Impact of Ripening and Fractions on Phenolic Composition and Antioxidant Capacity in Australian Jackfruit (Artocarpus heterophyllus L.)
Abstract
Artocarpus heterophyllus Lam., a tropical tree native to India and prevalent in Asia, Africa and parts of South America, boasts a phytochemical profile dominated by polyphenols. The composition of jackfruit phytochemistry changes during maturity stages due to enzymatic and biochemical reactions. Extraction module and conditions play a crucial role in polyphenol extraction. This investigation aimed to understand the impact of ripening and extraction methods on polyphenol concentration in different jackfruit fractions. Two fresh jackfruit samples, unripe jackfruit (JUR) and ripe jackfruit (JR), along with a commercially frozen sample (JC), were sourced from the local market of Australia. Samples were further processed into four different fractions (peel, core, seed and fruit), and extraction was carried out through conventional and ultrasonic extraction systems. Ripened jackfruit samples exhibited higher phenolic content and antioxidant activity than unripened ones. Among the jackfruit fractions, the highest phenolic contents and antioxidant activity were observed in the peel followed by core, seed and fruit. As far the extraction module is concerned, ultrasound extraction-based jackfruit samples exhibited overall higher phenolic content and antioxidant activity as compared to conventional. The LC–ESI–QTOF–MS/MS screening showed the presence of 65 compounds in different jackfruit samples showing the diversity of phenolics in this fruit. The majority of the compounds belonged to flavonoids (49), lignans (5), phenolic acids (7) and other polyphenols (4). The HPLC quantification showed that the gallic acid, catechin, syringic acid, epicatechin, coumaric acid, trans-ferulic acid, sinapic acid, procyanidin A2, quercetin, caffeic acid and kaempferol were present in an appreciable amount in all the samples. This study provides insight into the changes in the phenolic profile during different ripening stages in different fractions of the jackfruit. These changes may impart a significant impact on the physicochemical and nutritional properties of the jackfruit.
1. Introduction
Jackfruit (Artocarpus heterophyllus Lam.) is a tropical climacteric fruit known for its economic significance and versatility [1]. As a member of the Moraceae family, it is widely cultivated in tropical and subtropical regions of the world. Native to West India and Malaysia, jackfruit is also extensively grown in Sri Lanka, Bangladesh, Indonesia, Thailand and the Philippine [2]. Additionally, its cultivation has expanded to regions like Brazil and Florida in the United States. In Australia, jackfruit is primarily cultivated in the northern regions of Queensland and Darwin in the Northern Territory (NT). The two major varieties found in NT are soft flesh and crisp flesh. The jackfruit industry in Australia is projected to reach a gross production value of $5–$10 million by 2025 [3].
The Jackfruit tree is an evergreen species, can grow up to 20 m tall with a trunk diameter of 30–50 cm [4] and is capable of producing up to 50 kg of edible fruit [5]. The period between flowering and fruit ripening stage from 150 to 180 days [6] causes substantial shifts in the phytochemical profile of the fruit. The ripening stage significantly impacts the physical, chemical and nutritional composition of jackfruit [7]. Jackfruit has an inedible core, a pointy rind and edible fruit flesh between the core and rind, containing 100–500 seeds. Compositionally, jackfruit consists of fruit pulp (30%), seeds (18%) and rind (5%–55%) [8]. Underutilized, the seeds and rind are rich in nutritional values [9]. Notable biomolecules in jackfruit include carotenoids, flavonoids, volatile acids, sterols and tannins [10], and compounds such as artocarpin, artocarpetin and norartocarpetin [11]. Jackfruit is also rich in essential nutrients, containing higher levels of protein, calcium, iron and thiamine compared to other tropical fruits. Previous studies have shown that jackfruit exhibited the antidiabetic, antimicrobial, anti-inflammatory, wound healing and anticancer properties [12].
Phenolic compounds, constituting secondary plant metabolites, are widely distributed across various plant tissues. These compounds exhibit significant variation in types and concentrations across different plant varieties, maturity stages, seasons and geographical regions. Recent studies have highlighted compositions of phenolics are related with the ripeness and varieties of fruit [13]. Polyphenols have a variety of important biological properties, such as antioxidant activity, which contribute to the defence of the human body against oxidative stress and its adverse effects. Furthermore, polyphenols obtain the antimicrobial, immunomodulatory, anticancer and anti-inflammatory properties [14]. Previous investigations have revealed the therapeutic potential of jackfruit due to its array of phytochemicals dominated by polyphenols.
The extraction of polyphenols from plant sources like jackfruit is a critical step that significantly influences the yield and composition of the extracted compounds. Various extraction techniques, such as solvent extraction and ultrasound-assisted extraction, have been employed to enhance polyphenol extraction efficiency [15]. The choice of extraction method can impact the bioactivity and stability of the extracted polyphenols, as different techniques may selectively extract specific classes of polyphenolic compounds. Studies have shown that ultrasound-assisted extraction, in particular, enhances the extraction of certain polyphenols by breaking down cell walls and increasing the solubility of compounds [16].
The estimation and quantification of phytochemicals in jackfruit, especially the phenols, can provide insights into the therapeutic capacity. The advanced techniques like liquid chromatography–electrospray ionization–quadrupole time-of-flight mass spectrometry–tandem mass spectrometry (LC–ESI–QTOF–MS/MS) and high-performance liquid chromatography coupled with photodiode array detector (HPLC–PDA) could be essential for phenolic compounds identification and characterization [17]. Some studies have examined the phenolic profile of jackfruit using different advance analytical assays, and limited work has been conducted on Australian varieties and different parts of the fruit at various ripening stages. The current study aimed to bridge this gap by extracting and characterizing the polyphenols from different parts of jackfruit—seed, peel, core and fruit—at two ripening stages, to estimate the variations in the phenolic profile with respect to the ripening level.
2. Materials and Methods
2.1. Sample Preparation
Jackfruit (A. heterophyllus Lam.) samples were procured from the local market of Australia. The samples were categorized as unripe jackfruit (JUR) and ripe jackfruit (JR), based on physical characteristics such as colour, solidity and surface structure following the guidelines of BBCH scale [18], and the stages are 815 and 817, respectively, for screening and phenolic profiling to assess the changes in polyphenols at different maturation stages. Moreover, a freeze commercial sample was purchased from a local market as a comparison. First, the fruits were manually cleaned and divided into four parts (peel, core, seed and fruit). Each part was then cut into pieces of approximately 0.5 × 1 cm in size. All samples were frozen at −80°C overnight and subsequently lyophilized at −45°C under a pressure of 50 MPa for 48 h. The freeze-dried samples were then ground into a fine powder that been stored at −20°C until further analysis.
2.2. Extraction of Phenolic Compounds
For the extraction of phenolic compounds, a consistent quantity of 2.0 ± 0.5 g from each of the four parts (fruit, peel, core and seeds) of both ripened and unripen jackfruit powder and commercial product was used. The extraction was followed by previous research with some modification [19]. Briefly, these samples were mixed with 20 mL of 70% ethanol with 0.1% formic acid, followed by homogenization. For the extraction of phenolics, two methods have been employed: The first is a conventional extraction achieved by shaking in an incubator for 16 h at 120 rpm and 10°C; the second is an ultrasonication method, where phenolics were extracted at 40% amplitude for 5 min using a cell disruptor (Branson, model Digital Sonifier 450 model). Afterwards, the extracts were centrifuged at 5000 rpm, 4°C for 15 min. The supernatants were collected and stored at −20°C for further analysis. For LC–MS analysis, the extracts were filtered through a 0.45-μm syringe filter (Thermo Fisher Scientific Inc., Waltham, MA, USA) [20].
2.3. Determination of Spectrophotometric Assays
2.3.1. Folin–Ciocalteu Assay (FCA)
The total phenolic content (TPC) of the samples was determined by the FCA method of Severo et al. [21]. Gallic acid dissolved in analytical ethanol with 0–200 μg/mL concentration was employed as a standard curve. The Folin–Ciocalteu reagent was diluted with Milli-Q water with a 1:3 volume ratio and prepared 10% (w/w) sodium carbonate for later use. The whole assay needs to conduct in the environment avoiding light exposure as the light sensitive of FC reagent. Initially, 25 μL samples, 25 μL FC reagent and 200 μL water were mixed in a 96-well plate. Then, the mixture has a 5-min incubation at 25°C. Next, addition of 25 μL sodium carbonate solution with a 60-min incubation at the same condition. The final outcome was measured at a wavelength of 764 nm by spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and presented as the mg standard equivalent per gram of a sample (GAE mg/g).
2.3.2. Total Flavonoid Concentration (TFC)
The flavonoid content of the samples was determined by the method of Peng et al. [22], with minor modification. Quercetin dissolved in analytical methanol with 0–50 μg/mL gradient concentration was employed as a standard curve. The final outcome was present as the mg standard equivalent per gram of a sample (QE mg/g). 2% aluminium chloride and 50 g/L sodium acetate are the reagents for this assay. The combination of 80 μL extract, 80 μL of aluminium chloride solution and 120 μL of sodium acetate solution was incubated for 150 min in the 96-well plate under identical condition, followed by the measurement of absorbance at 440 nm.
2.3.3. Determination of Total Tannins Concentration (TTC)
This assay was performed based on the method of Zou et al. [23] with modifications. Catechin dissolved in analytical methanol with 0–1000 μg/mL concentration was employed to estimate the tannins content of samples. 4% (w/v) vanillin and 32% (v/v) sulphuric acid dilated by pure methanol were prepared. 20 μL of extract, 150 μL of 4% vanillin solution and 25 μL of 32% (v/v) sulphuric acid were combined in a 96-well plate with a 15-min period incubation in the dark at 25°C, followed by measuring absorbance at 500 nm. The results were expressed as mg of catechin equivalent of weight from samples dry weight (CE mg/g).
2.3.4. 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
The DPPH and following assays were employed to assess the antioxidant ability of samples. The mechanism of DPPH assay is the radicals scavenge ability, and the steps were followed by Duan et al. [24]. 4-mg DPPH powder was dissolved into 100 mL of pure methanol to prepare the DPPH solution. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) dissolved into pure ethanol to prepare the gradient standard curve range from 0 to 200 μg/mL, while the outcome present as mg Trolox equivalent per gram of samples dry weight (TE mg/g). 40 μL of the extract and 260 μL of 0.1 mM DPPH solution were added to a 96-well plate and incubated for 40 min in the absence of light exposure. Subsequently, the measurement of 517 nm was conducted.
