Long-Term Pretreatment With D-Limonene Protects Gastric Lipid Composition by Reducing Oxidative Damage and Inflammation in Indomethacin-Induced Gastric Injury
Abstract
D-Limonene is a monoterpene compound that is widely contained in citrus plants. It has been demonstrated to have several beneficial properties. The present study aimed to investigate the effect of D-limonene against indomethacin-induced gastric ulceration. D-Limonene was administered orally for 21 days. Gastric ulceration was induced by oral administration of 25 mg/kg indomethacin via gastric gavage, five minutes after the administration of Lim or ranitidine. The stomachs of the rats were examined macroscopically and histopathologically to assess gastric lesions. GR, MDA, caspase-3, TNF-α, PGE2 levels, and iNOS, catalase, and SOD activities were measured using the ELISA method. The lipid composition of gastric tissue was measured using a high-performance thin-layer chromatography method. The alteration observed in the lipid profile of the indomethacin group differed from that of the control group. In contrast, the lipid profile of the Lim-treated groups was similar to that of the control group. Although the L50 group demonstrated a significant difference compared to the Ind group regarding GR, catalase, caspase-3, and iNOS activity, no significant differences were observed in MDA, SOD, TNF-α, and PGE2 levels when compared to the Ind group. However, no indomethacin-induced gastric damage was observed in the macroscopic and microscopic evaluations of gastric tissues collected from the L100 and L250 groups. Additionally, the gastroprotective activity of these two groups was noted in all tests. This study’s results indicated that a 50 mg/kg dose of D-limonene was subeffective, while 100 and 250 mg/kg doses showed gastroprotective activity in rats.
1. Introduction
Gastritis, inflammation of the stomach lining, is one of the most frequent digestive tract diseases. If the etiological factor is not thoroughly eliminated or treated, this can lead to serious complications, such as ulceration of the stomach. The main reason for gastric injury is the imbalance between destructive and defensive factors [1]. The gastric mucosa is protected by mucus, growth factors, and adequate blood flow. The damaging factors affecting this barrier include acid, pepsin, ischemia, nonsteroidal anti-inflammatory drugs (NSAIDs), hypoxia, alcohol consumption, and Helicobacter pylori infection, all of which can impair defensive mechanisms [2]. The treatment approach to gastritis or gastric ulceration depends on the condition’s cause. The most common medication option is proton pump inhibitors, which reduce the stomach’s acid production. Also, histamine receptor antagonists reduce acid secretion by binding H2 receptors located on gastric parietal cells. In some cases, these drugs are also used with antibiotics in a multidrug regimen for Helicobacter pylori infections that cause stomach ulcers. Also, cytoprotective agents or elevation of defensive factors could help protect the stomach line in gastric ulceration [3].
There are several mechanisms of gastroprotection, including mucus secretion, adequate mucosal blood flow, stabilization of tissue lysosomes, inactivation of reactive oxygen species (ROS), maintenance of mucosal sulfhydryl compounds, and cell proliferation. Prostaglandins (PGs) also prevent gastric mucosal damage and ulceration induced by a wide variety of agents [3–5].
Limonene or 4-isopropenyl-1-methylcyclohexene (C10 H16) which is a monoterpene compound is a major component of citrus family plants. In addition to limonene, flavonoids (e.g., hesperidin, naringin, luteolin, and quercetin), limonoids (e.g., limonin and nomilin), and terpenes (e.g., myrcene) have been identified as the active compounds in citrus family plants [6–8].
There are two natural enantiomers, namely, R-limonene and S-limonene. The most common isomeric form of limonene is R-limonene, also known as D-limonene (Lim). While L- or S-limonene is used as a natural insect repellent, Lim is widely used as an additive in cleaning, cosmetics, and food products, due to its citrus-specific scent [6]. This chemical substance is perhaps the most widely used odorant in the cosmetics and cleaning products industry. However, Lim is frequently used as an additive in many food products due to its lemon flavor and is often consumed orally. In many geographical regions, citrus family plants and fruits are also applied for sedatives, pain relievers, and antimicrobial activity in traditional medicine methods [9–11]. Also, it has been reported to have anti-inflammatory, anticancer, gastroprotective, anxiolytic, and neuroprotective properties [6, 7, 10, 12, 13]. This study aimed to investigate the effect of various doses of Lim on indomethacin (Ind)-induced gastric damage in rats.
