Colla Corii Asini Prevents H2O2-Induced Cellular Aging and Skin Barrier Damage in HaCaT Keratinocytes
Abstract
Background: Aging weakens the skin barrier, causing dryness and inflammation. Colla Corii Asini (CCA) shows antioxidant and antiaging potential, possibly protecting against aging-related skin damage.
Objective: This study aims to evaluate the effects of CCA on H2O2-induced aging and skin barrier damage in human keratinocytes (HaCaT) cells and to explore its underlying mechanisms.
Method: We performed proteomics on CCA, simulated its digestion to obtain CCA intestinal absorption solution (CCA IAS), and tested its therapeutic effects on H2O2-induced skin aging and barrier damage in HaCaT cells.
Results: Proteomic analysis of CCA reveals its potential to prevent aging and repair skin barrier damage by regulating key protein domains and biological processes. CCA enhances intestinal absorption of amino acids, hydroxyproline, and proteins, with significant antioxidant capabilities in the absorption fluid. CCA intestinal absorption solution (CCA IAS) enhances the proliferation and migration of H2O2-induced HaCaT cells and reduces oxidative stress by regulating reactive oxygen species (ROS) levels and antioxidant enzyme activity. Additionally, CCA reduces gene expression of aging markers p21, p53, and senescence-associated secretory phenotype (SASP). CCA IAS modulates gene expression of differentiation markers FLG, IVL, K10, tight junction proteins CLD-1, ZO-1, E-cadherin, and AMPs, while inhibiting phosphorylation in the mitogen-activated protein kinase (MAPK) pathway.
Conclusion: This study demonstrates that CCA alleviates oxidative stress-induced aging and repairs H2O2-induced barrier damage in HaCaT cells, potentially through reducing phosphorylation levels in the MAPK pathway. These findings provide a foundation for further mechanistic and application-focused research on CCA.
1. Introduction
The global population is rapidly aging, leading to increasingly prominent issues of premature skin aging. Aging skin becomes dull, rough, and saggy; is more prone to impaired wound healing, dryness, and itching; and increases the risk of cancer [1]. Current antiaging strategies include topical care, laser treatments, and chemical exfoliation. However, their effects are limited, vary greatly among individuals, and have uncertain long-term outcomes, potentially even accelerating aging [2]. The weakening of the skin barrier function is a key feature of aging. Repair strategies often rely on topical drugs or metabolites [3], but these are not very effective and can cause side effects such as allergic reactions and skin intolerance due to individual differences. Therefore, a safe, effective, and widely applicable preventive skin antiaging strategy is urgently needed to meet the growing public demand.
As a traditional Chinese medicine, CCA has a long history of use in the field of traditional medicine [4]. It is sweet and mild, considered a superior tonic. It can be used as both food and medicine. Long-term use may replenish blood, combat aging, improve complexion, and boost immunity. Recent research highlights that CCA is rich in proteins and amino acids, with over 80% being collagen, which helps maintain skin elasticity and has antioxidant properties [5]. The amino acids in CCA promote collagen synthesis (e.g., hydroxyproline), remove free radicals, and repair and protect epidermal cells [6], playing a crucial role in skin health and antiaging. Despite CCA’s potential in preventing skin aging and repairing barrier damage, research on its mechanisms is limited. Exploring these mechanisms can lead to new antiaging strategies and improve skin conditions, holding significant clinical importance.
The epidermis uses a “brick wall structure” formed by keratinocytes and intercellular lipids to protect against external damage and maintain internal homeostasis. The aging of keratinocytes is a key factor in skin aging, leading to reduced expression of markers like filaggrin (FLG) and claudin-1 (CLD-1), which weakens barrier function. As age increases, the proliferation and differentiation ability of epidermal cells declines, and the epidermal calcium gradient is lost [7], leading to weakened barrier function and dry, rough skin [8]. Additionally, the repair mechanisms in aging skin are impaired. There is an increase in pro-inflammatory factors and a decrease in antimicrobial peptide genes (AMPs) expression. Abnormal activation of the mitogen-activated protein kinase (MAPK) signaling pathway exacerbates cell damage and inflammation [9], reducing defense capability, accelerating aging, and further damaging the barrier function.
To investigate whether CCA exerts antiaging effects by preventing barrier damage caused by skin aging, this study used H2O2 to induce oxidative damage in human keratinocytes (HaCaT) cells, evaluated the antioxidant activity of CCA, and explored its protective effects on HaCaT cell aging and skin barrier damage. The goal is to provide scientific evidence for the application of CCA in skin health, beauty, and antiaging, while laying the foundation for further studies on its mechanisms and potential applications.
2. Materials and Methods
2.1. Sample Protein Processing and Mass Spectrometry Analysis
In this study, sample proteins were prepared and analyzed using mass spectrometry. After reduction and alkylation, trypsin was added at a 1:50 mass ratio and digested at 37°C for 20 h. Digestion products were desalted, freeze-dried, reconstituted in 0.1% FA solution, and stored at −20°C.
For mass spectrometry analysis, Solution A was 0.1% formic acid in the water and Solution B was 0.1% formic acid in 84% acetonitrile water. The chromatographic column was equilibrated with 95% Solution A, and the sample was loaded via an automatic sampler. Full scans and MS2 scans collected 20 fragment spectra. Raw files were analyzed using Proteome Discoverer 1.4, yielding identified protein results.
2.2. Reagents and Antibodies
CCAs manufactured were provided by Dong-E-E-Jiao Co. Ltd., China. The antibodies used included FLG (c-66192, Santa Cruz Biotechnology, Inc., Europe), Ki67 (66009-1-Ig, Proteintech, America), Lamin B1 (A1910, ABclonal, China), E-cadherin (20874-1, Proteintech, America), tubulin (AB52866, Abcam, America), JNK (A22376, ABclonal, China), p-JNK (AP0631, ABclonal, China), P38(8690S, Cell Signaling, USA), p-P38 (4511S, Cell Signaling, USA), Erk (ABclonal, A4782, China), and p-Erk (4370S, Cell Signaling, USA).