2.3.5. 2,2′-Azino-Bis-3-Ethylbenzothiazoline-6-Sulphonic Acid (ABTS) Assay
ABTS assay determines the free radical scavenging activity of samples, which provides a reference for the DPPH assay. It was conducted from the method of Tang et al. [25]. The ascorbic acid was selected as a standard that dissolves in Milli-Q water with gradient concentration from 0 to 150 μg/mL. The result is expressed in mg of ascorbic acid equivalent of sample per gram (AAE mg/g). Making ABTS stock solution is complicated, 5 mL of 7 mM ABTS solution and 88 μL of 140 mM potassium persulphate were mixed and then incubated at room temperature for at least 16 h. Then, the stock solution was diluted with ethanol with 1:90 (v/v) ratio to make ABTS dye. In brief, 10 μL of extract and 290 μL of ABTS dye were mixed and incubated for 6 min without light exposure. Absorbance was measured at 734 nm.
2.3.6. Hydroxyl Radical Scavenging Activity (•OH-RSA) Assay
The •OH-RSA assay was carried out to determine the hydroxyl radical scavenge capacity in the present investigation based on the method described in Smirnoff and Cumbes [26]. The ascorbic acid selected as standard that dissolves in Milli-Q water with gradient concentration from 0 to 300 μg/mL. 6 mM FeSO4·7H2O, H2O2 and 3-hydroxybenzonic acid were prepared, respectively. In summary, the mixture of 50 μL extract, 50 μL FeSO4 7H2O solution and 50 μLH2O2 solution was incubated for 10 min. Then, 50 μL 3-hydroxybenzoic acid was added as a trapping agent. Absorbance readings were measured at 510 nm.
2.3.7. Ferric Reducing Antioxidant Power (FRAP) Assay
The capacity of reduction is another important mechanism for antioxidant ability, which has been determined by FRAP assay in this study and method from Benzie and Strain [27]. The standard curve and concentration are as same as the DPPH assay. The FRAP dye is the mixture in the volume ratio of 10:1:1 of 300 mM sodium acetate buffer (pH 3.6), 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) and 20 mM FeCl3 solution. The procedure was 20 μL of the extract and 280 μL of FRAP dye added to a 96-well plate, and the plate was incubated for 10 min at 37°C without light conditions, and absorbances were measured at 593 nm.
2.3.8. Reducing Power Assay (RPA)
The principle of this assay is the substance, which have reduction potential, react with potassium ferricyanide (Fe3+) to form potassium ferrocyanide (Fe2+), then react with ferric chloride to form ferric-ferrous complex. Sodium phosphate buffer (pH 6.6), 1% (w/v) K3[Fe (CN)6] solution, 10% (w/v) trichloroacetic acid (TCA) solution and 0.1% (w/v) FeCl3 solution. The standard curve is Trolox, and concentration is 0–500 μg/mL. Initially, 10 μL extract, 25 μL of sodium phosphate buffer and 25 μL K3[Fe(CN)6] were combined and incubated for 20 min. Then, 25 μL of TCA solution was added to stop further reaction, and 85 μL of water and 8.5 μL of FeCl3 solution were added with further for 15-min incubation under identical condition. Absorbance readings were measured at 750 nm.
2.3.9. Ferrous Ion Chelating Activity (FICA) Assay
This assay is to measure the chelating ability of ferrous ion of substance. Ethylenediaminetetraacetic acid (EDTA) dissolved in water under alkaline condition was employed as standard curve with range from 0 to 50 μg/mL, and outcomes were expressed as mg EDTA/g. In a no-light exposure environment, 15 μL of sample extract, 85 μL of water, 50 μL of ferrous chloride and 50 μL of ferrozine solution were incubated for 10 min at 25°C. Absorbance was measured at 562 nm.
2.3.10. Phosphomolybdenum Assay (PA)
The total antioxidant ability (TCA) was determined by the PA method described in Jan et al. [28]. TAC dye solution was mixture of 0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate with 1:1:1 volume ratio. 40 μL extract and 260 μL dye solution were added to a 96-well plate and wrapped in aluminium foil. The incubation occurred in an oven under 90°C for 90 min. After the samples were cooled to room temperature, the absorbance of the mixture was measured at 765 nm. Trolox dissolves in ethanol (0–200 μg/mL) as a standard curve.
2.4. Identification and Characterization of Phenolic Compounds in Jackfruit by LC–ESI–QTOF–MS/MS
Identification and characterization of phenolic compounds in jackfruit samples were conducted using LC–ESI–QTOF–MS/MS analysis, following the methodology outlined in Suleria et al. [29, 30]. The instrument consists of an Agilent 1200 series HPLC system coupled with an Agilent 6520 Accurate-Mass QTOF LC/MS (Agilent Technologies, Santa Clara, CA, USA) and ESI. The separation was carried out by a Synergi Hydro-RP 80A, LC column 250 × 4.6 mm (Phenomenex, Torrance, CA, USA). The mobile phase was composed of 0.5% (v/v) acetic acid in water (A), and a mixture of acetonitrile, water and acetic acid in a 100: 99: 1, v/v/v ratio (B). The flow rate was set at 0.8 mL/min with 5 μL of injection volume. Mass spectra in the m/z ranged from 50 to 1300, and the peak identification was employed in both negative and positive modes. Nitrogen gas was maintained at a temperature of 300°C with a flow rate of 5 L/min, while sheath gas was heated to 250°C with a flow rate of 11 L/min, and the nebulizer gas pressure was regulated to 45 psi. The capillary and nozzle voltage were set at 3.5 kV and 500 V, respectively.
2.5. Quantification of Phenolic Compounds by HPLC–PDA
Quantification of targeted phenolic compounds in jackfruit was carried out using an Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) with a PDA, adhering to the method outlined in Zhong et al. [17]. The same column and operational conditions as those described in the LC–ESI–QTOF–MS/MS protocol were used, with the only variation being increased sample injection volume of 20 μL. Phenolic compounds were detected at three distinct wavelengths (280 nm, 320 nm and 370 nm) to cater to the diverse absorption properties of the compounds.
2.6. Statistical Analysis
All analyses were conducted in triplicate, with results reported as the mean ± standard deviation (n = 3). Statistical differences between sample means were assessed using one-way analysis of variance (ANOVA), followed by Tukey’s honestly significant difference (HSD) test at a significance level of p < 0.05. Pearson’s correlation coefficient (at p < 0.05) and multivariate statistical analysis conducted by XLSTAT (2019.1.3, Addinsoft Inc. New York, NY, USA) were used to establish correlations between polyphenol content and antioxidant activities.
3. Results and Discussion
3.1. Estimation of Phenolic Compounds (TPC, TFC and TTC)
This investigation assessed the polyphenol content in extracts from various jackfruit fractions. The analysis of phenolic content in both JR and JUR, using conventional and ultrasonication extraction methods, revealed significant variations (p < 0.05) in their phenolic content. Ripe jackfruit displayed higher phenolic contents compared to their unripe counterparts. Among the different jackfruit fractions, the peel showed the highest phenolic content, followed by the core, seed and fruit. Notably, samples extracted using ultrasonication consistently displayed higher phenolic contents and antioxidant activity than those extracted conventionally. TPC contents ranged from 56.90 ± 1.55 mg GAE/g to 15.63 ± 0.68 mg GAE/g in ripened jackfruit, whereas 26.27 ± 1.61 mg GAE/g to 0.84 ± 0.05 mg GAE/g in unripe sample. Likewise, the recorded TPC contents in peel were 56.90 ± 1.55 mg GAE/g followed by core (18.76 ± 0.06 mg GAE/g), seed (17.91 ± 0.63 mg GAE/g) and fruit (15.63 ± 0.68 mg GAE/g). Additionally, commercial jackfruit samples exhibited very low TPC contents (0.36 ± 0.02 mg GAE/g).
The TFC results depicted a range of values, with ripe jackfruit samples presenting varied levels compared to unripe samples. Convention–extraction jackfruit fractions consistently exhibited higher TFC than their ultrasound-extracted counterparts. For instance, in the ripe jackfruit, TFC ranged from 0.78 ± 0.05 mg QE/g to 0.02 ± 0.06 mg QE/g, with the peel displaying the highest TFC, followed by the core and seed. TTC values displayed distinct patterns among different jackfruit fractions and ripening stages. Ripe jackfruit samples, particularly those extracted through ultrasonication, exhibited higher TTC compared to unripe samples as 85.04 ± 3.67, 18.05 ± 2.84, 24.00 ± 1.32 and 15.63 ± 0.68 mg CAE/g, respectively, in peel, core, seed and fruit (Table 1).
