Exploring the Role of Nutraceuticals in the Management and Prevention of Experimental-Induced Abdominal Aortic Aneurysms: Mechanisms and Therapeutic Potential
Abstract
A potentially fatal ailment, abdominal aortic aneurysms (AAAs) frequently result in death by dissection or rupture. AAA is caused by a histopathologic anomaly that includes smooth muscle cell loss, media degeneration, inflammatory cell infiltration, and elastic fiber damage in the aorta wall. Nevertheless, it is still unknown how AAAs form. Improved knowledge of how AAA develops and progresses might lead to the development of innovative, less intrusive treatment approaches for those suffering from this debilitating illness. Functional foods have a range of protective impacts, such as antioxidant, anti-inflammatory, antifibrotic, anticarcinogenic, and cardiovascular protective impacts, as shown by numerous human and animal research. Pretreatment with nutraceuticals can suppress the growth of AAA and pro-inflammatory markers. The use of nutraceuticals to treat ischemic-reperfusion cardiac cells has been shown to protect against ischemia–reperfusion damage through a diversity of mechanisms. This information was most recently reported. Thus, by inhibiting apoptosis, oxidative damage, c-Jun N-terminal kinase pathway, and inflammation, we investigated the impact of nutraceuticals on experimental-induced AAA in the current review.
1. Introduction
A complicated and multifaceted pathophysiology comprising a degenerative process of segmental weakening and dilatation characterizes abdominal aortic aneurysms (AAAs), which are usually asymptomatic until they burst [1, 2]. When the abdominal aortic wall is seen to be abnormally enlarged over 3 cm or to be expanded beyond 50% of the normal vessel diameter, AAA is diagnosed [3]. The placement of AAA in relation to the renal artery sources determines its classification. With 2% suprarenal, 80%–85% infrarenal, and the remaining portion pararenal, the majority are fusiform [4, 5]. Male gender, high blood pressure, atherosclerosis, smoking cigarettes, and a hereditary susceptibility are all linked to AAA [6, 7]. AAA was present in 1.34% of the 700,000 males screened for it [8, 9]. Since there are currently a few pharmacological treatments to slow down AAA enlargement [10], the best course of treatment for the condition is to optimize cardiovascular health, use extended intermittent imaging, and consider elective aneurysm surgery if the danger of rupture outweighs the risks of peri-surgical morbidity and death [11]. At present, the sole treatment for AAA involves either open surgery or endovascular repair. In many developed regions, AAAs are often detected early due to incidental imaging and screening initiatives. Randomized clinical trials (RCTs) have shown that early elective surgical repair of small AAAs does not provide any advantages, while for aneurysms greater than 5.5 cm in males and > 5.0 cm in females, surgery is the only option. Therefore, there is a significant clinical need to create medical therapies aimed at small AAAs that can restrict or prevent the aneurysm from growing and rupturing [12].
Nutraceuticals encompass a wide range of natural compounds that offer therapeutic and health-promoting benefits [13]. These components can be derived from various sources, including plants, herbs, and even certain animal products [14]. Common examples of nutraceuticals include dietary fibers, which aid in digestive health; flavonoids, known for their antioxidant properties; and polyphenols, which have been linked to reduced inflammation and improved heart health. Additionally, polyunsaturated fatty acids, particularly omega-3 and omega-6 fatty acids, play a critical role in maintaining cardiovascular health and supporting brain function [15].
Vitamins are essential micronutrients that contribute to numerous bodily processes, while spices like turmeric and ginger not only enhance flavor but also possess anti-inflammatory and antioxidant effects. Probiotics, the beneficial bacteria found in fermented foods, support gut health and enhance the immune system [15]. The growing interest in nutraceuticals is driven by their potential to prevent chronic diseases, promote overall well-being, and complement traditional medical treatments. As research continues to uncover the mechanisms behind these natural compounds, nutraceuticals are increasingly recognized as valuable tools in holistic health management and disease prevention strategies [16, 17]. Polyphenols are a great family of nutraceuticals, which are mainly categorized as phenolic acids, stilbenes, flavonoids, and lignans [18, 19]. Several pre- and clinical evaluations have exhibited remarkable anti-inflammatory impacts of “resveratrol” and “curcumin” [20, 21]. This effect is attributed to their ability to modulate several enzymes complicated in the metabolism of arachidonic acid, like phospholipase A2, cyclooxygenase-1 (COX-1), and COX-2, as well as their capacity to scavenge radicals. With great developments in the essentials of redox biology, many metabolic syndromes (MetS), such as cancer, diabetes mellitus, Alzheimer’s disease, and atherosclerosis, are brought on by elevated oxidative damage in the body [22, 23]. Studies using animal models have demonstrated that the body’s fat deposition can be reduced by supplementing with single or multiple polyphenolic compounds. These compounds work by preventing the overexpression of the adiponectin pathway, extracellular single kinase (ERK) 1/2, vascular endothelial growth factor (VEGF), peroxisome proliferator-activated receptor γ (PPRA-γ), and kinase insert domain receptor [24, 25].