2.3. Preparation of CCA IAS
After CCA was subjected to in vitro enzymatic digestion, CCA IAS was obtained through the intestinal sac everted experiment. The detailed procedure is described in the Supporting Information 1.
2.4. Cell Culture and Cell Viability Assay
HaCaT cells were sourced from Qingqi Shanghai Biotechnology Development Co., Ltd. China, and cultured in DMEM with 10% FBS and antibiotics. Cell viability was assessed using a CCK-8 assay. Cells were seeded in 96-well plates and treated with various CCA IAS concentrations. To test H2O2-induced toxicity, cells were cultured with 3.125%, 6.25%, and 12.5% CCA IAS for 24 h, and then treated with 400 μM H2O2 for 4 h. Cell viability was determined using the formula: cell viability (%) = (A − A1)/(A0 − A1) × 100%.
2.5. Cell Migration Assay
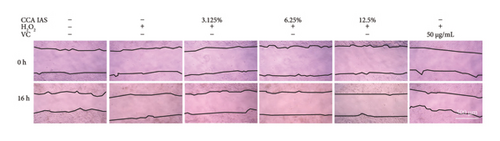
HaCaT cells were seeded in a 24-well plate at a density of 1 × 106 cells per well. After reaching confluence, the cells were pretreated with 3.125%, 6.25%, and 12.5% CCA for 24 h, followed by 400 μM H2O2 for 4 h. A scratch was made to create a wound, and cells were washed with PBS. The wound was replenished with drug-containing IAS, and cell migration was observed at 0 and 36 h. The migration area was calculated using ImageJ software with the formula: migration rate (nh) = (blank area at 0 h − blank area at nh hours)/blank group migration area [10].
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
HaCaT cells (2 × 105 cells/mL) plated for 24 h were exposed to CCA (3.125%, 6.25%, 12.5%). After 24 h, total RNA was prepared by general extraction method with TRIzol reagent. Complementary DNA was synthesized from total RNA using the ReverTra Aceq PCR RT Master Mix Kit (FSQ-201, TOYOBO, Japan). Subsequently, qRT-PCR analysis was performed employing the SYBR Green Real-time PCR Master Mix Kit (QPK-201, TOYOBO, Japan) and a PCR instrument. The fluorescence was measured at the end of each elongation step. Values were normalized to GAPDH mRNA expression both for HaCaT. All of the primers (Table S1) were designed by Sangon Biotech.
2.7. Immunocytochemistry
After drug treatment in a 96-well plate, cells were washed with PBS and fixed with methanol for 1 h, followed by three PBS washes. Cells were then treated with a permeabilization solution for 20–30 min and blocked with a blocking solution for 1 h. Primary antibody was added and incubated overnight at 4°C. After washing, a secondary antibody was added and incubated for 1 h, protected from light. Finally, cells were washed with PBS, treated with DAPI for 5 min, and observed.
2.8. Statistical Analysis
The experiment was performed with a minimum of three independent parallel replicates. Statistical analysis was conducted using GraphPad Prism 8.0. Statistical significance was determined using two-way ANOVA. The results are presented as mean ± standard error of the mean (SEM). A significance level of p < 0.05 or lower was considered statistically significant.
3. Result
3.1. Proteomics Research on the Role of CCA in Antiaging and Skin Barrier Repair Function
In this study, we analyzed the role of CCA in preventing skin aging and barrier damage through proteomic analysis. Results showed that CCA’s protein domains include intermediate filament protein, laminin EGF domain, keratin type II head, and collagen triple helix repeat (Figure 1(b)). These domains play crucial roles in delaying skin aging and maintaining skin barrier function. For example, intermediate filament protein and keratin type II head enhance the integrity of the skin barrier, while collagen triple helix repeat provides skin strength and elasticity, with its reduction being a key feature of skin aging, leading to wrinkles and sagging.
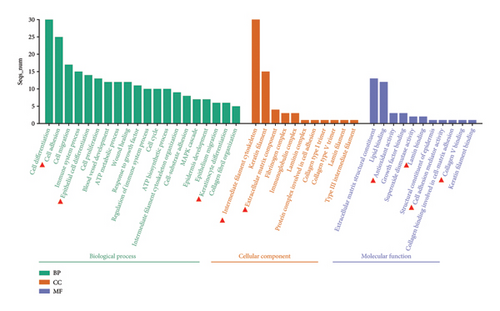
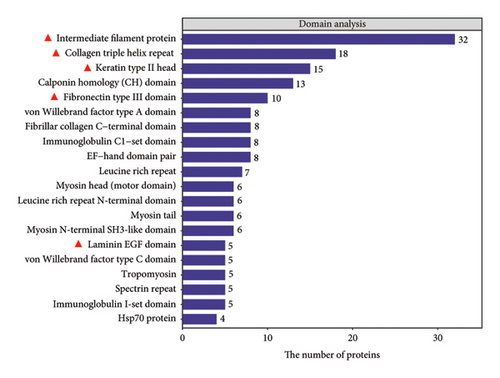
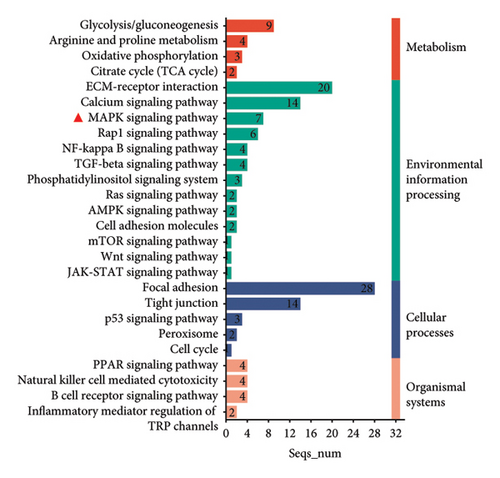
The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis revealed that CCA significantly affects key biological processes such as cell adhesion, keratinocyte differentiation, collagen binding, antioxidation, extracellular matrix formation, and keratinocyte cytoskeleton construction (Figure 1(a)). These processes are crucial for delaying epidermal aging and maintaining skin barrier function. Further analysis identified associations with key signaling pathways, including the MAPK signaling pathway, involved in cell growth, differentiation, and aging (Figure 1(c)). The above analysis provides insights into CCA’s role in preventing epidermal aging and skin barrier damage.