| Sample | TPC (mg GAE/g) | TFC (mg QE/g) | TCT (mg CE/g) | DPPH (mg TE/g) | ABTS (mg AAE/g) | FRAP (mg TE/g) | RPA (mg TE/g) | OH (mg AAE/g) | FICA (mg EDTA/g) | TAC (mg TE/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ultrasonication extraction | ||||||||||
| JRS | 17.91 ± 0.63aCD | 0.02 ± 0.06bCD | 19.47 ± 1.57aC | 27.73 ± 1.16aC | 45.01 ± 3.37aC | 66.50 ± 3.39aBC | 18.05 ± 2.84aCD | 24.00 ± 1.32aC | 2.88 ± 0.10bB | 3.54 ± 0.92aBC |
| JRC | 18.76 ± 0.06aC | — | 13.45 ± 1.82bD | 28.69 ± 2.53bBC | 44.82 ± 1.36bC | 76.04 ± 2.47aB | 25.35 ± 3.54bC | 19.00 ± 0.54aD | 2.16 ± 0.08bCD | 7.02 ± 0.43aA |
| JRP | 56.90 ± 1.55aA | 0.78 ± 0.05aA | 85.04 ± 3.67aA | 66.46 ± 1.65aA | 87.93 ± 7.54aA | 120.26 ± 8.51bA | 69.30 ± 6.71aA | 3.89 ± 0.30aF | 3.80 ± 0.49bA | 3.74 ± 0.32bB |
| JRF | 15.63 ± 0.68bD | — | 17.20 ± 1.08aCD | 32.38 ± 0.48aB | 52.13 ± 3.72aC | 61.86 ± 0.55aC | 11.61 ± 0.08bDE | 19.56 ± 1.76aD | 2.01 ± 0.10bD | — |
| JURS | 0.84 ± 0.05bG | — | — | 2.62 ± 0.16bF | 4.90 ± 0.31bF | 0.54 ± 0.05bG | — | 51.60 ± 0.57bB | 2.72 ± 0.31bBC | 1.20 ± 0.05bCD |
| JURC | 6.62 ± 0.22bF | 0.15 ± 0.12bBC | 33.61 ± 2.34aB | 18.09 ± 0.52aE | 18.37 ± 0.15bE | 15.99 ± 1.01bF | 8.63 ± 0.22bE | 55.43 ± 0.37aA | 2.05 ± 0.20bD | 1.91 ± 0.05bBCD |
| JURP | 26.27 ± 1.61aB | 0.29 ± 0.2aB | 33.35 ± 1.77aB | 31.75 ± 0.86bB | 66.64 ± 5.39bB | 41.82 ± 3.66bD | 35.46 ± 3.35aB | 14.99 ± 1.50aE | 2.02 ± 0.16bD | 2.32 ± 0.19aBCD |
| JURF | 12.05 ± 0.46bE | — | 3.09 ± 0.63E | 22.85 ± 2.12aD | 32.64 ± 1.38bD | 27.06 ± 0.08bE | 4.49 ± 0.27bEF | 23.22 ± 1.47aC | 1.69 ± 0.17aD | 1.43 ± 0.16BCD |
| JCF | 0.36 ± 0.02aG | 0.11 ± 0.09aBC | — | 0.68 ± 0.03aF | 0.54 ± 0.00bF | 0.45 ± 0.04aG | — | 1.26 ± 0.05aF | 0.56 ± 0.02aE | 0.8 ± 0.06aD |
| Conventional extraction | ||||||||||
| JRS | 15.41 ± 0.2bE | 0.34 ± 0.04aBC | 11.13 ± 0.82bCD | 24.96 ± 0.91bDE | 39.46 ± 0.50bD | 36.87 ± 2.74bC | 22.53 ± 1.19aD | 25.24 ± 0.14aB | 4.35 ± 0.31aB | 4.42 ± 0.99aC |
| JRC | 18.89 ± 0.19aD | 0.26 ± 0.08BC | 17.51 ± 1.62aBC | 61.91 ± 1.2aB | 49.64 ± 2.62aC | 35.70 ± 2.27bC | 37.31 ± 3.10aC | 14.24 ± 0.85bE | 8.64 ± 0.38aA | 6.51 ± 0.68aB |
| JRP | 49.29 ± 1.83bA | 0.75 ± 0.24aA | 73.61 ± 7.9aA | 69.51 ± 2.9aA | 94.30 ± 6.86aA | 138.90 ± 3.34aA | 71.56 ± 1.55aA | 3.87 ± 0.52aF | 8.00 ± 0.70aA | 13.70 ± 0.71aA |
| JRF | 22.60 ± 1.72aC | 0.09 ± 0.06CD | 13.05 ± 1.75bCD | 28.48 ± 2.13bD | 25.34 ± 1.65bE | 36.81 ± 2.48bC | 27.79 ± 0.73aC | 17.14 ± 0.40aD | 4.38 ± 0.25aB | 2.50 ± 0.29DE |
| JURS | 2.58 ± 0.11aF | — | 5.29 ± 0.47DE | 12.46 ± 0.17aF | 6.96 ± 0.19aF | 5.23 ± 0.10aD | 1.16 ± 0.25F | 53.59 ± 0.15aA | 3.69 ± 0.02aB | 13.14 ± 0.66aA |
| JURC | 13.65 ± 0.07aE | 0.5 ± 0.12aAB | 8.35 ± 1.06bD | 15.64 ± 0.68bF | 31.92 ± 0.33aE | 32.26 ± 2.67aC | 17.76 ± 1.24aE | 20.47 ± 0.81bC | 3.89 ± 0.26aB | 3.02 ± 0.22aCD |
| JURP | 26.27 ± 1.37aB | 0.74 ± 0.26aA | 21.75 ± 2.02bB | 35.03 ± 1.38aC | 75.81 ± 1.12aB | 94.77 ± 5.09aB | 41.41 ± 2.93aB | 4.04 ± 0.53bF | 8.23 ± 0.79aA | 1.30 ± 0.37bEF |
| JURF | 13.52 ± 0.24aE | 0.26 ± 0.02BC | — | 21.26 ± 1.52aE | 40.25 ± 0.75aD | 31.86 ± b1.05aC | 15.13 ± 0.58aE | 21.39 ± 0.89aC | 1.95 ± 0.18aC | — |
| JCF | 0.40 ± 0.04aF | 0.23 ± 0.01aBC | — | 0.39 ± 0.05bG | 0.65 ± 0.02aF | 0.45 ± 0.04aD | — | 1.43 ± 0.13aG | 0.16 ± 0.00bD | 0.80 ± 0.02aF |
- Note: Values with different letters (a, b) indicate significant differences between two different extractions within the same part of jackfruit. Values with different capital letters (A–G) along the row indicate significant statistical differences (p < 0.05) using one-way analysis of variance (ANOVA) and Tukey’s test between different parts of jackfruit with the same extraction.
- Abbreviations: JCF, jackfruit commercial fruit; JRC, jackfruit ripe core; JRF, jackfruit ripe fruit; JRP, jackfruit ripe peel; JRS, jackfruit ripe seed; JURC, jackfruit unripe core; JURF, jackfruit unripe fruit; JURP, jackfruit unripe peel; JURS, jackfruit unripe seed.
- ∗Values represented as mean ± standard deviation (n = 3).
Chavez-Santiago et al. [11] compared the TPC and TFC contents of jackfruit at two different ripening stages, finding higher values in ripened jackfruit, with TPC 1178 and 844 mg EAG/100 g and TFC of 68 and 37 mg QE/100 g in ripe and unripe samples, respectively. Likewise, Shafiq et al. [31] assessed TPC in jackfruit pulp and revealed a TPC of 239.87 mg GAE/100 g and TFC of 109.04 QE/100 g in methanolic extracts. Variations in TPC values across studies may be attributed to the intricate nature of polyphenols, the method of extraction employed, and various intrinsic and extrinsic factors, as discussed by [32].
Phenolic compounds in fruits, including phenolic acids, flavonoids and tannins, contribute to colour, flavour and antioxidant activities. But their content decreases as fruits mature due to chemical and enzymatic alterations, including glycoside hydrolysis, polyphenol oxidation and free phenol polymerization [33].
The higher phenolic content observed in jackfruit peel in this study, compared to the fruit, seed and core, may be due to the peel’s exposure to external environmental factors, rendering it more susceptible to abiotic stress. Conditions such as sunlight, ultraviolet radiation, high temperature, insect activity and desiccation are known to potentially stimulate the synthesis and accumulation of polyphenols [34]. Similar findings have been reported in the peels of other fruits. Zhang et al. [33] evaluated the total phenolics and total flavonoids and concluded that the peel demonstrated the highest values. Specifically, the phenolic content in the peel was found to be 4.65, 4.12 and 4.95 times higher than that of the pulp, flake and seed extracts, respectively. Similarly, Daud et al. [35] also observed the higher phenolics and flavonoids activity in jackfruit rind as compared to other fractions. The higher phenolics yield through ultrasound extraction is corroborated by the Cheng et al. [36], who investigated the effect of different extraction techniques and processing parameters on the phenolics and antioxidant profile of jackfruit pulp. They observed significant variations among the TPC due to extraction solvent and module. They found that ultrasound and microwave-assisted extraction resulted in a higher yield compared to conventional extraction methods. They also narrated that the mixture of organic solvent and water was more effective as compared to organic solvent alone and ethanol with water (60:40) considered as best solvent for extraction owing to its low toxicity.
Bioactive compounds are amassed within vesicles situated within plant cells, enveloped by sturdy cell walls. Ultrasound induces pressure changes that cause microscopic bubbles to form and collapse, creating tiny shockwaves. This phenomenon, known as cavitation, disrupts the plant cell structure and enhances the release of matrix from intracellular vesicles. Moreover, this facilitates improved penetration of the extracting solvent into the matrix, leading to an increased extraction yield of the desired compounds [37]. Earlier, Naeem et al. [38] observed higher phenolics with ultrasound extraction in different fruit and vegetable peels, delineating that green extraction technologies proved effectual as compared to conventional extraction module and extraction module and processing parameter played a pivotal role in the recovery of phenolics from plant matrices. The findings of this study align with trends observed in research involving alcoholic extractions. Primary alcohols, particularly methanol and ethanol, are effective solvents for phytochemicals due to their polarity [33].
The higher phenolic contents and antioxidant activity observed in ultrasound extraction compared to conventional methods. As ultrasonics wave causes the food change in internal structure [39], the potential mechanisms are fragmentation, erosion, capillarity, detexturation and sonoporation which could be achieved by cell disruption and mass transformation facilitation [40]. Another attribution is cavitation phenomenon, which could increase the bioactive compounds contact with solvent, causing less destruction and in thus more effective for higher extraction yield [41]. Jackfruit is a climacteric fruit, and ethylene biosynthesis increases during the mature period [42]. Meanwhile, the gaseous phytohormone ethylene (ETH) is a regulator in phenolic biosynthesis, which is confirmed by a research based on the Cabernet Sauvignon grapes [43]. The ETH promoted the biosynthesis for anthocyanin and nonanthocyanin, while it has the synergy effect with light expose. In addition, the ethylene production in jackfruit might increase the content of ascorbic acid content and high activity of cell wall hydrolases [44]. In sum, the changes in the phenolics concentration in jackfruit during different maturity stages may be due to chemical and enzymatic changes in the fruit, which result in hydrolysis of glycosides, polymerization of free phenols and oxidation of polyphenols.
3.2. Antioxidant Activity
Given the complexity of mechanisms within plant matrices, a mixture of different tests is often applied to comprehensively assess the antioxidant capacity of the tested plant. In the present study, ABTS, DPPH, FRAP, RPA, FICA, ⋅OH-RSA and TAC assays were adapted to estimate the antioxidant potential of different jackfruit samples and their fractions [45].
