Gelatin Extracted From Yanbian Cattle Skin Suppresses LPS-Induced Inflammation in RAW264.7 Cells
Abstract
Objective: To develop an efficient method for extracting gelatin from Yanbian cattle skin and to study its anti-inflammatory effects.
Methods: Gelatin was extracted using enzymatic hydrolysis and water extraction techniques. The basic structures of the prepared gelatin were analyzed using Fourier transform infrared spectroscopy (FT-IR), ultraviolet (UV) spectroscopy, amino acid analysis, scanning electron microscopy (SEM), and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). LPS established the RAW264.7 cell inflammation model in vitro. The therapeutic dose range of gelatin, which did not exhibit significant cytotoxicity, was determined using the CCK-8 assay for subsequent experiments. The nitric oxide (NO) content was measured using the Griess method. The content of proinflammatory cytokines was determined using ELISA. Reactive oxygen species (ROS) levels were detected using the DCFH-DA fluorescent probe method, and oxidative stress–related indicators were measured using a kit. The mRNA expression levels of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and proinflammatory cytokines were detected using qRT-PCR. Western blot (WB) was used to detect the protein expression of iNOS and COX-2 in each group.
Results: Characteristic absorption peaks of gelatin were observed in the FT-IR and UV spectra. Additionally, gelatin from Yanbian cattle skin significantly reduced NO content in cells, decreased the secretion of proinflammatory cytokines, inhibited ROS generation, reduced oxidative stress–related indicators, significantly lowered the mRNA expression levels of iNOS, COX-2, and proinflammatory cytokines in cells, and significantly reduced the protein expression of COX-2 and iNOS.
Conclusion: Enzymatic hydrolysis and water extraction efficiently prepare gelatin from Yanbian cattle skin. This gelatin can inhibit LPS-induced inflammation in RAW264.7 cells, potentially by inhibiting the secretion of proinflammatory cytokines and ameliorating oxidative stress.
1. Introduction
Yanbian cattle are considered a nationally recognized and protected breed, classified among China’s five premier cattle breeds. Additionally, Yanbian cattle are high-yield meat producers. This breed is characterized by its large body, excellent skin quality, and superior meat attributes, which contribute to its prominent market position in the beef industry and its substantial economic value. Furthermore, it is a globally renowned beef breed designated as protected by the Ministry of Agriculture of China [1, 2]. However, the processing of Yanbian cattle generates a substantial amount of by-products, such as skin. These by-products are often underutilized, leading to resource waste and environmental concerns. Currently, the processing technology for Yanbian cattle skin is relatively underdeveloped, resulting in low added value and economic benefits [3]. Gelatin, a macromolecular hydrophilic colloid derived from collagen hydrolysis, is produced from the connective tissues of animals, including skin, bones, and tendons. Gelatin has wide-ranging applications in food, medicine, and cosmetics [4, 5]. Presently, gelatin is mainly extracted from marine and terrestrial animals, utilizing raw materials such as fish skin, bones, fins, and the skin and bones of livestock and poultry [6]. The primary methods for extracting gelatin include acid, alkali, enzymatic, and hot water extraction. The choice of raw material significantly influences the gelatin’s extraction method, yield, and nutrient composition [7, 8]. For example, See et al. [9] used catfish skin as raw material, treating it with sodium hydroxide and acetic acid, which improved gelatin production, gel strength, viscosity, melting temperature, and gelation temperature. Abedinia et al. [10] investigated the effects of acid and alkali enzyme pretreatment on the yield of duck paw gelatin, finding that the average yields were 4.09%, 3.65%, and 5.75% for acid, alkali, and enzyme pretreatments, respectively. He et al. [11] employed high-pressure-assisted liquid extraction to prepare calfskin gelatin, demonstrating that this method significantly reduced processing time compared to traditional methods.
Inflammation is an adaptive response of the immune system triggered by pathogens, damaged cells, exogenous stimuli, and various other factors. It plays a crucial role in host defense and maintaining homeostasis. However, excessive inflammation can adversely affect the body, leading to tissue damage and disease [12]. Excessive inflammatory mediators constitute the foundational basis for an excessive inflammatory response. Macrophages are crucial immune cells and serve as the primary line of defense against pathogens such as bacteria, viruses, and fungi, which are closely linked to the onset and progression of inflammation [13]. Upon stimulation with lipopolysaccharide (LPS), macrophages become overactivated and release various cellular regulatory factors, including common inflammatory mediators such as NO, TNFα, IL-6, and IL-1β, which amplify the inflammatory response and disrupt the onset and progression of various inflammatory processes. Macrophages predominantly secrete TNF-α and play a pivotal role in regulating the function of immune cells. As an endogenous pyrogen, TNF-α can induce apoptosis. IL-6 can be produced by both lymphoid and specific nonlymphoid cells, including T lymphocytes, B lymphocytes, macrophages, dendritic cells, and other nonlymphoid cells [14]. Prostaglandins (PGs) belong to the eicosanoid family. The synthesis of PGs necessitates the involvement of COX. In macrophages, prostaglandin E2 (PGE2) plays a supportive role in their activation by interferon-gamma (IFN-γ) and TNF-α through the regulation of intracellular cyclic adenosine monophosphate (cAMP) levels. Regulating NO levels is regarded as a crucial strategy for treating inflammation, and NO is also viewed as a significant indicator of macrophage activation and participation in the immune response. NO serves as a significant inflammatory mediator produced by RAW264.7 cells upon stimulation. When NO concentrations become excessively elevated, they can lead to localized tissue damage and exacerbate the inflammatory response. Concurrently, the activation of iNOS can enhance the release of NO, which in turn can stimulate the secretion of proinflammatory factors such as TNFα, IL-6, and IL-1β. Additionally, it initiates the body’s immune response and is implicated in various inflammation-related diseases. COX-2 is an inducible isoenzyme of COX, and its catalytic products can enhance the release of NO and proinflammatory factors [15]. ROS, which are chemically active oxygen-containing molecules produced by oxygen metabolism in biological systems, primarily include superoxide anion, hydroxyl radical, and hydrogen peroxide; they can also stimulate the inflammatory response by expressing of proinflammatory factor genes. Furthermore, increased ROS accumulation results in oxidative stress, contributing to the senescence of critical cellular components (lipids, proteins, and DNA), with MDA as a typical indicator of lipid peroxidation. Simultaneously, the antioxidant defense system offers essential protection to biological systems by mitigating the harmful effects of ROS, including the antioxidant enzyme SOD. Additionally, the enzymatic antioxidant GSH plays a vital role in maintaining normal ROS levels. Therefore, excessive ROS production may be a primary factor contributing to the inflammatory response, and effective scavenging of ROS is beneficial for alleviating inflammation [16, 17]. Recent studies have shown that gelatin possesses various biochemical functions, including antioxidant activity, antibacterial effects, and antiaging properties. Nurilmala et al. [18] reported that gelatin and its derived peptides exhibit significant antioxidant activity. Chen et al. [19] demonstrated that yak skin gelatin promotes platelet membrane glycoprotein activity and enhances platelet function, contributing to hemostasis. Additionally, Chen et al. [20] found that cod skin gelatin peptides effectively prevent ultraviolet (UV)-induced skin photoaging by inhibiting matrix metalloproteinase (MMP) expression and activity. While many scholars have investigated the bioactive functions of gelatin from various sources, the anti-inflammatory effects of gelatin derived from Yanbian cattle hide are yet to be elucidated.
