Unveiling the Metabolic Function of Three Types of High-Temperature Daqu During Baijiu Fermentation
Abstract
White, yellow, and black Daqu are three typical types of Jiangxiangxing high-temperature Daqu, which result from differences in the microenvironment during Daqu culture. However, there is still limited research on the metabolic characteristics of three types of Daqu and their impact on the final products. In this work, headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME-GC-MS) and high-throughput sequencing were utilized to reveal volatile compounds and microbial genera in different types of Daqu. Furthermore, simulated fermentation of different types of Daqu was employed to verify their fermentation performance. The results revealed 64 representative volatile compounds and 21 differential microbial genera among three types of Daqu. The correlation analysis and prediction of microbial functional potential indicated that white Daqu was characterized by significant decomposition and utilization of bio-macromolecules, while yellow Daqu had more pronounced formation of aroma compounds. The content of relevant metabolites related to fatty acid and phenylalanine metabolism was significantly higher in yellow Daqu than in the other types. The results of the simulated fermentation of three types of Daqu demonstrated that all types of Daqu stably maintained their functional diversity during fermentation. Specifically, yellow Daqu promoted the production of alcohols and aldehydes, while white and yellow Daqu tended to generate esters and nitrogen-containing compounds such as pyrazines, whereas black and yellow Daqu produced more acids and ketones during fermentation. This study provides a theoretical basis for the rational use of different types of Daqu in Baijiu production.
1. Introduction
Jiangxiangxing Baijiu is a traditional distilled alcoholic beverage with an alcohol content typically around 53%. It has a long history in China and also represents the culmination of the industrious wisdom of the Chinese people. Its unique taste and distinct brewing process are mainly based on Jiangxiangxing high-temperature Daqu (HTD) starter [1], which provides a wide variety of microbes, enzymes, and microbial metabolites [2, 3]. HTD is produced in an open environment using wheat as the primary raw material, which undergoes shaping, fermenting, and ripening processes [4]. First, proportionally blended raw materials (crushed wheat grains, water) and Muqu (high-quality mature starter) are mixed and pressed. Then, starter bricks are stacked for fermentation, with each brick being separated by straw and sprayed with water to create an ideal environment for microbial growth and fermentation. In order to allow each brick to ferment evenly, starter bricks are relocated to modify the temperature and humidity after the fermentation core temperature rises above 60°C. Even with timely ventilation, it is still challenging to achieve even fermentation. Uneven fermentation significantly affects the microecology of Daqu, which in turn impacts the microbial metabolism and enzyme activity [5, 6]. Due to variations in microenvironmental factors such as temperature, humidity, and oxygen concentration in different locations of the same fermentation chamber, three distinct types of Daqu bricks are produced [7]. Usually, white Daqu is obtained from the top layer of brick piles as well as areas near a window or door, where moisture dissipation is faster. Yellow Daqu usually forms in the middle layer of brick piles, with even fermentation. Black Daqu is formed in the bottom layer, where heat and moisture are especially high. Finally, all types of Daqu are mashed and blended proportionally for manufacturing of Jiangxiangxing Baijiu after 3 to 6 months of maturation. At present, the differentiation of Daqu types mainly depends on the skill of artisans, who rely on their experience to select appropriate proportions for Baijiu production.
Nevertheless, the specific features of the three types of Daqu have received increasing attention in recent years. Relevant studies have mostly concentrated on the correlation between metabolites, physicochemical or enzymatic properties, and microbial community structure. Yang et al. [7] investigated the correlation between the physicochemical properties and metabolic profiles of different types of Daqu and discovered that variations of pH and hydrolytic activity were the main causes of changes in the metabolite profile. Deng et al. [8] found that yellow Daqu had the largest saccharification yield, red Daqu had the highest esterification yield, and white Daqu had the strongest protease activity. As a carrier that connects environmental factors with metabolic products, the microbes and their correlation with environmental factors are essential for the analysis of Baijiu brewing. Wang et al. [9] found that the bacterial community structure of different types of HTD varied significantly. Gan et al. [10] not only identified the functional genes and microorganisms related to starch and cellulose hydrolysis pathways but also provided fresh insights into how the microbial diversity varied between mature and immature Moutai starters, including yellow and white Daqu. In a recent study, Shi et al. [11] compared the differential characteristics of Daqu types during the stacking fermentation process. To date, the relevant studies focused on the distinction between the different types of Daqu in terms of physicochemical, microbiological, and metabolic profiles, as well as their correlation. However, it is still unknown how these variations affect subsequent fermentation and its final products.
This study therefore aimed to (1) unveil the metabolic differences of three types of Daqu and confirm how they impact the subsequent brewing process, through laboratory scale anaerobic fermentation experiments; (2) unravel the correlation between metabolites and core microbes through correlation analysis and identify metabolic pathways; as well as (3) consolidate existing sensory judgments on three types of Daqu through the introduction of a melanoidin index. Scientific evaluation of the function of different types of Daqu and their scientific use in brewing will be conducive to increasing the production yield and quality of final products, while also promoting the achievement of desired flavor profiles in Jiangxiangxing Baijiu.
2. Materials and Methods
2.1. Materials
Based on the sensory evaluation of experienced artisans, samples of white, yellow, and black Daqu were obtained after maturation from a distillery located in Moutai town in the northwest of Guizhou Province on March 2021. A total of 10 samples of each type of Daqu were pulverized for the determination of melanoidin, and the selected typical Daqu samples were stored at −20°C until further analysis.
2.2. Reagents
High-purity methyl acetate (> 98%) was purchased from Aladdin for use as an internal standard. Other chemicals used in this research were all of analytical grade and acquired from local companies.