As indicated in Table 1, there was a significant difference in the DPPH values between JR and JUR samples. The highest DPPH was observed in ripe sample (69.51 ± 2.9 mg TE/g), the highest in unripe is 35.03 ± 1.38 mg TE/g, and the least was observed in the commercial jackfruit sample as 0.39 ± 0.05 mg TE/g. Among the different fractions, the peel exhibited highest value, with the order of activity being peel > core > seed > fruit. Similarly, samples extracted using ultrasonication showed higher activity compared to those extracted conventionally. The ABTS radical scavenging activity followed a similar trend, with ripe jackfruit samples, particularly those subjected to ultrasonication, displaying higher activity. The peel consistently exhibited the highest ABTS scavenging activity among jackfruit fractions, followed by the core, seed and fruit. Ultrasonication consistently enhanced ABTS scavenging activity compared to conventional extraction. The recorded values for ABTS in the seed, core, peel and fruit of ripe jackfruit were 45.01 ± 3.37, 44.82 ± 1.36, 87.93 ± 7.54 and 52.13 ± 3.72 (mg AAE/g), respectively. The FRAP assay results indicated variations in the reducing power of different jackfruit fractions and ripening stages. A similar trend was observed for FRAP, and highest activity was noted in the peel of ripe jackfruit sample extracted through ultrasound (120.26 ± 8.51 mg TE/g) and lowest in the commercial jackfruit sample (0.45 ± 0.04 mg TE/g). Similar trends were observed in other assays, including RPA, OH, FICA and TAC, where ripe jackfruit samples and those extracted via ultrasonication consistently yielded higher values than unripe samples and those obtained through conventional extraction. The peel generally showed the highest values, followed by the core, seed and fruit. Notably, commercial jackfruit samples exhibited lower values across these assays (Table 1).
Evaluation of antioxidant activity in natural compounds demands a comprehensive approach, as antioxidant mechanisms operate through various pathways, including repairing biological damage, sequestering transition metal ions and scavenging free radicals. To gain a comprehensive understanding of the antioxidant capacity, it is crucial to employ multiple methods. Factors such as solvent choice, temperature, the chemical structure of phenolic compounds and pH can significantly affect antioxidant mechanisms, potentially influencing the precision of activity estimates. Acknowledging this complexity, a combination of methods was utilized to assess the antioxidant activity of the samples [45].
DPPH is a widely used free radical scavenger employed in antioxidant assays. Its colour changes from purple to yellow allow for the quantitative measurement of antioxidant activity in various substances. The DPPH activity record for jackfruit in this study was in line with the earlier findings of Shafiq et al. [31], who observed DPPH value of jackfruit in the range of 17–89 mg TE/g. Their finding highlighted that the antioxidant activity depended on the solvents used, and a mixture of solvents proved more effective than a single, pure solvent. Jackfruit contains a variety of bioactive compounds, including flavonoids and phenolic acids, which possess potent free radical scavenging properties. These compounds found in jackfruit neutralize reactive oxygen species, thereby protecting cellular structures and biomolecules from oxidative damage [46].
Likewise, Jagtap and Bapat [47] conducted an in vitro analysis of the antioxidant activity of jackfruit fruit pulp and found the methanolic extract displayed promising DPPH radical scavenging activity with an IC50 of 0.4 mg/mL in dose-dependent manner. The higher DPPH activity observed in the current study is also supported by Zhang et al. [33], who observed a higher DPPH activity in the peel compared to the seed, pulp and flakes. In a separate study, Calderón-Chiu et al. [48] observed notable DPPH and ABTS radical scavenging activities in A. heterophyllus leaf protein. Interestingly, when hydrolysed with pancreatin and pepsin, the leaf protein concentrate exhibited varying radical quenching activities, with pancreatin hydrolysis showing superior effects. The differences were attributed to peptide length and amino acid configurations, suggesting that hydrolysates with smaller peptides from higher degrees of hydrolysis could possess significant antioxidant properties.
The FRAP assay measures a substance’s ability to reduce Fe3+ to Fe2+ ions in the presence of a reagent. This reduction leads to a change in colour, and the degree of colour change is proportional to the reducing power, reflecting the antioxidant capacity of the sample. FRAP and other antioxidant tests are crucial for assessing the overall antioxidant potential of fruits. It provides valuable insights into the concentration of reducing agents, such as vitamin C and polyphenols, which help protecting cells from oxidative stress. Therefore, these various assays are instrumental in understanding and comparing the antioxidant contributions of different fruits, aiding in nutritional assessments and the development of health-promoting food products [49]. Chavez-Santiago et al. [11] observed higher ORAC and FRAP values in ripe jackfruit as compared to the unripe sample, in line with the current study findings. In addition, based on the results from Morelos-Flores et al. [50], which explore the antioxidant ability of different storage periods with different genotype jackfruit pulp from Mexico by DPPH, ABTS and FRAP. The range of DPPH, ABTS and FRAP is from 2.67 ± 1.26 to 11.74 ± 0.2, 0.60 ± 0.33 to 10.43 ± 0.36 and 3.99 ± 0.09 to 10.21 ± 0.86 mmol TE/g DW, respectively. The genotype named ‘Agüitada’ jackfruit showed an increase in DPPH, FRAP and ABTS scavenger ability during the 11 days of storage, while ‘Licenciada’ showed the highest ABTS scavenger ability at Day 11, which was 10.43 ± 0.36 mmol TE/g DW. The potential reason might be the highest concentration of shikimic acid. In another research conducted by the same group [51], three assays were used to determine the antioxidant capacity of 4 genotype jackfruits for total soluble phenols (TSP) and total carotenoids (TC). ‘Licenciada’ exhibited the highest value in DPPH within TSP and TC, which were 11.975 and 4.01 μmol TE/g DW, respectively. The genotype jackfruit also showed the highest value, 3.04 μmol TE/g DW, in the ABTS method. In sum, this genotype jackfruit has the greatest amount of phytochemicals for antioxidant ability, which has the potential for commercialization. The FICA is to determine the chelate ferrous ions ability of extracts. The previous research explores the chelate ferrous ions ability for jackfruit leaves [52], with the value of 0.09 ± 0.01 μmol EDTA equivalent. This is significantly lower than other parts of jackfruit that were conducted in this project, with the range from 1.69 ± 0.17 to 8.64 ± 0.38 mg EDTA/g DW.
3.3. Correlation of Polyphenols and Antioxidant Activities
Pearson’s correlation analysis was carried out to explore the correlation between polyphenols and antioxidant capacity for both ultrasound and conventional extraction (Table 2). For ultrasound extraction, TPC shows a strong correlation (p < 0.05) with TFC, DPPH, ABTS, FRAP, RPA and FICA. A similar trend was exhibited in the conventional extraction. TFC has a significant correlation with TCT and RPA in both ultrasound and conventional extraction. However, it is notable that TFC and DPPH have a correlation only for ultrasound, while TFC showed a significant correlation with ABTS and FRAP conventional extraction. This might ascribe to the low concentration of TFC by ultrasonication extracts. The correlation of TCT also lied parallel tendency in two extraction way, strong correlation for DPPH, ABTS, FRAP and RPA. But TCT only has a significant correlation with FICA (r = 0.691) in ultrasonication extraction. DPPH, ABTS, FRAP, RPA and FICA exhibited strong intercorrelations (p < 0.05). The r is almost larger than 0.75. OH is generally negatively correlated with all assays, with two exceptions, OH with FICA (0.134) in ultrasound and OH with TAC (0.396) in conventional. Additionally, TAC showed generally weak positive correlations with other assays, indicating its lower association with the other antioxidant measurements.
| Variables | TPC | TFC | TCT | DPPH | ABTS | FRAP | RPA | OH | FICA |
|---|---|---|---|---|---|---|---|---|---|
| Ultrasonication extraction | |||||||||
| TFC | 0.856∗∗ | ||||||||
| TCT | 0.905∗∗ | 0.929∗∗ | |||||||
| DPPH | 0.968∗∗ | 0.751∗ | 0.876∗∗ | ||||||
| ABTS | 0.931∗∗ | 0.662 | 0.782∗ | 0.954∗∗ | |||||
| FRAP | 0.898∗∗ | 0.594 | 0.745∗ | 0.935∗∗ | 0.894∗∗ | ||||
| RPA | 0.98∗∗ | 0.881∗∗ | 0.917∗∗ | 0.92∗∗ | 0.896∗∗ | 0.86∗∗ | |||
| OH | −0.498 | −0.408 | −0.259 | −0.424 | −0.476 | −0.478 | −0.472 | ||
| FICA | 0.704∗ | 0.545 | 0.691∗ | 0.697∗ | 0.622 | 0.691∗ | 0.685∗ | 0.134 | |
| TAC | 0.419 | 0.172 | 0.283 | 0.396 | 0.384 | 0.578 | 0.494 | −0.159 | 0.385 |
| Conventional extraction | |||||||||
| TFC | 0.716∗ | ||||||||
| TCT | 0.924∗∗ | 0.671∗ | |||||||
| DPPH | 0.845∗∗ | 0.496 | 0.807∗∗ | ||||||
| ABTS | 0.915∗∗ | 0.846∗∗ | 0.817∗∗ | 0.829∗∗ | |||||
| FRAP | 0.955∗∗ | 0.849∗∗ | 0.908∗∗ | 0.759∗ | 0.954∗∗ | ||||
| RPA | 0.979∗∗ | 0.735∗ | 0.915∗∗ | 0.914∗∗ | 0.94∗∗ | 0.943∗∗ | |||
| OH | −0.473 | −0.627 | −0.391 | −0.341 | −0.480 | −0.500 | −0.510 | ||
| FICA | 0.729∗ | 0.558 | 0.665 | 0.873∗ | 0.801∗∗ | 0.721∗ | 0.825∗∗ | −0.228 | |
| TAC | 0.358 | 0.034 | 0.612 | 0.466 | 0.245 | 0.332 | 0.364 | 0.396 | 0.405 |
- ∗Represents significant correlation at p < 0.05.
- ∗∗Represents a highly significant correlation at p < 0.01.