2. Materials and Methods
2.1. Animals, Groups, and Experimental Procedure
Adult male Sprague Dawley rats were obtained from the Karadeniz Technical University animal research facility. All experiments were carried out following the National Guidelines for the Care and Use of Mammals in Animal Research, and experimental protocols were approved by the university animal ethics committee (permission number: 2018-16). All animals used in the experiment were approximately 10–12 weeks of age. Only those with a similar body weight (285.91 ± 36.4 g) and a healthy appearance were included. The health status of the animals was regularly monitored by experts (DVM) at the Animal Research Facility, and exclusion criteria were based on expert evaluations of health status or a weight loss exceeding 10%. Animals were housed in polypropylene cages and fed with standard chow and tap water ad libitum. Animals were housed in a cage and maintained on a 12-h light/dark cycle in a temperature-controlled (25 ± 1°C) and humidity-controlled (50%–55%) environment. After 7 days of the acclimatization period in the Animal Research Facility (Giresun University D.H.M., Giresun), all animals were randomly (using https://www.graphpad.com/quickcalcs/randomize1) divided into six groups (n = 8) as (i) control (c), (ii) Ind, (iii) ranitidine used in a reference treatment group (Ran), (iv) Lim 50 mg/kg (L50), (v) Lim 100 mg/kg (L100), and (vi) Lim 250 mg/kg group (L250). Each dose of Lim (50, 100, and 200 mg/kg) was prepared daily by dissolving it in 0.25% Tween-80 (polyoxyethylene 80 sorbitan monooleate) in water and administered orally for 21 days [14]. On the final day of the experiment, the rats fasted for 24 h; however, their access to water was not restricted. In the reference treatment group (iii), Ran was administered orally at a dose of 30 mg/kg before the gastric injury, according to the protocol described in the previous study [15]. Gastric injury was induced by oral administration of 25 mg/kg Ind by gastric gavage five minutes after Lim or Ran administration [16].
Six hours after the administration of Ind, animals in all groups were sacrificed using high-dose anesthesia, and their stomachs were removed. The stomachs were opened along with major curvature, washed with saline, and examined macroscopically. Samples obtained from the stomach tissues of all rat groups were placed in a 10% formalin solution and stored at −20°C. All histopathological and biochemical analyses were conducted by blinded experts, using tissue samples labeled with subject numbers known only to the research group.
2.2. Chemicals and Drugs
D-Limonene (C10H16) (CAS number: 5989-27-5) was purchased from Sigma-Aldrich, USA. Gastric ulceration was induced by indomethacin (Endol) obtained from Deva ilaç/Turkey ranitidine (Ranitab) also obtained from Deva ilaç/Turkey. Rat TNF-α (Cat. No.: E-EL-R2856), rat inducible nitric oxide synthase (iNOS), (Cat. No.: E-EL-R0520), rat caspase-3 (Cat. No.: E-EL-R0160), rat PGE2 (Cat. No.: E-EL-0034), and catalase (CAT) activity (Cat. No.: E-BC-K031-M) assay ELISA kits were purchased from Elabscience (Houston, Tx., USA).
2.3. Macroscopic and Microscopic Examination of Stomach Tissue
Rat stomachs were examined macroscopically for gastric lesions, and the number and location of ulcers were recorded. In order to quantify the extent of damage to the mucosa, the damaged areas were marked on a scale of millimeters using clear graph paper. These areas were expressed as mm2 [17]. After assessing the macroscopic appearance of the stomach, two sections were taken from the fundus, corpus, and antrum, particularly from the pathological areas and subsequently embedded in paraffin blocks following routine tissue preparation. Then, 5-micron sections were taken from the tissue and stained with hematoxylin–eosin, and the gastric tissue was examined under a light microscope. The lesions detected in the stomach were graded according to the criteria of Esplugues et al. [18]. Histological scoring for each field was performed on a scale from 0 to 4, based on the following criteria: (0) indicates normal histology; (1) corresponds to epithelial cell damage; (2) represents glandular disruption, vasocongestion, or edema in the upper mucosa; (3) denotes mucosal disruption, vasocongestion, or edema in the mid-lower mucosa; and (4) signifies extensive mucosal disruption affecting the full thickness of the mucosa.
2.4. Biochemical Analysis
Stomach tissues were ground with liquid nitrogen, and 0.025 g of each rat group’s tissues was weighed and diluted 1/60 (w/v) by adding 1.5 mL of buffer solutions (a different buffer system for each parameter). These ground tissues were homogenized using a homogenizer on ice for 10 min. The homogenates were filtered through filter paper and centrifuged at 4°C using a refrigerated centrifuge (the speeds specified in the literature for each enzyme). Biochemical analyses were carried out on these supernatants.
2.5. Measurement of Glutathione Reductase (GR), Malondialdehyde (MDA) Levels, Superoxide Dismutase (SOD), and CAT Activity
Protein concentrations of all tissues studied were determined by the Bradford protein assay using bovine serum albumin as a standard [19]. The stomach homogenates were used for the determination of glutathione according to the methods of Sedlak et al. and expressed as mmol/g protein [20]. Lipid peroxidation was assessed by using the standard method of Okhawa, Ohishi, and Yagi and expressed as nmol of MDA formed/min/mg protein [21]. Homogenates were also used for the determination of SOD according to Nishikimi, Appaji, and Yagi [22] and CAT activity according to a method of Aebi [23]. The method is based on measuring the decomposition of hydrogen peroxide using the CAT enzyme. The results were compared to the protein amounts and were expressed as EU/g protein.
