The Genoprotective Role of Laurocerasus officinalis: Study on Genotoxic/Antigenotoxic and Cytotoxic/Anticytotoxic Effects in Human Lymphocytes
Abstract
Laurocerasus officinalis, a valuable medicinal plant, has rich flavonoid and anthocyanin content, providing benefits for various diseases. It also has ethnopharmacological uses such as diuretics and antidiabetics. Phenolic compounds exhibit their anticarcinogenic effects directly by repairing DNA damage and reducing chromosomal abnormalities. This study aims to investigate genotoxicity/antigenotoxicity and cytotoxicity/anticytotoxicity of L. officinalis extract in human peripheral lymphocytes by the methods of chromosomal aberration (CA), cytokinesis-block micronucleus (CBMN) test, and mitotic index value. The doses of L. officinalis extract were determined as 125, 250, 500, and 1,000 μg/mL for the genotoxicity test, and mitomycin C (MMC) was added to induce DNA damage for the investigation of antigenotoxicity. In both applications (24 and 48 h), all doses, positive control (MMC) and negative control (sterile dH2O), were used. In CA and CBMN tests for genotoxicity, no significant differences existed between all doses and the solvent control group (p > 0.05). In antigenotoxic activity, doses added with MMC were significantly lower than positive control (p < 0.05). For both the 24-hour and 48-hour periods, the percentage of abnormal cells and the CAs per cell ratio were significantly lower at all L. officinalis extract concentrations compared to the positive control. Additionally, as the treatment concentration increased, the CA ratio showed a decreasing trend. These differences were strongly dose-dependent compared to the positive control (r = −0.799). In all concentrations in the CBMN test, the frequencies were significantly lower than the positive control, and the higher the L. officinalis extract concentrations, the lower the micronuclei (MN). There was a strong dose-dependent relationship in MN formation compared to positive control (r = −0.925). Therefore, L. officinalis extract has no genotoxic effect on human lymphocytes and also has antigenotoxic and protective effect to the damage of MMC. In the mitotic index result, the L. officinalis extract was cytotoxic at only one dose (125 μg/mL) but anticytotoxic at all other doses against MMC exposure. In conclusion, consuming L. officinalis medicinally benefits and may protect against exposure to genotoxic agents.
1. Introduction
Laurocerasus officinalis M.Roem., synonym of Prunus laurocerasus L., is grown in Turkey, the Balkans, Northern Ireland, Iran, and some Mediterranean countries for its fruit, known to have medicinal benefits, and for use in the landscape such as anti-erosion and windbreak [1, 2]. L. officinalis has ethnopharmacological uses including its diuretic and anti-diabetic properties and for the treatment of stomach ulcers, digestive system problems, bronchitis, eczemas, and hemorrhoids. The most well-known benefit of L. officinalis, an ethnographically important fruit, is for diabetic patients [3–5]. There are studies in the literature about the positive effects of L. officinalis fruit on neurodegenerative diseases such as Alzheimer’s and Parkinson’s, and cardiovascular disease due to its high antioxidant content. Moreover, it is considered to have analgesic and antispasmodic effects [6–8]. According to biochemical analysis, L. officinalis fruit contains minerals such as magnesium, sodium, potassium, calcium, ferric, and zinc; and carbohydrates, protein, and lipid such as linoleic acid [9, 10]. The phenolic acid content of the L. officinalis fruit provides high antioxidant activity, consisting of chlorogenic acid, vanillic acid, hydroxybenzoic acid, citric acid, and catechin [9–11].
Anthocyanins, water-soluble red or purple color pigments, are one of the most important subgroups of phenolic acids, well-known as strong antioxidants. L. officinalis was focused on in many scientific studies because of its high anthocyanin and phenolic acid content and ethnopharmacological uses. In a study with type 2 diabetic rats, the group treated with L. officinalis extract had higher glutathione peroxidase (GPx), superoxide dismutase (SOD), serum insulin, paraoxonase, and aryl esterase activity compared to the control group. Also, glucose, triglyceride, and malondialdehyde levels were significantly lower in the L. officinalis treated group. Therefore, the antilipidemic and antihyperglycemic effects of L. officinalis were shown clearly [12]. The increase in blood insulin level was observed in diabetic rats by stimulating beta cell and pancreatic secretion [13], and the glucose level increase in hypoglycemic rats and decrease in hyperglycemic rats were also detected by treating with L. officinalis extract [14]. The anti-inflammatory and antinociceptive activity of L. officinalis extract was proved with in vitro studies on rats [15, 16]. Moreover, L. officinalis extract decreased the damage of MTX on the testis tissue and increased sperm motility in rats [17]. The bacteriostatic effect of L. officinalis extract on Escherichia coli (O157:H7) and Salmonella Enteritidis was proved [18].