3.2. Preparation and Analysis of CCA IAS: Amino Acids, Protein, and Antioxidant Capacity
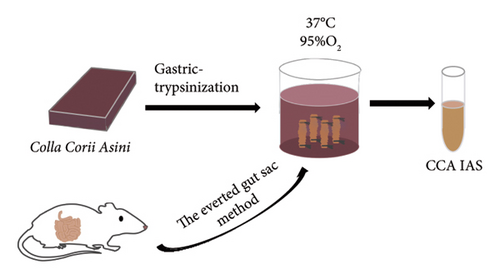
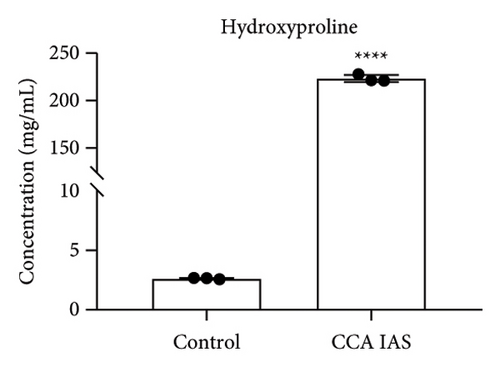
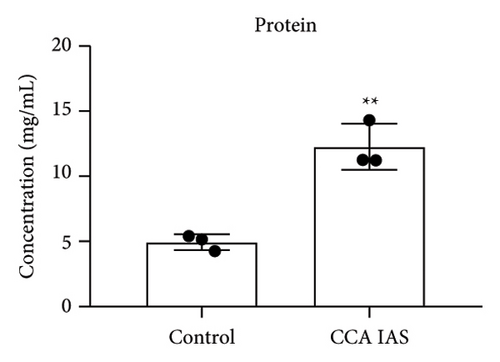
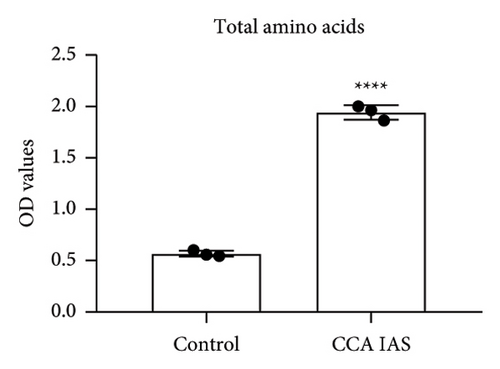
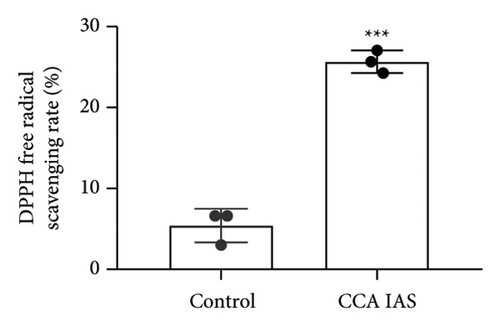
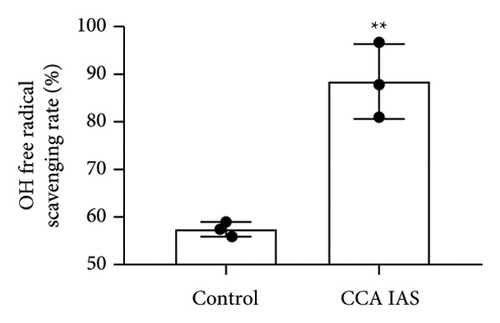
In this study, we used the traditional oral administration method for CCA. To simulate gastrointestinal digestion and absorption, CCA was subjected to in vitro enzymatic hydrolysis using pepsin and trypsin [11], and absorption was simulated using a rat gut sac model to obtain CCA IAS (Figure 2(a)). The obtained CCA IAS was analyzed for amino acid, hydroxyproline, and protein content. Results showed that the total amino acid, hydroxyproline, and protein content in CCA IAS were significantly higher than in the blank control (Figures 2(b), 2(c), and 2(d)), indicating successful preparation of CCA IAS for subsequent in vitro cellular efficacy and mechanism studies. Additionally, the antioxidant activity of CCA IAS was evaluated using DPPH [12] and hydroxyl radical scavenging assays. Results showed that CCA IAS exhibited strong radical scavenging ability (Figures 2(e) and 2(f)), indicating good in vitro antioxidant activity.