Experimental in vitro and in vivo studies propose the beneficial effects of several medicinal nutraceuticals in reducing or preventing the progression of AAA [26–28]. To address all these issues related to AAA, the current review will highlight the function of nutraceuticals in terms of apoptosis, oxidative damage, c-Jun N-terminal kinase (JNK) pathway, and inflammation in experimental-induced AAA.
2. Pathogenesis of AAA
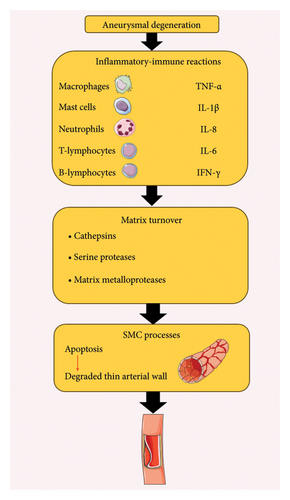
A complicated degenerative process known as “aneurysmal degeneration” influences all layers of the arterial wall and involves inflammatory-immune reactions, damaging remodeling of the aortic connective tissue, and advanced dilatation (this process is also shown in Figure 1) [29, 30]. The inflammatory features of AAA are defined by an infiltrate of macrophages, mast cells, neutrophils, T- and B-lymphocytes, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-8, IL-6, and interferon-γ, as well as systemic and local effects that support the recruitment and proliferation of additional inflammatory cells, encourage neo-angiogenesis, and improve matrix turnover [31, 32]. The process of matrix turnover involves the stimulation of constitutive as well as inducible proteases, like cathepsins, serine proteases, and matrix metalloproteinases, which are subsequently activated by pathways regulated by cytokines [33, 34]. These molecular mediators, which come from the cells of the lymphomonocytic infiltrate or intrinsic vessel wall components, are part of an enhanced proteolytic cascade that gradually breaks down the structural matrix proteins, especially elastin and collagen. This results in a thin, degraded medium that has a substantial loss of elastic component and an adventitia that is inflammatory or fibrotic [35]. Furthermore, cytokines increase the elastic media tissue damage and decrease the quantity of smooth muscle cells by initiating apoptotic cell death pathways [36].
3. Polyphenols and AAA: Current Evidence
Given the advantageous effects of polyphenols and their numerous roles in cellular and molecular processes, they are also considered as great candidate for treating AAA. This section reviews the current evidence about the mechanisms by which polyphenols like curcumin, quercetin, and resveratrol affect AAA (these mechanisms are also summarized in Figures 2 and 3).
3.1. Curcumin
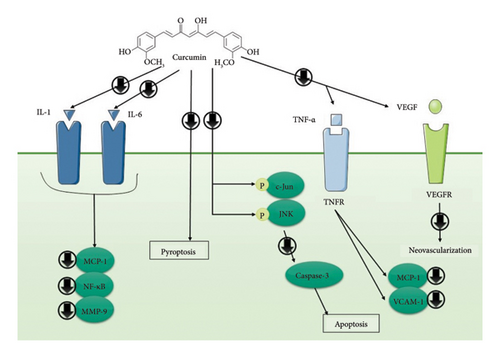
Numerous effects of curcumin on angiogenesis, apoptosis, tumorigenesis, and signal transduction pathways have been investigated in the past. Curcumin’s potential to regulate angiogenesis and inflammatory signaling in thoracic aortic aneurysms (TAAs) was investigated by the collection of human TAA samples and the formation of a TAA model with the use of CaCl2 applied periodically. Four weeks following surgery, TAA rats treated with either 1% carboxymethyl cellulose or curcumin were killed. In comparison to normal aorta walls, human TAA samples showed enhanced neovascularization and VEGF expression. Curcumin therapy decreased the size of the aneurysm and restored the elastic lamellae’s wavy form in rat tissues. Furthermore, curcumin lowered VEGF expression and neovascularization. According to an immunohistochemical study, curcumin dramatically reduced CD68+ and CD3+ clusters of differentiation cells’ ability to infiltrate TAA. Moreover, curcumin therapy reduced the expression of TNF-α, vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1, and monocyte chemoattractant protein-1 (MCP-1) [37]. According to recent research, JNK may be involved in the progress of AAA. Aortic aneurysm advancement may be efficiently stopped by directly blocking JNK with certain inhibitors. A different study looked at curcumin’s potential to stop apoptosis and JNK pathways in AAA. In the animal model of AAA generated by CaCl2, oral gavage with vehicle alone or 100 mg/kg of curcumin was administered every day for 4 weeks. The findings showed that curcumin greatly inhibited both the structural maintenance of medial elastin fibers and the increase of the thoracic aorta diameter caused by CaCl2. Most notably, curcumin therapy dramatically reduced the phosphorylation of c-Jun and JNK in AAA tissues, which was associated with a decrease in cell apoptosis. Furthermore, caspase-3 expression and Bax/Bcl-2 ratio were markedly decreased in the aortic walls of curcumin-treated rats [38] (Table 1 and Figure 2).