Therefore, our research aims to develop methods for processing Yanbian cattle skin to efficiently convert them into gelatin, thereby addressing the issue of resource waste and promoting resource utilization. By constructing an in vitro cell inflammation model, we aim to elucidate the anti-inflammatory effects of gelatin, which could lead to the development of new anti-inflammatory drugs or health products using gelatin as a raw material. Ultimately, this research seeks to enhance the utilization value of Yanbian cattle skin, provide scientific insights for the deep processing of the cattle hide industry, and improve economic benefits.
2. Materials and Methods
2.1. Reagents and Equipment
Yanbian cattle skin was provided by the Engineering Research Center of North-East Cold Region Beef Cattle Science & Technology Innovation, Ministry of Education. Pepsin (1:3000) was purchased from Solaibao Technology Co., LTD. (Beijing, China). PBS buffer, DMEM high glucose medium, fetal bovine serum (FBS), and 0.25% trypsin EDTA were obtained from Gibco (San Diego, CA, USA). LPS and dimethyl sulfoxide (DMSO) were also acquired from Solaibao Technology Co., LTD. (Beijing, China). Kits for SOD, MDA, GSH-Px, and NO were sourced from the Nanjing Jiancheng Biological Engineering Research Institute (Nanjing, China). ELISA assay kits for IL-6 (ml098430), TNF-α (mIC50536-1), IL-1β (ml028595), and PGE2 (ml037542) were purchased from Shanghai Enzyme Linked Biotechnology Co., LTD. (Shanghai, China). CCK-8 and ROS kits were obtained from Shanghai Biyuntian Company (Shanghai, China). Rabbit anti-GAPDH, rabbit anti-COX-2, and rabbit anti-iNOS antibodies were procured from Thermo Fisher Scientific (Shanghai, China). Total RNA extraction kit (adsorption column method) was purchased from Beijing Tiangen Biochemical Technology Co., Ltd. (Beijing, China); first strand cDNA synthesis kit was purchased from Nanjing Novizan Biotechnology Co., Ltd. (Nanjing, China). The CO2 incubator was purchased from Shanghai Hengscientific Instrument Co., LTD. (Shanghai, China). Synergy HTX multifunctional microplate reader was purchased from Boten Instruments Co., LTD. (USA). ABI 7500 Fast Fluorescent Quantitative PCR System was purchased from ABI Corporation (USA). The inverted microscope Ts2 was purchased from Nikon Precision Machinery Shanghai Co., LTD. (Shanghai, China). UV visible spectrometer (UV-2550) was acquired from Shimadzu Corporation (Japan). Fourier transform infrared spectrometer (IRTRACER-100) was acquired from Shimadzu Corporation (Japan). Matrix-assisted laser resolution ionization time-of-flight mass spectrometer was purchased from Bruker Corporation (USA). Cold field emission scanning electron microscope (SU 8010) was acquired from Hitachi Corporation (Japan). Hitachi LA8080 automatic amino acid analyzer was purchased from Hitachi Corporation (Japan).
2.2. Preparation and Characterization of Gelatin From Yanbian Cattle Skin
2.2.1. Preparation of Gelatin From Yanbian Cattle Skin
2.2.2. Analysis of the Physicochemical Properties of Gelatin
Moisture content was assessed through direct drying and the use of gelatin (2 g) in a vacuum oven for 5 h at 100°C. Protein and ash contents were determined in accordance with the AOAC standards.
2.2.3. Determination of Gelatin by FT-IR and UV
Samples of 2 mg of cattle skin gelatin and commercial gelatin were each mixed with 200 mg of KBr to form tablets and scanned using a Fourier transform infrared spectrometer in the range of 4000 to 400 cm−1. Solutions of cattle skin gelatin and commercial gelatin at 1 mg/mL were prepared and scanned in the range of 400 to 200 nm using UV spectroscopy.
2.2.4. Determination of Amino Acid Content of Gelatin
The amino acid composition was determined according to the method of Xu et al. [22]. Weigh 0.1 g of gelatin (accurate to 0.0001 g) in a clean digestion tube. After weighing the sample, 10–15 mL of 6 mol/L hydrochloric acid solution was added to the digestion tube. Subsequently, it was placed in an electric heating constant-temperature drying oven at 110°C, where it was hydrolyzed for 23 h, removed, and cooled to room temperature. The solution was transferred to a 50 mL cuvette, diluted to 50 mL with purified water, and mixed. 1 mL of the above sample diluent was transferred into a nitrogen blow tube and placed in an automatic nitrogen blow concentrator for drying. The temperature of the nitrogen blower was set to 45°C. Nitrogen was introduced, and the sample was redissolved to 10 mL with buffer solution and then diluted according to the desired concentration for measurement. The samples were filtered through a 0.22 μm aqueous-phase filter membrane and prepared for analysis.
2.2.5. Gelatin Was Analyzed by SEM
Gelatin was adhered to the sample stage using conductive adhesive and coated with gold; subsequently, the microstructure was examined at various magnifications.
2.2.6. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) Analysis of Gelatin
The α-Cyano-4-hydroxycinnamic acid (CHCA) substrate was dissolved in a 50:50 (v/v) mixture of 50% acetonitrile (ACN) and 0.1% trifluoroacetic acid (TFA) in water until the solution was saturated. Following the application of 1 μL of CHCA matrix solution onto the MALDI target plate and allowing it to dry at ambient conditions, 1 μL samples from the reaction system were subsequently applied to the target plate for detection.