The L. officinalis fruit can be a very useful dietary product with a wide range of uses, a food that supports the treatment or acts as a preventive measure for many diseases. For this, L. officinalis must be tested in terms of its effects on human health, and its reliability must be proven. Because of the high anthocyanin content, there are many studies on the antioxidant properties of L. officinalis extract and oxidative stress-related disease, but there is limited evidence for the protective role of these substances against DNA damage. Therefore, this work aims to evaluate genotoxic/antigenotoxic and cytotoxic/anticytotoxic effects of L. officinalis extract using mitomycin C (MMC) as a mutagen applying in vitro chromosomal aberrations (CA) and cytokinesis-block micronucleus (CBMN) tests in human peripheral blood lymphocytes.
2. Materials and Methods
2.1. Laurocerasus officinalis Extract (LOE) and Chemicals
In this study, the classical extraction method was applied with some modifications to obtain total anthocyanin and phenolic substances from L. officinalis fruits. The fruits collected from Yuvalıdere village in Karasu district of Sakarya province during August–September were stored at −20°C. The fleshy part of the frozen fruit was separated from the seeds, and 100 g of fruit was blended using a blender. Milled fruit with methanol (250 mL) was taken to an Erlenmayer flask and placed in the dark at room temperature for 48 h. After the mix was filtrated with Whatman paper, methanol was removed from the filtrate by using rotary evaporator under vacuum at 40°C–43°C for purification of total anthocyanins. The chemicals were used for genotoxicity and antigenotoxicity tests: Chromosome medium B (cas no: F 5023) was obtained from Biochrome (Berlin, Germany), and MMC (cas no: 50-07-7), colchicine (cas no: 64-86-8), cytochalasin B (C29H37NO5, cas no: 14930-96-2), potassium chloride (cas no: 7447-40-7), methanol (cas no: 67-56-1), and acetic acid (cas no: 64-19-7) were obtained from Sigma (St. Louis, MO, USA). Also, giemsa stain (cas no:3204-19-00), tampon A (KH2PO4, cas no: 7778-77-0), tampon B (Na2HPO4, cas no: 7558-79-04), and heparin were used.
2.2. Collection of Blood Samples
For genotoxicity and antigenotoxicity tests, four healthy donors (two male and two female) were selected voluntarily by signing the informed consent form to collect peripheral venous blood. Criteria for selecting the donors were aged between 20 and 25 years, not smoking and not using alcohol, not having any chronic diseases, not using drugs in the last 2 months, not being exposed to diagnostic and therapeutic radiation in the last 6 months, and with no history of chromosome fragility and recent viral infection. This research was approved by Sakarya University Medicine Faculty Non-drug Clinical Research Ethics Committee (Ethic Committee no: 16214662/050.01.04/75).
2.3. Dose Selection
The highest application concentration for LOE was determined as 1,000 μg/mL. The other three concentrations were calculated as 1/2, 1/4, and 1/8 of the highest concentration. As a result, 1,000, 500, 250, and 125 μg/mL concentrations were used for the test substance. In addition, positive, solvent, and negative controls were included. LC50 values were considered in the selection of the dose.
2.4. Chromosome Aberration (CA) Assay
Peripheral blood samples (2 mL) (with 10% heparin) of four healthy donors were added to 2.5 mL culture medium (chromosome medium B) containing a mitogen (phytohemagglutinin). For the genotoxicity test, the cultures were treated with 125, 250, 500, and 1,000 μg/mL concentrations of LOE, and negative control (sterile dH2O), solvent control (methanol), and positive control (MMC, 0.20 μg/mL) groups were prepared. For the antigenotoxicity test, all lymphocyte cultures were treated with MMC plus 125, 250, 500, and 1,000 μg/mL concentrations of LOE. Lymphocyte cells were subjected to two treatments with the test substance in a culture medium for 24 and 48 h. In both tests, the lymphocyte culture was incubated at 37°C for 72 h, and colchicine (0.06 μg/mL) was added to each one at the 70th hour. The cultures were harvested by centrifugation (1,200 rpm for 10 min), and the pellet was treated with 0.075 M KCl (hypotonic solution) for 30 min at 37°C. The culture tubes were centrifugated again and treated with fixation solution (3 methanol: 1 acetic acid) at 4°C for 15 min and repeated three times. The supernatant was removed and the pellet was homogenized with the pipette, dropped on the slides, stained with 5% Giemsa (pH: 6.8) in Sorensen buffer (with tampon A and tampon B) for approximately 20 min, washed with distilled water, let to dry at room temperature, and mounted with entellan [19].