2.3. Methods
2.3.1. Determination of Melanoidins
In melanoidins quantification, the definition and key parameters such as absorption wavelength followed the method described by Martins and Van Boekel [12]. We determined through orthogonal experiments that the optimal extraction conditions were as follows: an extraction temperature of 60°C, an extraction time of 6 h, an alcohol concentration of 0% in the extraction solvent, and a liquid-to-solid ratio of 20 mL/g. Water bath extraction and appropriate dilution were carried out prior to measurement. The absorbance at 470 nm was determined using a U-3010 spectrophotometer (Hitachi, Japan). The melanoidin content was calculated using the formula: C = A × d/ε × L, where C is the melanoidin concentration (mmol/L), A is the absorbance (470 nm), ε is the molar extinction coefficient of melanoidins (0.64 L/mmol·cm), L is the path length (cm), and d is dilution factor.
2.3.2. Determination of Volatiles
Headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME-GC-MS) was used to determine the volatile components of three types of Daqu and the corresponding fermented grains (Trace 1310-ISQ, Thermo Scientific, Germany). One gram of sample along with the appropriate quantity of internal standard was placed into a 20 mL vial, combined with 5 mL of saturated sodium chloride solution (dissolve more than 35.9 g of sodium chloride in 100 mL of water until insoluble particles begin to appear), capped tightly, and left to equilibrate for 15 min at 60°C. Thereafter, the sample was extracted for 45 min using a conditioned SPME fiber (Supelco, Bellefonte, PA, USA) coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) (50/30 μm), which was then immediately inserted into the GC injection port for injection at 250°C for 5 min in splitless mode. The analytes were separated on an HP-FFAP capillary column (50 m × 0.200 mm × 0.33 μm, Agilent Technology, USA). The detailed GC conditions were as follows: Helium (99.999%) carrier gas flow rate of 1 mL/min; the oven temperature was set at 40°C for 3 min, then increased to 150°C at a rate of 3°C/min, held for 2 min, then increased to 230°C at a rate of 5°C/min, and held for 10 min. Mass spectrometry conditions: EI ionization mode with an electron energy of 70 eV. Full scan mode was employed for signal acquisition, with a scan range of 35–500 m/z. The ion source and transmission line temperatures were set at 250 and 230°C, respectively. After comparing with the NIST library, only volatiles with similarity (SI) > 800 were kept for further analysis.
2.3.3. Microbial Community Analysis Based on High-Throughput Sequencing
The DNeasy PowerSoil Kit (Mo Bio/QIAGEN) was used to extract total DNA in accordance with the manufacturer’s instructions. The quality of the DNA was evaluated by electrophoresis on a 1% agarose gel. The PCR amplification and construction of a sequencing library were completed by Personal Bio (Shanghai Personal Biotechnology Co., Shanghai, China). The sequencing was completed on an Illumina Nova platform using sequencing strategy PE-250. Qiime dada2 denoise-paired software was utilized to process the sequencing data, including trimming of PCR primers, quality filtering, denoising, splicing, and removing chimeric sequences [13]. Following the aforementioned procedures, the longest sequences were chosen as representative for taxonomic identification by aligning them with sequences in the UNITE (Release 8.0, https://unite.ut.ee/) and Silva (Release 132, https://www.arb-silva.de) databases, which allowed the high-quality sequences with similarity greater than 97% to be clustered into different operational taxonomic units (OTUs). The relative abundances of the bacterial and fungal taxa in different types of Daqu were compared according to the sequence number of each corresponding OTU in each sample.
2.3.4. Solid-State Fermentation and Its Evaluation
Different types of Daqu were used to inoculate previously liquefied, saccharified, and sterilized sorghum grains to avoid interference. To assure optimal fermentation, an additional solution containing Saccharomyces cerevisiae was added to the mixture at an initial concentration of 106 CFU/g. Then, 190 g of the mixture was loaded into a 250 mL Erlenmeyer flask containing a fermentation plug and airlock-filled with 5 mol/L sulfuric acid solution for anaerobic fermentation at 30°C. The endpoint of fermentation was defined as the time when the daily production of CO2 fell below 0.2 g. Two samples from each type of HTD (W1, W5, Y6, Y7, B1, and B5) were randomly selected for solid-state fermentation. Each fermentation was carried out in triplicates. The ethanol content of the fermented grains was determined using an alcoholmeter (Yaohua Instrument Factory, Hebei Province, China) and the content of total acids was measured using the titrimetric method after distillation. The detailed protocols were in accordance with the national standards of the People’s Republic of China “GB12456-2021” and “GB/T 10345-2022,” respectively.
2.3.5. Data Analysis
Partial least squares discriminant analysis (PLS-DA) and principal coordinates analysis (PCoA) were conducted in Simca 14.1 (Umetrics, Sweden) or through an online platform (https://www.genescloud.cn/) to classify and discriminate the microbial community composition and metabolites in different types of Daqu. The statistical significance (p < 0.05) was evaluated using single-factor analysis of variance (one-way ANOVA). Linear discriminant analysis (LDA) effect size (LEfSe) was calculated to analyze the variance, with LDA > 3.5 and p < 0.05 as the threshold. Venn diagrams were plotted to visualize the unique and shared taxa at the genus level among the three types of Daqu using a free online tool (https://www.ehbio.com/test/venn/#/) [14]. The quantitative results were presented as the averages ± standard deviations. Each analysis was conducted in triplicate. Raw data of amplicon sequencing was submitted to NCBI SRA metadata under accession code SRP354289.