3.3. CCA Inhibits the Cytotoxic Effects of H2O2 on HaCaT Cell Proliferation and Promotes Cell Migration
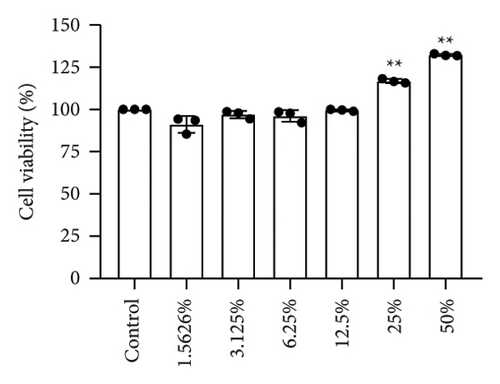
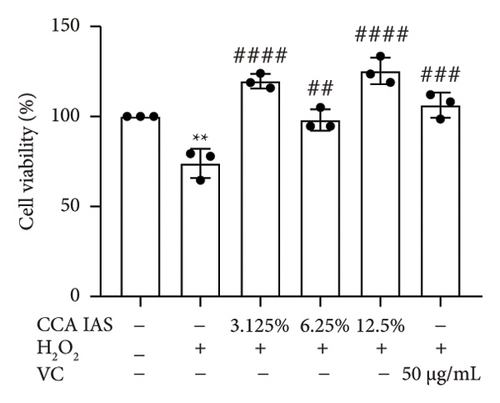
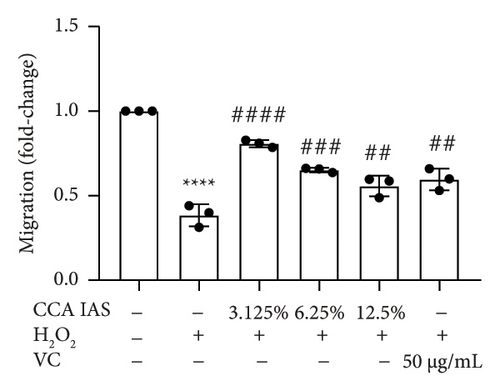
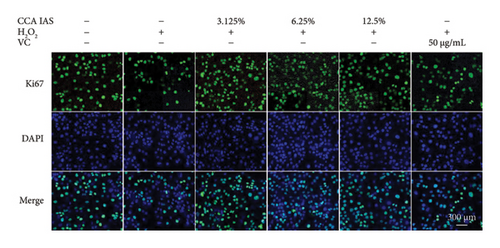
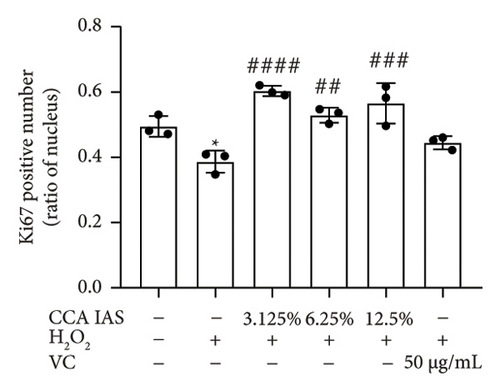
After treatment with different concentrations of CCA IAS, the survival rate of HaCaT cells remained above 90%, indicating that CCA IAS has no toxic effect on HaCaT cells (Figure 3(a)). Further investigation on the effect of CCA IAS on H2O2-induced HaCaT cell damage showed that Ki67 protein is expressed in the nuclei during all cell cycle phases except G0, peaking during cell division [13]. CCK-8 and Ki67 immunofluorescence results showed that CCA IAS significantly increased the number of Ki67-positive cells after H2O2 exposure (Figures 3(b), 3(c), and 3(d)), indicating that CCA inhibits the cytotoxicity induced by H2O2. Subsequently, the cell migration assay showed that CCA IAS significantly promoted cell migration (Figures 3(e) and 3(f)), and its effect was superior to the positive control group. These findings confirm that CCA has a significant protective effect against H2O2-induced cell damage.
3.4. CCA Alleviates Oxidative Stress Damage Induced by H2O2 in HaCaT Cells
When the concentration of free radicals in the body is too high, it leads to oxidative stress, which can disrupt metabolism and function. The aging process itself can increase oxidative stress levels in cells. To study the effect of CCA IAS on reactive oxygen species (ROS) levels in H2O2-induced HaCaT cells, we used the DCFH-DA fluorescence probe to measure ROS content in HaCaT cells. Results showed that CCA could reduce the increase in ROS levels induced by H2O2 (Figures 4(a) and 4(b)). SOD can catalyze the conversion of superoxide radicals, preventing damage to cells [14]. MDA is a lipid peroxidation product caused by oxygen free radicals, and its level can serve as an indicator of oxidative stress [15]. GSH is an important antioxidant that reacts with oxygen free radicals to reduce oxidative stress damage to cells. The results showed that CCA significantly reduced MDA and SOD levels while enhancing GSH activity, comparable to the improvements in the positive drug group (Figures 4(c), 4(d), and 4(e)).
