | 425.28 ± 51.22 | 441.34 ± 25.37 | 4.09 ± 0.87 | 2.00 ± 0.56 |
| ChCl:OA | 35.59 ± 9.53 | 21.55 ± 1.40 | 3.05 ± 0.53 | 2.33 ± 0.16 |
| ChCl:MA | 268.09 ± 26.35 | 656.92 ± 72.85 | 2.04 ± 0.19 | 1.20 ± 0.11 |
| ChCl:LA | 308.06 ± 56.38 | 1930.44 ± 140.29 | 5.30 ± 1.40 | 1.67 ± 0.47 |
| ChCl:U | 176.71 ± 16.82 | —a | 13.15 ± 4.70 | 1.36 ± 0.34 |
| ChCl:U:W | 111.91 ± 25.06 | —a | 2.98 ± 0.72 | 1.32 ± 0.13 |
- aAntioxidant effects were not observed.
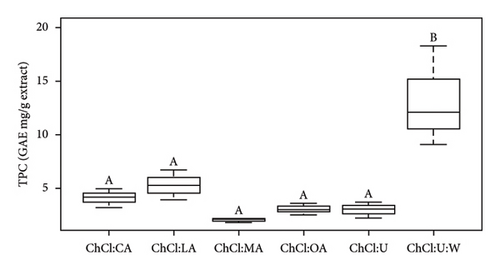
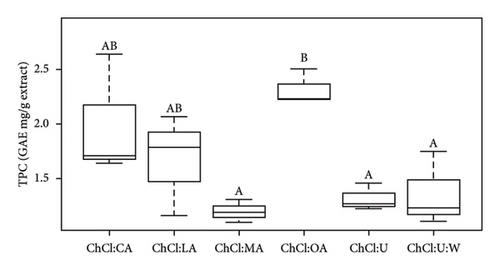
Notably, ChCl:U with extracts showed the highest TPC, while ChCl:MA with extracts showed the lowest. The variation in TPC values between the literature and our case study could be attributed to several factors, including variations in biomass composition (due to climatic environment and variety) and differences in the extraction process [19, 43]. In addition, the impact of pH on the stability of phenolic compounds cannot be overlooked, except for anthocyanins. Anthocyanins degrade at pH levels above 7. In this study, the low pH maintained their stability. Previous studies, such as the work by Ruesgas-Ramón et al., have highlighted the potential for phenolic compound degradation under low pH conditions [15]. This could potentially explain the higher TPC observed in ChCl:U with extracts, given its relatively higher pH values compared to pure acid-based NADES, which ranged from 0.35 to 1.70. In addition, the polarity index of the solvent as pointed out by Deniz et al. has an influence on the extraction of phenolic compounds [25]. Urea, with its relatively high polarity index of 89.63 kJ/mol compared to oxalic acid (the most efficient HBD compound of one of the NADES studied) of 78.59 kJ/mol, may contribute to the higher TPC observed in ChCl:U extracts [25]. Despite the high viscosity of pure ChCl:U (104.3 ± 7.8 mPa.s), its superior extraction capacity, as reflected in the TPC results, highlights the influence of polarity index on phenolic compound extraction. This indicates that, in addition to viscosity, solvent polarity is a key factor influencing extraction efficiency. Golmohamadi et al. extracted high-value-added molecules from red raspberry puree using ultrasound-assisted extraction [44]. They observed that anthocyanidins had minimal impact on TPC results. This explains why TPC values were highest for ChCl:U extracts, but antioxidant test values were lowest.
EC50 values obtained from the DRSC antioxidant assay ranged from 35.59 to 425.28 mgExtracts/mL (Table 3). As a reminder, EC50 represents the half effective concentration required to decrease the initial concentration of DPPH to 50% at equilibrium. Lower EC50 values indicate higher biological activity of the sample tested. According to Figures 2(a) and 2(b), there are 4 different groups of NADES with bioactive compounds. ChCl:LA and ChCl:MA with extracts were insignificantly different. Extract ChCl:CA was significantly different from the other DRSC values as ChCl:OA. Urea-based NADES-containing extracts were insignificantly different. In Figure 1(b), pure ChCl:OA, ChCl:U, and ChCl:U:W were similar. However, pure ChCl:CA, ChCl:LA, and ChCl:MA exhibited significant differences from one another. Additionally, significant differences were observed between NADES-containing bioactive compounds and pure NADES, except for ChCl:CA (Figure S2).