2.5. CBMN Assay
Peripheral blood samples (2 mL) (with 10% heparin) of four healthy donors were added to 2.5 mL culture medium (chromosome medium B) containing a mitogen (phytohemagglutinin). For the genotoxicity test, the cultures were treated with 125, 250, 500, and 1,000 μg/mL concentrations of LOE, and negative control (sterile dH2O), solvent control (methanol), and positive control (MMC, 0.20 μg/mL) groups were prepared. For the antigenotoxicity test, all lymphocyte cultures were treated with MMC plus 125, 250, 500, and 1,000 μg/mL concentrations of LOE for 48 h. In both tests, the lymphocyte cultures were incubated at 37°C for 72 h and cytochalasin B (5.2 μg/mL) was added to each one at the 44th hour to arrest cytokinesis. The cultures were harvested by centrifugation (1,000 rpm for 10 min), and the pellet was treated with 0.075 M KCl (hypotonic solution) for 5 min at 4°C. The culture tubes were centrifugated again and treated with fixation solution (3 methanol: 1 acetic acid) at 4°C for three times. In the last fixative step, 1% formaldehyde was added to preserve the cytoplasm. The supernatant was removed, and the pellet was homogenized with the pipette, dropped on the slides, stained with 5% Giemsa (pH: 6.8) in Sorensen buffer for approximately 15 min, washed with distilled water, let to dry at room temperature, and mounted with entellan [19].
2.6. Slide Evaluation
For the CA assay, 100 well spread included 46 chromosome in the metaphase stage for per donor were analyzed. In total, 400 metaphase stages were examined from each dose.
2.7. Statistical Analysis
For obtaining the percentage of the abnormal cell, CA/cell, MI, CBMN, and the z-test were used, and the concentration–response relationships were determined from the regression coefficients for the percentage of the abnormal cell, CA/cell, and MN. Statistical analysis was done by using the software program SPSS 20.0 (developed by SPSS, Chicago, IL, USA).
3. Results
3.1. Genotoxic Profile of the Laurocerasus officinalis Extract
CA and CBMN assays were performed to assess the genotoxic profile of LOE in human lymphocytes in vitro. For cytotoxic effect evaluation, mitotic index values were assigned. The results of the CA analysis and the mitotic index values were reported in Table 1.
| Test substance | Treatment | Structural aberrations | Abnormal cell ± SE (%) | CAs/Cell ± SE | MI ± SE (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period (h) | Concentration (μg/mL) | ctb | csb | f | cte | scu | dc | ||||
| Negative control | 1 | 1 | 0.50 ± 0.35 | 0.005 ± 0.004 | 5.55 ± 0.21 | ||||||
| Solvent control | 24 | 10 µL | 1 | 2 | 2 | 1.25 ± 0.56 | 0.018 ± 0.007 | 4.65 ± 0.19 | |||
| Positive control | 24 | 0.20 | 54 | 66 | 38 | 2 | 9 | 1 | 38.00 ± 2.43 | 0.620 ± 0.024 | 2.70 ± 0.15 |
| LOE | 24 | 125 | 8 | 1 | 1 | 1 | 2.75 ± 0.82 ∗ | 0.033 ± 0.009 ∗∗ | 4.93 ± 0.20 ∗ | ||
| 250 | 4 | 1 | 2 | 1 | 2.00 ± 0.70 | 0.025 ± 0.008 ∗ | 4.49 ± 0.19 ∗∗∗ | ||||
| 500 | 9 | 1 | 2 | 3.00 ± 0.85 ∗∗ | 0.033 ± 0.009 ∗∗ | 4.40 ± 0.19 ∗∗∗ | |||||
| 1,000 | 7 | 3 | 1 | 2.27 ± 0.77 ∗ | 0.035 ± 0.009 ∗∗ | 4.38 ± 0.19 ∗∗∗ | |||||
| Negative control | 1 | 1 | 0.50 ± 0.35 | 0.005 ± 0.004 | 5.55 ± 0.21 | ||||||
| Solvent control | 48 | 10 µL | 8 | 2 | 2 | 3.00 ± 0.85 | 0.035 ± 0.009 | 4.85 ± 0.20 | |||
| Positive control | 48 | 0.20 | 59 | 56 | 27 | 15 | 3 | 33.75 ± 2.36 | 0.585 ± 0.025 | 3.45 ± 0.17 | |