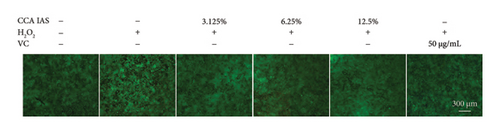
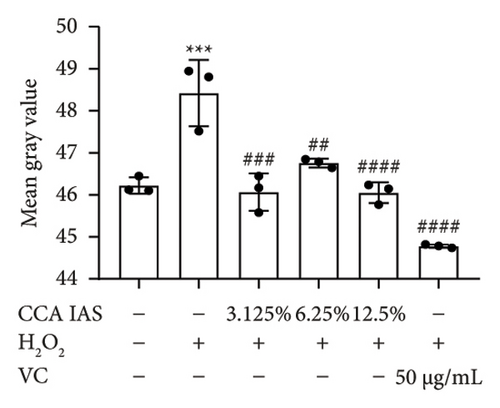
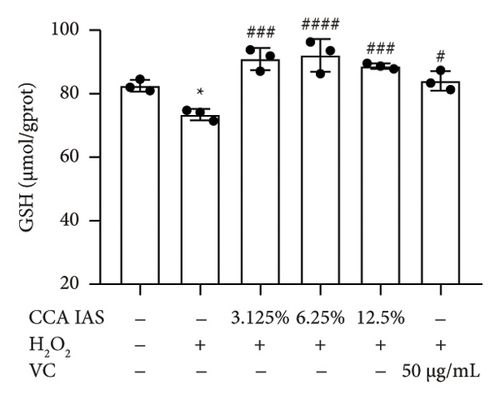
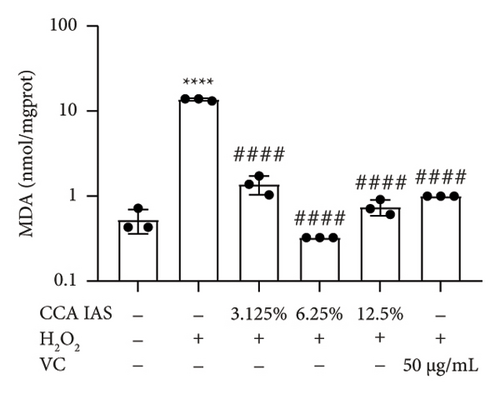
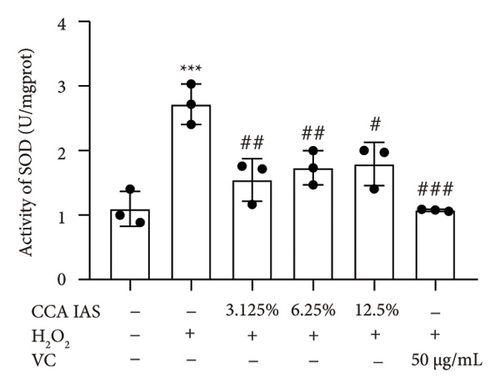
The above results indicate that CCA IAS can enhance GSH activity, which helps in scavenging ROS, thereby reducing MDA and SOD levels and alleviating oxidative stress damage caused by H2O2 to cells.
3.5. CCA Prevents H2O2-Induced Aging in HaCaT Cells
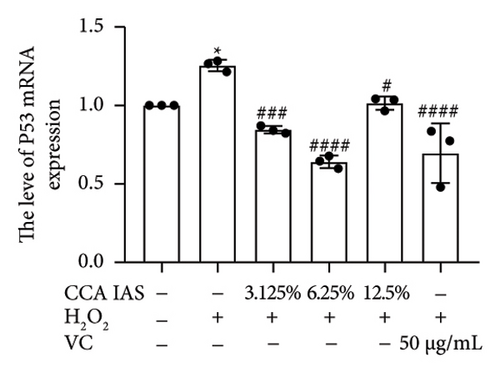
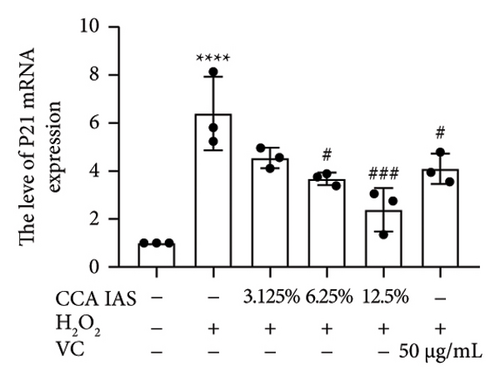
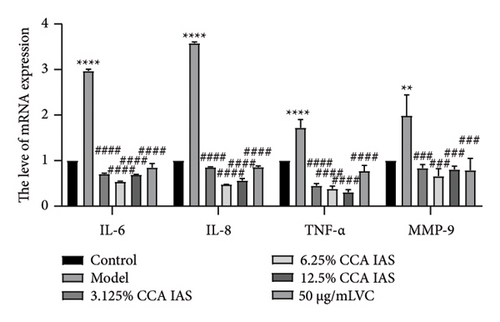
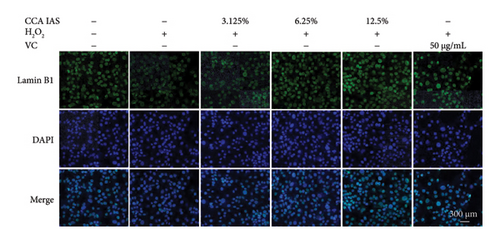
Cellular aging is a complex biological process associated with various aging-related pathological processes. In the DNA damage response pathway, p53 is a key regulator involved in controlling the cell cycle and cellular aging. This study used HaCaT cells as a model, inducing oxidative stress with H2O2 and analyzing the expression of aging-related genes. Results showed that after H2O2 induction, the expression of p53 and p21 was significantly upregulated, which is considered a biomarker of cellular aging [16]. Further studies found that after treatment with CCA IAS, the expression levels of p53 and p21 in H2O2-induced cells were significantly reduced (Figures 5(a) and 5(b)), suggesting that CCA IAS may inhibit cellular aging by suppressing p53 and p21 expression. Furthermore, treatment with CCA IAS enhanced the reduction of Lamin B1 caused by H2O2 treatment (Figures 5(d) and 5(e)), a nuclear morphology marker protein. Additionally, CCA IAS significantly reduced the expression levels of IL-6, IL-8, TNF-α, and MMP-9 secreted by senescent cells (Figure 5(c)), comparable to the improvements in the positive drug group. In summary, CCA has the ability to prevent H2O2-induced cellular aging, possibly by reducing oxidative stress damage to cells.