2.6. TNF-α, PGE2, Caspase-3, and iNOS Measurements
For ELISA measurements, supernatants obtained by centrifugation (5000 × g for 5 min) of tissue homogenates were stored at −80°C until assayed by ELISA. TNF-α, PGE2, caspase-3, and iNOS were performed according to the product instructions. In the last step, following the termination of the enzyme–substrate reaction, the optical density was measured spectrophotometrically with an ELISA reader (AccuReader M965+, Metertech, Inc., Taipei, Taiwan) at a wavelength of 450 nm. The concentration of each molecule in the samples was calculated through the specific standard curves of the ELISA kits.
2.7. Measurements Lipid Composition of Gastric Tissue by High-Performance Thin-Layer Chromatography (HPTLC)
500 μL of n-hexane–isopropanol 3:2 (v/v) mixtures was added to 1000 μL of homogenized samples in an Eppendorf tube. After vigorous vortexing, tubes were centrifuged at 5000 x g, +40 C for 5 min and the phase was used for tissue lipid chromatographic analysis. HPTLC silica gel 60 plates (20 × 10 cm) were used to separate and identify lipids. The HPTLC plates were loaded with a standard lipid mixture (triolein, cholesterol [COL], palmitate, 2-palmitoylglycerol, glycerol di-palmitate, and L-α-phosphatidylcholine) and tissue lipid extracts. Lipids were developed with n-hexane: diethyl ether: formic acid; 80:20:2 (v/v) up to 15 cm above the point of application. After development, the entire plate was immersed in charring reagent (10% copper(II) sulfate (CuSO4) (w/v) in 8% phosphoric acid (H3PO4) (v/v)), and lipid classes were visualized by incubation at 180°C. Tissue lipids were separated into the following classes: triacylglycerols (TAGs), free fatty acids (FFAs), sterols (STE), monoacylglycerols (MAG), diacylglycerols (DAGs), and phospholipids (PLs). HPTL chromatograms were scanned with a photo scanner and analyzed with TotalLab TL 120 software (Informer Technologies, Inc. DE, USA), and the results were expressed as the percentage of each lipid class in the total lipid composition [24].
2.8. Statistical Analysis
The study employed G∗Power software Version 3.1.94 (Heinrich Heine University, Düsseldorf, Germany) to estimate the required sample size. A one-way fixed model analysis of variance (ANOVA) was conducted with an alpha value of 0.05 and a power of 80% (1-β err. prob). The actual power was 0.81. Based on these values, a minimum sample size of 48 subjects was required. The data were presented as the mean ± SEM. Statistical analyses were conducted using IBM Corp.’s SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: USA). The normal distribution assumption was evaluated using the Shapiro–Wilk test, and the homogeneity of variances was confirmed using Levene’s test. An absolute skewness value ≤ 1.5 or an absolute kurtosis ≤ 1.5 was used as reference values for determining considerable normality [25]. The differences between groups for ordinal variables related to gastric damage scores were assessed using the Kruskal–Wallis test. The measured and calculated data were analyzed using one-way ANOVA, followed by Tukey’s post hoc multiple comparison tests. Statistical significance was set at p < 0.05.
3. Results
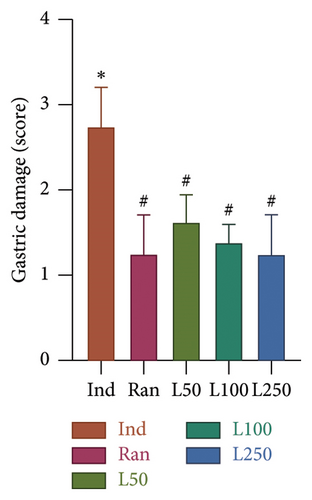
Macroscopic examination of gastric mucosa was evaluated between the groups. The macroscopic scoring procedure applied in the previous study was also utilized in the present study [17]. There was a significant difference in the ulcerative areas of the gastric tissues (F[5, 42] = 25.41, p < 0.001). In the post hoc evaluations between the groups, it was observed that there was a statistical difference between the scores of the Ind group and the scores of all groups (p < 0.05). As shown in Figure 1(a), the ulcerations in the Ind group are notably penetrating. A comparison between the Ran and Lim groups revealed no significant difference (p > 0.05). This result shows that the gastroprotective effect of Lim, particularly at doses of 100 and 250 mg/kg, is similar to that of the reference treatment (Ran) group.