2.3. Cell Experimental Study
2.3.1. Cell Culture
The recovered RAW264.7 cells were transferred into sterile culture flasks, and a high-glucose DMEM medium supplemented with 10% FBS and 1% antibiotics (streptomycin and penicillin) was added. The cells were incubated at 37°C with 5% CO2, and the culture medium was regularly replaced.
2.3.2. The Effect of Gelatin on RAW264.7 Cell Viability Was Detected by CCK-8 Method
After the cells reached the logarithmic growth phase, 100 μL of the uniform cell suspension was added to each well of a 96-well plate; the density was 3000 cells per well, and the surrounding wells were sealed with PBS and incubated for 24 h. After adding 0, 0.1, 0.3, 0.5, 0.7, 1.0, 1.5, and 2.0 mg/mL gelatin solution for 24 h, the culture medium was aspirated and the wells were washed twice with PBS. The CCK-8 solution was prepared by diluting it with a complete medium. 100 μL of CCK-8 solution was added to each well, and cell viability was assessed after incubating for 3 h. Absorbance values were measured at a wavelength of 450 nm.
2.3.3. LPS-Induced Inflammation in RAW264.7 Cells and Experimental Grouping
RAW264.7 cells (1.5 × 104 cells per well) were seeded in six-well plates and incubated for 24 h. Except for the blank group, all other groups were treated with 1 μg/mL LPS for 24 h. After the treatment, low (0.7 mg/mL), medium (1.0 mg/mL), and high (1.3 mg/mL) doses of gelatin were incubated for 24 h.
2.3.4. Cell Morphological Observation
After cell incubation and processing, place the six-well plates under an inverted microscope to observe and take photos.
2.3.5. Effect of Gelatin on NO Release From RAW264.7 Cells Stimulated by LPS
The cells were incubated as described above. NO content was determined with reference to previous studies [23]. Griess Reagents I and II were removed and allowed to equilibrate at room temperature. Standards (1–100 μM) were diluted in a DMEM and 10% FBS solution. Standards and samples were added to 96-well plates at a volume of 50 μL per well. Room temperature Griess Reagent I was added to each well at a volume of 50 μL. Room temperature Griess Reagent II was added to each well at a volume of 50 μL. Therefore, NO content was subsequently determined.
2.3.6. Effect of Gelatin on LPS-Stimulated TNF-α, IL-1β, IL-6, and PGE2 in RAW264.7 Cells
The cells were incubated as described above. Remove the kit from the refrigerator at 4°C and equilibrate it at room temperature for a minimum of 30 min. The washing solution provided by the kit is 25 times concentrated and must be diluted with ultrapure water at a ratio of 1:25 prior to use as a working solution. Serial standard concentration solutions of TNF-α, IL-1β, IL-6, and PGE2 were prepared through gradient dilution of the standards. No sample was added to the blank wells; only chromogen A, chromogen B, and the termination solution were included for baseline measurement. 50 μL of diluted standard solution at each concentration was added to each well of quasi-quality wells, followed by the addition of 50 μL of sample dilution to the blank wells, and then 50 μL of HRP reagent was added. 50 μL of the sample was added to the sample wells, followed by the addition of 50 μL of HRP reagent. The microplate was gently shaken to ensure even mixing of the liquid within the wells, followed by incubation in a cell incubator at 37°C for 60 min. Discard the liquid from the wells, gently shake to dry, fill each well with washing solution, and allow to stand. After 30 s, discard the washing solution, repeat this process five times, and pat the wells dry using absorbent paper. Each well was initially treated with 50 μL of chromogenic agent A, followed by 50 μL of chromogenic agent B; the mixture was then gently shaken and incubated at 37°C in the dark for 10 min for color development. The reaction was terminated by rapidly adding 50 μL of termination solution to each well, with the blank well set as the baseline for measurement. The absorbance of each well at a wavelength of 450 nm was measured using a microplate reader, and the corresponding optical density value was recorded. The concentrations of TNF-α, IL-1β, IL-6, and PGE2 were calculated based on their respective concentrations and optical density values.
2.3.7. Effect of Gelatin on ROS Content in RAW264.7 Cells Stimulated by LPS
The cells were incubated as described above. According to the ROS kit instructions, the DCFH-DA fluorescent probe method was used to detect ROS levels, using fluorescence microscope images and ImageJ software for semi-quantitative analysis of fluorescence intensity.
2.3.8. Effect of Gelatin on SOD, MDA, and GSH-Px Levels in RAW264.7 Cells Stimulated by LPS
The cells were incubated as described above. The levels of SOD, MDA, and GSH-Px in the cells were measured according to the kit instructions.
2.3.9. Effect of Gelatin on the mRNA Expression of iNOS, COX-2, IL-1β, IL-6, and IL-10 in RAW264.7 Cells Stimulated by LPS
The mRNA expression levels of iNOS, COX-2, IL-1β, IL-6, and IL-10 were detected by qRT-PCR. After cell incubation and processing, cells were collected and total RNA was extracted using an RNA extraction kit. According to the reagent instructions, RNA was reverse transcribed into cDNA. PCR amplification was then performed under the following conditions: predenaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s, and annealing and extension at 60°C for 30 s. The expression levels of iNOS, COX-2, IL-1β, IL-6, and IL-10 in cells were calculated using the 2−ΔΔCt method with GAPDH as the reference gene. The primers for the target genes were synthesized by Sangon Bioengineering Co., LTD (Shanghai, China) according to the design. The primer sequences and product sizes are shown in Table 1.