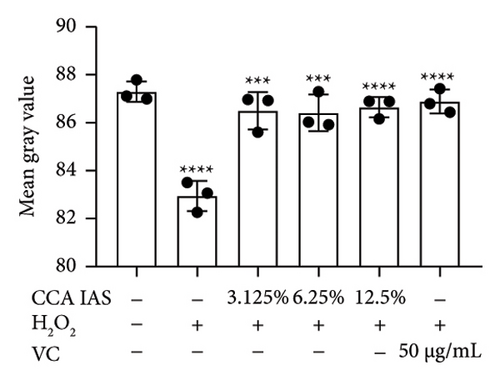
3.6. CCA Prevents Damage to the Skin Barrier in H2O2-Induced Senescent HaCaT Cells
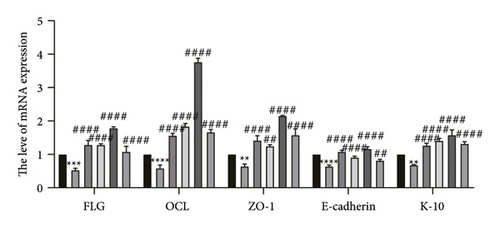
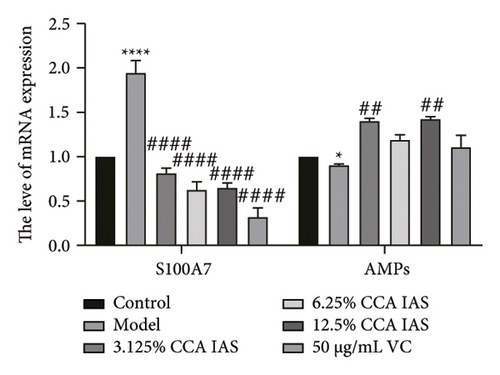
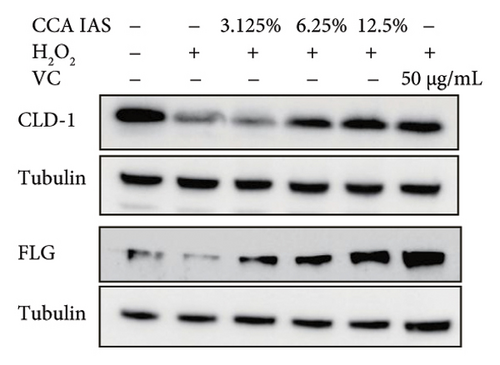
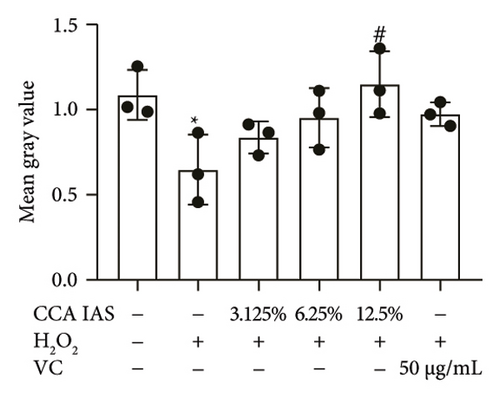
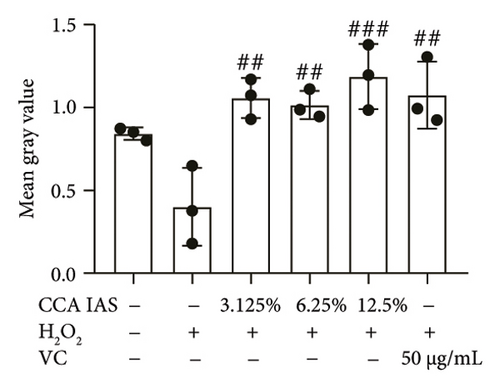
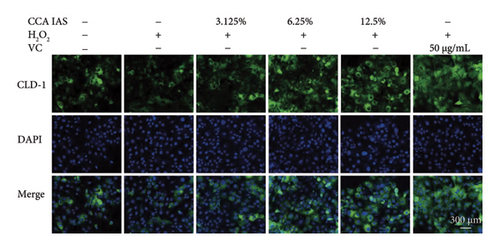
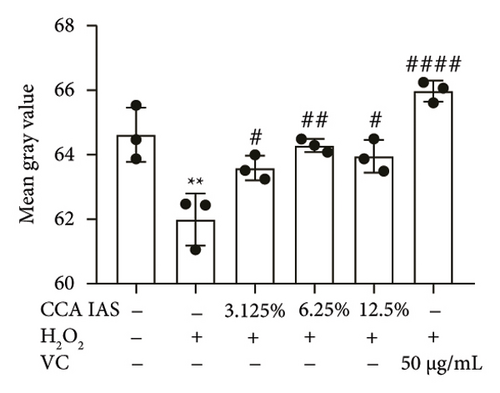
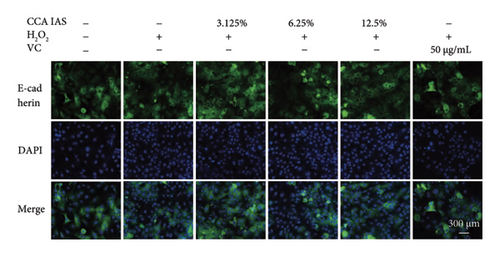
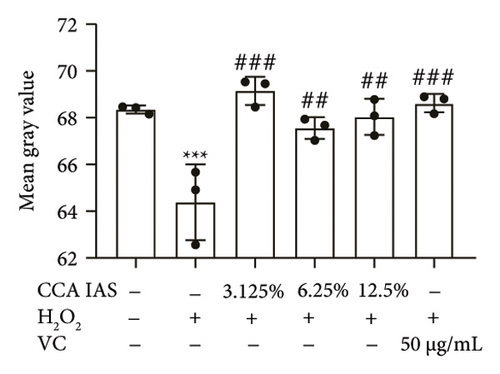
The skin barrier is the body’s primary defense against external harmful factors, primarily relying on the formation of the stratum corneum. Proteins in the stratum corneum, such as keratinocyte differentiation proteins (FLG, IVL, etc.) [17], tight junction proteins (CLD-1, ZO-1, etc.), and E-cadherin, which regulates the integrity and function of epithelial tissues, play crucial roles in constructing the skin barrier. To further determine whether CCA can enhance the barrier function of aged HaCaT, we measured skin barrier–related proteins such as FLG and E-cadherin. The results showed that CCA IAS significantly increased the levels of these proteins induced by H2O2 treatment (Figures 6(a), 6(c), 6(d), 6(e), 6(f), 6(g), 6(h), and 6(i)). Additionally, AMPs can inhibit the expression and release of pro-inflammatory factors, further enhancing skin immunity. The study found that after H2O2 treatment, CCA IAS significantly increased the levels of AMPs and reduced S100A7 levels, comparable to the improvements observed in the positive drug group (Figure 6(b)). This indicates that CCA IAS can regulate the skin’s immune defense mechanisms, thereby enhancing skin immunity. In summary, CCA has the function of repairing skin barrier damage caused by aging.