3.4. Distribution of Phenolic Compounds
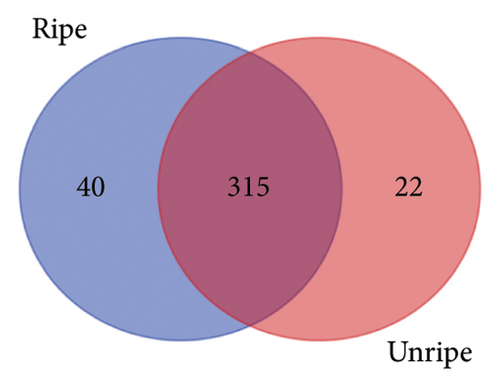
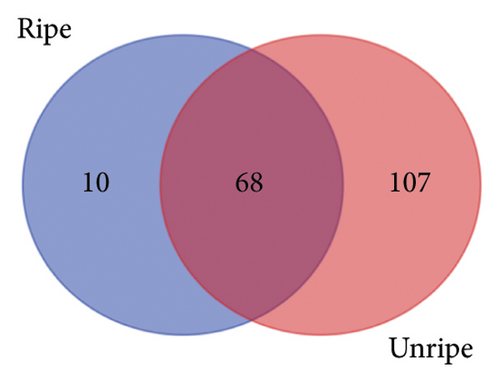
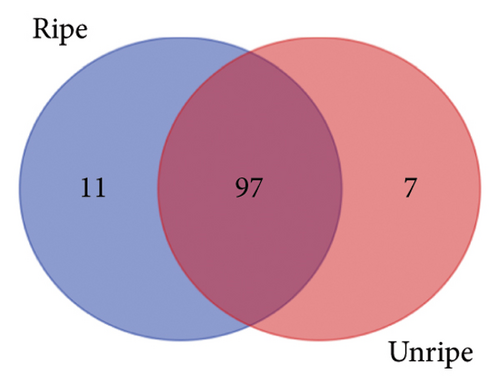
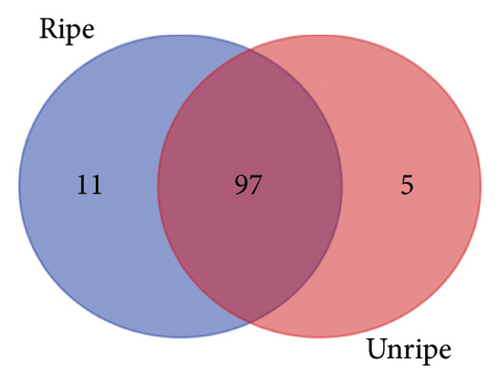
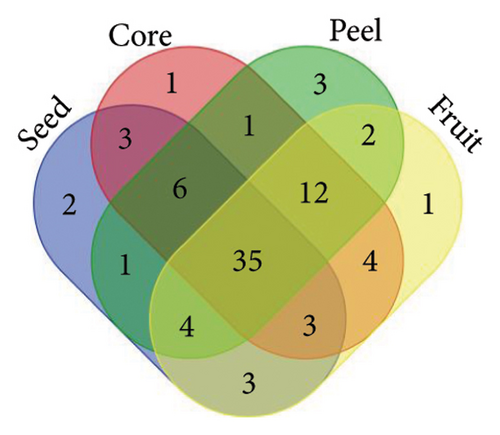
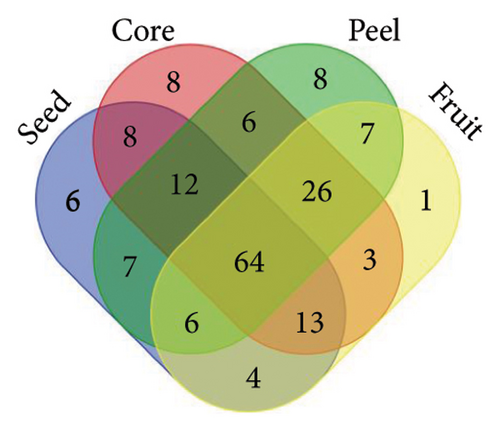
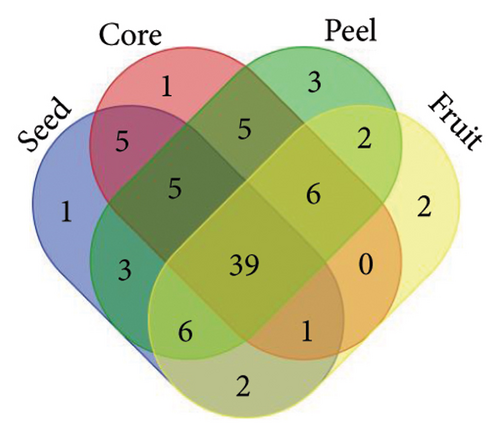
From the analysis of JR and JUR samples, a total of 377 compounds were identified (Figure 1(a)), with a majority of the polyphenols (83.55%) being common across all samples. Ripe jackfruit samples exhibited a higher percentage of total phenolic compounds (94.16%) compared to unripe samples (89.3%). Out of the 185 phenolic acids identified, 36.7% were common across all jackfruit samples (Figure 1(b)). Interestingly, JUR samples contained a higher proportion of phenolic acids (57.83%) compared to ripe samples (5.40%). Regarding flavonoids, 6.08% were unique to the JUR profile, while 93.91% were found in the ripe samples, as shown in Figure 1(c). Likewise, 113 other polyphenols were detected and 85.84% were found common in both samples (Figure 1(d)). Furthermore, ripe samples had a higher quantity of polyphenols compared to unripe ones.
A similar observation was noted in Figure 2, where various jackfruit fractions (seed, core, peel and fruit) exhibited a diversity in total phenolics, phenolic acids, flavonoids and other polyphenols, with the majority being common across all fractions. However, the peel exhibited higher concentration as compared to other fractions. The trend regarding the higher polyphenols in peels is aligned with earlier findings of Zhang et al. [33], who observed a higher phenolic concentration in the peel of jackfruit in comparison with other fractions. The accumulation of phenolics in the peel is a result of evolutionary adaptations to environmental challenges and a strategy to enhance the overall defence mechanisms of the plant. Daud et al. [35] also observed an abundance of polyphenols in peel of jackfruit as compared to other parts. One possible explanation for the lower concentration of phenolics in unripe samples is that their levels change during different fruit maturity stages, and concentration of other phytochemicals might be higher in the early stages of maturity.

3.5. LC–MS Characterization
Through LC–ESI–QTOF–MS/MS technique, a total of 65 phenolic compounds were tentatively identified in various fractions of both JR and JUR. Details about the screening of these phenolic compounds are summarized in Table 3. The detected compounds were classified as phenolic acids, flavonoids, lignans, stilbenes and other polyphenols.
| No. | Proposed compounds | Molecular formula | RT (min) | Ionization (ESI+/ESI−) | Molecular weight | Theoretical (m/z) | Observed (m/z) | Error (ppm) | MS2 product ions | Jackfruit by-products |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acid | ||||||||||
| Hydroxybenzoic acids | ||||||||||
| 1 | 4-O-Methylgallic acid | C8H8O5 | 27.750 | [M+H]+ | 184.0386 | 185.0459 | 185.0460 | 0.5 | 170, 142 | JCF-C |
| 2 | Ellagic acid acetyl-arabinoside | C21H16O13 | 40.053 | [M-H]− | 476.057 | 475.0497 | 475.0496 | −0.2 | 300 | JURC-U |
| Hydroxycinnamic acids | ||||||||||
| 3 | 1-Sinapoyl-2-feruloylgentiobiose | C33H40O18 | 11.049 | ∗∗[M-H]− | 724.2212 | 723.2139 | 723.2111 | −3.9 | 529, 499 | ∗∗JURC-C, JURC-U, JRF-C, JRS-U |
| 4 | 5-5′-Dehydrodiferulic acid | C20H18O8 | 27.111 | ∗∗[M-H]− | 386.0971 | 385.0898 | 385.0905 | 1.8 | 369 | ∗∗JRS-U, JURC-U, JRC-C, JCF-C, JRF-U, JRF-C |
| 5 | Ferulic acid 4-O-glucuronide | C16H18O10 | 34.99 | [M+H]+ | 370.0882 | 371.0955 | 371.0959 | 1.1 | 193 | ∗JRS-U, JRF-C, JCF-C, JRC-U, JCF-U |
| 6 | p-Coumaroyl tyrosine | C18H17NO5 | 56.57 | [M+H]+ | 327.1094 | 328.1167 | 328.1169 | 0.6 | 282 | JURS-U |
| 7 | Rosmarinic acid | C18H16O8 | 59.49 | [M+H]+ | 360.0822 | 361.0895 | 361.0902 | 1.9 | 179 | ∗JRP-U, JRF-C |
| Flavonoids | ||||||||||
| Flavanols | ||||||||||
| 8 | Procyanidin trimer C1 | C45H38O18 | 38.359 | ∗∗[M-H]− | 866.2081 | 865.2008 | 865.1983 | −2.9 | 739, 713, 695 | ∗∗JURF-U, JRS-C |
| 9 | (−)-Epicatechin | C15H14O6 | 34.508 | ∗∗[M-H]− | 290.0779 | 289.0706 | 289.0702 | −1.4 | 289, 169 | ∗∗JURC-U, JRP-C, JURF-U, JURC-C |
| 10 | Procyanidin dimer B1 | C30H26O12 | 48.235 | ∗∗[M-H]− | 578.1412 | 577.1339 | 577.1348 | 1.6 | 451 | ∗∗JRP-C, JURS-C, JRF-C, JURP-U, JURC-C, JURF-C, JURF-U |
| Flavones | ||||||||||
| 11 | Apigenin 7-O-apiosylglucoside | C26H28O14 | 59.38 | [M+H]+ | 564.1461 | 565.1534 | 565.1536 | 0.4 | 296 | ∗JCF-C, JURS-U |
| 12 | Gardenin B | C19H18O7 | 62.38 | [M+H]+ | 358.1044 | 359.1117 | 359.1119 | 0.6 | 344, 329, 311 | ∗JRP-U, JURF-U, JURP-C, JURP-U, JRP-C |
| 13 | Chrysoeriol 7-O-(6″-malonyl-apiosyl-glucoside) | C30H32O18 | 60.24 | [M+H]+ | 680.1579 | 681.1652 | 681.1659 | 1.0 | 445, 427, 409, 381 | JURP-U |
| 14 | Rhoifolin | C27H30O14 | 49.518 | ∗∗[M-H]− | 578.1635 | 577.1562 | 577.1569 | 1.2 | 413, 269 | ∗JURS-C, JURC-C, JCF-C, JRF-C, JURC-U, JRC-U, JRF-U, JURS-U, JRS-U, JURF-C, JRP-C, JURP-U |
| 15 | 3-Methoxysinensetin | C21H22O8 | 67.66 | [M+H]+ | 402.1328 | 403.1401 | 403.1407 | 1.5 | 388, 373, 355, 327 | ∗∗JRP-C, JRC-C, JRC-U, JRF-U, JURF-U, JURP-U, JRF-C, JURP-C, JURF-C, JRP-U |
| 16 | Luteolin 7-O-(2-apiosyl-glucoside) | C26H28O15 | 45.406 | ∗∗[M-H]− | 580.1411 | 579.1338 | 579.1352 | 2.4 | 301 | ∗∗JRP-U, JURC-U, JURP-C, JURP-U |
| 17 | Cirsilineol | C18H16O7 | 60.87 | [M+H]+ | 344.0894 | 345.0967 | 345.098 | 3.8 | 330, 312, 297, 284 | ∗JCF-U, JCF-C |
| 18 | 3′-Hydroxygenistein | C15H10O6 | 11.67 | [M+H]+ | 286.0463 | 287.0536 | 287.055 | 4.9 | 269, 259 | ∗JURP-U, JURC-U, JRC-C, JURP-C, JRF-C, JRF-U, JRP-U, JRP-C, JURF-C, JRS-C, JURF-U, JCF-U, JRS-U |
| Flavanones | ||||||||||
| 19 | Narirutin | C27H32O14 | 48.011 | ∗∗[M-H]− | 580.1823 | 579.175 | 579.1737 | −2.2 | 271 | ∗∗JRF-C, JRS-U, JURF-C, JRC-C, JURF-U, JRF-U, JURC-C, JURP-U, JURC-U, JRF-U, JRP-U, JURP-C, JRS-C, JRP-C |
| 20 | Hesperetin 3′-sulphate | C16H14O9S | 26.035 | [M-H]− | 382.0368 | 381.0295 | 381.0296 | 0.3 | 301, 286, 257, 242 | ∗JCF-C, JURF-U, JRP-C, JURC-U, JCF-U, JURP-C, JRC-U, JURS-C, JURF-C |
| 21 | Dihydrobiochanin A | C16H14O5 | 43.532 | ∗∗[M-H]− | 286.0855 | 285.0782 | 285.0784 | 0.7 | 269, 203, 201, 175 | ∗∗JRC-U, JURC-U, JRP-U, JURP-C, JRS-U, JRS-C |