| Primer | Primer sequence (5′-3′) | Length (bp) |
|---|---|---|
| iNOS | F: CAACAGGAACCTACCAGUTCACT | 253 |
| R: AGUCTGAAGTCATGTTTGUCG | ||
| COX-2 | F: GAAATATCAGGTCATTGGTGGAGA | 205 |
| R: ATGUTCCTGUTTGAGTATGTCG | ||
| IL-1β | F: CACTACAGGCTCCGAGATGAACAAC- | 145 |
| R: TGTCGTTGCTTGGTTCTCCTTGTAC- | ||
| IL-6 | F: CTTCTTGGGACTGATGCTGGTGAC | 91 |
| R: TCTGTTGGGAGTGGTATCCTCTGTG | ||
| TNF-α | F: AGACCCAGGAGTGTTCACAGACC | 141 |
| R: GTCACCAGGCGAGTTATAGCTTCAG- | ||
| GAPDH | F: CCTCGTCCCGTAGACAAAATG | 133 |
| R: TGAGGTCAATGAAGGGGTCGT | ||
2.3.10. Western Blot (WB) Was Used to Detect the Expression of Related Proteins
Protein quantification was conducted by measuring the protein concentration of each group in accordance with the instructions provided by the BCA protein quantification kit. The corresponding volumes of 5x Loading Buffer and PBS were added to the protein samples from each group, which were subsequently incubated at 100°C for 10 min and stored at −20°C. The samples were loaded at 20 μg, subjected to SDS-PAGE electrophoresis, and subsequently transferred to PVDF membranes. The PVDF membrane was rinsed with 1x TBST for 5 min, repeated four times, and subsequently blocked with skim milk powder for 2 h. The membrane was incubated with diluted primary antibodies (COX-2 and iNOS) overnight at 4°C on a shaker. The following morning, the membranes were washed with 1x TBST for 5 min, repeated four times, and then incubated with goat anti-rabbit IgG secondary antibody at room temperature in the dark for 1.5 h (dilution ratio 1:10,000) on a shaker. The ECL-developed solution was employed to analyze the protein band patterns of the WB using ImageJ software.
2.3.11. Statistical Analyses
All test data are presented as the mean ± standard deviation from three experiments (mean ± SD, n = 3). One-way analysis of variance was performed using GraphPad Prism 8.02 software to compare the data. p values of < 0.01 and < 0.05 were considered indicative of significant differences between groups. Significant differences were also illustrated using this software.
3. Results and Discussion
3.1. Cattle Skin Gelatin Extraction and Characterization
3.1.1. Yield, Protein, Ash, and Moisture Content of Gelatin
According to the data presented in Table 2, the yield of gelatin is 35.47 ± 1.21%, the protein content of the gelatin is 89.77 ± 2.01%, the moisture content is 4.35 ± 0.09%, and the ash content is 0.32 ± 0.01%. Gelatin production in cattle skin has been shown to increase following protease treatment. Ahmad et al. [24] also found that pepsin treatment increased gelatin production. It is hypothesized that proteases represent a class of enzymes that hydrolyze peptide chains by catalyzing the degradation of proteins into smaller peptides or individual amino acids. During gelatin preparation, proteases catalyze the hydrolysis of peptide bonds in collagen molecules, thereby breaking them down into individual polypeptide chains or smaller molecular fragments. Furthermore, gelatin is a product of the partial hydrolysis of collagen. Under the influence of protease, collagen molecules undergo hydrolysis into polypeptide fragments with a specific molecular weight distribution, which constitute the primary components of gelatin. Therefore, protease treatment significantly increases the yield of gelatin, primarily by facilitating the hydrolysis of collagen molecules.
| Yanbian cattle skin gelatin | |
|---|---|
| Yield (%) | 35.47 ± 1.21 |
| Protein content (%) | 89.77 ± 2.01 |
| Moisture content (%) | 4.35 ± 0.09 |
| Ash content (%) | 0.32 ± 0.01 |
3.1.2. Results of Fourier Infrared Spectrum and UV Spectrum of Gelatin
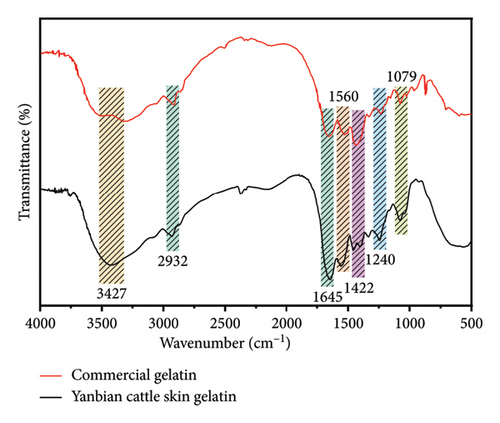
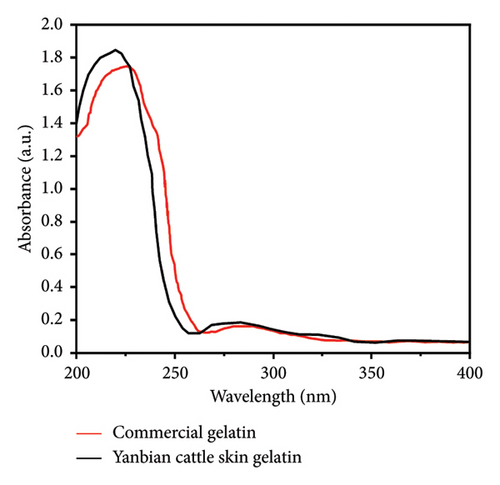
As observed in the infrared spectrum in Figure 1(a), cattle skin gelatin exhibits distinct absorption peaks characteristic of gelatin. The amide A band at 3367 cm−1 represents the absorption peak produced by the stretching vibration of O-H and N-H bonds, indicating the presence of numerous amino acid residues containing hydroxyl and amino groups. The absorption peak at 2932 cm−1 corresponds to the amide B band, which is the stretching vibration peak of the aliphatic C-H bond, indicating the presence of a C-H skeleton in the gelatin. The stretching vibration peak of the C = O bond at 1645 cm−1 corresponds to the amide I band, one of the main characteristic peaks of proteins and peptides. The peak of N-H bending vibration and C-N stretching vibration at 1560 cm−1 corresponds to the amide II band. The absorption peak at 1457 cm−1 is related to the bending vibration of the C-H bond and may represent vibrations in methyl or methylene groups. The absorption peak at 1404 cm−1 is related to the carboxylate’s symmetric -COO- stretching vibration, indicating that the gelatin contains a deprotonated carboxyl group. The absorption peak at 1337 cm−1 is related to the bending vibration of the C-H bond. The absorption peak at 1246 cm−1 corresponds to the amide III band, mainly composed of N-H bending vibration and C-N stretching vibration. The absorption peak at 1079 cm−1 corresponds to the stretching vibration of the C-O bond, indicating the presence of an alcohol group in the amino acid residues in this gelatin. Comparative analysis indicated that the secondary structure of the gelatin we prepared was consistent with that reported in a prior study [25]. The results of He et al. [11] align with our findings; the peak shapes of cattle skin gelatin were similar, and both exhibited the characteristic vibrational modes of proteins. Additionally, the secondary structure of Yanbian cattle skin gelatin resembles that of commercial gelatin. As seen in Figure 1(b), the UV spectrum diagram shows an absorption peak near 230 nm, indicating the presence of peptide bonds. This is consistent with the FT-IR results.