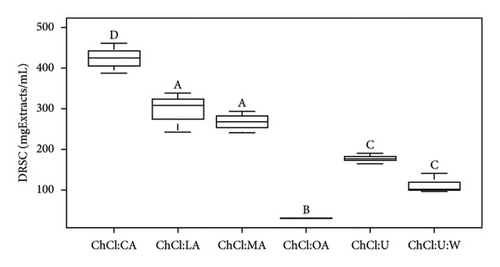
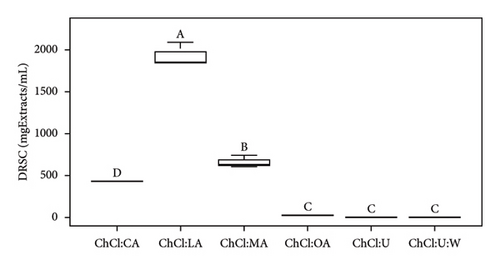
According to Table 3, the ChCl:OA extract is the most effective extract in terms of antioxidant activity. However, it should be noted that NADES solvents are primarily responsible for antioxidant activity [45]. EC50 values (ChCl:OA) for extracts and pure NADES are 35.59 ± 9.53 and 21.55 ± 1.40 mgSamples/mL, respectively. Notably, all acid-based solvents show antioxidant activity, while urea-based solvents do not, which is consistent with the existing literature [25, 42, 46]. Radošević et al. have previously suggested that ChCl and urea lack antioxidant properties, which was confirmed by the results of the oxygen radical absorbance capacity test (antioxidant test) and corroborated by the results of our DRSC assay [42].
Interestingly, both urea-based NADES extracts displayed notable EC50 values, with ChCl:U and ChCl:U:W extracts reaching 176.71 ± 16.82 and 111.91 ± 25.06 mgExtracts/mL, respectively. The difference between these two samples is the addition of 20% water, which results in a decrease in viscosity (from 104.3 ± 7.80 to 9.32 ± 0.13 mPa.s) [24]. In addition, pure NADES do not exhibit antioxidant activity. This reduction in viscosity improves diffusion rates and mass transfer, thereby enhancing antioxidant activity. Extracted molecules in ChCl:LA and ChCl:MA solvents show similar EC50 values, 308.06 ± 56.38 and 268.09 ± 26.35 mgExtracts/mL, respectively. However, the EC50 values of their solvents vary significantly; the ChCl:MA solvent shows a higher antioxidant property (656.92 ± 72.82 mgSolvent/mL) than the ChCl:LA solvent (1930.44 ± 140.29 mgSolvent/mL). This variation suggests that the extracts in ChCl:LA may possess higher antioxidant properties than the ChCl:MA with extracts. In contrast, the extracts in ChCl:CA showed lower antioxidant activity (425.28 ± 51.22 mgExtracts/mL), mainly due to its solvent (441.34 ± 25.37 mgSolvent/mL). As previously mentioned, viscosity plays a crucial role during extraction and, consequently for antioxidant activity. The EC50 values appear to correlate with the pH of pure acid-based NADES, except for ChCl:CA solvents, for which lower pH values correspond to lower EC50 values, indicating better antioxidant activity. Overall, the order of antioxidant activity of the NADES observed is as follows: ChCl:OA > ChCl:CA > ChCl:MA > ChCl:LA (Table 3), with corresponding pH values of the solvent: ChCl:OA < ChCl:MA < ChCl:LA < ChCl:CA (Table 2). The exception of ChCl:CA may be attributed to the high viscosity of this solvent. The results presented in Table 3 are statistically analyzed in Figures 1 and 2.
As preliminary tests for future experiments using Amberlite XAD-16 (resin) to remove solvents, we were able to measure the EC50 of extracts, even in the presence of residual solvent (Table S1). Remarkably, after partial purification of extracts, the solvent had no noticeable effect on antioxidant activity. These results suggest that the antioxidant activity observed is mainly attributed to the bioactive compounds extracted from the AP rather than the solvent itself.