| Nutraceuticals | Participants | Key outcomes | Ref |
|---|---|---|---|
| Curcumin | Rats | Reduced expression of vascular cell adhesion molecule-1, intracellular adhesion molecule-1, monocyte chemoattractant protein-1, and tumor necrosis factor-α | [37] |
| Curcumin | Rats | Inhibition of JNK and apoptosis | [38] |
| Curcumin | Rats | Effects of anti-inflammation, antioxidative stress, and downregulation of ERK signaling pathway | [20] |
| Curcumin | Humans | No effects on urine IL-18, N-terminal pro-B-type natriuretic peptide, serum creatinine, and high-sensitivity C-reactive protein | [26] |
| Curcumin | Rats |
|
[27] |
| Curcumin | Rats |
|
[39] |
| Resveratrol | Rats | Increase SIRT1 expression, reducing inflammatory cells, inhibiting the inflammation response | [40] |
| Resveratrol | Rats | Upregulated ACE2 and inhibited AAA growth | [41] |
| Resveratrol | Rats | Inhibiting apoptosis, extracellular matrix degradation, autophagy, and inflammation of VSMCs via HMOX1 upregulation | [42] |
| Resveratrol | Rats | Reducing oxidative stress and apoptosis | [43] |
| Resveratrol | Rats | Reduced macrophage differentiation, monocyte-dependent inflammatory response, and aortic lumen enlargement | [21] |
| Quercetin | Rats |
|
[44] |
| Quercetin | Rats | Reduced oxidative stress and MMP activation, and modulation of JNK/AP-1 signaling | [45] |
| Quercetin | Rats | Reduced the proteases and blocking the inflammatory response | [46] |
| Pentamethylquercetin | Rats | Inhibited angiotensin II-induced AAA formation by bounding to C/EBPβ at Lys253 | [47] |
| Green tea | Rats |
|
[48] |
| Carotenoids | Rats | Protective effects against Ang II-induced AAA through ameliorating macrophage recruitment | [49] |
| All-trans retinoic acid (ATRA) | Rats | ATRA attenuated the progression of Ang II-induced AAAs through downregulating MMP2, MMP9, and AT-1 expression | [50] |
| Omega-3 | Rats | Inhibited aortic and macrophage-mediated inflammation | [51] |
| Omega-3 | Humans |
|
[52] |
| Omega-3 | Humans | Decreased PWV and heart rate | [53] |
| Omega-3 | Rats | Anti-inflammatory effects through the modulation of MMPs and TIMPs | [54] |
| Omega-3 | Rats | Inhibited critical remodeling pathways | [55] |
| Omega-3 | Rats | Inhibited Tak-1-JNK pathway through activating Gpr-120/Ffar-4 | [56] |
Researchers looked at how curcumin affected the growth of AAA. Three groups of ApoE (−/−) mice were randomly assigned: the AngII group, the control group, and the AngII + curcumin (AngII + Cur) group (100 mg/kg/day). ApoE (−/−) mice received subcutaneous miniosmotic pump implants to administer AngII for 28 days. The outcomes exhibited that treating with curcumin considerably reduced the incidence of AAA. The AngII + Cur group had substantially reduced levels of macrophage infiltration, TNF-α, and MCP-1 compared with the AngII group. Compared with the AngII group, superoxide dismutase (SOD) concentrations in the AngII + Cur group were considerably greater. Also, compared with the AngII group, the ERK1/2 phosphorylation in the AngII + Cur group was considerably lesser [20].