3.1.3. Amino Acid Content of Gelatin
The amino acid content of cowhide gelatin is presented in Table 3, demonstrating that gelatin is rich in amino acids, particularly high in glycine, proline, hydroxyproline, and glutamic acid, but lacking in tryptophan. The glycine content constitutes approximately one-third of the total amino acids, suggesting its derivation from collagen. Glycine is regarded as an anti-inflammatory nutrient effective in treating inflammation associated with conditions such as arthritis, colitis, and alcoholic liver disease. Research indicates that hydroxyproline is the principal component of gelatin, and both hydroxyproline and proline are critical factors influencing gelatin stability. The present study found that the content of proline and hydroxyproline in cowhide gelatin constitutes approximately 15% of the total amino acids. Previous studies have shown that the amino acids in cowhide gelatin are primarily proline and hydroxyproline; the pyrrole ring structure of imino acids generates steric resistance conducive to stabilizing the helical structure. Both the nitrogen atom in the pyrrole ring and the hydroxyl group in hydroxyproline participate in the formation of hydrogen bonds, thereby enhancing intermolecular forces. Meanwhile, the glutamic acid content in the prepared oxhide gelatin was high, whereas the levels of methionine, isoleucine, and histidine were low [26]. These findings are consistent with previous research.
| Amino acid | Content (g/100 g protein) |
|---|---|
| Asp | 5.10 ± 0.03 |
| Thr | 1.56 ± 0.03 |
| Ser | 3.08 ± 0.02 |
| Glu | 9.20 ± 0.05 |
| Gly | 19.92 ± 0.02 |
| Ala | 8.09 ± 0.05 |
| Cys | 0.00 ± 0.00 |
| Val | 1.74 ± 0.05 |
| Met | 0.75 ± 0.05 |
| Ile | 1.52 ± 0.03 |
| Leu | 2.59 ± 0.03 |
| Tyr | 7.56 ± 0.05 |
| Phe | 1.69 ± 0.02 |
| Lys | 3.01 ± 0.02 |
| His | 0.55 ± 0.02 |
| Arg | 6.83 ± 0.04 |
| Pro | 9.38 ± 0.02 |
| Hypro | 4.85 ± 0.04 |
| NH3 | 1.91 ± 0.02 |
| Total amino acids | 87.42 ± 0.23 |
3.1.4. SEM Results of Gelatin
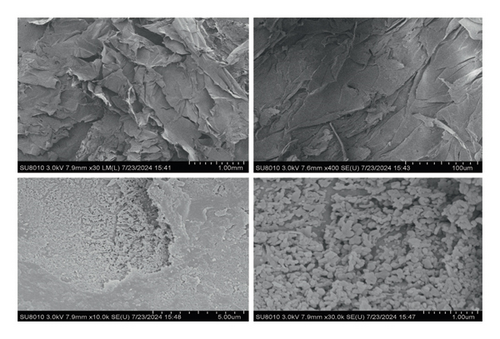
The SEM results of gelatin are presented in Figure 2. The structure of gelatin was observed at different magnifications, revealing a spherical, compact, and uniform structure. Gelatin molecules possess numerous polar groups, including hydroxyl and amine groups, capable of attracting and binding to one another through hydrogen bonds. This physical interaction facilitates the tight binding of gelatin molecules, resulting in the formation of a stable network structure. During gelatin formation, in addition to physical cross-linking primarily via hydrogen bonds, covalent bonds may also be formed through chemical cross-linking. The denser microstructure of gelatin indicates an enhanced formation of intermolecular hydrogen bonds and covalent bonds, thereby improving its water-binding capacity.
3.1.5. Analysis of Gelatin by MALDI-TOF MS
Iwasaki et al. [27] found that low-molecular-weight gelatin significantly increased Hyp peptide absorption levels in human plasma compared to high-molecular-weight gelatin. This finding suggests that low-molecular-weight gelatin may confer additional health benefits. Consequently, we analyzed gelatin ranging from 0 to 20,000 m/z using MALDI-TOF MS. The results are presented in Figure 3. The results demonstrate that cowhide gelatin exhibits a wide molecular weight distribution. We focused on the distribution of gelatin in the low molecular weight range and found that its content is higher in the range of 200 Da to 1200 Da. Thus, we hypothesize that the gelatin derived from Yanbian cowhide may possess significant anti-inflammatory effects.

3.2. Inhibitory Effect of Gelatin on LPS-Induced Inflammation of RAW264.7 Cells
3.2.1. Effect of Gelatin on the Viability of RAW264.7 Cells
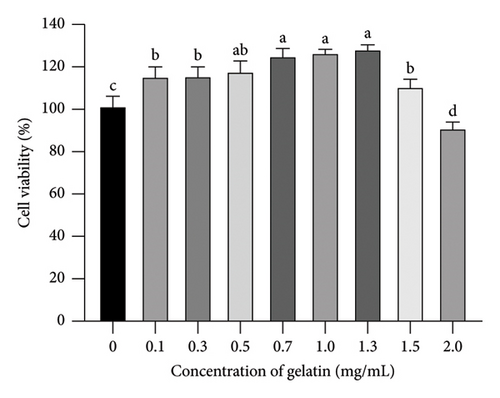
The cell viability assay is a widely used indicator to determine whether cells cultured in vitro can grow normally under specific conditions, making it essential in drug research and development. Figure 4 illustrates the impact of gelatin on the survival rate of RAW264.7 cells. Figure 4 shows that cell viability was higher at gelatin concentrations between 0.7 and 1.3 mg/mL compared to other concentrations. Therefore, gelatin concentrations of 0.7, 1.0, and 1.3 mg/mL were chosen as low, medium, and high doses for subsequent experiments in this study.
3.2.2. Effect of Cattle Skin Gelatin on the Morphology of RAW264.7 Cells Stimulated by LPS
Figure 5 illustrates the effect of LPS and cattle skin gelatin on the morphology of RAW264.7 cells. Figure 5 shows that the cells in the blank group were round, while after LPS stimulation, the cells exhibited polygonal or irregular shapes accompanied by numerous pseudopodia. However, different doses of gelatin treatment significantly reduced the number of pseudopodia, resulting in rounder cells, with the effect being dose-dependent. The morphological state of cells can reflect their health. The study showed that under normal conditions, RAW264.7 cells were small and round, lacking pseudopodia. After LPS stimulation, the cells exhibited polygonal or irregular shapes accompanied by numerous pseudopodia [28]. This suggests that our findings are in line with previous studies.