3.5. TAC Determination
The TAC of the solvents was measured to assess their impact. However, no significant responses were detected. It should be noted that acid-based NADES are well-known for their ability to extract anthocyanins [20, 24, 47]. Among them, ChCl:OA extracts exhibited the highest TAC, with a value of 64.81 ± 4.65 M3GE μg/mL (Figure 2). Interestingly, ChCl:LA and ChCl:U:W extracts gave similar results, with TAC values of 33.46 ± 3.87 and 31.41 ± 11.13 M3GE μg/mL, respectively. Similarly, extracts from ChCl:MA and ChCl:U produced comparable results, with TAC values of 18.89 ± 5.56 and 16.63 ± 2.44 M3GE μg/mL, respectively. Notably, ChCl:CA extracts presented the lowest TAC, with a value of 9.68 ± 2.23 M3GE μg/mL.
According to Figure 3, ChCl:OA, ChCl:CA, and ChCl:LA extracts are significantly different from each other (p < 0.05). However, ChCl:MA, ChCl:U, and ChCl:U:W containing bioactive compounds are not significantly different from ChCl:LA and ChCl:CA extracts.
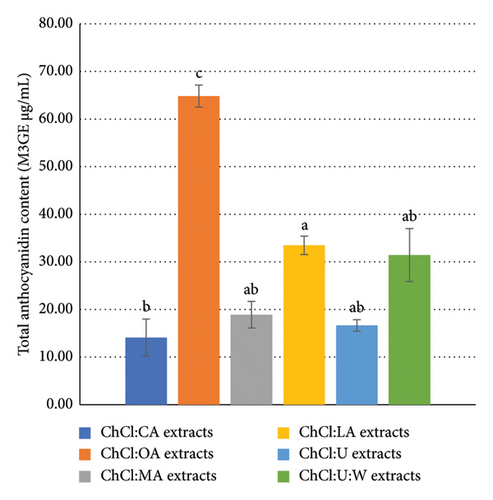
Pure ChCl:CA exhibits the highest viscosity (162.6 ± 21.2 mPa·s), the highest EC50, and the lowest TAC value among NADES tested. This observation points to the limitation of high-viscosity NADES, which poses a significant problem for anthocyanidins extraction by reducing mass transfer [24]. Moreover, the high viscosity of pure NADES may dissuade manufacturers from using them [19, 24]. High viscosity increases power requirements for stirring, for instance.
The results show a clear correlation between TAC and the EC50 of extracts, except for ChCl:U extracts (Table 4). The relatively low TAC of ChCl:U extracts could be attributed to the absence of additional water and the high viscosity (104.3 ± 7.8 mPa·s) of the pure NADES. Similarly, ChCl:CA extracts showed poor anthocyanidin extraction due to the highly viscous nature of the solvent, which is in accordance with the literature [24]. Furthermore, pure ChCl:U is not commonly used for anthocyanidin extraction compared to acid-based NADES, as anthocyanidins are more easily extracted with low pH solvents [20, 24, 47]. The pure ChCl:U seems to be more suited for extracting polyphenol (such as phlorizin and quercetin), which explains the higher TPC despite its high viscosity and low TAC in its extracts. The variation in TAC between the ChCl:U extract (16.63 ± 2.44 M3GE μg/mL) and the ChCl:U:W extract (31.41 ± 11.13 M3GE μg/mL) is explained by the addition of water, anthocyanins being water-soluble molecules [32].
| Sample | TAC (M3GE μg/mL)a | DRSC EC50 (mgExtracts/mL)a | Dynamic viscosity (mPa·s)b |
|---|---|---|---|
| ChCl:CA | 9.68 ± 2.23 | 425.28 ± 51.22 | 162.6 ± 21.2 |
| ChCl:OA | 64.81 ± 4.65 | 35.59 ± 9.53 | 13.64 ± 1.05 |
| ChCl:MA | 18.89 ± 5.56 | 268.09 ± 26.35 | 33.46 ± 0.18 |
| ChCl:LA | 33.46 ± 3.87 | 308.06 ± 56.38 | 49.14 ± 15.14 |
| ChCl:U | 16.63 ± 2.44 | 176.71 ± 16.82 | 104.3 ± 7.8 |
| ChCl:U:W | 31.41 ± 11.13 | 111.91 ± 25.06 | 9.32 ± 0.13 |
- aNADES with extracts.
- bPure NADES.