| 22 | 6-Prenylnaringenin | C20H20O5 | 31.616 | ∗∗[M-H]− | 340.1298 | 339.1225 | 339.1229 | 1.2 | 323, 137 | ∗∗JRF-C, JRP-C, JURP-U, JURP-C, JRF-U, JRP-U, JRC-U, JURF-U, JRC-C, JURF-C, JURC-U, JRS-C, JURC-C |
| 23 | Hesperidin | C28H34O15 | 57.1 | [M+H]+ | 610.1889 | 611.1962 | 611.1972 | 1.6 | 593, 465, 449, 303 | ∗JURP-U, JRC-U |
| 24 | Eriocitrin | C27H32O15 | 55.8 | [M+H]+ | 596.1752 | 597.1825 | 597.1842 | 2.8 | 431, 287 | ∗JRP-U, JRF-U, JRC-C, JRP-C, JURP-U, JURF-C, JRS-U, JRS-C, JRF-C, JURS-C |
| 25 | Hesperetin 3′-O-glucuronide | C22H22O12 | 56.87 | [M+H]+ | 478.1106 | 479.1179 | 479.1193 | 2.9 | 301, 175, 113, 85 | ∗JRF-U, JURS-U, JURC-U, JURP-U, JRS-U, JURF-C, JRC-C, JURS-C |
| Flavonols | ||||||||||
| 26 | Quercetin 3-O-(6″-malonyl-glucoside) | C30H32O20 | 62.856 | [M-H]− | 712.1504 | 711.1431 | 711.1399 | −4.5 | 303 | ∗JRP-C, JRS-C |
| 27 | Kaempferol 3-O-glucosyl-rhamnosyl-galactoside | C33H40O20 | 8.359 | [M-H]− | 756.2143 | 755.207 | 755.2045 | −3.3 | 285 | ∗JRC-C, JURC-U |
| 28 | Quercetin 3′-O-glucuronide | C21H18O13 | 43.393 | ∗∗[M-H]− | 478.0795 | 477.0722 | 477.0709 | −2.7 | 301 | ∗∗JCF-C, JURS-U |
| 29 | 3-Methoxynobiletin | C22H24O9 | 62.1 | [M+H]+ | 432.1444 | 433.1517 | 433.1509 | −1.8 | 403, 385, 373, 345 | ∗JURP-U, JRP-C, JRC-U, JRP-U, JURP-C, JRC-C |
| 30 | Quercetin 3-O-(6″-malonyl-glucoside) | C24H22O15 | 11.07 | [M+H]+ | 550.0981 | 551.1054 | 551.1054 | 0.0 | 303 | ∗JURC-U, JCF-U, JURF-U, JRC-C, JCF-C, JURF-C, JRC-U, JURP-U, JURC-U, JRF-C, JRP-U, JURP-C, JRF-U, JURC-C |
| 31 | Myricetin 3-O-galactoside | C21H20O13 | 52.16 | [M-H]− | 480.0911 | 479.0838 | 479.0845 | 1.5 | 317 | ∗JRS-U, JURP-C, JCF-C |
| 32 | Quercetin 3-O-xylosyl-glucuronide | C26H26O17 | 10.04 | [M+H]+ | 610.1194 | 611.1267 | 611.1279 | 2.0 | 479, 303, 285, 239 | ∗JURS-C, JURC-U, JURF-U, JRF-C, JURP-U, JRP-U, JRS-C, JURF-C, JURS-U, JCF-U, JRC-C, JURC-C, JURP-C, JCF-C, JRC-U, JRF-U |
| 33 | Spinacetin 3-O-glucosyl-(1 ⟶ 6)-glucoside | C29H34O18 | 64.089 | [M-H]− | 670.1721 | 669.1648 | 669.1673 | 3.7 | 345, 330 | ∗JRP-U, JURP-C |
| 34 | Spinacetin 3-O-(2 | C43H48O24 | 65.871 | [M-H]− | 948.2486 | 947.2413 | 947.2456 | 4.5 | 741, 609, 301 | JRS-C |
| Dihydroflavonols | ||||||||||
| 35 | Dihydroquercetin | C15H12O7 | 59.36 | [M+H]+ | 304.0561 | 305.0634 | 305.0636 | 0.7 | 285, 275, 151 | ∗JURC-C, JRC-U, JURF-C, JRP-U, JURF-U, JRF-U, JURC-U, JRC-C |
| Anthocyanins | ||||||||||
| 36 | 4-O-Methyldelphinidin 3-O-D-glucoside | C22H23O12 | 56.29 | [M+H]+ | 479.1184 | 480.1257 | 480.1245 | −2.5 | 317, 303, 285, 271 | JRC-C |
| 37 | Cyanidin 3-O-(6″-p-coumaroyl-glucoside) | C30H27O13 | 55.8 | [M+H]+ | 595.1406 | 596.1479 | 596.1476 | −0.5 | 287 | ∗JURS-U, JURP-C |
| 38 | Isopeonidin 3-O-arabinoside | C21H21O10 | 36.28 | ∗∗[M-H]− | 433.1138 | 432.1065 | 432.1065 | 0.0 | 271, 253, 243 | ∗∗JRP-C, JRC-U, JRF-C, JRP-U, JURC-U |
| 39 | Cyanidin 3,5-O-diglucoside | C27H31O16 | 66.756 | ∗∗[M-H]− | 611.1609 | 610.1536 | 610.154 | 0.7 | 449, 287 | ∗∗JRS-U, JURP-U |
| 40 | Cyanidin 3-O-(6″-malonyl-3″-glucosyl-glucoside) | C30H33O19 | 67.823 | [M-H]− | 697.1618 | 696.1545 | 696.1556 | 1.6 | 271, 209, 393 | ∗JURF-U, JRF-C |
| 41 | Peonidin 3-O-sambubioside-5-O-glucoside | C33H41O20 | 54.58 | [M+H]+ | 757.2224 | 758.2297 | 758.2315 | 2.4 | 595, 449, 287 | ∗JURS-U, JRS-C, JRS-U, JURS-C, JRC-C |
| 42 | Delphinidin 3-O-glucosyl-glucoside | C27H31O17 | 46.05 | ∗∗[M-H]− | 627.154 | 626.1467 | 626.1483 | 2.6 | 465, 3030 | ∗∗JRC-U, JURS-C |
| 43 | Pelargonidin 3-O-rutinoside | C27H31O14 | 57.17 | [M+H]+ | 579.172 | 580.1793 | 580.1817 | 4.1 | 271, 433 | ∗JCF-C, JURP-C, JURC-U, JURC-C, JURF-U, JRC-C, JRC-U, JRF-U, JRP-C, JRP-U, JRS-C, JRS-U, JURP-U, JRF-C, JURF-C |
| Isoflavonoids | ||||||||||
| 44 | Tectorigenin 7-sulphate | C16H12O9 | 42.569 | [M-H]− | 380.0174 | 379.0101 | 379.0084 | −4.5 | 299 | JCF-C |
| 45 | Glycitin | C22H22O10 | 65.16 | [M+H]+ | 446.1218 | 447.1291 | 447.1287 | −0.9 | 285 | JRC-U |
| 46 | 5,6,7,3′,4′-pentahydroxyisoflavone | C15H10O7 | 62.65 | [M+H]+ | 302.042 | 303.0493 | 303.0494 | 0.3 | 285, 257 | ∗JRC-C, JURC-U, JURF-C, JRP-U, JRF-U, JRP-C, JURP-U |
| 47 | 2′-hydroxyformononetin | C16H12O5 | 67.28 | [M+H]+ | 284.0679 | 285.0752 | 285.0755 | 1.1 | 270, 229 | ∗JRP-C, JRF-C, JURF-C, JURF-U, JURP-U, JURC-U, JRC-C, JRF-U, JURP-C, JRP-U, |
| 48 | 3′-Hydroxydaidzein | C15H10O5 | 65.28 | [M+H]+ | 270.0544 | 271.0617 | 271.062 | 1.1 | 253, 241, 225 | ∗JRP-C, JURC-U, JRF-C, JURF-C, JRS-C, JRF-U, JURF-U, JCF-U, JRC-C, JRP-U, JURP-U, JURP-C, JRF-U |
| 49 | 6″-O-Malonylgenistin | C24H22O13 | 65.12 | [M+H]+ | 518.1045 | 519.1118 | 519.1125 | 1.3 | 271 | JRC-U |
| Other polyphenols | ||||||||||
| Hydroxycoumarins | ||||||||||
| 50 | Coumarin | C9H6O2 | 13 | [M+H]+ | 146.0367 | 147.044 | 147.044 | 0.0 | 103, 91 | ∗JURS-C, JURS-U, JURP-U, JURF-U, JRF-U, JURP-C, JRP-C, JRF-C, JRP-U, JURS-C |
| 51 | Scopoletin | C10H8O4 | 16.664 | ∗∗[M-H]− | 192.0417 | 191.0344 | 191.034 | −2.1 | 176 | ∗∗JURP-C, JRF-U, JRP-U |
| Hydroxybenzaldehydes | ||||||||||
| 52 | p-Anisaldehyde | C8H8O2 | 55.64 | [M+H]+ | 136.0537 | 137.061 | 137.0611 | 0.7 | 122, 109 | ∗JRC-U, JURC-U |
| Curcuminoids | ||||||||||
| 53 | Demethoxycurcumin | C20H18O5 | 38.414 | ∗∗[M-H]− | 338.1132 | 337.1059 | 337.1047 | −3.6 | 217 | ∗∗JRF-C, JRC-U, JURF-U, JURP-C, JRF-C, JRF-U, JURP-U, JURC-U, JCF-C, JRP-U, JRP-C, JRC-C, JURF-C, JCF-U |
| Furanocoumarins | ||||||||||
| 54 | Isopimpinellin | C13H10O5 | 56.54 | [M+H]+ | 246.0537 | 247.061 | 247.0612 | 0.8 | 232, 217, 205, 203 | JURC-U |
| Phenolic terpenes | ||||||||||
| 55 | Rosmanol | C20H26O5 | 61.86 | [M+H]+ | 346.1808 | 347.1881 | 347.1887 | 1.7 | 301, 241, 231 | ∗JRS-C, JURS-C |
| Tyrosols | ||||||||||
| 56 | Demethyloleuropein | C24H30O13 | 62.099 | ∗∗[M-H]− | 526.1687 | 525.1614 | 525.1616 | 0.4 | 495 | ∗∗JURS-C, JRF-C, JURC-C, JCF-U, JURC-U, JURP-C, JCF-C |
| Other polyphenols | ||||||||||
| 57 | Salvianolic acid C | C26H20O10 | 10.37 | [M+H]+ | 492.1074 | 493.1147 | 493.1137 | −2.0 | 311, 267, 249 | JRS-C |
| 58 | 4-Vinylphenol | C8H8O | 13.92 | [M+H]+ | 120.0573 | 121.0646 | 121.0647 | 0.8 | 119, 93, 91 | ∗JRC-C, JURC-U, JURP-U, JRP-C, JURP-C, JRS-C, JURS-C |
| 59 | Salvianolic acid B | C36H30O16 | 52.903 | [M-H]− | 718.1559 | 717.1486 | 717.1513 | 3.8 | 519, 339, 321, 295 | JRS-C |
| 60 | Salvianolic acid C | C26H20O10 | 52.903 | [M-H]− | 718.1559 | 717.1486 | 717.1513 | 3.8 | 311, 267, 249 | JRS-C |