3.2.3. Effect of Gelatin on NO Release From RAW264.7 Cells Stimulated by LPS
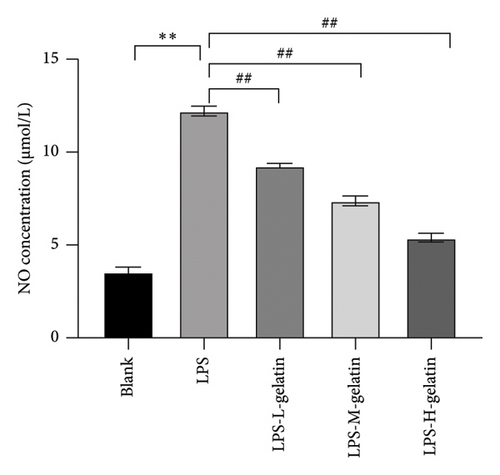
NO is an endogenously synthesized gas signal molecule, synthesized in the cytoplasm of cells, and quickly spreads across the cell membrane. Other free radicals quickly react to generate highly active oxidant peroxidase and other reactive nitrogen species; these substances are closely related to various diseases [29]. Figure 6 shows that the NO content in RAW264.7 cells significantly increased after LPS stimulation (p < 0.01) and significantly decreased after gelatin treatment (p < 0.01), indicating that cattle skin gelatin could inhibit NO release and thus alleviate inflammation.
3.2.4. Effect of Gelatin on TNF-α, IL-6, and IL-1β Contents in RAW264.7 Cells Stimulated by LPS
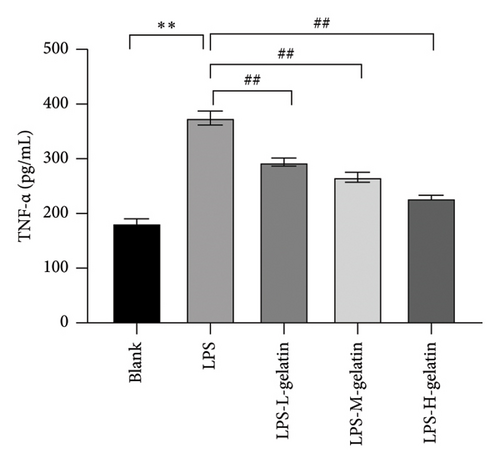
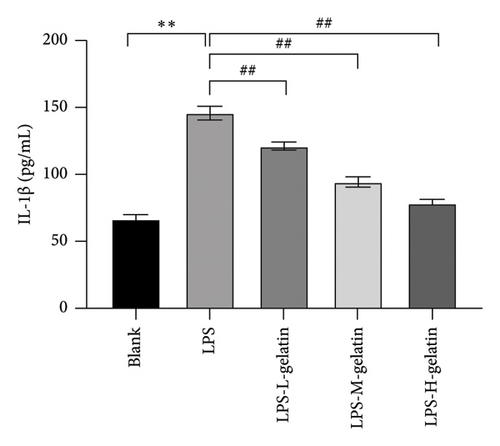
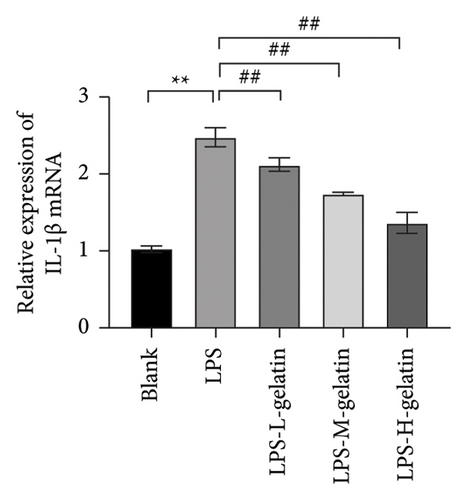
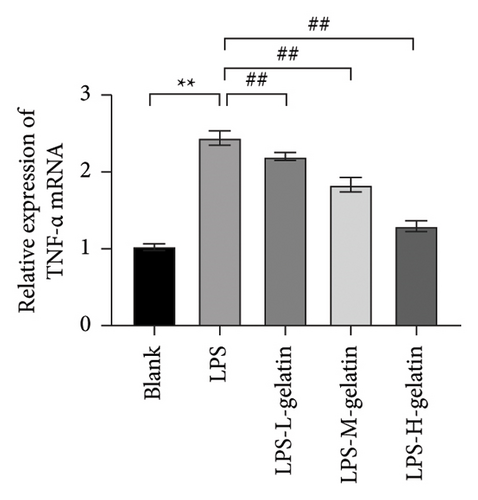
As shown in Figure 7, the levels of IL-1β, IL-6, and TNF-α in the LPS group significantly increased compared to the blank group (p < 0.01). In contrast, gelatin concentrations of 0.7, 1.0, and 1.3 mg/mL significantly decreased the secretion of IL-1β, IL-6, and TNF-α (p < 0.01). The study by Xing et al. [30] found that bone gelatin peptides can reduce the levels of IL-6, NO, and TNF-α in RAW264.7 cells induced by LPS, thereby inhibiting inflammation; our results also confirmed this. IL-1β, IL-6, and TNF-α are widely distributed in various tissues and organs of organisms, transmitting information within and between cells. As biological messenger molecules, they play an irreplaceable role in various physiological and pathological activities in the body. IL-1β mediates the important process of inflammation; it is produced by various cells. Under physiological conditions, its levels are particularly low, but in many pathological conditions, they increase significantly [31]. As an initiating factor in the inflammatory response, TNF-α promotes the adhesion of neutrophils, induces a respiratory burst, and enhances the expression of proinflammatory cytokines. When produced in excess, IL-6, a multifunctional inflammatory cytokine with both proinflammatory and anti-inflammatory functions, can cause inflammatory damage, leading to various diseases. IL-1β, IL-6, and TNF-α produced by RAW264.7 cells stimulated by LPS are key cytokines that promote the inflammatory response [32, 33]. The results showed that cattle skin gelatin could inhibit the secretion of IL-1β, IL-6, and TNF-α induced by LPS in RAW264.7 cells, thereby reducing inflammation.