| LOE | 48 | 125 | 6 | 2 | 1 | 1 | 2.50 ± 0.78 ∗ | 0.033 ± 0.009 ∗∗ | 4.13 ± 0.18 ∗∗∗† | ||
| 250 | 6 | 3 | 2 | 2.75 ± 0.82 ∗ | 0.035 ± 0.009 ∗∗ | 4.80 ± 0.20 ∗∗ | |||||
| 500 | 6 | 1 | 1 | 2.00 ± 0.70 | 0.023 ± 0.007 ∗ | 4.46 ± 0.19 ∗∗∗ | |||||
| 1,000 | 3 | 3 | 2 | 2.00 ± 0.70 | 0.028 ± 0.008 ∗ | 4.40 ± 0.19 ∗∗∗ | |||||
- Note: Four hundred metaphases were scored for each treatment for CAs, and 12,000 metaphases were scored for each dose level for the MI.
- Abbreviations: csb, chromosome break; ctb, chromatid break; cte, chromatid exchange; dc, dicentric; f, fragment; LOE, Laurocerasus officinalis extract; scu, sister chromatid union; SE, standard error.
- ∗Significantly different from negative control; p < 0.05 (z-test).
- ∗∗Significantly different from negative control; p < 0.01 (z-test).
- ∗∗∗Significantly different from negative control; p < 0.001 (z-test).
- †Significantly different from solvent control; p < 0.01 (z-test).
The LOE induced five types of structural aberrations for the 24-h period and four types for the 48-h period. In both treatment periods, chromatid breaks were the most frequently observed chromosomal aberrations, followed by chromosome breaks and fragments, respectively. Moreover, the dicentric chromosome was observed at 125 μg/mL for both period treatments, whereas sister chromatid union was only at 250 μg/mL in the 24-h period. Although some treatments were significantly different from negative control for abnormal cell (%) and the ratio CA/Cell, there was no significant increase or decrease when compared with solvent control. When examining all treatments in terms of dose-dependent relation, a strong relation was revealed only in abnormal cell percent for the 48-h period (r = −0.840).
According to the mitotic index results, all concentrations in 24 and 48 h periods were significantly lower than the negative control, whereas only 125 μg/mL concentration in 48 h was significantly lower compared with solvent control. For dose-dependent relation, none of the concentrations had a strong relation with solvent control.
The cytokinesis-block MN test was applied to investigate the clastogenic and/or aneugenic effects of LOE in human lymphocytes, with the results shown in Table 2.
| Test substance | Treatment | BN cells scored | Distribution of BN cells according to the number of MN | MN (%) ± SE | |||
|---|---|---|---|---|---|---|---|
| Period (h) | Concentration (μg/mL) | (1) | (2) | (3) | |||
| Negative control | 48 | 0 | 4,000 | 7 | 2 | — | 0.275 ± 0.08 |
| Solvent control | 48 | 10 µL | 4,000 | 9 | 1 | — | 0.275 ± 0.08 |
| Positive control | 48 | 0.20 | 4,000 | 130 | 8 | 1 | 3.725 ± 0.30 |
| LOE | 48 | 125 | 4,000 | 16 | — | — | 0.400 ± 0.09 |
| 250 | 4,000 | 11 | — | — | 0.275 ± 0.08 | ||
| 500 | 4,000 | 10 | — | — | 0.250 ± 0.07 | ||
| 1,000 | 4,000 | 6 | 1 | — | 0.200 ± 0.07 | ||
- Abbreviations: BN, binucleated; LOE, Laurocerasus officinalis extract; MN, micronucleus; SE, standard error.
According to the MN test results, all concentrations of LOE for 48 h of treatment resulted in BN cell with one micronucleus, while the BN cell with two MN was observed in only 1,000 μg/mL. There was no significant increase or decrease in micronucleus frequencies for any concentration compared with negative and solvent control. Considering the dose-dependent criterion, a slight relation was found (r = −0.719).