3. Results and Discussion
3.1. Selection of Typical Samples
To objectively select typical samples for subsequent analysis, we introduced the method of melanoidin determination in addition to traditional color-based classification. In the production of Jiangxiangxing Baijiu, the utilization of high-protein raw materials, hot and humid environment including heap fermentation, pit fermentation, and distilling process provide favorable conditions for the Maillard reaction [15]. When reducing sugars and amino acids or proteins are heated, they produce dark compounds called melanoidins, which endow Daqu with a brown color and strong soy sauce–like flavor [16, 17]. However, different positions of Daqu bricks result in variations of the temperature and humidity of fermentation, which leads to very different degrees of Maillard reaction, which are reflected in different levels of melanoidin accumulation.
The melanoidin content of the three types of Daqu is shown in Supporting Table 1. There was an obvious difference in melanoidin levels among the three types of Daqu. As expected, white Daqu (3.48 ± 0.33 mmol/L) had the lowest, and black Daqu (6.76 ± 1.01 mmol/L) the highest melanoidin content, whereas yellow Daqu (4.98 ± 0.62 mmol/L) was intermediate (Figure S1). This was in agreement with previous reports that the color depth of Daqu is proportional to its content of melanoidins [17]. Hence, the melanoidin content offered us an objective judgment of different types of Daqu. As a result, a total of 22 Daqu samples, including 10 white Daqu, 7 yellow Daqu, and 5 black Daqu, were selected as typical samples for subsequent analysis.
3.2. Flavor Substances
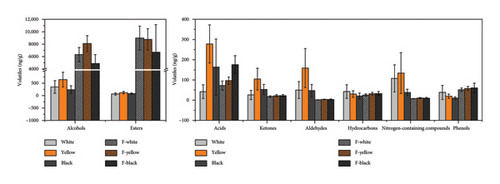
As a carrier of flavor substances or precursors, Daqu has a significant impact on the final flavor of Baijiu [18]. In our study, a total of 143 volatile compounds were identified in three different types of Daqu and clustered into 9 different groups, including 27 alcohols, 24 esters, 20 acids, 17 ketones, 15 aldehydes, 15 nitrogen-containing compounds, 12 hydrocarbons, 5 phenols, and 8 others. The total contents of various volatiles varied among three types of Daqu. Alcohols and aldehydes were prevalent in yellow Daqu, significantly higher than in the other two types. Acids, esters, and ketones in yellow and black Daqu were also significantly higher than in white Daqu, while nitrogen-containing compounds in yellow and white Daqu were significantly higher than in black Daqu. Hydrocarbons and phenols were nonsignificantly higher in white Daqu (Table 1). This result was consistent with a previous finding [7] that despite the greatest diversity of esters and alcohols in white Daqu, yellow Daqu had the highest concentration.
| Volatiles | White Daqu | Yellow Daqu | Black Daqu |
|---|---|---|---|
| Alcohols | 347.26 ± 255.372b | 642.99 ± 298.133a | 237.86 ± 157.488b |
| Esters | 66.15 ± 44.534b | 118.08 ± 52.135a | 79.47 ± 26.624ab |
| Acids | 41.87 ± 34.176b | 277.98 ± 294.333a | 163.59 ± 138.55a |
| Ketones | 25.96 ± 22.765b | 104.32 ± 53.465a | 53.21 ± 25.1ab |
| Aldehydes | 49.79 ± 42.134b | 159.00 ± 96.238a | 47.19 ± 30.354b |
| Hydrocarbons | 42.57 ± 33.936a | 30.3 ± 16.281a | 20.98 ± 13.758a |
| Nitrogen-containing compounds | 107.59 ± 117.102ab | 133.76 ± 100.916a | 37.93 ± 16.233b |
| Phenols | 38.41 ± 65.072a | 19.72 ± 10.336a | 10.21 ± 5.923a |
- Note: The different letters (a, b, c) indicate significant differences, while same letters indicates no significant difference.
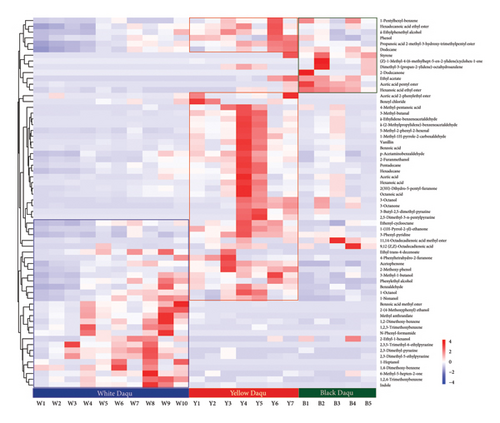
Next, the three different types of Daqu were screened for differential volatiles using PLS-DA, and the results demonstrated distinct profiles, indicating that flavor substances differed between the three types of Daqu (Figure S2). Volatiles with variable importance projection (VIP) values exceeding 1.0 and p-values below 0.05, were selected as potential biomarkers for differentiation of volatile profiles among the types of Daqu. Based on a heat map of 64 differential volatiles of the three types of Daqu, the metabolic characteristics could be easily distinguished (Figure 1). Dimethoxy-benzene, trimethoxy-benzene, indole, alcohols (1-octanol, 1-nonanol), esters (methyl benzoate, ethyl decanoate), and pyrazines (2,3,5-trimethyl-6-ethylpyrazine, 2,3-dimethyl-pyrazine and 2,3-dimethyl-5-ethylpyrazine) were the primary volatile compounds found in white Daqu. According to a prior study [1], white Daqu had substantially higher abundance of enzymes for the synthesis of acetoin, a precursor of pyrazines, than did yellow and black Daqu. Some acetate and ethyl esters, long-chain fatty acids and their esters, 2-dodecanone, alkanes, and olefins appeared to be more prevalent in black Daqu than in the other types. Yellow Daqu, being a transitional type, exhibited partial overlap of flavor components with both black and white Daqu, in addition to retaining its own unique volatiles. More substances were accumulated in yellow Daqu, including aldehydes, long-chain fatty acid esters, ketones, phenols, furanones, pyridines, pyrazines, alcohols (1-octanol, 1-nonanol), and aromatic amino acid metabolites (phenyl ethanol, acetophenone, benzaldehyde, benzoic acid, and vanillin). The fruit-like and floral aroma of Jiangxiangxing Baijiu stems from aromatic compounds such as esters and phenylalanine metabolites, including phenylacetic acid, phenylacetaldehyde, and related derivatives [19]. White Daqu is fermented at a relatively low temperature, which results in a higher diversity of microbes and flavor compounds. However, yellow Daqu contains most of the aroma substances and accounts for the largest proportion in the brewing process. Thus, its contribution to the production of Jiangxiangxing Baijiu has been highly regarded.