| Lignans | ||||||||||
| 61 | Enterolactone | C18H18O4 | 57.03 | [M+H]+ | 298.1184 | 299.1257 | 299.1267 | 3.3 | 281, 187, 165 | ∗JRC-C, JURS-C |
| 62 | 7-Oxomatairesinol | C20H20O7 | 58.06 | [M+H]+ | 354.1118 | 355.1191 | 355.1183 | −2.3 | 358, 343, 328, 325 | ∗JRF-C, JURP-C, JRF-U, JRP-U, JRP-C, JURS-U, JURP-U, JRS-U, JURF-C, JRC-C, JRC-U |
| 63 | 7-Hydroxymatairesinol | C20H22O7 | 58.12 | [M+H]+ | 354.1119 | 355.1192 | 355.1184 | −2.3 | 343, 313, 298, 285 | ∗JURP-U, JRS-C, JRC-U, JURS-C, JURS-U |
| 64 | Schisandrin C | C22H24O6 | 56.61 | [M+H]+ | 354.1112 | 355.1185 | 355.1178 | −2.0 | 370, 315, 300 | ∗JURC-U, JURF-C, JURS-U, JURS-C, JURF-U, JURC-C, JRS-C, JRS-U, JURP-U |
| 65 | Schisandrin | C24H32O7 | 56.55 | [M+H]+ | 354.1127 | 355.12 | 355.1193 | −2.0 | 455 | ∗JURP-U, JURC-C, JRP-U, JRC-U, JCF-U, JURF-U, JURP-C, JRF-C |
- Note: Conventional extraction (-C) and ultrasound extraction (-U).
- Abbreviations: JCF, jackfruit commercial fruit; JRC, jackfruit ripe core; JRF, jackfruit ripe fruit; JRP, jackfruit ripe peel; JRS, jackfruit ripe seed; JURC, jackfruit unripe core; JURF, jackfruit unripe fruit; JURP, jackfruit unripe peel; JURS, jackfruit unripe seed.
- ∗Compound was detected in more than one jackfruit samples, and data presented in this table are from the asterisk sample.
- ∗∗Compounds were detected in both negative [M-H]− and positive [M+H]+ modes of ionization, while only single mode data were presented.
17 components were identified in both positive and negative ionization modes, including compounds such as 1-sinapoyl-2-feruloylgentiobiose (m/z 723.2111), 5-5′-dehydrodiferulic acid (m/z 385.0905), procyanidin trimer C1 (m/z 865.1983) and (−)-epicatechin (m/z 289.0702). Additionally, 37 compounds were exclusively identified in the positive ionization mode and 11 in the negative ionization mode. The primary component identified across various jackfruit samples was tentatively identified as narirutin (compound 19), characterized by the precursor ion [M-H]− at m/z 579.1737. A detailed description of the specific compounds identified in each group is provided below.
3.5.1. Phenolic Acids
In the analysis of jackfruit by-products, a total of seven phenolic compounds have been identified, which were classified into two categories: hydroxybenzoic acids and hydroxycinnamic acids. Among the hydroxybenzoic acids, compound 1, identified as 4-O-methylgallic acid (m/z 185.0460), was detected in the conventional extract of the fruit fraction from commercial jackfruit (JCF-C). Meanwhile, compound 2, ellagic acid acetyl-arabinoside (m/z 475.0496), was found in the core fraction of ultrasound-extracted unripe jackfruit (JURC-U). Within the hydroxycinnamic acid category, five compounds (numbered 3, 4, 5, 6 and 7) were identified. Among these, compound 4, identified as ferulic acid 4-O-glucuronide (m/z of 370.0882 [M+H]+), was present in JRS-U, JRF-C, JCF-C, JRC-U and JCF-U. In contrast, compound 6, p-coumaroyl tyrosine (m/z of 327.1094 [M+H]+), was exclusively detected in JURS-U.
Gallic acid and ferulic acid, being the most abundant compounds in jackfruit, exhibit therapeutic potential due to their dual roles as antioxidants and anti-inflammatory agents. Its ability to neutralize free radicals and modulate inflammatory responses suggests a role in cellular protection and inflammation management [53]. The presence of gallic acid and ferulic acid in the current study is supported by the findings of Zhang et al. [33] and detects the ferulic acid 4-O-glucuronide at m/z of 356.1107 at [M+H]+. This compound formation may be due to the loss of neutral glucosyl moiety and CO2 between feruloyl glucose and caffeoyl glucose, respectively. Similar findings were also reported by Jiménez-Sánchez et al. [54]. However, Vázquez-González et al. [37] observed different phenolic acids in jackfruit as compared to the present study. They conducted a detailed structural analysis on jackfruit through HPLC–DAD–ESI–MS and observed various phenolic acids including caffeic acid and quinic acid. The discrepancies with the current study may be attributed to factors such as extraction assisted technology, genetic variation, environmental conditions and developmental stages of the fruit. 4-O-Methylgallic acid, a derivative of quinic acid, is formed through methylation at the 4th position, resulting in a phenolic compound. The inclusion of a phenolic structure in its composition suggests potential antioxidant properties and implicates its role in plant defence mechanisms [35].
3.5.2. Flavonoids
A total of 42 compounds identified as flavonoids, categorized into various subgroups based on their chemical structures. Within the flavanol subgroup, three compounds were identified across various jackfruit samples: procyanidin trimer C1 (m/z 865.1983), (−)-epicatechin (m/z 289.0702) and procyanidin dimer B1 (m/z 577.1348). The flavone subgroup consisted of eight compounds. In flavanones, seven compounds were identified across various jackfruit by-product samples. The flavanols subgroup included nine compounds, notably quercetin 3-O-(6″-malonyl-glucoside) (compound 30; m/z 551.1054), kaempferol 3-O-glucosyl-rhamnosyl-galactoside (compound 27; m/z 755.2045) and myricetin 3-O-galactoside (compound 31; m/z 479.0845). Compound 35, identified as dihydroflavonol, had an ionization mode of [M+H]+ and m/z of 305.0636. In the anthocyanin subgroup, eight compounds were identified. Finally, in the isoflavonoid subgroup, six compounds were identified, including tectorigenin 7-sulphate (compound 47; m/z 379.0084) and 3′-hydroxydaidzein (compound 48; m/z 271.062). The degree of maturity had a substantial effect on the phytochemistry, and the notable compounds identified in ripe jackfruit samples were (−)-epicatechin (m/z 289.0702), chrysoeriol 7-O-(6″-malonyl-apiosyl-glucoside) (m/z 681.1659), luteolin 7-O-(2-apiosyl-glucoside) (m/z 579.1352), quercetin 3-O-(6″-malonyl-glucoside) (m/z 711.1399), myricetin 3-O-galactoside (m/z 479.0845), 3-methoxynobiletin (m/z 433.1509), 2′-hydroxyformononetin (m/z 285.0755) and 3′-hydroxydaidzein (m/z 271.062). Morelos-Flores et al. [50] detected the seven flavonoids in Mexico jackfruit, which were gallocatechin, epigallocatechin, epicatechin, rutin, myricetin, quercetin and naringenin, which consisted of the results of the current project.