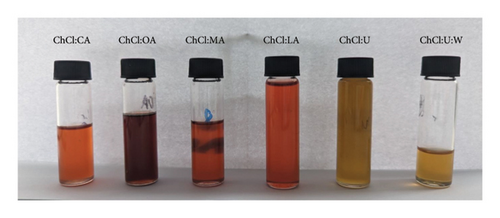
The color of the extracts provides additional information (Figure 4). Extracts in acid-based NADES, particularly the extract in ChCl:OA (with the highest TAC), show a dark red color. In contrast, extracts in ChCl:U:W and ChCl:U appear yellowish-orange. Given that the apple variety used in the study is Story, known for its dark red color, the observed color of the extracts is in agreement with the results obtained for TAC [48]. In addition, the color of anthocyanins is pH-dependent. Under acidic conditions, anthocyanins are red or pink, and in basic solutions, these pigments are blue or purple [32]. Extracts in ChCl:U and ChCl:U:W are under alkaline conditions and neither of them is blue or purple, reflecting the low concentration of anthocyanins in the solvents. According to Golmohamadi et al., the solvent is darker when there are more anthocyanidins extracted [44]. This observation is in good agreement with our results.
As previously indicated, there appears to be a correlation between TAC and EC50, suggesting that the antioxidant activity of extracts is influenced by both the solvent used and the concentration of anthocyanidins extracted. Similar results were observed by Zannou and Koca who also noted a relationship between antioxidant activity and TAC [24].
TAC also appears to correlate with viscosity (Table 4). As noted by Zannou and Koca, high viscosity limits the anthocyanidin extraction capacity [24]. Among acid-based NADES, ChCl:CA has the highest viscosity (162.6 ± 21.2 mPa·s) and a low TAC (9.38 ± 2.23 M3GE μg/mL), consistent with the findings of Zannou et al. In contrast, the extract in ChCl:OA, the least viscous NADES (13.64 ± 1.05 mPa·s), exhibits the highest TAC (64.81 ± 4.65 M3GE μg/mL). Although NADES ChCl:LA (49.14 ± 15.14 mPa·s) has a NADES higher viscosity than ChCl:MA (33.46 ± 0.18 mPa·s), it extracts anthocyanidins more efficiently (33.46 ± 3.87 and 18.89 ± 5.56 M3GE μg/mL, respectively). This may be attributed to the ability of NADES ChCl:LA to readily form hydrogen bond networks with polyphenols, thus facilitating anthocyanidin extraction despite its higher viscosity than NADES ChCl:MA [24].
3.6. Antibacterial Activity
The antibacterial activity of the extracts is evident from the EC50 and DIZ values presented in Tables 5 and 6. All extracts show antibacterial activity against E. coli, L. innocua, and B. cereus. Extracts in ChCl:CA and in ChCl:MA exhibited larger DIZ than their respective solvents for all strains studied (Table 5). Furthermore, the extract in ChCl:LA leads to larger DIZ than pure ChCl:LA for L. innocua and B. cereus, while the extract in ChCl:OA and pure ChCl:OA shows this trend only for E. coli (Table 5). The concentration of 300 mgSample/mLPBS was chosen according to preliminary tests. The results presented in Table 5 are statistically analyzed in Figures S7, S8, and S9.
| Sample | Diameter inhibition zone (mm) | |||||
|---|---|---|---|---|---|---|
| Escherichia coli | Listeria innocua | Bacillus cereus | ||||
| With extract | Pure | With extract | Pure | With extract | Pure | |
| ChCl:CA | 16.2 ± 1.0 | 14.7 ± 2.1 | 16.7 ± 2.1 | 13.3 ± 1.5 | 14.8 ± 2.0 | 12.7 ± 0.6 |
| ChCl:OA | 18.0 ± 0.5 | 13.3 ± 0.6 | 14.2 ± 1.0 | 20.0 ± 1.0 | 14.3 ± 1.5 | 19.7 ± 0.6 |
| ChCl:MA | 17.3 ± 1.3 | 15.3 ± 0.6 | 16.0 ± 1.0 | 13.2 ± 0.3 | 16.7 ± 0.8 | 16.3 ± 1.5 |
| ChCl:LA | 14.8 ± 0.3 | 15.0 ± 2.0 | 13.2 ± 0.3 | 12.3 ± 0.6 | 14.5 ± 1.5 | 13.8 ± 0.8 |
| ChCl:U | —b | —b | —b | —b | —b | —b |
| ChCl:U:W | —b | —b | —b | —b | —b | —b |
| ChCl | —b | —b | —b | |||
| CA | 17.0 ± 1.0 | 21.3 ± 0.6 | 23.7 ± 2.1 | |||
| OAa | 20.7 ± 0.6 | 20.0 ± 1.0 | 24.3 ± 1.5 | |||
| MA | 19.7 ± 0.6 | 15.3 ± 2.1 | 24.5 ± 0.5 | |||
| LA | 12.7 ± 0.6 | 16.0 ± 1.0 | 22.0 ± 1.0 | |||
| U | —b | —b | —b | |||
| Ampicillin | 21.0 ± 2.0 | 28.7 ± 0.6 | 12.3 ± 1.7 | |||
| PBS in waterc | —b | —b | —b | |||
- aConcentration at 150 mg/mLPBS.