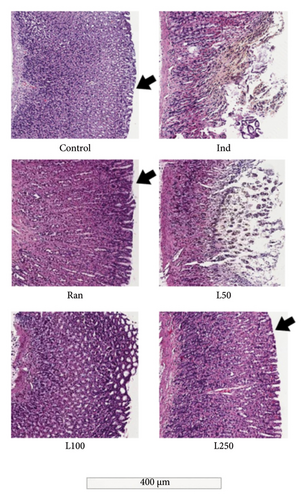
The gastric tissues from the damaged mucosal areas obtained from the animals are shown in Figure 2. The intact appearance of the upper mucosa is prominent in the C, Ran, and especially the L250 groups. In contrast, mucosal areas with ulcerative lesions were observed in the Ind group and, to a lesser extent, in the L50 group. Additionally, some findings observed in the histological examination but not demonstrated by statistical analysis included granulocytic cell infiltration, which was observed in all Ind-treated groups but was relatively reduced in the Lim-treated groups. However, we did not use this as a criterion in our calculations (data not shown). As observed in histological sections and scored accordingly, mucosal damage and mucosal disruption were observed in the Ind group but not in any of the other groups (p < 0.05). The most notable observation was that mucosal damage in the L100 and L250 groups was similar to that in the Ran group, with no statistical difference (p > 0.05). The edematous and ulcerative appearance of the upper mucosa observed in the L50 group was not seen in the L100 and L250 groups.
The most important finding of our study was that the lipid profile altered by Ind administration in the Lim pretreated groups was in a similar range to the C group for almost all parameters (Table 1). In detail, a statistical difference was observed between the groups regarding COL levels, an important element in the fluidity of the cell membrane (p < 0.005). In the post hoc analysis between the groups, the highest COL level was observed in the Ind group and was significantly reduced in the L250 group (p < 0.001). Moreover, no statistically significant difference was found between the C and L250 groups (p > 0.05). Similarly, FFA levels, which were elevated in the Ind group, were found to decrease significantly in all three Lim-treated groups (p < 0.001). The ratios of TAGs in the gastric tissue were evaluated, and a statistical difference was found between the groups (p < 0.005). The Lim-treated groups had higher TAG levels compared to the Ind group in a dose-dependent manner (p < 0.001). Besides, there was no difference between the L250 group and the C group (p > 0.05). DAG levels, an important structure involved in intracellular signaling, were analyzed, and according to ANOVA results, there was a difference between the groups (p < 0.005). In all three groups treated with Lim, DAG levels were higher than those treated with Ind (p < 0.001). ANOVA results showed a statistical difference between the groups in the PL level, the main component of the cell membrane in gastric tissue (p < 0.001). Similarly, PL levels were lower in all groups treated with Lim compared to the Ind (p < 0.001).
| % | C | Ind | Ran | L50 | L100 | L250 |
|---|---|---|---|---|---|---|
| TAG | 82.1 ± 2.2∗∗ | 51.5 ± 2.2 | 66.1 ± 1.9# | 75.1 ± 2.7∗ | 76.9 ± 2.2∗ | 79.0 ± 2.5∗ |
| FFA | 6.7 ± 1.1∗∗ | 25.6 ± 2.1 | 18.5 ± 0.7∗ | 11.9 ± 2.7∗∗ | 10.4 ± 1.6∗∗ | 9.5 ± 1.4∗∗ |
| DAG | 1.65 ± 0.08∗∗ | 0.42 ± 0.05 | 0.65 ± 0.04# | 1.97 ± 0.11∗∗ | 1.96 ± 0.11∗∗ | 1.75 ± 0.10∗∗ |
| MAG | 0.23 ± 0.01∗∗ | 2.99 ± 0.47 | 2.53 ± 0.41 | 0.66 ± 0.05∗∗ | 0.48 ± 0.04∗∗ | 0.26 ± 0.03∗∗ |
| COL | 6.6 ± 0.8∗∗ | 11.7 ± 0.7 | 7.9 ± 0.4∗ | 7.7 ± 0.3∗ | 7.6 ± 0.4∗ | 6.4 ± 0.3∗∗ |
| PL | 2.8 ± 0.4∗∗ | 7.8 ± 0.7 | 4.4 ± 1.0# | 2.7 ± 0.8∗∗ | 2.7 ± 0.8∗∗ | 3.1 ± 0.9∗∗ |
- Note: Group values are expressed as mean ± SD of the percentage of lipid composition.
- Abbreviations: COL, cholesterol; DAG, diacylglycerol; FFA, free fatty acids, Ind, indomethacin; L50, limonene 50 mg/kg; L100, limonene 100 mg/kg; L250, limonene 250 mg/kg groups; MAG, monoacylglycerol; PL, phospholipids; Ran, ranitidine; TAG, triacylglycerol.
- ∗A significance level of p < 0.005 versus the Ind group.
- ∗∗A significance level of p < 0.001 versus the Ind group.