Apigenin-7-O-glycosides (compound 11) is a apigenin moiety, which is O-glycosidically linked to carbohydrate moiety at the C7 position. Apigenin has exhibited promising anticancer effects through both in vivo and in vitro studies, through mediating five pathways, including apoptosis, autophagy, immune response, cell cycle, cell migration and invasion [55].
Narirutin (compound 19), rich in citrus fruit, is the bioactive compound in the mandarin peel. It has great potential in the treatment of obesity and its metabolic complications [56]. Eriocitrin (compound 24) exhibited significant pharmaceutical effects ascribed to its antioxidant, antitumour, antiallergic, antidiabetic and anti-inflammatory activities. Furthermore, it carried out more effective in inhibiting oxidative stress in chronic diseases caused by excessive oxidative stress, such as diabetes mellitus [57]. These compounds also found in the peel fraction of jackfruit, proving the further research direction.
Kaempferol 3-O-glucosyl-rhamnosyl-galactoside (compound 27) were identified by Pm et al. [58] from jackfruit, which exhibit antioxidant ability. They also detected myricetin from jackfruit by LC–QTOF–MS/MS. Myricetin has the antioxidant and anti-inflammatory ability, while improved cognitive and motor function in mice treated with pentyl tetrazole, which leads to seizures, and significantly reduced seizure severity and mortality [59]. Myricetin 3-galactoside (compound 31) is a glycosyl oxyflavone that is myricetin with a beta-D-galactosyl residue attached at position 3. It is one of neuroprotective substances and has potential to develop drug to alleviate Alzheimer’s disease [60].
Regarding anthocyanins, such as compound 36 tentatively identified as 4-O-methyldelphinidin 3-O-D-glucoside (m/z 480.1245) was only found in core of ripe jackfruit. Compound 39, observed in [M-H]- ionization mode at m/z of 432.1065, was tentatively identified as cyanidin 3,5-O-diglucoside is in a glycosylated state. Earlier, Lyu et al. [61] also observed the similar ionization mode (∗∗[M-H]−) and m/z (612.1664) for cyanidin 3,5-O-diglucoside. Moreover, all of the compounds they detected in anthocyanins were glycosylated. Anthocyanins are vibrant pigments found in plants, contributing to red, purple, and blue hues in fruits and vegetables. Renowned for their antioxidant properties, major anthocyanins include cyanidin, delphinidin, and malvidin, which have been associated with various health benefits [62].
Compound 44, identified as tectorigenin 7-sulphate in negative ionization mode and at m/z 379.0101, was detected in a commercial jackfruit sample. This might be the first instance of its identification in this fruit. During the formation of tectorigenin 7-sulphate, the substitution of a hydroxyl group at the seventh position of tectorigenin with a sulphate group occurred. Tectorigenin, a key component in traditional medicinal practices, exhibits notable anti-inflammatory properties [62].
In general, isoflavonoids, prominent in soybeans and other legumes, offer diverse health benefits. Known for their hormone-balancing effects, they may alleviate menopausal symptoms and support cardiovascular health by improving lipid profiles. Additionally, isoflavonoids exhibit antioxidant and anti-inflammatory properties, contributing to overall well-being and potential protection against chronic diseases [63].
3.5.3. Other Polyphenols
Under the ‘Other Polyphenols’ category, we identified eleven compounds, each representing different subclasses. These subclasses include hydroxycoumarins (2 compounds), hydroxybenzaldehydes (1 compound), curcuminoids (1 compound), furanocoumarins (1 compound), phenolic terpenes (1 compound) and tyrosols (5 compounds).
Compound 51, identified as scopoletin, was predominantly found in the peel and fruit of the jackfruit. It was detected in both mode ∗∗[M-H]− at 191.034. The ionization mode and mass-to-charge ratio (m/z) for scopoletin in our study align with the findings of Lyu et al. [61], who also reported this compound’s prevalence at similar m/z (191.0358) and in the same ionization mode ([M-H]−). Compound 53, identified as demethyloleuropein (a tyrosol), was consistently detected in the majority of the samples. It is a mature indicator of olives [64], and the role in jackfruit has not been explored.
Scopoletin’s formation involves the substitution with a hydroxyl group (OH) at the 7th position on the benzopyran ring. This hydroxyl group contributes to its biological activities and potential pharmacological properties. Scopoletin is notable for its diverse biological effects, including antioxidant, anti-inflammatory and antimicrobial activities, as documented in Tiwari et al. [65].
3.5.4. Lignans
Within jackfruit samples, five lignans have been identified, adding to the overall chemical diversity of these by-products. Compound 61, identified as ‘Enterolactone’ (C18H18O4), was consistently present in most of the analysed jackfruit samples, particularly in JRC-C and JURS-C. Enterolactone was characterized by a mass-to-charge ratio (m/z) of 299.1267 and an ionization mode of [M+H]+. The prevalence of this compound in ripe jackfruit suggests its conversion during ripening stages, as it is predominantly found in ripened samples. Enterolactone is a lignan and is formed through the metabolism of dietary lignans by gut bacteria. During its formation, deglycosylation and dehydroxylation reactions lead to the production of enterodiol which is then metabolized into enterolactone through dehydrogenation, highlighting its therapeutic potential [66].
Observations indicate that the levels of polyphenol constituents in jackfruit vary depending on the maturity stages and ion detection methods used. This trend could be explained by the numerous metabolic reactions occurring during the ripening and developmental stages, leading to the disparate release and generation of various free phenols and bioactive components in different jackfruit fractions.
3.6. HPLC Quantification
HPLC analysis identified 11 phenolic compounds in various parts of both JR and JUR samples, including gallic acid, catechin, syringic acid, epicatechin, coumaric acid, trans-ferulic acid, sinapic acid, procyanidin A2, quercetin, caffeic acid and kaempferol. Quercetin and gallic acid were identified as the predominant polyphenols in all samples, though gallic acid was absent in the peel of ripe jackfruit.
Polyphenol concentrations varied between samples extracted using conventional methods and those extracted using ultrasound. A hierarchical heatmap had been constructed to further analyse the HPLC–PDA data for these 11 phenolic compounds (Figure 3), revealing three clusters. Variations in clustering and colour intensity within the heatmap indicated differences in the concentration of these phenolic compounds. The HPLC analysis showed that ripening stage, extraction method and fruit fraction significantly influenced phenolic concentration.
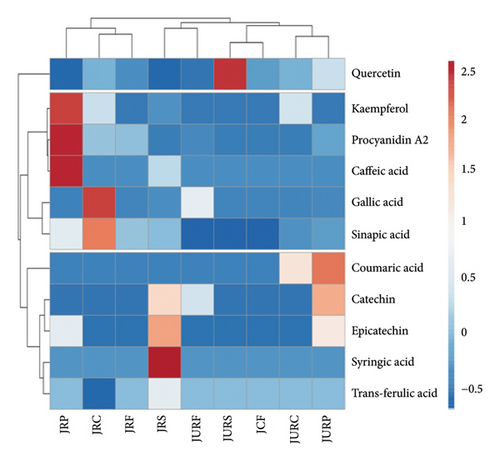

Previous study [67] reported that notable polyphenols in jackfruit included ferulic acid, gallic acid and tannic acid, along with flavonoids such as rutin, catechin and myricetin [68]. Moreover, Singh et al. [69] observed the difference in phenolic level between the jackfruit samples at different maturity levels. The raw jackfruit containing gallic, ferulic, caffeic and tannic acids, while ripe fruit which showed only ferulic and gallic acid.
Polyphenol level depends on factors such as extraction module, solvent choice, extraction temperature, particle size and pH. In this study, ultrasound extraction yielded higher polyphenol concentrations compared to conventional extraction method, may be attributed to variations in extraction.
4. Conclusion
In conclusion, this study provides valuable insights into the dynamic changes in the phenolic profile of jackfruit during various ripening stages and across different fractions. Ripened jackfruit samples had higher phenolic contents and exhibited greater antioxidant activity compared to their unripened counterparts. Among fractions analysed, the peel consistently displayed the highest phenolic content and antioxidant activity, surpassing the core, seed and fruit. Ultrasound extraction proved more effective than conventional methods in extracting phenolics. The LC–ESI–QTOF–MS/MS analysis identified a diverse array of 65 compounds, with flavonoids, lignans and phenolic acids. HPLC quantification highlighted the presence of specific compounds, such as gallic acid and quercetin. The ripening of jackfruit involves complex biochemical processes that significantly influence the behaviour and composition of phenolics compounds. Understanding these changes is crucial for optimizing the utilization of jackfruit in food and nutraceutical development. Future research should focus on analysing the phenolic profile of various jackfruit cultivars at different maturity stages, supporting the development of jackfruit-based commercial products.
Ethics Statement
The study does not involve any human or animal testing.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Shujun Ye: conceptualization, writing – original draft and investigation. Ali Imran: writing – review and editing and supervision. Osman Tuncay Agar: writing – review and editing and supervision. Dakshina Yadav: writing – review and editing, resources, project administration and validation. Chelsea Moore: resources, project administration and validation. Hafiz A. R. Suleria: conceptualization, investigation, resources, writing – review and editing, supervision, funding acquisition, project administration and validation.
Funding
The research was funded by the Australian Research Council under ‘Discovery Early Career Award’ (Grant No. ARC-DECRA-DE220100055), funded by The University of Melbourne under the ‘Future Food Hallmark Research Initiative Funds (Grant No. UoM 21/23)’, ‘Collaborative Research Development Grant (Grant No. UoM-21/23)’ and funded by AgriFutures, Australia (Grant ID. TA108561).
Acknowledgements
We thank the AgriFutures, Australia, for supporting our study by funding the Project on Jackfruit (ID TA108561). We would like to thank Nicholas Williamson, Shuai Nie and Michael Leeming from the Mass Spectrometry and Proteomics Facility, Bio21 Molecular Science and Biotechnology Institute, the University of Melbourne, VIC, Australia, for providing access and support for the use of LC-DAD and LC–ESI–QTOF–MS/MS and data analysis. We would like to thank the Master/PhD and postdoctoral researchers of the Dr. Hafiz Suleria group from the School of Agriculture, Food and Ecosystem Sciences, Faculty of Science (the University of Melbourne), for their incredible support.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