3.2. Antigenotoxic Profile of Laurocerasus officinalis Extract
CA and MN assays were performed to assess the antigenotoxic profile of LOE in human lymphocytes in vitro. For anticytotoxic effect evaluation, mitotic index values were assigned. The results reported in Table 3 show the inhibition of CA after treatment with increasing concentrations of LOE on human lymphocytes treated with the genotoxic agent, MMC.
| Test substance | Treatment | Structural aberrations | Abnormal cell ± SE (%) | CAs/cell ± SE | MI ± SE (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period (h) | Concentration (μg/mL) | ctb | csb | f | cte | scu | dc | ||||
| Positive control | 24 | 0.20 | 54 | 66 | 38 | 2 | 9 | 1 | 38.00 ± 2.43 | 0.620 ± 0.0242 | 2.70 ± 0.15 |
| MMC + LOE | 24 | 125 + 0.20 | 48 | 28 | 14 | 6 | 2 | 22.40 ± 2.08 ∗∗∗ | 0.335 ± 0.024 ∗∗∗ | 3.23 ± 0.16 ∗ | |
| 250 + 0.20 | 37 | 21 | 12 | 4 | 1 | 18.25 ± 1.93 ∗∗∗ | 0.253 ± 0.022 ∗∗∗ | 3.75 ± 0.17 ∗∗∗ | |||
| 500 + 0.20 | 24 | 18 | 13 | 2 | 14.25 ± 1.75 ∗∗∗ | 0.192 ± 0.020 ∗∗∗ | 3.69 ± 0.17 ∗∗∗ | ||||
| 1000 + 0.20 | 25 | 13 | 6 | 2 | 11.50 ± 1.60 ∗∗∗ | 0.153 ± 0.018 ∗∗∗ | 3.56 ± 0.17 ∗∗∗ | ||||
| Positive control | 48 | 0.20 | 59 | 56 | 27 | 15 | 3 | 33.75 ± 2.36 | 0.585 ± 0.025 | 3.45 ± 0.17 | |
| MMC + LOE | 48 | 125 + 0.20 | 47 | 25 | 21 | 10 | 24.25 ± 2.14 ∗∗ | 0.345 ± 0.024 ∗∗∗ | 2.92 ± 0.15 | ||
| 250 + 0.20 | 39 | 15 | 14 | 6 | 18.00 ± 1.92 ∗∗∗ | 0.238 ± 0.0213 ∗∗∗ | 3.38 ± 0.16 | ||||
| 500 + 0.20 | 30 | 9 | 12 | 13 | 1 | 15.50 ± 1.81 ∗∗∗ | 0.220 ± 0.0207 ∗∗∗ | 3.57 ± 0.17 | |||
| 1000 + 0.20 | 31 | 6 | 4 | 6 | 4 | 1 | 12.75 ± 1.67 ∗∗∗ | 0.173 ± 0.0189 ∗∗∗ | 3.38 ± 0.16 | ||
- Note: Four hundred metaphases were scored for each treatment for CAs, and 12,000 metaphases were scored for each dose level for the MI.
- Abbreviations: csb, chromosome break; ctb, chromatid break; cte, chromatid exchange; dc, dicentric; f, fragment; LOE, Laurocerasus officinalis extract; scu, sister chromatid union; SE, standard error.
- ∗Significantly different from positive control; p < 0.05 (z-test).
- ∗∗Significantly different from positive control; p < 0.01 (z-test).
- ∗∗∗Significantly different from positive control; p < 0.001 (z-test).