- bAntibacterial effects were not observed.
- cPBS: phosphate-buffered saline, pH 7.4.
| Sample | ChCl:CA | ChCl:OA | ChCl:MA | ChCl:LA | ChCl:U | ChCl:U:W | ||
|---|---|---|---|---|---|---|---|---|
| Bacillus cereus | MIC (mgSample/mL) | Pure NADES | 50 | 25 | 50 | 75 | —b | —b |
| With extracts | 50 | 25 | 50 | 75 | —b | —b | ||
| EC50 (mgSample/mL) | Pure NADES | 17.4 ± 1.2 | 5.8 ± 0.1 | 20.3 ± 0.7 | 36.5 ± 3.8 | —a | —a | |
| With extracts | 17.2 ± 1.3 | 6.0 ± 0.3 | 19.4 ± 0.8 | 30.5 ± 0.8 | —a | —a | ||
| Listeria innocua | MIC (mgSample/mL) | Pure NADES | 50 | 50 | 50 | 100 | —b | —b |
| With extracts | 50 | 50 | 50 | 100 | —b | —b | ||
| EC50 (mgSample/mL) | Pure NADES | 20.3 ± 0.9 | 17.2 ± 0.7 | 24.3 ± 0.4 | 36.9 ± 3.4 | —a | —a | |
| With extracts | 22.5 ± 0.2 | 19.4 ± 1.7 | 25.5 ± 1.1 | 40.8 ± 0.7 | —a | —a | ||
| Escherichia coli | MIC (mgSample/mL) | Pure NADES | 50 | 50 | 50 | 75 | 150 | 150 |
| With extracts | 50 | 50 | 50 | 75 | 150 | 150 | ||
| EC50 (mgSample/mL) | Pure NADES | 24.1 ± 0.3 | 18.8 ± 1.5 | 30.7 ± 1.4 | 35.8 ± 1.7 | 106.4 ± 8.8 | 130.5 ± 10.7 | |
| With extracts | 29.9 ± 3.0 | 23.2 ± 3.2 | 28.9 ± 1.0 | 34.3 ± 9.9 | 47.2 ± 11.5 | 90.3 ± 1.1 | ||
- aAntibacterial effects were not observed (when 50% of inhibition).
- bAntibacterial effects were not observed (when ≥ 90% of inhibition).
Analysis of the MIC and DIZ values (Tables 5 and 6) shows that B. cereus is the least sensitive of the strains studied. Interestingly, E. coli and L. innocua exhibit similar MIC values, except for extract in NADES ChCl:LA and pure NADES, where the MIC for L. innocua is higher than that of E. coli, at 100 and 75 mgSample/mL, respectively. It should be noted that E. coli is Gram-negative bacteria, while L. innocua and B. cereus are Gram-positive bacteria. Previous studies have indicated that Gram-positive bacteria are generally more sensitive than Gram-negative bacteria due to differences in their cell wall structure. Gram-negative bacteria have an additional lipopolysaccharide membrane, which forms a protective layer for the cell [42, 49].
The antibacterial properties observed are mainly attributed to the NADES used. According to the existing literature, pure acid-based NADES can exhibit a certain toxicity to organisms due to their pH, which denatures bacterial membranes [42]. The optimum pH for bacterial growth lies between 6.5 and 7.5 [49]. In particular, oxalic acid shows the highest antibacterial activity, followed by malic acid and citric acid [42, 49]. In our study, pure ChCl:CA displays greater antibacterial activity than pure ChCl:MA, with EC50 values of 24.1 ± 0.3 and 30.7 ± 1.4 mgSolvent/mL for E. coli, respectively (Table 6). This difference in activity could be attributed to variations in the formulation method of the NADES [49]. Indeed, Bedair et al. stated that the same NADES can exhibit different toxicities depending on its method of preparation. The higher antibacterial activity observed with pure ChCl:CA may be attributed to antibacterial impurities present in the solvent, or conversely, pure ChCl:MA may contain impurities that promote bacterial growth. Furthermore, Radošević et al. previously reported that malic acid exhibited greater antibacterial activity than citric acid. However, the preparation methods differ between their study and ours; they employed a vacuum preparation technique, whereas we used a heating method.