- #A significance level of p < 0.05 versus the Ind group.
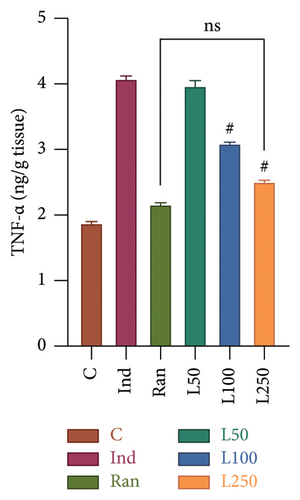
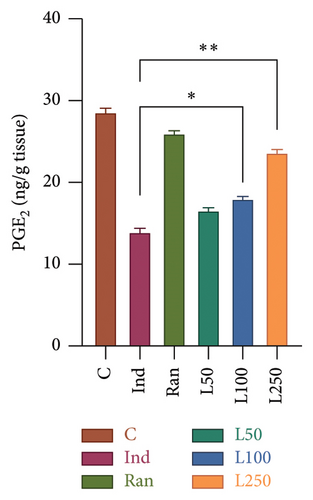
The TNF-α level, an important mediator of acute inflammation, was evaluated, and a significant difference was found between the groups (F[5, 42] = 23.73, p < 0.001). It was observed that proinflammatory processes were increased in the Ind group compared to the other groups, except the L50 group (Figure 3(a)). However, no significant difference was found between the L50 group and the Ind group (p > 0.05). Furthermore, the results indicate that there is no statistically significant difference between the C group and either the L250 group or the reference treatment (Ran) (p > 0.05). A comparison of PGE2 levels, which inhibit gastric acid and elevate mucus secretion, revealed a statistically significant difference between the groups (F[5, 42] = 34.76, p < 0.001] (Figure 3(b)). The results showed that there was a statistically significant difference between the Ind group and the L100 (p = 0.011) and L250 groups (p < 0.001), but not with the L50 group (p > 0.05). However, the PGE2 level was higher in the Ran group (25.81 ± 0.52) than in the L250 group (23.59 ± 0.43) (p < 0.05).
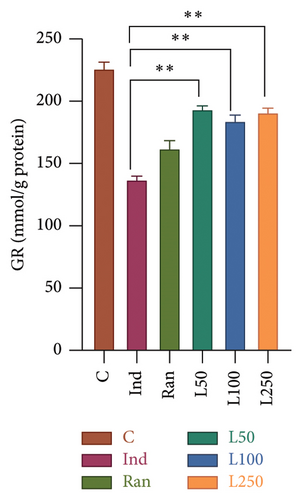
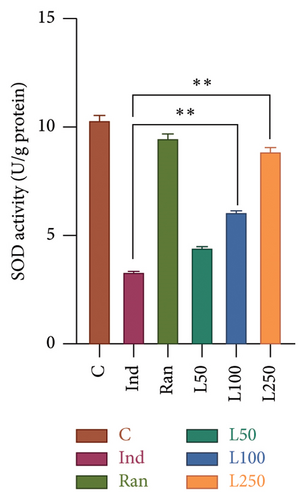
Significant statistical differences in GR levels were observed among the groups (F[5, 42] = 39.08, p < 0.001). In post hoc analyses, a statistical difference was found between the Ind group and all the remaining groups. Figure 4(a) shows that all Lim groups were higher than the reference treatment group as Ran group. Besides, the GR levels of the L50 (193.11 ± 3.30), L100 (183.89 ± 5.0), and L250 (190.68 ± 4.22) groups were higher than that of the Ind group (136.33 ± 3.60) in detail. When comparing the SOD activity between the groups, a difference was found among the groups (F[5, 42] = 49.52, [p < 0.001]). In the post hoc evaluations between the groups, the Ind group was compared with the other groups, and a difference was observed between the Ran group as reference treatment and the L100 (p < 0.001) and L250 groups (p < 0.001), except for the L50 group (p > 0.05). There was no difference between the C group and the Ran, and L250 groups (p > 0.05) (Figure 4(b)).