The impact of curcumin on the risk of complications and the inflammatory response following elective AAA repair was examined in a different investigation. During surgery, subjects in the therapy group were given oral curcumin (2000 mg, eight times) for four days. The outcomes demonstrated that no biomarker (urine N-terminal pro-B-type natriuretic peptide, IL-18, serum high-sensitivity C-reactive protein, and creatinine) was significantly impacted by either placebo or curcumin. Furthermore, there was no significant change between the groups in terms of the median duration of hospital admissions or the risk of clinical events, although there was an enhanced risk of acute renal damage with curcumin compared with placebo [26]. An animal model was used in another investigation to assess the impact of curcumin nicotinate (CurTn) on AAA. In vascular smooth muscle cells (VSMCs), CurTn inhibited pyroptosis, decreased Ang-II-induced AAA-associated proteins, and lowered the production of inflammatory markers. The overexpression of plasmacytoma variant translocation 1 (PVT1) sponging miR-26a inhibited the inhibitory effect of CurTn on AngII-induced pyroptosis and inflammation in VSMCs. miR-26a directly targeted and inhibited the expression of krüppel-like factor 4 (KLF4), ultimately leading to the deactivation of the PI3K/AKT signaling pathway. Furthermore, the PVT1/miR-26a/KLF4 pathway reversed the regulatory impact of CurTn on the pyroptosis of VSMCs caused by Ang-II [27]. C57Bl/6 mice were given transient elastase perfusion of the abdominal aorta to create AAAs, and then they were given with either water alone or 100 mg/kg of curcumin for 14 days to see if curcumin consumption affected aneurysmal degeneration. The results showed that while the groups’ aorta wall inflammation was comparable, the mice treated with curcumin had substantially higher medial elastin structural integrity. Curcumin-treated animals also showed considerably decreased aortic tissue concentrations of IL-1beta, IL-6, matrix metalloproteinase-9 (MMP-9), and MCP-1, as well as relative reductions in nuclear factor kappa B (NF-κB) and aortic tissue activator protein-1 DNA binding activities [39].
3.2. Resveratrol
A naturally occurring polyphenolic compound with anti-inflammatory and cardiovascular benefits is resveratrol. The beneficial influences of resveratrol on β-aminopropionitrile-induced aortic dissection (AD) in mice were examined in a work. The findings suggested that resveratrol could stop AD from happening. More importantly, the protective effect included decreasing the recruitment of inflammatory cells through endothelial cells, suppressing the inflammatory response, and increasing the gene expression of sirtuin 1 (SIRT1) in endothelial cells to aid in the rebuilding of their structure, all of which suppressed the progress of AD [40]. An investigation looked at the impact of angiotensin-converting enzyme 2 (ACE2) impairment on the development of AAA and the effectiveness of resveratrol in upregulating ACE2 in experimental AAA. An increased production of markers for inflammation and proteolytic enzymes were linked to ACE2 deletion in mice lacking in apolipoprotein. ApoE−/− animals given a high-fat diet and continuously injected with angiotensin II (Ang II) for 56 days showed growth inhibition of pre-established AAAs in response to resveratrol supplements (0.05/100-g chow). Elevated ACE2 and enhanced suprarenal aorta tissue levels of SIRT1 and ACE2 activities were linked to reduced suprarenal aorta dilatation in mice given resveratrol. Furthermore, there was a significant reduction in the gene expression of encoding the NF-κB light metallopeptidases 2 and 9, polypeptide gene enhancer in B cells 1, and the Ang I receptor within the suprarenal aorta tissue. In vitro, resveratrol upregulated ACE2 expression [41]. The purpose of another study was to assess the potential therapeutic benefits of resveratrol for AAA and the function of HMOX1 in these benefits. To confirm the impact and method of action of resveratrol, a rat model of AAA was developed. The findings showed that resveratrol reduced Ang II-induced VSMC dysfunction, stimulation of Ang II increased MMP-2, the messenger RNA (mRNA), and MMP-9 levels, reduced elastin expression, and elevated the secretion of inflammatory factors, apoptosis, and autophagy in VSMCs. Furthermore, in VSMCs pretreated with resveratrol as opposed to Ang II treatment alone, there was a considerable increase in heme oxygenase-1 and HMOX1 mRNA levels. HMOX1 silencing eliminated resveratrol’s impact on VSMC dysfunction. Also, resveratrol inhibited the progress of AAA in rats by elevating HMOX1 expression [42].