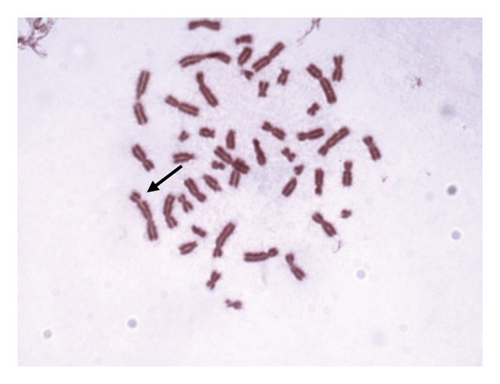
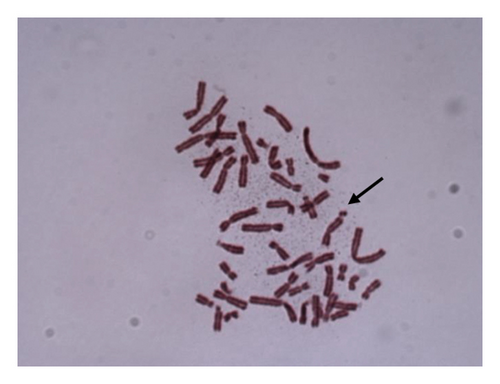
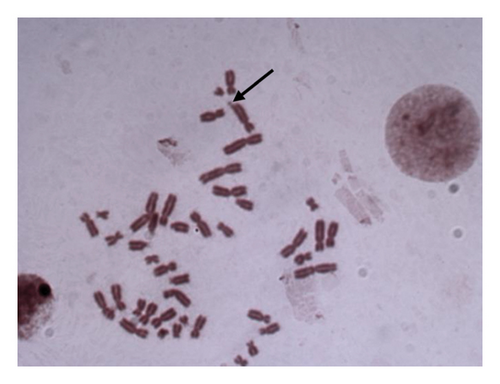
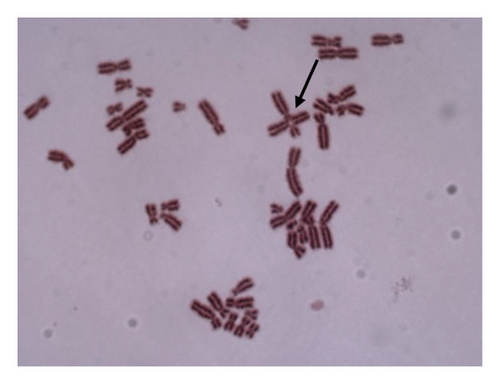
For 24 and 48 h treatment of MMC + LOE, chromatid breaks (Figure 1(a)) were observed as the most common aberrations, and chromosome breaks (Figure 1(b)), fragments (Figure 1(c)), and chromatid exchange (Figure 1(d)) followed, respectively. Sister chromatid union (Figure 1(e)) was shown in 125 and 250 μg/mL in the 24-h period. When the 48-h period was examined, the dicentric chromosome (Figure 1(f)) was observed in 500 and 1,000 μg/mL, and sister chromatid union was found only in 1,000 μg/mL.
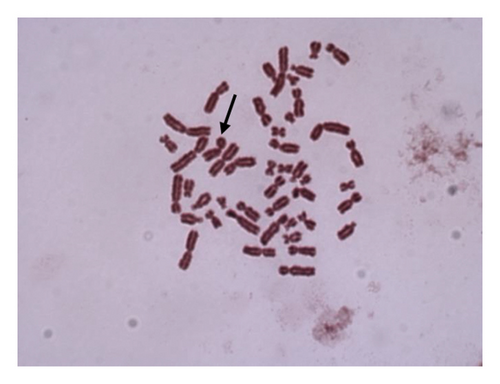
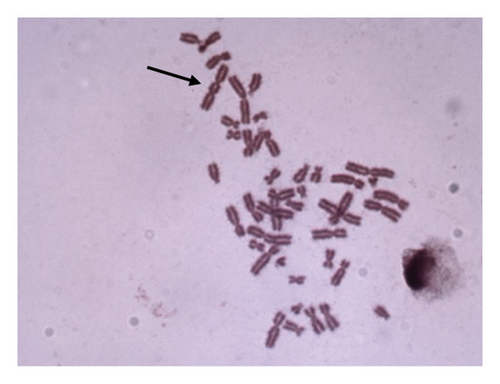
For both the 24-hour and 48-hour periods, the percentage of abnormal cells and the CAs per cell ratio were significantly lower at all LOE concentrations compared to the positive control. Additionally, as the treatment concentration increased, the chromosome aberration ratio showed a decreasing trend. These occurring differences were strongly dose-dependent compared to the positive control (r = −0.799).
Considering the mitotic index score, for 24 h of treatment, all concentrations of LOE with MMC were significantly higher than the positive control with a slight dose-dependent relation (r = 0.581). On the other hand, none of the concentrations of LOE had significant differences with the positive control for the 48-h treatment.