Several factors may contribute to bacterial mortality, including pH, solvent viscosity, osmolality, chelation of membrane-bound divalent cations, and interactions involving ChCl in aqueous media, such as electrostatic interactions or hydrogen bonding with polysaccharide chains [42, 49, 50]. In this study, various concentrations of NADES extracts in PBS were tested against bacterial strains. PBS was used to maintain a pH of 7.4, thereby minimizing pH-related effects on bacterial viability. However, PBS alone was not sufficient to neutralize the acidic pH of NADES extracts, as observed for pure acidic NADES (Figure S3). A correlation was found between the EC50 of solvents and pH, where lower pH values in pure NADES were associated with lower EC50 values, as shown in Tables 2 and 6.
According to Radošević et al., pure ChCl:U exhibits low toxicity, as this solvent only demonstrates antibacterial activity against E. coli (37 ± 5 mm with ChCl:U and 10% of water) and no antibacterial activity against Proteus mirabilis, Salmonella typhimurium, Pseudomonas aeruginosa, and Staphylococcus aureus [42]. We agree with this statement from Tables 5 and 6. In our case study, whether pure or containing an extract, ChCl:U showed no antibacterial activity against L. innocua, and B. cereus, in solid broth from 150 mgSolvent/mL, in liquid broth against E. coli (Tables 5 and 6). The difference in results could be due to the bacterial strains used [46]. To corroborate our data, ampicillin (antibiotic) was tested and led to different DIZs (Table 5) depending on the strains. For instance, B. cereus (12.3 ± 1.7 mm) is more resistant to the antibiotic than E. coli or L. innocua (21.0 ± 2.0 and 28.7 ± 0.6 mm, respectively), whose bacterial resistance depends on the sample tested, in this specific case, the antibiotic. Furthermore, B. cereus seems to be more resistant to pure or extract-containing NADES than other strains (Table 6). ChCl:U and ChCl:U:W pure or containing an extract does not exhibit antibacterial activity with respect to their MIC (when %inhibition ≥ 90%) and their EC50 (when %inhibition = 50%) against B. cereus (Table 6). The percentage inhibition of pure NADES and NADES with extracts is from 0% to 30% for concentrations between 5 and 300 mgSamples/mL. The fact that urea-based solvents are not toxic to bacteria can be attributed to the components. Indeed, ChCl and U are essential for the development of living cells [19]. A second study mentions that pure NADES such as ChCl:U can help maintain enzyme stability. This stability is attributed to the hydrogen bond networks, which maintains NADES activities, even though urea is used as a protein-denaturing agent [46, 51]. In the case of pure ChCl:U, no antibacterial activity is cited in the literature, indicating that pure NADES does not denature bacterial protein membranes. Our investigations suggest similar results with pure ChCl:U and ChCl:U:W on B. cereus and L. innocua (Tables 5 and 6). However, pure ChCl:U demonstrates antibacterial activity against E. coli at a concentration of 150 mgSolvent/mL (Table 6). According to Table 5, pure or extract-containing ChCl:U does not exhibit any antibacterial activity. On the other hand, EC50 of ChCl:U containing extract is lower than the EC50 ChCl:U of pure NADES, respectively, at 47.2 ± 11.5 mgExtract/mL and 106.4 ± 8.8 mgSolvent/mL (Table 6). EC50 values indicate antibacterial activity of the extracted bioactive compounds. However, according to Table 4, ChCl:U with extracts demonstrates no antibacterial activity (no DIZ). Table 6 indicates antibacterial activity (MIC at 150 mgSamples/mL). The difference between these two tests can be explained by the fact that ChCl:U with extract and pure NADES does not necessarily kill bacteria but only inhibits their growth. They are bacteriostatic and no bactericidal. The results presented in Table 6 are statistically analyzed in Figures 5, S4, S5, and S6.