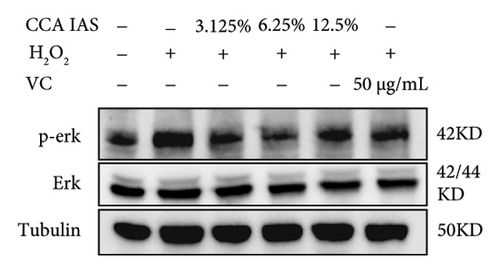
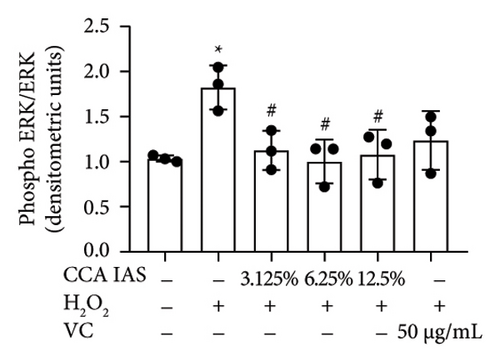
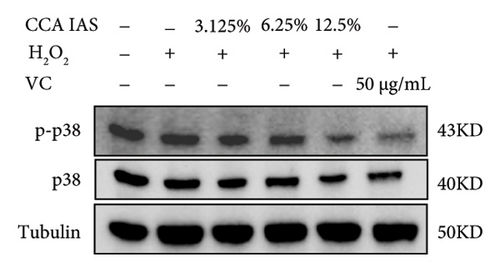
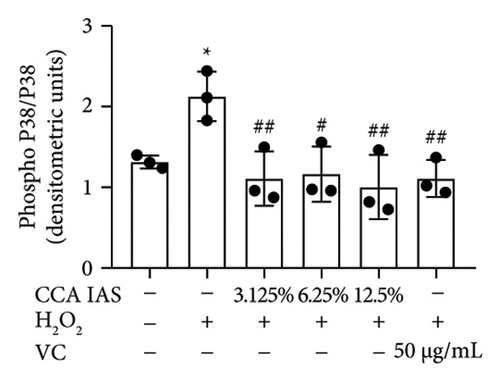
3.7. The Effect of CCA on the MAPK Signaling Pathway in H2O2-Induced HaCaT Cells
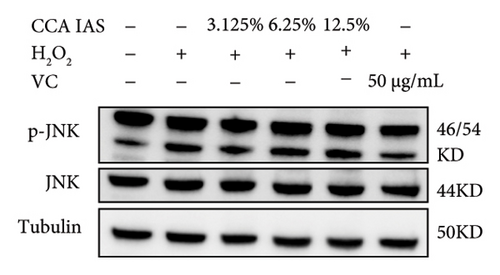
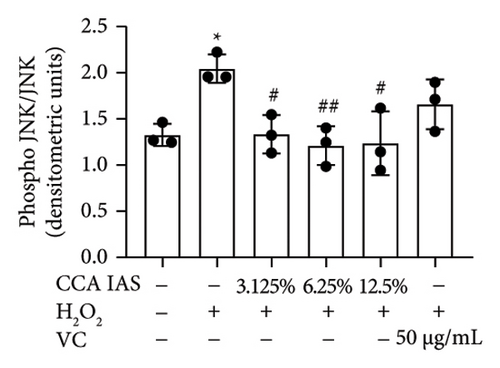
The MAPK pathway is a signal transduction pathway activated by external stimuli, regulating intracellular oxidative stress responses and closely related to immune responses. H2O2-induced excessive ROS can activate the MAPK signaling pathway [18]. To investigate whether CCA prevents keratinocyte aging and enhances the skin barrier via the MAPK pathway in H2O2-exposed HaCaT cells, we used Western blotting to evaluate the effect of CCA on MAPK pathway protein levels. The results showed that H2O2 treatment significantly increased the phosphorylation levels of MAPKs proteins (p-p38, p-ERK, and p-JNK) in HaCaT cells, while pretreatment with CCA IAS significantly reduced these protein levels, comparable to the improvements observed in the positive drug group (Figures 7(a), 7(b), 7(c), 7(d), 7(e), and 7(f)). These results indicate that CCA pretreatment inhibits abnormally activated MAPK signals by reducing the phosphorylation levels of MAPK pathway proteins. Therefore, we speculate that CCA may attenuate H2O2-induced oxidative stress damage and impaired differentiation in HaCaT cells through the MAPK pathway.
4. Discussion and Conclusion
Based on the structural and functional damage to the skin barrier caused by aging, this study investigates the antioxidant effects of CCA to explore its role in preventing epidermal aging and barrier damage.
As the global population ages, people seek new antiaging solutions, including natural products. CCA, a traditional Chinese medicine, has been analyzed through proteomics to reveal its potential in preventing epidermal aging and skin barrier damage. Components like intermediate filament proteins, collagen, keratin, and laminin, along with CCA’s antioxidant activity, suggest its role in slowing aging and maintaining skin health. These proteins are crucial for keratinocyte differentiation, cell adhesion, and forming an effective skin barrier. The MAPK pathway is key in responding to oxidative stress by regulating inflammation, antioxidant gene expression, and promoting cell survival and repair [19]. This study explores CCA’s material basis and antioxidant activity to understand its role in preventing epidermal aging and repairing skin barrier damage via the MAPK pathway.