3.3. Microbial Community Structure
During Jiangxiangxing Baijiu brewing, microbes in HTD play crucial roles in the degradation of macromolecules and the formation of aromatic compounds [20]. Therefore, it was also necessary to investigate the microbial community structure of different types of Daqu.
3.3.1. Alpha and Beta Diversity
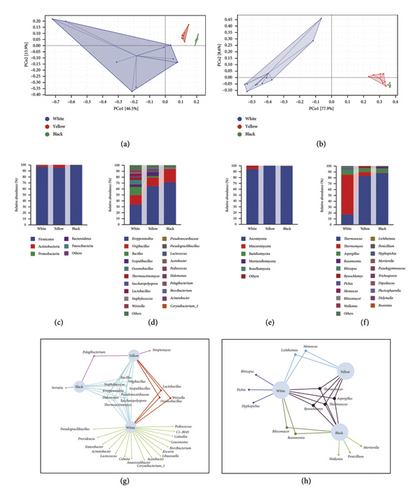
A total of 1,210,081 high-quality reads assigned to bacteria and 1,313,872 assigned to fungi were obtained from three types of Daqu. For the V3–V4 region of the bacterial 16S rRNA genes, there were 55,003 reads on average, ranging from 30,126 to 82,272 (N = 22). For the ITS1(a) region of the fungal rRNA gene, there were 59,721 reads on average, ranging from 53,890 to 65,928 (N = 22). The rarefaction curve was almost parallel to the x-axis and reached saturation, indicating that the numbers of analyzed bacterial and fungal OTUs in our samples were nearly completely identified and had met the requirements of subsequent analysis (Figure S3). To assess the alpha diversity of microbial communities across the three types of Daqu, Chao 1 richness, observed species, as well as the Shannon and Simpson indices were analyzed (Supporting Table 2). Regarding bacterial alpha diversity, the Chao 1 richness and observed species were highest in white Daqu, followed by yellow Daqu, and lowest in black Daqu. The Shannon and Simpson indices were significant higher in white and yellow Daqu than in black Daqu, indicating that as the color of Daqu deepens, the bacterial richness and diversity show a decreasing trend. In terms of fungal alpha diversity, Chao 1 richness and observed species remained relatively stable among the three types of Daqu, but Shannon and Simpson indices were significantly higher in yellow and black Daqu than in white Daqu, demonstrating a more prevalent fungal diversity in dark colored Daqu. This was consistent with a previous report [21] that high amounts of melanoidins could inhibit bacterial growth or even have a bactericidal effect. Therefore, we hypothesize that in addition to an excessively high fermentation temperature, the accumulated melanoidins may be another factor causing a lower bacterial richness and diversity in black Daqu. Overall, yellow Daqu had a more advantageous balanced microbial richness and diversity. Differences of microbial community structure among the three types of Daqu were assessed via PCoA based on Bray–Curtis distances. The beta diversity results revealed structural disparities of both the bacterial (PCo1 = 46.5%, PCo2 = 15.9%) and fungal communities (PCo1 = 77.9%, PCo2 = 8.6%) (Figures 2(a) and 2(b)). The fungal communities of yellow and black Daqu clustered closely together, and further from white Daqu, demonstrating a similar fungal structure between yellow and black Daqu.
3.3.2. Microbial Community Structure
The composition of microbial communities was analyzed at the phylum and genus levels to reveal the taxonomic hierarchy of the microbial community structures of different types of Daqu (Figures 2(c), 2(d), 2(e), 2(f)). At the phylum level, Firmicutes predominated the bacterial community, with a relative abundance of 96.69%, 95.25%, and 99.47% in white, yellow, and black Daqu, respectively (Figure 2(c)). Actinobacteria was also identified as a major bacterial phylum, with relative abundance of 2.63%, 4.46%, and 0.40% in the three types of Daqu, respectively. Among the three types, yellow Daqu had relatively higher abundance of Actinobacteria. The relative abundance of the top 20 bacterial genera is shown in Figure 2(d). Kroppenstedtia constituted the predominant taxon at the genus level, with relative abundance of 33.39%, 64.86%, and 71.05% in white, yellow, and black Daqu, respectively. Kroppenstedtia was initially reported by von Jan et al. in 2011 and is known for its excellent heat resistance [22, 23]. Virgibacillus was also dominant, accounting for 16.48%, 14.56%, and 22.22% in the three types of Daqu, respectively. Aside from the above-mentioned two dominant genera, there were more bacterial genera with relative abundance exceeding 1% in white Daqu. Bacillus (13.47%), Oceanobacillus (7.42%), Lactobacillus (4.74%), Thermoactinomyces (4.48%), Scopulibacillus (4.32%), Weissella (3.25%), Saccharopolyspora (1.89%), and Staphylococcus (1.54%) also made significant contributions to the microbial community of white Daqu. This indicated that the fermentation environment of white Daqu is more conducive to the growth and reproduction of diverse microorganisms. As the fermentation temperature increases, bacterial diversity gradually decreases, which may be the reason for the relatively concentrated microbial compositions of black and yellow Daqu, as well as the more scattered microbial composition of white Daqu. Scopulibacillus (7.11%), Saccharopolyspora (3.39%), Bacillus (2.23%), Thermoactinomyces (1.13%), and Pseudonocardiaceae (1.03%) were also among the main bacterial genera in yellow Daqu, as was Staphylococcus (1.58%) in black Daqu. The most abundant bacterial orders were Bacillales, Lactobacillales, and Pseudonocardiales, which was consistent with previous research [24]. Consistently, our results shared almost all of the top 20 bacterial and fungal genera with the research of Deng et al. [8], but their proportions were somewhat different.