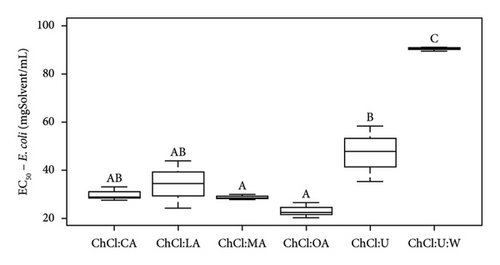
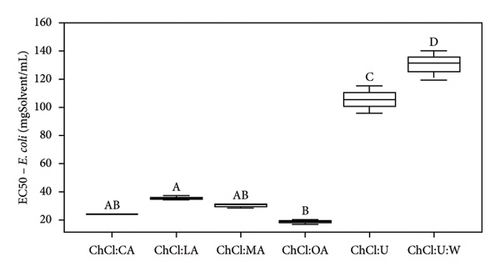
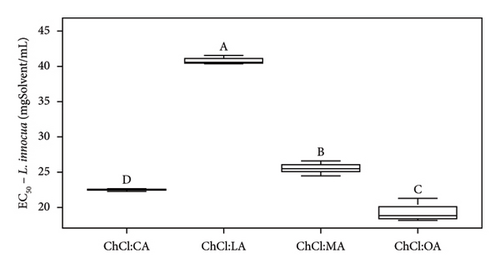
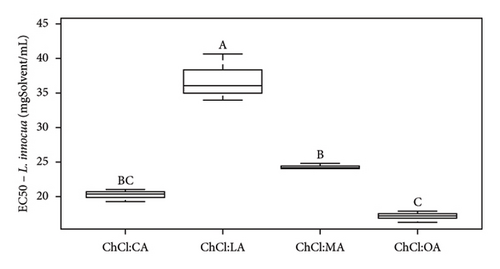
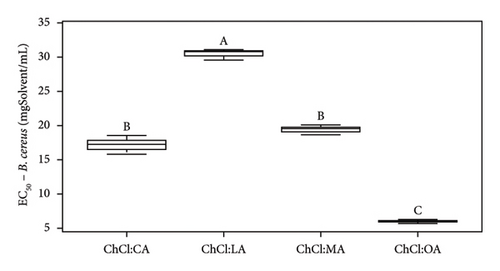
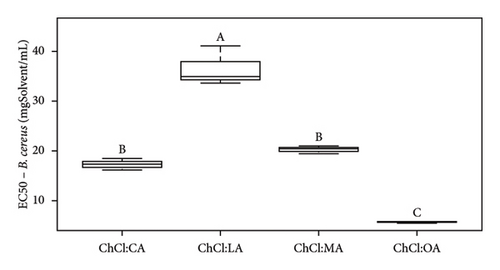
According to Figure 5(a), ChCl:OA and ChCl:MA, and ChCl:U and ChCl:U:W extracts are significantly different from each other (p < 0.05). However, ChCl:CA and ChCl:LA-containing bioactive compounds are not significantly different from ChCl:OA and ChCl:MA, ChCl:U, and ChCl:U:W extracts. Pure ChCl:CA and ChCl:MA are not significant different from ChCl:LA and ChCl:OA according to Figure 5(b). All the MIC values against L. innocua are significantly different from each other (Figure 5(c)). However, only ChCl:CA is not statically different from ChCl:LA and ChCl:MA in Figure 5(d). Against B. cereus, ChCl:CA and ChCl:MA extracts exhibit significant differences compared to ChCl:LA and ChCl:OA extracts (Figures 5(e) and 5(f)). The same trend is observed for pure NADES.
For statistical analysis, please refer to Figures 5, S4, S5, and S6 in ESI.