Another research used resveratrol to lessen the oxidative stress-induced liver damage brought on by reperfusion and ischemia from aortic cross-clamping during surgery. Forty mice were separated into four groups at random: I/R + resveratrol, I/R + glycerol + ischemia/reperfusion (I/R) (sham), and I/R. All groups slated for I/R had a 60-min shock phase followed by a 60-min ischemia period. Resveratrol (10 mg/kg) was given intraperitoneally 15 min before ischemia and right before reperfusion in the I/R + resveratrol group. Additionally, all groups received 120 min of anesthetic-assisted reperfusion following ischemia. Administering resveratrol improved GSH levels while decreasing edematous fields, MDA levels, cleaved caspase-3 positive, intralobular and interlobar necrosis, and vascular congestion [43]. In a model of AAA, resveratrol has been tested for its ability to prevent the growth of aneurysms and the monocyte-dependent inflammatory response. From 7 days before the AAA induction with elastase until 14 days post-induction, male rats were given either vehicle (ethanol) alone or resveratrol (10 mg/kg/die). Five littermates served as the untreated control group. The results exhibited that resveratrol inhibited the increase of CD62L-monocyte subset, the expression of CD143 monocytes, and MMP-9 and TNF-α levels in the bloodstream linked to the progress of AAA. Likewise, resveratrol therapy markedly reduced the expression of TNF-α, AAA enlargement, VEGF, vascular wall macrophage infiltration, and MMP-9 [21].
3.3. Quercetin
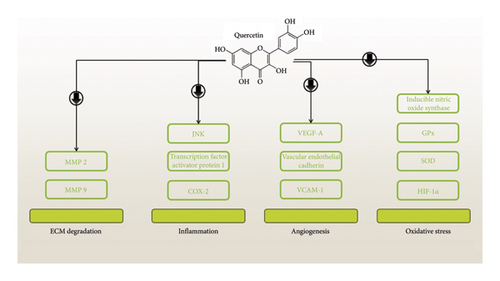
Quercetin’s antiangiogenic properties were examined in relation to experimental abdominal aneurysms. Periaortic administration of CaCl2 in an in vivo AAA mouse model was given to investigate quercetin’s ability to prevent angiogenesis. On the day of the AAA induction, quercetin was given once daily at a dosage of 60 mg/kg for 6 weeks. The findings showed that quercetin greatly reduced the development of aneurysms and medial neovascularization in AAA mice. As a result, quercetin reduced the production of proangiogenic mediators such as VEGF-A, VCAM-1, and vascular endothelial cadherin. Moreover, quercetin administration reduced hypoxia-inducible factor 1α (HIF-1α) and COX-2 expression. Additionally, it was shown that in aortic vascular smooth muscle cells isolated from AAA animals, quercetin-3-glucuronide reduced COX-2, VEGF-A, and HIF-1α expression, and MMP activity [44]. Another study set out to investigate if quercetin’s ability to suppress the formation of aneurysms is due to any involvement from an antioxidant mechanism. Quercetin was administered consistently to male C57/BL6 mice via extraluminal CaCl2 for 2 weeks before and 6 weeks after the AAA induction. According to data, quercetin reduced the incidence of AAA and prevented the aortic tissue’s synthesis of nitrotyrosine, reactive oxygen species, and lipid peroxidation during AAA development. Furthermore, mice administered with quercetin showed a markedly reduced expression of p47phox and inducible nitric oxide synthase. Additionally, there was a coordinated downregulation of the glutathione peroxidase (GPx) 1 and 3 expression, as well as SOD activity. Additionally, quercetin reduced the activation of the transcription factor activator protein (AP) 1 and decreased the production of phospho-JNK and JNK. Moreover, quercetin prevented MMP 9 and MMP 2 activation during the progress of AAA [45] (Figure 3).