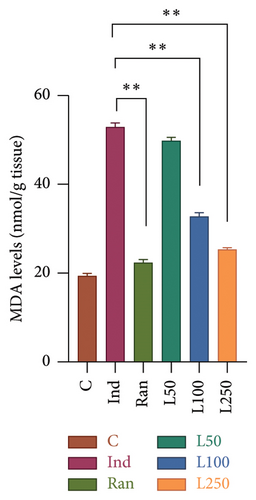
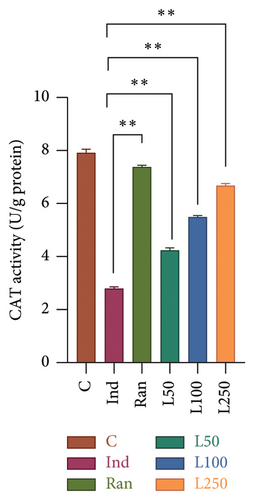
The levels of MDA in the stomach tissue, another product of oxidative stress, were compared between the groups. According to the results, a significant difference was found between the groups (F[5, 42] = 17.87, p < 0.001). In the post hoc evaluations, no difference was observed between the L50 group and the Ind group (p > 0.05). However, a difference was found when the reference treatment group (Ran) (p < 0.001) and the other Lim doses (L100 and L250) were compared with the Ind group (p < 0.001). There was no difference between the C group and the Ran, and L250 groups (p > 0.05) (Figure 5(a)). The activity of CAT, one of the key enzymes protecting against oxidative damage in stomach tissue, was also evaluated. Statistically significant differences were found in comparisons between the groups (F[5, 42] = 40.47, p < 0.001). When the groups were evaluated using the post hoc multiple comparisons, the mean of the Ind group was statistically different from the Ran group (p = 0.002) and all other groups (p < 0.001). Comparisons were made with the C group, no statistical difference was observed with the Ran and L250 groups (p > 0.05), while a statistical difference was found with the L50 and L100 groups (p < 0.05) (Figure 5(b)).
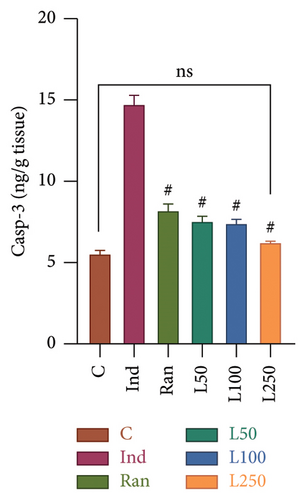
A statistically significant difference was observed in Casp-3 levels, an apoptosis marker when the groups were compared (F[5, 42] = 82.62, p < 0.001) (Figure 6(a)). In multiple comparisons among the groups, there was a difference between the Ind group and the other groups (p < 0.001). However, no significant difference was observed between the L250 group and the C group (p > 0.05). Similarly, no difference was found between the reference treatment group (Ran group) and the L50 and L100 groups (p > 0.05). These findings indicate that the L50 and L100 groups demonstrated similar efficacy to the Ran group.
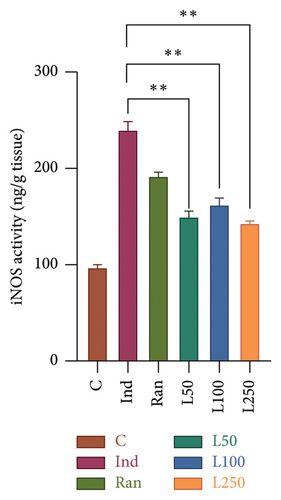
In this study, the activity of iNOS, an important proinflammatory enzyme, was compared between the study groups to determine mean differences and to identify whether there were differences between the groups (Figure 6(b)). A significant difference was observed (F[5, 42] = 54.15, p < 0.001). The difference between the Ind group (238.49 ± 9.8) and the L50 (149.07 ± 6.4), L100 (161.65 ± 7.8), and L250 (141.64 ± 3.7) groups was found to be statistically significant (p < 0.001).
4. Discussion
Several studies have demonstrated that limonene has antioxidant, anti-inflammatory, antidepressant, and beneficial effects in some types of cancer [6, 7, 10, 26]. Also, our study demonstrated that Lim has neuroprotective properties on chronic restraint stress models in rats [26]. In this study, we aimed to investigate whether Lim is effective in an Ind-induced gastric ulceration model in rats.
Gastric injury or ulcerations are usually caused by factors that increase gastric damage, such as Helicobacter pylori, heavy alcohol consumption, and the use of NSAIDs, or by the suppression of defensive factors that reduce mucus production, such as stress, and decreased PG synthesis.
NSAIDs are the most widely used group of drugs worldwide. They inhibit PG synthesis in the stomach by competitively inhibiting the cyclooxygenase pathway [2, 15, 18, 27, 28]. While the decrease in PG synthesis increases acid production, it also reduces mucus formation and bicarbonate production of the gastric mucosa, that is, the formation of the gastric barrier [2, 18, 29]. In this study, we used Ind, a type of NSAID, to investigate a model of gastric damage. Ind contains polar lipids with high affinity for lipophilic regions of cell membranes, and its polar groups trigger membrane disruption with loss of structural PLs and membrane proteins [2, 30]. This leads to decreased hydrophobicity of the mucosal coating adhering to the mucosal cell surface. This loss of hydrophobicity makes it easier for water-soluble damaging agents, such as acid and pepsin, to enter the parenchyma [2, 18]. These drugs induce lipid peroxidation, leading to changes in membrane lipids, which in turn affect membrane fluidity and contribute to the development of gastric mucosal lesions [31]. Our study showed that the gastric tissue in the Ind-treated group suffered from bleeding, edema, and lesions at both macroscopic and microscopic levels. However, in all three groups treated with Lim, the damage was reduced compared to the group that received Ind alone (Figure 1). Furthermore, the macroscopic damage score observed in the examined tissues showed a gradually decreased dose-dependent Lim administration.