Micronucleus results, which is another method to determine the antigenotoxic effect of LOE, are given in Table 4.
| Test substance | Treatment | BN cells scored | Distribution of BN cells according to the number of MN | MN (%) ± SE | |||
|---|---|---|---|---|---|---|---|
| Period (h) | Concentration (μg/mL) | (1) | (2) | (3) | |||
| Positive control | 48 | 0.20 | 4000 | 130 | 8 | 1 | 3.725 ± 0.30 |
| MMC + LOE | 48 | 125 + 0.20 | 4000 | 93 | 3 | — | 2.475 ± 0.25 ∗ |
| 250 + 0.20 | 4000 | 83 | 2 | — | 2.175 ± 0.23 ∗∗ | ||
| 500 + 0.20 | 4000 | 51 | — | — | 1.275 ± 0.18 ∗∗ | ||
| 1,000 + 0.20 | 4000 | 25 | 1 | — | 0.675 ± 0.13 ∗∗ | ||
- Abbreviations: BN, binucleated; LOE, Laurocerasus officinalis extract; MN, micronucleus; SE, standard error.
- ∗Significantly different from positive control; p < 0.01 (z-test).
- ∗∗Significantly different from positive control; p < 0.001 (z-test).
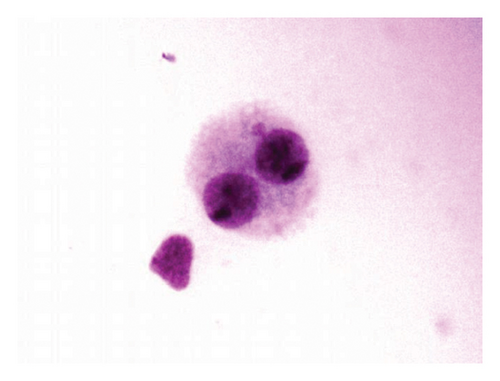
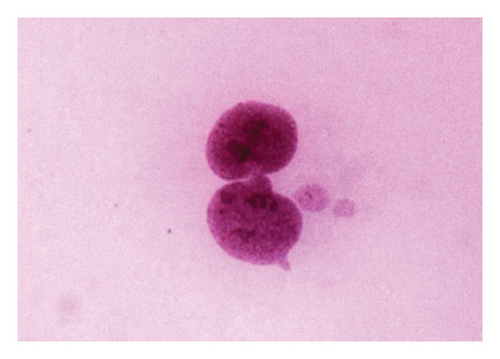
According to the results of the MN test for the 48-h period, binucleated cell with one micronucleus (Figure 2(a)) and two MN (Figure 2(b)) was observed in MMC + LOE treatments. Binucleated cell with three MN (Figure 2(c)) was seen as the only positive control. In all concentrations in this treatment, the frequencies were significantly lower than the positive control, and the higher the LOE concentrations, the lower were the MN. There was a strong dose-dependent relationship in MN formation when compared to positive control (r = −0.925).

4. Discussion
Considering the side effects of the drugs used in the treatment of diseases and the financial and moral burden of the treatment on the country’s health sector, preventive medicine has become an increasingly important issue in the scientific world today. Preventive treatment starts with a healthy life, maintained with a healthy diet comprising natural plants. Considering this situation, alternative medicine is becoming the focus of attention of scientists. The criteria aimed here are less use of chemicals and fewer side effects to protect against diseases and support the treatment of diseases.
Since flavonoids and anthocyanins are compounds with high antioxidant activity, they play a protective role against all the negative effects of oxidative stress-related disease. Intake of anthocyanin-containing foods with diet enhances the treatment of many chronic diseases such as cardiovascular disease, obesity, and diabetes mellitus [21, 22]. L. officinalis, grown in the Black Sea Region in Turkey, is believed to be beneficial for diabetic patients ethnographically. The L. officinalis fruit has plenty of anthocyanin and other flavonoids. The phenolic compound content in 1 g of L. officinalis extract, analyzed using the classical extraction method, the method applied in our study, includes 35.17 mg chlorogenic acid, 1.58 mg vanillic acid, 1.12 mg caffeic acid, and 0.82 mg rutin [11]. It is essential to learn whether a plant extract is a genotoxic agent so as to determine its effect-confidence interval or to determine its damage-reducing property against genotoxic agents; so, this plant can be categorized as a medicinal plant. Therefore, the genotoxic/antigenotoxic and cytotoxic/anticytotoxic effects of LOE were assessed firstly in this work by performing CA and CBMN tests in human peripheral lymphocytes as endpoints.