Quercetin’s effects on inflammatory cell infiltration, cytokine production that follows, and protease activation in experimental AAA were investigated. In C57/BL6 mice, aneurysms were generated by administering calcium chloride intraluminally. One daily dose of quercetin (60 mg/kg) was given for 8 weeks, starting 2 weeks formerly AAA induction. The aorta diameter of mice given quercetin treatment shrank. Preventing AAA was linked to the maintenance of the medial structure, a decrease in the infiltration of macrophages and CD3(+) T cells in the aortic tissue, the release of inflammatory cytokines, and NF-κB activation. In aortic tissue, quercetin raised the expression of the gene for tissue inhibitors of (TIMP)-1 while reducing cathepsin B, MMP-9, MMP-2, and cathepsin K expression [46]. Another study looked at the underlying mechanism and the preventive influences of pentamethylquercetin (PMQ) against the progress of AAA. To create the AAA model, Ang II was continuously fed into ApoE−/− mice for 4 weeks. Five days prior to Ang II infusion, intragastric PMQ administration was started and sustained for 4 weeks. The incidence of Ang II-induced AAA, elastin degradation, VSMC phenotypic shift, aneurysm diameter expansion, and apoptosis were all dose-dependently decreased by PMQ. In Ang II-stimulated VSMCs, PMQ also prevented phenotypic transition and apoptosis. The therapeutic benefits of PMQ were reduced by PTEN overexpression mediated by AAV. The PMQ prevented VSMCs from changing their phenotype or going through apoptosis. It also reduced the development of AAA caused by Ang II [47].
3.4. Green Tea Polyphenols
The impact of epigallocatechin-3-gallate (EGCG) on the advancement of AAA was examined in an animal study. Extraluminal CaCl2 and intraluminal elastase were used to produce AAA. Two groups of rats—one for EGCG and the other for control—were assigned at random. In the EGCG group, each rat received an oral dose of EGCG (20 mg/day) beginning 2 weeks before AAA induction and continuing for an additional 4 weeks after induction. The findings demonstrated that on day 28, the abdominal aortic diameter was much lower in the EGCG group compared with that of the control group. Compared with the control group, the EGCG group had higher elastin content and medial layer wall thickness. The EGCG group had greater concentrations of lysyl oxidase and tropoelastin gene expression right before AAA induction. IL-1β and TNF-α expression, among other pro-inflammatory cytokines, were markedly downregulated in the EGCG group. On day 7, the EGCG group had considerably reduced gelatinolytic activity of MMP-9, while the group had significantly increased tissue inhibitors of MMP-1 expression [48].
4. Carotenoids and AAA: Mechanisms
The effects of α-tocopherol and β-carotene intake were evaluated in a study to determine the underlying processes of AAA. Ang II was infused intraperitoneally into 4-month-old Apoe (−/−) mice to induce aneurysms, and the animals were then given an oral diet supplemented with α-tocopherol and β-carotene for 6 days. The mice treated with Ang II showed a significant increase in circulating inflammatory cells, LDL, triglycerides, and cholesterol. The aneurysm-induced mice’s aorta showed increased VCAM-1, MMP-12, ICAM-1, M-CSF, MCP-1, MMP-9, and MMP-2 expression. There were large plaques, aneurysms, and inflammatory cells diffusive into the tunica intima. When an aneurysm was generated in the animals, the aorta’s size increased considerably. It is interesting to note that circulation and macrophage diffusion into the aortic tunica intima were significantly regulated by β-carotene. Additionally, it prevented atheromatous plaque from forming. The aneurysm-induced mice’s aorta diameter was dramatically reduced by β-carotene. After treatment, it also decreased the expression of ICAM-1, MCP-1, M-CSF, VCAM-1, MMP-9, MMP-2, PPAR-α, MMP-12, and PPAR-γ. Additionally, it was noted that inflammatory cells were diffusing into the tunica intima [49]. Another investigation examined the potential impact of all-trans retinoic acid (ATRA) on the progression of experimental AAA produced by Ang II. Mice devoid of apolipoprotein E (ApoE−/−) were randomized into four groups. For 28 days, mice assigned to the ATRA and AAA groups received continuous subcutaneous Ang II infusion, whereas the mice in the control and Sham groups received saline infusion. The findings demonstrated that the ATRA group’s abdominal aortic diameter was considerably lower than that of the AAA group and that on days 7, 14, and 28, the ATRA group’s blood pressure was lower. In the abdominal aortic tissue of the ATRA group compared with that of the AAA group, there was a notable decrease in the breakdown of elastic fibers, as indicated by the low expression of MMP9, MMP2, Ang II receptor type 1 (AT1), and EVG staining. Analysis revealed that ATRA therapy had a significant impact on the protein levels of MMP2, MMP9, retinoic acid receptor α (RARα), and AT1 [50].