Previous studies have shown that Ind changes regional blood flow and reduces PG synthesis [2, 16, 27, 32]. Inhibition of PGE2 in the gastric mucosa by Ind leads to increased inflammatory factors, resulting in increased vascular permeability and inflammatory cell infiltration, e.g., neutrophils [2, 33]. TNF-α plays a pivotal role in neutrophil infiltration in Ind-induced gastric injury [33]. Neutrophil activation is known to be associated with the release of ROS. Biologically, this is very important for the antimicrobial role of neutrophils. However, these free radicals have also been implicated in pathological conditions of gastric tissue [32]. de Souza et al. showed that the lowest dose of Lim, 50 mg/kg, reduced the level of TNF-α and had a gastroprotective effect by modulating the inflammatory response [34]. The present study demonstrated that Lim acts as a protective agent against Ind-induced gastric injury by increasing PGE2 and enhancing defensive factors. It was also shown to reduce inflammation by lowering levels of TNF-α, as shown in Figure 3(a).
Gürbüz et al. reported that increased inflammation in the Ind-induced gastric ulcer model caused iNOS production and that high levels of NO increased oxidative stress and mucosal damage by generating reactive nitrogen derivatives [27]. It is widely accepted that increased NO produced by iNOS in the digestive tract is cytotoxic [27, 35]. Also in our study, as in previous studies, iNOS levels were increased in the Ind group [27, 34–36]. On the contrary, iNOS levels were decreased in all groups treated with Lim (Figure 6(b)). As observed in our findings, Yu et al. have also reported that Lim displays anti-inflammatory and antioxidant properties via the iNOS and extracellular signal-related kinase (ERK) signaling pathways [37]. Yoon, Lee, and Hyun reported that Lim decreased lipopolysaccharide-induced iNOS production and PGE2 levels in a series of RAW 264.7 macrophages [38]. Rehman et al. also showed that Lim inhibits doxorubicin-induced oxidative stress and inflammation mediated by COX and iNOS pathways in the rats [36].
It is well established that enzymatic (SOD, CAT, GPx, and GR) and nonenzymatic antioxidant systems (GSH) prevent oxidative damage caused by ROS. SOD facilitates the conversion of superoxide radicals into hydrogen peroxide (H2O2) and oxygen (O2) molecules. Subsequently, hydrogen peroxide is transformed into H2O by enzymes such as CAT or glutathione peroxidase (GPx), thereby eliminating its potential for damage. In addition, GR plays a critical role in the reutilization of glutathione, maintaining the cell in a redox state and preventing apoptosis [15, 27]. Studies have shown that Ind or other NSAIDs lead to an increase in ROS, resulting in gastric damage [1, 2, 15, 18, 28]. Ind increases oxidative damage by acting as an oxidant and suppressing antioxidant systems [15, 28, 29]. Our results showed that CAT activity decreased with Ind administration, and Lim administered at doses of 50, 100, and 250 mg/kg increased CAT activity in a dose-dependent manner (Figure 5(b)). de Souza reported that Lim did not change SOD and GR levels in ethanol-induced gastric damage [34]. On the contrary, in our study, we found an increase in GR activity in all three Lim-treated groups compared to the Ind group, and SOD activity increased in the 100 and 250 mg/kg Lim-treated group.