4. Conclusion
In conclusion, bioactive compounds from AP were successfully recovered using an efficient green extraction system using six ChCl-based NADES with four organic acids (citric, oxalic, malic, and lactic acid) and two ureas (one supplemented with 20% water). The highest content of TPC was obtained with ChCl:U with extracts (13.15 ± 4.70 GAE mg/g extract). Nevertheless, the highest antioxidant (DRSC) and antibacterial activities were obtained for ChCl:OA with extract 35.59 ± 9.53 mgExtract/mL (EC50) and 25 mgExtract/mL (MIC), respectively, on B. cereus. A correlation between DRSC, TAC, and viscosity was observed. At the lowest viscosities, the highest antioxidant properties and TAC were observed. Several parameters improve the solvent extraction capacity of (1) the addition of water improves the extraction yield of biomolecules, as evidenced by the TAC and DRSC of ChCl:U and ChCl:U:W with extracts (16.63 ± 2.44 and 31.41 ± 11.13 M3GE μg/mL, and 176.11 ± 16.82 and 111.91 ± 25.06 mgExtracts/mL, respectively) due to decreased viscosity (increased of mass transfer); (2) acidic conditions allow more selective extraction than alkaline conditions, especially for anthocyanins, as shown by the TAC values: 64.81 ± 4.65 M3GE μg/mL for ChCl:OA extracts and 31.41 ± 11.13 M3GE μg/mL for ChCl:U:W extracts. For acidic NADES, the biological properties of the extracts are mainly attributed to the solvents (urea-based solvents do not exhibit antibacterial activity against B. cereus). The current work focuses on removing the solvent to investigate the activities of pure bioactive molecules. Parts of this work were previously presented and discussed in the doctoral thesis of Bruna [52], which served as a foundation for the development of the present manuscript.
Nomenclature
-
- ANOVA
-
- Analysis of variance
-
- AP
-
- Apple pomace
-
- B. cereus
-
- Bacillus cereus
-
- BHI
-
- Brain–heart infusion
-
- CA
-
- Citric acid
-
- ChCl
-
- Choline chloride
-
- ChCl:CA
-
- Choline chloride–citric acid
-
- ChCl:LA
-
- Choline chloride–lactic acid
-
- ChCl:MA
-
- Choline chloride–malic acid
-
- ChCl:OA
-
- Choline chloride–oxalic acid
-
- ChCl:U
-
- Choline chloride–urea
-
- ChCl:U:W
-
- Choline chloride–urea–water
-
- DES
-
- Deep eutectic solvent
-
- DIZ
-
- Diameter inhibition zone
-
- DRSC
-
- DPPH radical scavenging capacity
-
- EC50
-
- Effective concentration at 50%
-
- E. coli
-
- Escherichia coli
-
- GAE
-
- Gallic acid equivalent
-
- HBA
-
- Hydrogen bond acceptor
-
- HBD
-
- Hydrogen bond donor
-
- LA
-
- Lactic acid
-
- L. innocua
-
- Listeria innocua
-
- MA
-
- Malic acid
-
- M3GE
-
- Malvidin-3-glucoside equivalent
-
- MIC
-
- Minimum inhibitory concentration
-
- NADES
-
- Natural deep eutectic solvent
-
- OA
-
- Oxalic acid
-
- OD
-
- Optical density
-
- o/n
-
- Overnight
-
- PBS
-
- Phosphate-buffered saline
-
- PD
-
- Petri dish
-
- RT
-
- Room temperature
-
- SC-CO2
-
- Supercritical CO2
-
- TAC
-
- Total anthocyanin content
-
- TPC
-
- Total polyphenol content
-
- U
-
- urea
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization, methodology, validation, data curation, writing – original draft, visualization: L.B.; investigation and formal analysis: L.B., C.F.; resources and funding acquisition: Gregory Chatel, Giancarlo Cravotto; writing – review and editing: L.B., M.D., E.L., G.G., Gregory Chatel, Giancarlo Cravotto; supervision, project administration: M.D., Gregory Chatel, Giancarlo Cravotto.
Funding
This study was funded by the French Agence Nationale de la Recherche, ANR 20-GURE-0015; the European Union Next-GenerationEU, CN00000022; Erasmus+ Programme; and the European University UNITA, Agritech National Research Center.
Acknowledgments
The authors thank the French Agence Nationale de la Recherche for its support of this work through the Programme d’investissement d’avenir (Grant No. ANR 20-GURE-0015) and the Agritech National Research Center and received funding from the European Union Next-GenerationEU (Piano Nazionale di Ripresa e Resilienza (PNRR)—missione 4 com-ponente 2, investimento 1.4—D.D. 1032 17/06/2022, CN00000022). The authors also extend our thanks to the Erasmus + Programme of the European Commission and the European University UNITA for awarding the PhD scholarship to Lauriane Bruna.
Supporting Information
An electronic Supporting Information provides some details on experimental part: (1) Sample clean-up to remove NADES; (2) antioxidant activity protocol (DDPH assay); (3) statistical analysis of TPC; (4) statistical analysis of DRSC; (5) results from DPPH assay; (6) measurement of pH of NADES solutions; (7) statistical analysis of EC50.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