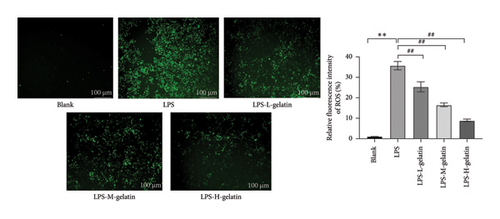
3.2.5. Effect of Gelatin on ROS Level From RAW264.7 Cells Stimulated by LPS
The intracellular ROS content of RAW264.7 cells treated with different concentrations of gelatin is presented in Figure 8. Compared to the blank group, the average fluorescence intensity of ROS in RAW264.7 cells stimulated by LPS was 35.75%. However, when treated with different concentrations of cattle skin gelatin, ROS levels in RAW264.7 cells were significantly lower (p < 0.01). ROS is a family of oxygen-derived species, including superoxide anion and hydroxyl radicals, produced by macrophages and neutrophils stimulated by various biologically active proinflammatory mediators. ROS plays a key role in inflammation and wound healing processes. Due to their lack of specificity for bacteria and their ability to be secreted outside the cell to exert cytotoxic effects on normal cells, it is crucial to remove harmful ROS from tissues and cells [34]. Fluorescent probes, which have the advantages of high sensitivity, simplicity, and reproducibility, are used to detect ROS. The most commonly used fluorescent probe is DCFH-DA. Nonfluorescent lipophilic DCFH-DA can diffuse through the cell membrane. The fluorescent DCF model can be used to indirectly measure intracellular ROS content [35]. Ma et al. [36] found that LPS-stimulated RAW264.7 cells produced a large amount of ROS, but LPS-induced inflammation was attenuated by inhibiting the ROS-mediated activation of the JAK1-STAT1/3 signaling pathway. These results are consistent with those of our study.
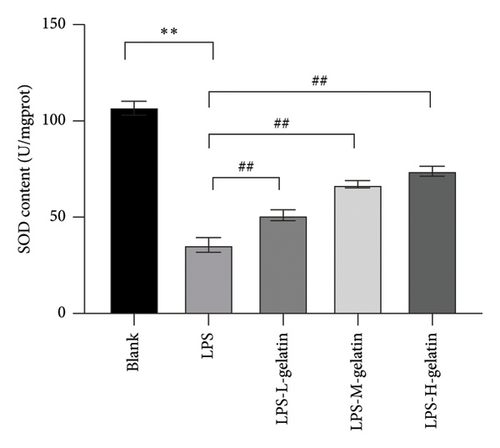
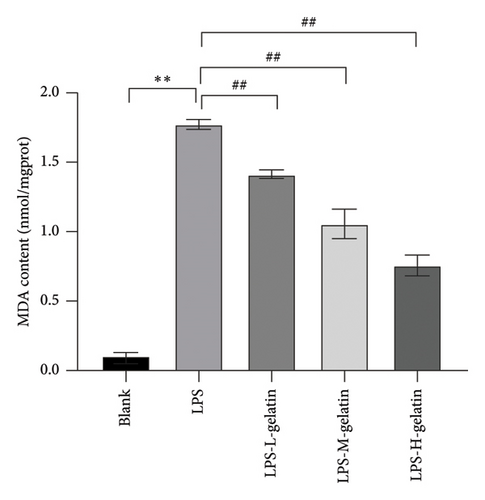
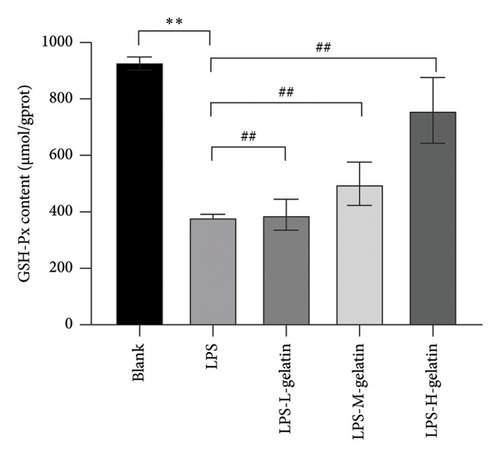
3.2.6. Effect of Gelatin on SOD, MDA, and GSH-Px Levels From RAW264.7 Cells Stimulated by LPS
Figure 9 illustrates the changes in levels of SOD, MDA, and GSH-Px in cells. The results indicate that LPS stimulation significantly reduces the GSH-Px and SOD content in cells (p < 0.01), while the formation of MDA significantly increases (p < 0.01). Moreover, cattle skin gelatin could significantly ameliorate oxidative damage in a dose-dependent manner (p < 0.01). SOD, MDA, and GSH-Px are important indicators reflecting the extent of oxidative stress in the body. Oxidative stress is characterized by an imbalance between oxidation and antioxidation within the body, resulting in excessive production of ROS and an increase in MDA, a by-product of membrane lipid peroxidation. Simultaneously, the body utilizes antioxidants, including GSH and the antioxidant enzyme SOD, to eliminate ROS and preserve cellular homeostasis. The reduction status of GSH and the activity of SOD serve as crucial indicators for assessing the body’s antioxidant capacity, collectively providing defense against oxidative stress–induced cellular damage. SOD catalyzes the dismutation reaction of superoxide anion radicals, resulting in the generation of harmless oxygen and hydrogen peroxide. This process significantly reduces the concentration of superoxide anion free radicals, thereby effectively mitigating their detrimental effects on cells. SOD not only protects the cell membrane from free radical attack but also enhances immune function, promotes the proliferation and differentiation of immune cells, and improves the body’s resistance to infectious diseases. In contrast, GSH-Px is a selenium-containing enzyme that catalyzes the reduction of hydrogen peroxide and glutathione, thereby scavenging peroxyl radicals. This reaction not only reduces the concentration of peroxyl radicals and prevents their damaging effects on cells but also safeguards the integrity of the cell membrane from lipid peroxidation. Additionally, GSH-Px functions as a scavenger of hydroxide ions, further protecting cells from oxidative stress [37, 38]. Our results indicate that gelatin may alleviate oxidative stress by enhancing SOD and GSH activity, while simultaneously inhibiting MDA production.