Among the fungal taxa, Ascomycota was the predominant phylum, accounting for 94.14%, 99.97%, and 99.22% of the relative abundance in white, yellow, and black Daqu, respectively. In addition to Ascomycota, white Daqu also contained 3.56% Mucoromycota (Figure 2(e)). The fungal profile was quite similar between yellow and black Daqu. The relative abundance of the top 20 fungal genera is shown in Figure 2(f). Thermoascus, a genus of heat-resistant fungi, was predominant in yellow and black Daqu, respectively accounting for 83.10% and 87.99%, while Thermomyces was predominant in white Daqu (67.85%). Nevertheless, Thermoascus ranked as the second most prevalent fungal genus in white Daqu, with a relative abundance of 17.30%. The two main fungal genera, Thermoascus and Thermomyces, were reported to be able to secrete various thermostable hydrolases [25]. Aspergillus was also a major fungal genus, with relative abundance of 6.35%, 6.77%, and 7.48% in white, yellow, and black Daqu, respectively. In addition to the above-mentioned dominant fungal genera, some exhibited increased abundance in HTD, such as Rhizopus (2.89%) and Pichia (1.55%) in white Daqu, Thermomyces (6.58%) and Byssochlamys (2.52%) in yellow Daqu, as well as Rasamsonia (2.78%) in black Daqu.
3.3.3. Differential Microorganisms
Among the taxa with average relative abundance above 0.01%, nine bacterial genera (Bacillus, Virgibacillus, Staphylococcus, Scopulibacillus, Kroppenstedtia, Pseudonocardiaceae, Halomonas, Saccharopolyspora, and Thermoactinomyces) and four fungal genera (Thermomyces, Aspergillus, Byssochlamys, and Thermoascus) were common to different types of Daqu (Figures 2(g) and 2(h)). Given that the same raw materials and procedure were used for the making of three types of Daqu, it is unsurprising that they shared some core microbial components. Nevertheless, the varying microenvironments in the same fermentation chamber resulted in significant differences of microbial community structure. Fermented at a relatively low temperature, white Daqu provides suitable habitat for a wide diversity of bacteria, which was reflected in more diversified bacterial genera, suggesting that most bacteria may be more susceptible to the harsh environment of Daqu, which was not true for many fungi. The vulnerability of the succession of bacterial communities to environmental factors was also recorded in another analysis [26]. High temperature, humidity, and acidity lead to the extinction of most microbes, with a few microorganisms becoming dominant in black and yellow Daqu due to their tolerance of the extreme environment.
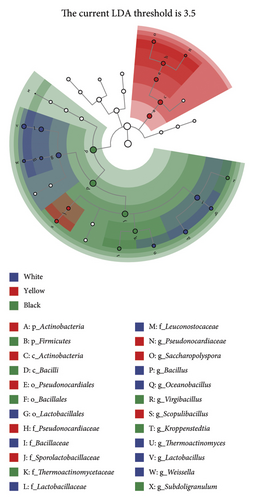
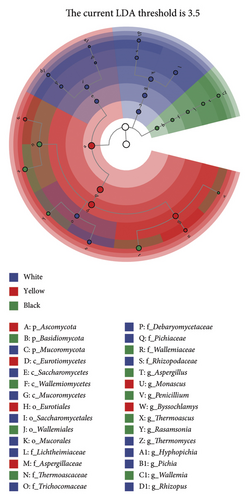
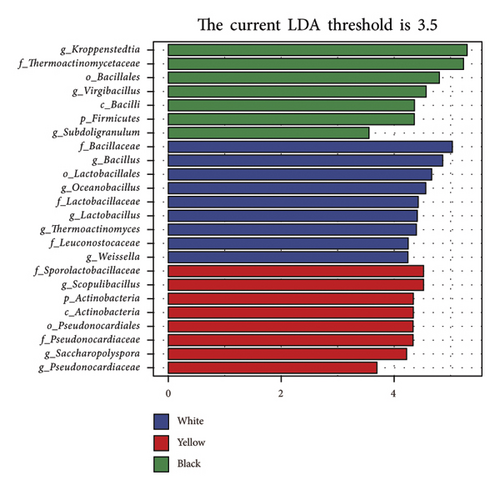
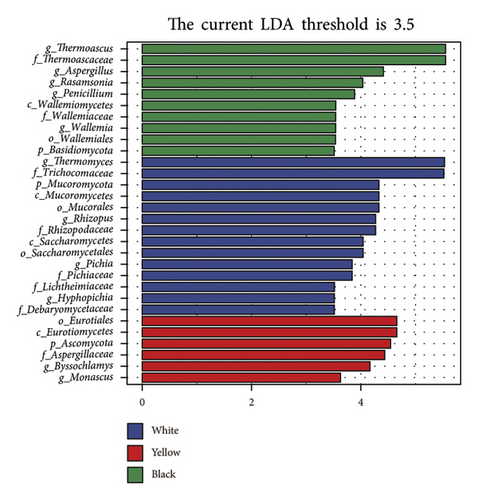
To further evaluate the differential bacterial and fungal genera in each type of HTD, we used the LEfSe algorithm. Differential microbial genera were determined based on LDA > 3.5 and p < 0.05. Differential taxa at the genus level included Bacillus, Oceanobacillus, Thermoactinomyces, Lactobacillus, Weissella, Thermomyces, Rhizopus, Pichia, and Hyphopichia in white Daqu, Scopulibacillus, Saccharopolyspora, Pseudonocardiaceae, Byssochlamys, and Monascus in yellow Daqu, as well as Kroppenstedtia, Virgibacillus, Thermoascus, Aspergillus, Rasamsonia, Wallemia, and Penicillium in black Daqu (Figures 3(a), 3(b), 3(c), 3(d)).