An elevation in MDA levels is regarded as an indicator of aldehyde production, which also reflects lipid peroxidation. [15]. Chattopadhyay et al. demonstrated that lipid peroxidation increased MDA levels, while antioxidant activity decreased in Ind-induced gastric injury [28]. Our results showed that MDA levels increased with Ind administration and decreased with 100 and 250 mg/kg doses of Lim. As mentioned above, lipid peroxidation disrupts membrane stability by modifying the structure of PLs, the main component of cell membranes [39]. The stomach mucosa’s hydrophobic characteristic helps protect the mucosa by limiting hydrogen ions. This characteristic has been related to the stomach epithelium’s PL layer. PLs play an important role in the protection of the stomach lining. Also, exogenous PL treatment has been demonstrated to have protective benefits against high acidity-induced gastric hemorrhages [4]. The disruption of PLs, such as phosphatidylcholine, by lipid peroxidation increases membrane permeability and inevitably leads to mucosal cell damage, inflammation, and elevation of apoptosis [30]. Lichtenberger and colleagues also suggested that phosphatidylcholine and phosphatidylethanolamine play a key role in increasing the hydrophobicity involved in PG-mediated cytoprotection of the gastric mucosa [4]. In our results, the PL level, which increased with Ind administration, was the same as the reference treatment and the C group with Lim administration. Lipid peroxidation during gastric injury leads to the release of FFAs from cell membranes. Increased MDA levels in the gastric mucosa can induce lipid peroxidation, leading to oxidative damage of FFAs in the cell membrane, and disrupting membrane integrity and function. This oxidative stress may impair enzymatic activities and increase membrane permeability, contributing to mucosal barrier dysfunction [28, 39]. As demonstrated in our study results, the FFA ratio increased with Ind administration. On the other hand, FFA levels were decreased in all groups pretreated with Lim, and the results were similar to those observed in the reference treatment group. As mentioned in the results, COL is an essential structural element for the stability and fluidity of the cell membrane [4, 30]. Inflammation and increased oxidative processes after gastric injury cause peroxidation and damage to COL and its esters. As a result, levels of oxidized COL, called oxysterols, increase [30, 39]. In our study, the COL ratio that changed with Ind administration was reduced in the Lim-treated groups. An increase in MAG levels due to the degradation of TAG, one of the lipid degradation products, is observed during oxidative stress, particularly during cell membrane damage [4, 30, 31]. In our results, the decrease in TAG levels and the increase in MAG levels in the Ind group were reversed by Lim administration.
Caspase-3 activation, which plays a role in the apoptotic process in gastric tissue, has been studied and demonstrated in Ind-induced gastric injury [29]. Gebril et al. report that IND can induce apoptotic and autophagic cell death of parietal cells in a time-dependent manner [40]. Lu et al. reported the efficacy of Lim in preventing gastric cancer in a metastatic mouse model. Lim was administered orally for 7 weeks and the tumor inhibition rate decreased by 46.06% when combined with Lim compared to the group administered fluorouracil, a chemotherapeutic agent alone. It has been suggested that Lim may act via apoptosis-inducing enzymatic pathways with Casp-3 and has antiangiogenic effects [41]. The present study demonstrated a dose-dependent reduction in caspase-3 levels following Lim administration, compared to the Ind-administered group.
5. Conclusion
Our findings demonstrated that Lim administration led to a reduction in the macroscopic damage associated with Ind exposure. Moreover, there was a dose-dependent increase in PGE2 levels, which had shown a reduction following Ind administration. Additionally, significant decreases in TNF-α and iNOS activity, as well as reduced Casp-3 levels, were observed. These results suggest that Lim protects against Ind-induced gastric injury by enhancing natural defense mechanisms and modulating the inflammatory response. Furthermore, Ind administration significantly elevated lipid peroxidation (MDA) while reducing the enzymatic antioxidant activity (SOD, CAT, and GR) in the gastric mucosa of Ind-treated rats. However, in the Lim groups receiving 100 and 250 mg/kg doses, MDA levels decreased, whereas GR, SOD, and CAT activities increased, and caspase-3 levels, an apoptosis marker, were reduced. Lim also stabilized the lipid composition that was altered by Ind administration. These results show that Lim inhibits oxidative stress by reducing the production of ROS and inhibits NSAID-induced damage by reducing the inflammatory response.
The limitation of this study is that additional immunological cytokines were not measured. However, the key findings highlight that our results demonstrate the gastroprotective properties of Lim through multiple measurements. Furthermore, the literature review up to the date of this study did not identify any studies investigating changes in lipid composition after Lim administration. In this study, we aimed to demonstrate the gastroprotective effect of Lim pretreatment through various parameters in rats with Ind-induced gastric injury. Future studies could explore the potential clinical applications of Lim, particularly in the prevention or treatment of peptic ulcers in humans. Given its anti-inflammatory and gastroprotective properties, Lim may also be beneficial in other gastrointestinal disorders such as gastroesophageal reflux disease or functional dyspepsia. As the properties of Lim and its derivatives become better understood, it may be considered for use in combination with other nutraceuticals or biotherapeutics for the effective management of gastrointestinal conditions associated with oxidative stress or inflammation.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Mehmet Alkanat: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, writing – original draft, and writing – review and editing. Hafize Özdemir Alkanat: funding acquisition, methodology, investigation, project administration, validation, and writing – review and editing.
Funding
This research was supported by the Science Foundation Program of Giresun University (Grant number: SAĞ-BAP-A-250221-25).
Acknowledgments
The advice and comments of Prof. Dr. Özgür Kaynar and the support of Dr. Aslıhan Alpaslan are gratefully acknowledged. The Science Foundation Program of Giresun University supported this study (Grant number: SAĞ-BAP-A-150219-41). All stages of the study were planned, organized, and completed according to “The ARRIVE Essential 10” checklist.
Supporting Information
All stages of the study were planned, organized, and completed according to “The ARRIVE Essential 10” checklist.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