5. Omega-3 Fatty Acids Affect AAA
Intake of omega-3 fatty acids through diet slows the advancement of atherosclerosis and averts more cardiovascular incidents. Ang II was infused into mice lacking apolipoprotein E to create the AAA model. Docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA) supplements were given to the mice. Following the delivery of DHA and EPA, there was a considerable increase of AAA formation and suppression of macrophage infiltration. In the aortas, transforming growth factor-β, MMP-9, TNF-α, MMP-2, MCP-1, and VCAM-1expression were considerably reduced with the administration of DHA and EPA. Following the delivery of DHA and EPA, there was a large increase in Ym1 expression and a significant reduction in arginase 2 expression. Following DHA and EPA infusion, peritoneal macrophages showed similar patterns [51]. Assessing the impact of DHA on antioxidant defense in macrophages from subjects with AAA was the goal of a different investigation. Males with age-matched men’s cells and small AAA were used, and they were treated with DHA for 1 hour before being exposed to 0.1 μg/mL of lipopolysaccharide (LPS) for 24 hours. Supplementing with DHA reduced the levels of IL-6 and TNF-α in the supernatants of macrophages. Heme oxygenase-1 mRNA expression and GPx activity were both elevated by DHA [52].
The impacts of omega-3 fatty acids (1.8 g/day) for 12 weeks on vascular stiffness markers in AAA subjects was assessed in a study [53]. Patients with AAA saw a substantial reduction in PWV and heart rate after a 12-week omega-3 fatty acid intake. Participants who received placebo pills showed no change at all. Although research suggests that individuals with AAA may benefit from omega-3 by having their aortic stiffness improved, the therapeutic consequences need to be thoroughly understood [53]. Another study looked at how an 8-week diet high or deficient in omega-3 affected the production of MMP and the breakdown of elastin in ApoE (−/−) mice. Eight weeks of a diet high or deficient in omega-3 were given to ApoE (−/−) mice before they received a 2-day infusion of Ang II. According to the findings, the omega-3 index for diets high in PUFA values and low in PUFA values was 13.03% and 3.78%, respectively. In inflammatory and endothelial cells, MMP-9 was lower in mice given the high-than-low omega-3 diet. Mice fed the high-oleic acid diet exhibited lower TGF-β1 stain intensity and TIMP-1 in inflammatory cells than those fed the low-oleic acid diet. Also, MMP-2 was not affected by diet [54].
In another research, the aortas of BALB/c mice given an EPA diet showed less inflammation, had a much smaller width than those of animals fed a control diet, and the aortic elastic lamina was mostly preserved. Interestingly, after receiving EPA therapy, CT imaging also showed much less aortic calcification. Mechanistically, following EPA therapy, the levels of MMP9, MMP2, and TNFSF11 in the aortas decreased. In agreement with this discovery, EPA-treated RAW264.7 macrophages had reduced Mmp9 levels following TNF-α simulation [55]. The goal of the study was to look into the mechanism by which EPA given orally stops severe AAAs from forming in mice lacking the osteoprotegerin (Opg) gene. The increased development of AAAs in Opg-KO mice, like the increase in aortic width with the degradation of elastic fibers in the media, was mitigated by EPA in the CaCl2-induced AAA model. The results demonstrated that EPA decreased the expression of MMP-9 in the aortic medium, as well as the phosphorylation of c-JNK and TGFβ-activated kinase-1/Map3k7 (Tak-1). EPA prevented the activation of the Tak-1–JNK pathway and upregulation of MMP-9 expression in smooth muscle cell cultures caused by rh-TRAIL [56].
6. Challenges and Limitations
The use of polyphenols, such as curcumin and resveratrol, in human models presents several challenges that researchers must navigate to better understand their potential health benefits. Despite their promising biological activities, the translation of these findings from laboratory settings to human applications is fraught with complexities. One of the primary challenges in utilizing polyphenols like curcumin and resveratrol is their poor bioavailability [57]. Curcumin, for instance, is known for its low solubility and rapid metabolism, leading to minimal amounts reaching systemic circulation after oral ingestion. Similarly, resveratrol is quickly metabolized and eliminated, limiting its effective concentration in the bloodstream [57]. This raises questions about the appropriate dosages needed to achieve therapeutic effects and whether higher doses might be necessary to overcome these bioavailability issues [57, 58]. Carotenoids are believed to play a significant role in the health benefits associated with higher vegetable intake. Several dietary factors influence the bioavailability of carotenoids, with the type of food matrix being a key element [59]. Specifically, the bioavailability of β-carotene from vegetables is relatively low (14% from mixed vegetables) compared to that of purified β-carotene added to a simple food matrix. In contrast, the difference for lutein is much less pronounced, with a relative bioavailability of 67% from mixed vegetables. Processing methods like mechanical homogenization or heat treatment can significantly improve the bioavailability of carotenoids from vegetables, potentially increasing it from 18% to six times higher [59]. The amount of dietary fat needed for carotenoid absorption appears to be minimal (approximately 3–5 grams per meal), although this can vary based on the physicochemical properties of the carotenoids consumed. Therefore, studies on the functional benefits of carotenoids should take into account that the bioavailability of β-carotene is significantly greater when it is provided as a pure compound in foods compared to its natural occurrence in food sources [59].