3.4. Correlation of Differential Microbes With Flavor Substances
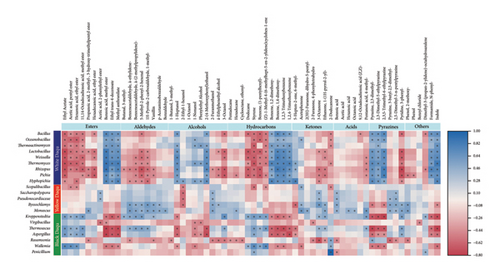
Tracking the microbial community structure and metabolites in different types of Daqu can provide us with a scientific basis for investigating the mechanism of flavor compound formation in Jiangxiangxing Baijiu brewing. Only by understanding the specific characteristics of the different types of Daqu and unraveling the underlying mechanisms that cause the differences, can we make full use of them to achieve consistent fermentation. The relationship between the above-mentioned 21 differential microbial genera corresponding to three types of Daqu and 64 potential biomarker volatiles was visualized in the form of a heat map to unravel the relationship between dominant microbes and flavor compounds (Figure 4). The results revealed that the differential genera in yellow (Monascus, Saccharopolyspora, and Pseudonocardiaceae) and black Daqu (Kroppenstedtia, Thermoascus, Aspergillus, and Wallemia) were positively correlated with a majority of fatty acid esters. Monascus was previously reported as a vital microbe for fatty acid ester synthesis [19]. Kroppenstedtia was noted for its potential in the biosynthesis of organic acids, providing sufficient substrates for esterification during Daqu fermentation [23], explaining the highest acidity observed in black Daqu during maturation. Aspergillus is known for producing amylolytic enzymes for starch degradation, contributing to lytic enzyme secretion and flavor substance synthesis in medium-temperature Daqu [27–30]. Aspergillus, which was equally abundant in all three types of Daqu, plays a key role in macromolecule decomposition and utilization, as well as flavor compound formation during Daqu making. Most acids and ketones showed significant positive correlations with the characteristic genera of yellow Daqu. The quality of Baijiu was previously found to be impacted by fatty acids, which serve as precursors of flavor compounds such as esters [23]. Fatty acids, being the main components in Chinese Baijiu, could eliminate the bitter taste, accelerate the liquor aging, and provide substrate for esterification, enriching the flavor of Baijiu [31]. Acetic acid, hexanoic acid, ethyl hexadecanoate, benzeneacetic acid, 1,2-propanediol, and lactic acid were selected as potential markers of empty cup aroma of Jiangxiangxing Baijiu in a previous study [32]. Here, we also observed significant positive correlations of benzoic acid methyl ester, ethyl trans-4-decenoate, and methyl anthranilate with all differential genera in white Daqu (Bacillus, Oceanobacillus, Thermoactinomyces, Weissella, Thermomyces, Rhizopus, Pichia, and Hyphopichia). In addition to lactic acid bacteria (LAB), Bacillus and Rhizopus were also reported as lactic acid–producing genera [33]. Pichia is considered a nonalcoholic yeast during Baijiu fermentation, mainly producing volatile compounds [34]. Most of the differential genera in yellow Daqu and some in black Daqu, particularly Byssochlamys, Monascus, Kroppenstedtia, and Thermoascus, exhibited positive correlations with most aldehydes. Most alcohols were positively correlated with differential genera in white Daqu, while 2-furanmethanol showed a correlation with differential genera in yellow Daqu (Saccharopolyspora, Pseudonocardiaceae, Byssochlamys, and Monascus). Among the differential genera in white Daqu, Thermoactinomyces was identified as a producer of a variety of enzymes, including α-amylase, glucoamylase, dehydrogenase, polyphenol oxidase, and protease [35]. Lactobacillus, which is abundant in white Daqu, facilitates protein hydrolysis and flavor precursor or compound synthesis [36]. Byssochlamys was reported to produce ethanol- and heat-resistant β-glucosidase [37], which is conducive to the accumulation of alcohols. In addition, 3-octanol showed a positive correlation with two differential genera of black Daqu (Kroppenstedtia and Thermoascus). Pyrazines (dimethyl-pyrazine, 2,3,5-trimethyl-pyrazine) and other nitrogen-containing compounds such as indole showed a positive correlation with the differential genera of white Daqu. Bacillus, which is abundant in white Daqu, was reported as a contributor to the content of pyrazine and pyridine compounds in Jiangxiangxing Baijiu [11]. However, some other pyrazines (3-butyl-2,5-dimethyl- pyrazine and 2,5-dimethyl-3-n-pentylpyrazine), as well as other nitrogen-containing substances (3-phenyl-pyridine) were more closely associated with the differential genera of yellow and black Daqu. Due to the variation in the fermentation microenvironment, the different types of Daqu differed in microbial community structure, which leads to differences in the utilization of bio-macromolecules, and the differential generation of precursors of aroma compounds in Baijiu.