As an animal-derived medicine primarily used orally, CCA differs from simple in vitro enzymatic hydrolysis methods [20]. We obtained the CCA IAS through in vitro digestion and subsequent intestinal absorption, better simulating its absorption in the gastrointestinal tract. After in vitro enzymatic hydrolysis, CCA significantly enhances protein absorption in the intestine, supplements skin collagen, promotes keratinocyte proliferation, and accelerates skin cell renewal. Additionally, CCA improves the absorption efficiency of total amino acids, helps maintain skin hydration, and enhances skin barrier function. Specific amino acids, such as lysine and arginine, possess antioxidant properties that reduce free radical damage, protect skin cells, and delay aging [21]. CCA also increases the levels of hydroxyproline in the intestine. This characteristic amino acid of collagen participates in the synthesis of extracellular matrix and the formation of the stratum corneum, promoting collagen maturation and deposition, thereby providing a material foundation for preventing skin aging. Additionally, CCA can scavenge free radicals, indicating that its active components absorbed through gastrointestinal digestion exhibit strong antioxidant activity. Recent studies show that enzymatically hydrolyzed CCA protects dermal cells from oxidative stress and UV damage [22], indicating its potential in antiaging and preventing epidermal barrier damage.
Oxidative stress is one of the main causes of skin aging and can lead to skin barrier damage. Therefore, in this study, we used H2O2 to treat HaCaT cells to induce oxidative damage. When ROS accumulate in epidermal cells, the body increases the levels of antioxidant enzymes SOD and MDA to eliminate excess free radicals. After CCA pretreatment, it can scavenge excess free radicals, thus protecting HaCaT cells from H2O2-induced oxidative damage. In summary, CCA not only has free radical scavenging ability but also exerts antioxidant effects by regulating the levels of antioxidant enzymes. Moreover, compared to single antioxidant substances, the various active components in CCA can synergize to provide more comprehensive antioxidant effects, better meeting the demands of modern consumers for health products. Therefore, CCA, as an antioxidant functional medicine, has potential for further development in preventing aging and repairing the skin barrier.
According to the free radical theory, excessive ROS is a major cause of cellular aging, damaging protein structures, triggering inflammation and apoptosis, and accelerating skin aging [23]. Studies show creatine and niacinamide can prevent H2O2-induced aging in human fibroblasts [24]. Our results indicate that CCA reduces aging markers p53, p21, and SASP [25], suggesting it can prevent H2O2-induced aging in HaCaT cells by scavenging free radicals. Changes in the skin barrier are a significant characteristic of skin aging, making skin more susceptible to irritation and infection. Protecting and repairing the skin barrier is crucial for preventing skin aging. Studies show CCA repairs the skin barrier by promoting fibroblast migration and type I collagen expression [26]. This study notes that aging leads to stratum corneum damage and impaired differentiation. We found that CCA maintains the skin barrier by increasing the expression of structural proteins FLG, IVL, CLD-1, E-cadherin, and keratin K-10, reducing water loss. Given that CCA’s main components are proteins and amino acids, it may enhance skin barrier function by supplementing structural proteins. Additionally, CCA enhances anti-inflammatory and antibacterial capabilities, reducing oxidative stress damage and maintaining skin barrier integrity. These results reveal CCA’s protective effects on the aging skin barrier. Our study also found that CCA increases H2O2-induced MAPK expression, inducing protein phosphorylation and inhibiting abnormal MAPK signals. Thus, CCA has broad potential applications in skin beauty and therapy.
However, this study has certain limitations. First, the specific mechanisms by which CCA prevents aging and repairs the skin barrier remain unclear and require further investigation. Additionally, the skin models used to study the effects of the drug have certain limitations; for instance, 3D bioprinted skin models better simulate the natural structure of the skin [27]. The study found that the pharmacological effects of CCA IAS did not exhibit clear concentration dependence within a certain range, possibly due to saturation during absorption and synergistic effects among its complex components, which warrant further investigation. In conclusion, while this study provides valuable insights into the potential of CCA in preventing skin aging and repairing skin barrier damage, its limitations require addressing in future research.
In summary, this study revealed the potential mechanisms of CCA in preventing oxidative stress–induced skin aging and barrier damage. It not only provides experimental evidence for the potential applications of CCA in antiaging and skin barrier repair but also offers critical guidance for developing innovative CCA-based skincare products and therapeutic strategies.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Xinyu Gao and Shuhua Ma: project design, data collection, and manuscript drafting; Yanan Sun and Lishuang Li: support in design, data collection, and method formulation; Yi Wang and Huiyuan Gao (corresponding author): overall project supervision, guidance, data analysis, and manuscript writing. Xinyu Gao and Shuhua Ma are co-first authors, among whom Xinyu Gao is the main first author.
Funding
This study was funded by Shandong Dong-E-E-Jiao Co., Ltd. (HX2023001), the National Natural Science Foundation of China (No. 82205294), the Central Public Welfare Research Institutes (Nos. JJPY2022006, RXRC2022004, and XTCX2021002), and Taishan Industrial Experts Program (No. tscy20180234), and Guidelines for Central Government Funding Projects in Local Scientific and Technological Development (YDZX2023090).
Acknowledgments
We thank the Morphology Laboratory of the Experimental Research Center, China Academy of Chinese Medical Sciences, for providing the experimental platform and technical guidance.
Supporting Information
Supporting file: Preparation of CCA IAS and sequences of primers used in qRT-PCR.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