According to the results of the CAand CBMN tests for genotoxic profile determination, there are no significant differences between doses (125–1,000 μg/mL) and solvent control. When Table 1 is examined, some significant differences are observed between the negative control and the treatment doses. However, these differences are not evidence of the genotoxic effect of LOE. These chromosomal abnormalities could be because of solvent control. Therefore, the comparison between solvent control and treatment doses should be considered to determine the genotoxic profile of LOE. Therefore, LOE has no genotoxic effect on human lymphocytes in vitro. In antigenotoxicity analyses, all treated doses were significantly decreased than positive control in CA and MN tests; so, it is determined that LOE has antigenotoxic and protective effect of genotoxic agents on human lymphocytes in vitro.
The studies on the genotoxicity and antigenotoxicity of L. officinalis are limited in the existing literature. In a research carried out by Eken, Endirlik, and Bakir, rat hepatoma cell line was treated with only LOE and LOE + dimethoate (genotoxic agent), and the DNA damage was examined using the comet method. While there was no significant difference in tail length in the group given LOE only, a significant shortening was observed in the tail length in the group treated with both LOE and dimethoate. Similar to the results in our study, these results showed that LOE has no genotoxic effect but has antigenotoxic effect on hepatoma cell in rats [23]. Our results were parallel with the studies conducted with other plant extracts, closely related to L. officinalis and containing anthocyanins. The antigenotoxic effect of Prunus avium extract was tested on human lymphocyte cells by induced DNA damage with MMC by using CA, MN, and comet methods; so, a significant decrease in DNA damage was observed in cultures with the extract, and so it was concluded that the extract has antigenotoxic effect [24]. Human lymphocyte cells were treated with an extract from Zataria multiflora containing high anthocyanin, and a significant decrease in MN count was observed [25]. In another study, protective effects of the anthocyanin extracted from boysenberry and blackcurrant were shown in human neuroblastoma and promyelocyte cells against H2O2-induced toxicity effects and DNA damage [26]. Moreover, it was determined that Prunus persica and Prunus serrulate extracts, rich in anthocyanins, reduced DNA damage [27, 28].
In our study, cytotoxicity was evaluated by the mitotic index. All concentrations treated with LOE for 24 -and 48-h period were observed to be significantly lower than the negative control, and there were no differences between solvent control, except 125 μg/mL for 48 h. These results showed that LOE had hardly ever a cytotoxic effect on human lymphocytes. A study by Aydın et al. demonstrated that L. officinalis extract had a slightly antiproliferative effect on cancer cell lines such as HT29, HeLa, Vero, and C6, similar to our results [29]. Moreover, in other studies with anthocyanin-containing substances, the effect of cell growth was found in various cell lines [30, 31]. According to anticytotoxic analysis, all concentrations were significantly higher than positive control in 24-h periods; however, there were no differences for the 48-h periods. Even if it is not statistically significant, in the long-term period, that is, 48 h, there is a little anticytotoxic effect at higher doses of LOE. There is no study related to the anticytotoxic effect of LOE in literature; but, other extracts including anthocyanins show an anticytotoxic effect to MMC on human lymphocytes [24], supporting our work.
Looking at the results of treatment with the genotoxic agent, MMC, both chromosomal abnormality and micronucleus frequency were observed to be significantly decreased as the doses increased. Therefore, it can be concluded that LOE has not only anticlastogenic but also antianeugenic effect in human peripheral lymphocyte.
5. Conclusion
Examining the CA and micronucleus results in our study, it is observed that the extract treated on lymphocyte cells from a healthy donor has no genotoxic effect. When LOE is used with a mutagenic agent, significant decrease in DNA abnormalities occurs. As a result of the study, the genotoxic and antigenotoxic profile of the LOE believed to be a medicinal plant was examined in vitro, and it was found that it did not cause a genotoxic effect and showed antigenotoxic properties against the toxic effect of MMC. The observed genoprotective activity of LOE in this study can be supported by in vivo studies and so, it will contribute to the disclosure of the effectiveness of the L. officinalis extract.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Esra Yıldız was responsible for writing–original draft, visualization, methodology, investigation, formal analysis, and data curation. Guncha Meredova played a role in methodology and experiment setup. Hüseyin Aksoy was involved in writing–review and editing, project administration, and conceptualization. The final version of the article was reviewed and approved by all authors.
Funding
This work was supported by the Scientific Research Projects Unit, Sakarya University (Project number: 2018-2-9-342).
Acknowledgments
This work was financially supported by Sakarya University.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