3.5. Differences of Fermentation Performance
3.5.1. Fermentation Characteristics
To clarify the impact of the different types of Daqu on the brewing process, we conducted solid-state fermentation with the three types of Daqu. Throughout the solid-state fermentation of different types of Daqu, the daily production of CO2 was measured to assess the variance in their fermentation rates. As shown in Supporting Table 3, the fermentation of white, yellow, and black Daqu, respectively needed 8, 7, and 7.5 days on average to complete. After distillation, the physicochemical parameters of the distillate such as the alcohol content and total acids were determined and compared. The total acid content was highest when using black Daqu (0.0642 ± 0.02 g/L), followed by yellow Daqu (0.0432 ± 0.01 g/L), and lowest with white Daqu (0.0378 ± 0.01 g/L). By contrast, the alcohol concentration was the highest when using yellow Daqu (11.40 ± 0.15%, v/v), followed by black Daqu (10.68 ± 1.99%, v/v), and lowest with white Daqu (9.91 ± 0.08%, v/v). These results indicated that black Daqu resulted in the highest total acid content, and yellow Daqu resulted in the highest alcohol content, consistent with the results of volatiles analysis. Thus, yellow Daqu has significant advantages in both fermentation speed and ethanol generation. Therefore, we speculated that the microorganisms and enzymes provided by yellow Daqu might be more conducive to the decomposition and utilization of the available biomass.
3.5.2. Volatiles of Fermented Grains
Due to differences in microbial structure and aroma precursor accumulation, different types of Daqu exhibited different metabolic characteristics when acting on fermentation substrates. A total of 118 volatile compounds (including 18 alcohols, 57 esters, 12 acids, 6 ketones, 2 aldehydes, 3 nitrogen-containing compounds, 12 hydrocarbons, 3 phenols, and 5 others) were detected in the grains separately fermented using the three different types of Daqu. Figure 5 shows a comparison of the volatiles in the three types of Daqu and the corresponding fermented grains, which demonstrates that the fermentation properties of different types of Daqu before and after solid-state fermentation were consistent. This means that different types of Daqu influenced aroma formation during fermentation in their own specific way, and were good carriers of microbes with stable biological activity for the fermentation. The abundant production of alcohols was an indisputable advantage of yellow Daqu, which significantly influenced the Baijiu yield. Yellow Daqu was also conducive to aldehyde formation. While yellow and white Daqu tended to generate esters and nitrogen-containing compounds such as pyrazines, black, and yellow Daqu had advantages in the formation of acids and ketones during fermentation.
3.5.3. Metabolic Characteristics
Microbial functional potential prediction using PICRUSt2 showed that there were differential metabolic pathways (Log2FC > 1, p < 0.05) across different types of Daqu. The metabolic pathways of different types of Daqu were constructed based on data from the MetaCyc database (Figures S4A and S4B). Notably, the metabolic pathways were more active at lower fermentation temperatures. The metabolic pathways tend to be less active, as the color of Daqu deepens (white > yellow > black Daqu) (Figure S4C). A recent analysis of the microbial meta-transcriptome of HTD also demonstrated that the expression of genes associated with carbohydrate metabolic pathways was downregulated when the temperature increased during fermentation [38]. Thus, the metabolic characteristics and distribution of metabolites in different types of Daqu appear to be influenced by different fermentation temperatures. Hence, different types of Daqu have distinct fermentation characteristics. During Baijiu fermentation, bio-macromolecules such as starch and cellulose are degraded and converted into ethanol and volatiles by the action of microbes and their diverse enzymes. The metabolism of microbes active during Daqu making usually includes the conversion of carbohydrates, amino acids, fatty acids, and related metabolites. While the main role of carbohydrate metabolism is to provide carbon and energy for the growth of microorganisms, the main function of amino acid metabolism is to regenerate amino acids or form organic acids, aldehydes, and other flavor compounds. Functional microbes metabolize bio-macromolecules from the substrates into pyruvate, which is subsequently converted into acetyl-CoA, a crucial precursor for the synthesis of fatty acids, acetate, and ethanol [39]. In addition to being a necessary amino acid for microbes, phenylalanine is also an important precursor of aromatic compounds. Various aromatic organic acids and other aromatics, including phenylacetic acid, phenylethanol, phenylacetaldehyde, and vanillin, are produced via phenylalanine metabolism and imbue Baijiu with pleasant scents reminiscent of fruits and flowers [40]. In a previous report, it was found that aromatic substances, such as phenylethyl alcohol and ethyl phenylacetate, contributed rose and honey flavors to Baijiu [41].
The upregulation of carbohydrate metabolic pathways in microorganisms from white Daqu enhanced its ability to decompose the cereal-based substrate and provide more nutrients, which in turn promoted species richness. Enzymes related to the hydrolysis of bio-macromolecules including alpha-glucosidase EC 3.2.1.20, alpha-amylase EC 3.2.1.1, beta-glucosidase EC 3.2.1.21, and 4-alpha-glucanotransferase EC 2.4.1.25 were all upregulated in white Daqu (Figure 6(a)). At the same time, black and especially yellow Daqu had an obvious advantage in enriching most compounds that influence the output and quality of Baijiu. Most of the relevant enzymes in the key metabolic pathways of flavor formation, such as pyruvate metabolism (pyruvate oxidase EC 1.2.3.3, pyruvate dehydrogenase EC 1.2.4.1, dihydrolipoyl lysine-residue acetyltransferase EC 2.3.1.12, and acylphosphatase EC 3.6.1.7), fatty acid metabolism (palmitoyl-protein hydrolase EC 3.1.2.22), and phenylalanine metabolism (primary-amine oxidase EC 1.4.3.21 and phenylacetaldehyde dehydrogenase EC 1.2.1.39), were all upregulated in yellow Daqu, which accordingly resulted in a significant enrichment of relevant flavor substances or their precursors (Figure 6). As can be seen in Figure 6, ethanol from pyruvate metabolism, short- or medium-chain fatty acids from the fatty acid biosynthesis pathway, as well as phenylacetate, phenylacetaldehyde, and phenylethyl alcohol from phenylalanine metabolism, including alpha-ethyl phenylacetate methyl ester, ethyl phenylacetate, benzyl alcohol, benzeneacetic acid, acetophenone, vanillin, alpha-ethyl phenylethanol, alpha-ethylidene phenylacetaldehyde, 2-methylpropylidene phenylacetaldehyde, alpha-methylphenylethanol, and alpha-vinyl-alpha-methylphenylethanol, were all significantly higher in yellow Daqu than other two types.