Determining the optimal dosage of nutraceuticals for therapeutic effects is another significant challenge. Most preclinical studies that demonstrate beneficial effects are conducted using concentrations of curcumin or resveratrol that far exceed what can realistically be achieved in human subjects through dietary intake or supplementation. The disparity between these concentrations raises concerns about the practicality of translating findings from animal models to human applications. Additionally, individual variations in metabolism, age, health status, and genetic predispositions can further complicate dosage recommendations [58]. While short-term studies may highlight the potential benefits of polyphenols, long-term safety remains an area requiring thorough investigation. Concerns about toxicity and adverse effects associated with the prolonged use of high doses of curcumin or resveratrol have yet to be fully addressed [60]. Some studies have reported gastrointestinal discomfort, liver toxicity, or interactions with medications at high doses. Establishing a clear understanding of the long-term safety profile of these compounds is crucial before recommending widespread use for health benefits [58, 60].
7. Conclusions
Aortic rupture is a risk factor for AAA, an inflammatory illness that progresses over time. Currently, patients are maintained with careful monitoring of the growth of their aneurysms and, for those with bigger aneurysms, repair surgery, as drug treatments have not proven to be effective in treating them. The theory posits that the pro-resolving, antioxidant, and anti-inflammatory properties of nutraceuticals and their substances will lessen the inflammatory infiltration load into the aorta wall, thereby delaying the advancement of the illness. Animal model studies have offered several possible insights into the effectiveness of therapies including the pharmaNutrition delivery of these bioactive substances, even though this theory has not yet been assessed in clinical trials. Reduced cytokine production, inflammatory cell infiltration, MMP activity and expression, and elastin degradation are among the advantages that have been reported. It is reassuring to see data that are consistent with several well-established animal models, such as aortic hypoperfusion, subcutaneous Ang II perfusion, periaortic CaCl2 administration, and intraluminal elastase perfusion. While much of the research on nutraceuticals has been done on rodent models of AAA, the results presented here should provide the field with the push it needs to go toward clinical trials.
8. Future Research
The exploration of nutraceuticals as potential therapeutic agents for AAA is an emerging field that warrants further investigation. However, the number of human studies investigating the role of nutraceuticals in the management and prevention of AAA was low; conducting further well-designed clinical trials is essential to establish the efficacy and safety of nutraceuticals in AAA management. RCTs should be implemented to assess the impact of specific nutraceuticals, such as curcumin, resveratrol, and fish oil, on AAA growth rates and clinical outcomes. These studies should include diverse populations to evaluate the effects across different demographics, including age, sex, and genetic predispositions. Furthermore, we suggest that longitudinal cohort studies could provide valuable data on the long-term effects of nutraceutical consumption on AAA. By tracking individuals with known risk factors for AAA over time, researchers can assess dietary patterns and supplement use in relation to AAA development. This approach could help identify critical windows for intervention and inform dietary recommendations. More importantly, research should also focus on establishing dose–response relationships for various nutraceuticals in the context of AAA. Understanding the optimal dosages required to achieve therapeutic effects without adverse outcomes is crucial. This can be achieved through both preclinical studies using animal models and subsequent human trials that assess different dosages and formulations. Other than this, investigating the synergistic effects of combining multiple nutraceuticals may yield enhanced benefits compared to single-agent therapies. Unfortunately, we could not find any evidence in this regard, and therefore, future studies should explore how combinations of antioxidants, anti-inflammatory agents, and other nutraceuticals can work together to modulate AAA development and progression more effectively than individual compounds. As the understanding of the role of nutraceuticals in managing AAAs continues to evolve, addressing these research suggestions will be crucial in determining their efficacy and safety. By focusing on mechanistic insights, clinical trials, longitudinal studies, and innovative formulations, researchers can pave the way for evidence-based dietary strategies that may significantly impact AAA prevention and management.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
The data gathering, writing, methodology, editing, evaluation, and text drafting were helped by Yi Yang, Kun Chen, Jun Chen, and Sima-Sadat Sabihi. The final paper was confirmed for submission by all authors.
Funding
The authors received no specific funding for this work.
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.