3.2.7. Effect of Gelatin on iNOS and COX-2 mRNA Expression From RAW264.7 Cells Stimulated by LPS
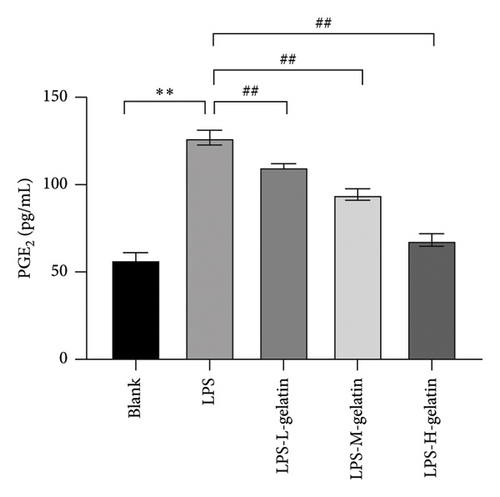
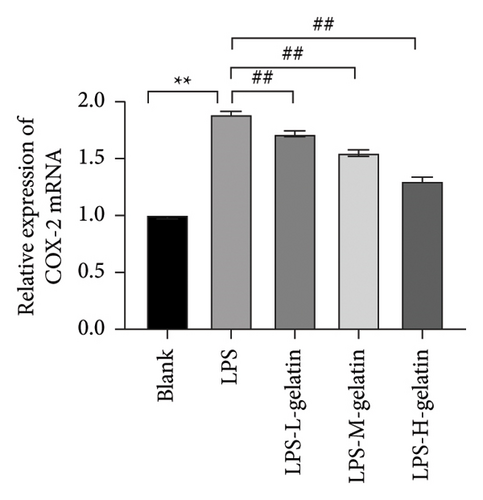
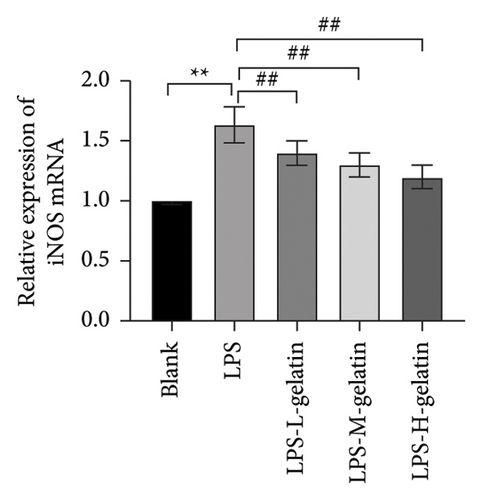
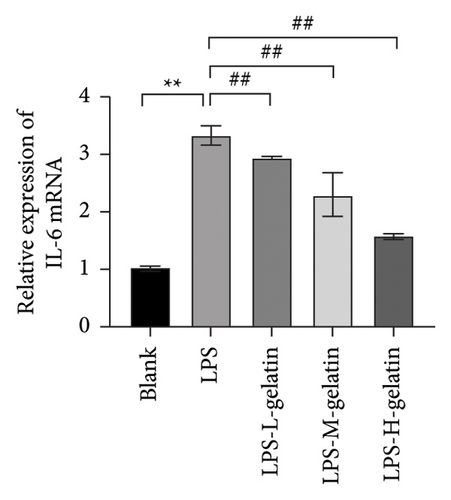
As shown in Figure 10, LPS significantly increased the mRNA expression levels of iNOS and COX-2 in RAW264.7 cells (p < 0.01), while cattle skin gelatin significantly reduced their expression (p < 0.01). Studies have shown that excessive expression of iNOS can lead to sustained and excessive NO generation, which is consistent with the above NO test results. The expression of iNOS is closely related to the secretion of NO; therefore, we examined the effect of gelatin on the level of iNOS mRNA expression. Our results also confirmed that cattle skin gelatin can reduce the expression of iNOS, thereby decreasing the secretion of NO and exerting its anti-inflammatory effects. The mRNA expression level of COX-2 is typically low but can be rapidly induced by growth factors and cytokines to participate in the inflammatory response. The expression of COX-2 increases during inflammation, causing excessive production of PGE2 and leading to an excessive inflammatory response [39]. Therefore, we examined the effect of different concentrations of gelatin on the expression of COX-2 in RAW264.7 cells induced by LPS. The results showed that cattle skin gelatin could inhibit the mRNA expression of COX-2 and alleviate inflammation.
3.2.8. Effect of Cattle Skin Gelatin on the Protein Expression of COX-2 and iNOS Induced by LPS in RAW264.7 Cells
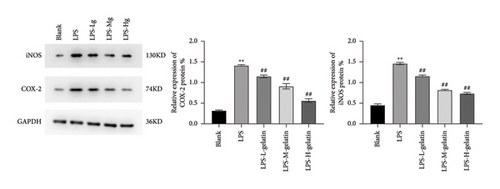
In comparison to the blank group, the protein expressions of COX-2 and iNOS in the LPS group were significantly increased (p < 0.01). In comparison to the LPS group, the expression of intracellular COX-2 and iNOS proteins was significantly decreased following cowhide gelatin treatment (p < 0.01), as illustrated in Figure 11. Baek et al. [40] also found that LPS stimulation of RAW264.7 cells significantly increased the protein expression of iNOS and COX-2, thereby increasing inflammation.
4. Conclusion
The results indicated that the yield of cattle skin gelatin was 35.47 ± 1.21% through enzymatic hydrolysis and water extraction. Yanbian cowhide gelatin exhibits a significant anti-inflammatory effect on RAW264.7 cells induced by LPS in vitro. The underlying mechanism may be associated with the inhibition of proinflammatory cytokine secretion and the enhancement of antioxidant capacity. Although we have confirmed the considerable anti-inflammatory effect of gelatin extracted from Yanbian cattle skin through extensive experimentation, the precise mechanism of action remains unclear. In future studies, we will further investigate the underlying molecular mechanisms and associated signaling pathways. This study provides scientific data to support the development of food, medicine, and cosmetics utilizing gelatin extracted from Yanbian cattle skin as a raw material.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: Xuanying Xin, Yihui Liu, Huaina Jin, Sungkwon Park, and Sun Jin Hur. Data curation: Xuanying Xin and Zhaohui Ruan. Formal analysis: Huaina Jin and Sungkwon Park. Methodology: Xuanying Xin and Seong-Ho Choi. Software: Huaina Jin, Sun Jin Hur, and Xuanying Xin. Validation: Seong-Ho Choi, Sungkwon Park, and Xiangzi Li. Investigation: Zhaohui Ruan. Writing–original draft: Xuanying Xin. Writing–review and editing: Seong-Ho Choi, Sungkwon Park, Sun Jin Hur, and Xiangzi Li.
Funding
This study was supported by the National Natural Science Foundation of China (grant number: 32060767), the Research Fund of Engineering Research Center of North-East Cold Region Beef Cattle Science & Technology Innovation, Ministry of Education, and the “111” Project (D20034), China.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