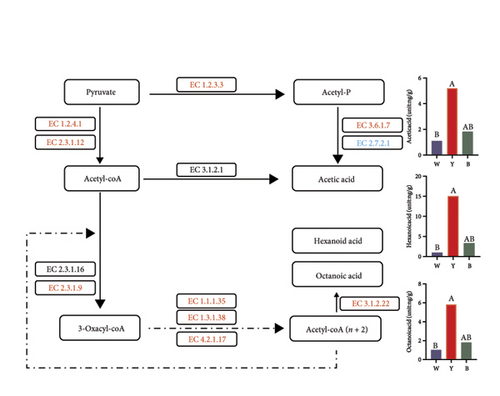
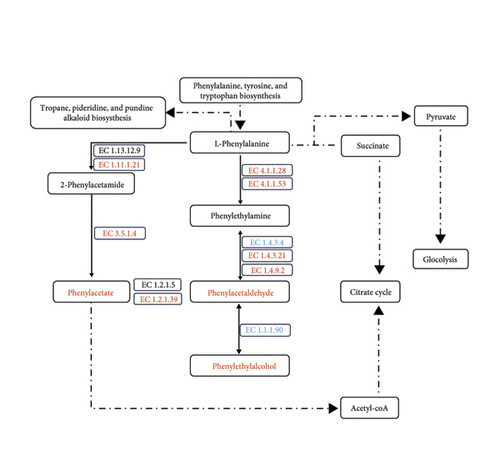
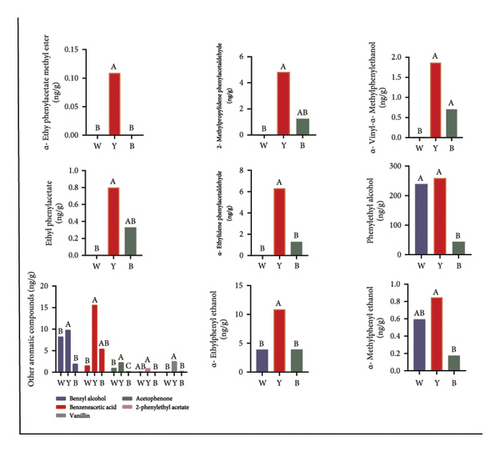
A somewhat higher fermentation temperature seems to be more conducive to flavor formation through pathways such as the Maillard reaction, explaining the aroma-formation advantages of yellow Daqu. Overall, the different types of Daqu exhibited functional complementarity and influenced the fermentation in their own way, whereby all were good carriers for metabolically active and stable microbes. Among them, the ability of yellow Daqu to generate a variety of flavor compounds was comparatively balanced and comprehensive, indicating that its fermentation conditions were more advantageous. Notably, this also scientifically explains the traditionally high regard for yellow Daqu and its expected productivity of over 80% in Baijiu fermentation.
4. Conclusions
The differential analysis of Daqu types remains a focus of research on Jiangxiangxing Baijiu. This study comprehensively analyzed the differences of microbial community structure and flavor compounds among three different types of HTD. Notably, we experimentally determined the differences and stability of their metabolic activity through simulated anaerobic fermentation for the first time. In addition, a melanoidin index was utilized to assist the existing sensory judgments of three types of HTD. The results scientifically explain the precise roles of different types of Daqu during fermentation and provide a theoretical basis for their rational application in the Jiangxiangxing Baijiu industry. Nevertheless, the determination of the effects of different types of Daqu on large-scale pit fermentation and its final products require further in-depth study.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ping Tang, Changwen Li, and Jun Lu designed the study and interpreted the results. Shanqimuge, Minsha Qiao, Fan Wang, Ying Guo, Li Wang, Rongyu Bi, Shuyue Hao, Xinyue Kang, and Liqin Qin performed the relevant experiments, collected data, and drafted the manuscript.
Funding
This research was supported by the Guizhou Provincial Science and Technology Plan Project (grant number Qian Ke He [2023]150). The authors thank Guizhou Guotai Digital-Intelligence Liquor Group Co., Ltd. and Tasly Pharmaceutical Group Co., Ltd. for financial support and for providing all the samples used in this study.
Acknowledgments
The authors thank Guizhou Guotai Digital-Intelligence Liquor Group Co., Ltd. and Tasly Pharmaceutical Group Co., Ltd. for financial support and for providing all the samples used in this study.
Supporting Information
Supporting Table 1: Melanoidin content of three types of Daqu. Supporting Table 2: The alpha diversity of microbial communities in white, yellow, and black Daqu samples. Supporting Table 3: Average fermentation time. The fermented grains of white, yellow, and black Daqu were abbreviated as F-white, F-yellow, and F-black, respectively. Supporting Figure 1: The melanoidin contents of three different types of Daqu (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001). Supporting Figure 2: The PLS-DA plot of volatiles of three types of HTD. Supporting Figure 3: The rarefaction curve (A: bacteria; B: fungi). Supporting Figure 4: Metabolism of three types of HTD. Relative abundance of MetaCyc pathways in bacteria (A) and fungi (B) of three types of HTD; (C). Volcano plot of metabolic pathways of three types of HTD. Red indicates upregulated and blue downregulated pathways.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.




