Antioxidant, Antibacteria, and Anti-Inflammatory Effects of Cordyceps militaris Extracts and Their Bioactive Compounds
Abstract
This study presents the bioactive compounds, antioxidant, antibacteria, and anti-inflammation of C. militaris fruiting body (FB) and FB with substrate (FBS). C. militaris FB and FBS were extracted by water, ethanol, and methanol. The chemical composition analysis of C. militaris extracts from FB and FBS showed bioactive compounds including adenosine, cordycepin, and carotenoids using HPLC. Moreover, the aqueous extract of FB and FBS showed the highest antioxidant activity against DPPH and ABTS radicals, supported by the presence of adenosine, cordycepin, and carotenoid compounds. Moreover, the ethanolic and methanolic extracts of C. militaris and the bioactive compounds, cordycepin and carotenoids, exhibited the greatest bactericidal activity against enteric pathogenic bacteria. In addition, C. militaris extracts and bioactive compounds were confirmed as new agents to prevent the adhesion and invasion of enteric pathogenic bacteria on Caco-2 colon cells. This finding also demonstrated the anti-inflammatory activity found in the aqueous extract of C. militaris and bioactive compounds on the LPS-stimulated Caco-2 cell model, which had the efficacy to suppress inflammatory moderators including iNos, Cox-2, NF-κB, TNF-α, AP-1, TLR-4, IL-1ß, and IL-6. Therefore, C. militaris extract and its bioactive compounds, cordycepin and carotenoids, impeded the adhesion and invasion of enteric pathogenic bacteria on colonic epithelial cells and also promoted anti-inflammation mechanisms. This study attests to C. militaris as an alternative therapeutic agent to prevent enteric pathogenic bacterial infection and inflammation due to its proven health benefits and high level of antioxidants.
1. Introduction
Enteric bacteria are generally found in the intestines of animals and humans. Some forms of enteric bacteria are harmless, such as normal flora in the gut, but others can lead to infection as enteric pathogenic bacteria [1]. Enteric pathogenic bacteria can cause diarrheal diseases, which are the leading cause of mortality and morbidity across the globe, and estimated to cause approximately 1.6 million deaths per year, across all ages [2]. Moreover, diarrhea is the fourth leading cause of death in children under 5 years, with an estimated 446,000 deaths per year [3]. Enteric bacterial pathogens have several genera, including Escherichia, Salmonella, Shigella, Yersinia, Vibrio, and Campylobacter, and thrive in distinct environmental habitats and within wild animals [4]. E. coli groups are categorized into seven groups, most of them falling under enteropathogenic E. coli (EPEC) or enterohaemorrhagic E. coli (EHEC), and are all linked to contamination in food and water [5]. EPEC is associated with diarrheal disease and is a major cause of diarrhea in infants. The EHEC strain, known as E. coli O157:H7, has been a source of many infectious outbreaks in the United States [6]. The symptoms of this strain can manifest after an infection with lower than 100 bacteria and can cause hemolytic–uremic syndrome (HUS) disease [7]. Another bacterial strain, Shigella dysenteriae, is well known for causing shigellosis disease. These bacteria are related to HUS disease because it can produce the Shiga toxin, which induces the disruption of the glomerular endothelial cells, resulting in renal failure [8, 9]. The Salmonella strain is able to contaminate undercooked chicken and eggs. Salmonella is associated with enteric fever, including typhoid and paratyphoid fever, which are caused by two S. enterica subspecies, enterica serovar Typhi (S. Typhi), and S. enterica serovars Paratyphi A, B, and C [10]. Moreover, diarrheal illness is often caused by the Vibrio cholerae strain, which is transmitted via the fecal–oral route through contaminated water and food [11].
Enteric pathogenic bacteria have displayed several mechanisms of infection that can adhere and multiply in the epithelial cells before entering internal tissues, leading to severe diseases in humans [12]. A vital step before bacterial infection is the adhesion of the pathogens to the host cells. Thus, investigating the interference and adhesion-blocking agents is a productive way to prevent bacterial infection. Antibiotics have been used successfully for the treatment of bacterial infections; however, they are synthesized from chemical agents and may induce many side effects in patients, as well as produce resistant strains after prolonged use [13]. Moreover, enteric pathogenic bacterial infection can penetrate the epithelial lining of the gastrointestinal tract and stimulate aberrant intestinal inflammation, which can progress to chronic inflammatory diseases, such as inflammatory bowel disease (IBD) [14]. One of the factors prompting IBD disease is lipopolysaccharides (LPS) of Gram-negative bacteria, especially in enteric pathogenic bacteria. LPS can stimulate the inflammatory mechanisms through the activation of Toll-like receptor (TLR)-4 in the transmembrane protein family, as well as a pattern-recognition receptor [15]. Furthermore, epithelial cells produce proinflammatory cytokines to disrupt tight junctions, leading to increased gut permeability [16].
To date, many people are concerned about the health drawbacks of synthetic drugs and attempt to find natural products to replace them. In this study, Cordyceps militaris, a medicinal mushroom, is evaluated for its biological activity to treat diseases. C. militaris is an entomopathogenic fungus that is parasitic on insects, attach to the underground pupae and larvae of lepidopteran insects, and capable of forming a fruiting body (FB). C. militaris is classified into the phylum Ascomycota, class Ascomycetes, order Hypocreales, and family Clavicipitaceae. It has a long traditional history as it can be used as medicinal mushrooms and folk remedy in East Asia [17].
C. militaris contains several bioactive substances, including γ-aminobutyric acid (GABA), glycolipids, glycoproteins, cordycepin, carotenoids, sterols, statins, phenolic compounds, flavonoids, vitamins, and biominerals, and is used presently for various medicinal purposes [18–20]. Recent research has reported the extract of C. militaris having many pharmacological benefits, such as improving insulin resistance and secretion [21], antifibrotic [22], antiangiogenetic [23], anti-inflammatory [24], anticancer [25], and antiviral effects [26]. The extract of C. militaris was also reported to have antibacterial effects on skin pathogenic bacteria, such as Staphylococcus aureus, Pseudomonas aeruginosa, Cutibacterium acnes, and methicillin-resistant S. aureus (MRSA) [27]. Cordycepin (3′-deoxyadenosine), a secondary metabolite produced by C. militaris (Hypocreales and Cordycipitaceae), was found in insects that were susceptibility to entomopathogenic fungi infection. Thus, cordycepin could suppress insect immune response during fungal infection [28]. Cordycepin and heterocycle 5,5′-dibuthoxy-2,2′-bifuran, fractioned from C. militaris extract, exhibited antibacterial activity against Bacillus subtilis and E. coli [29]. Additionally, the yellow pigment of C. militaris was characterized to be carotenoids (xanthophylls and cordyxanthin), which are known for their anticancer properties [30, 31]. C. militaris showed a high antioxidant activity and cytotoxicity effects on human colorectal HT-29 cancer cells due to the extract that revealed a high phenolic and flavonoid contents [32]. However, previous studies were unclear on the ability of C. militaris extract to inhibit the growth and adhesion of enteric pathogenic bacteria. Therefore, the main purpose of this study is to investigate functional activity between C. militaris FB and FB with substrate (FBS) extracts since the substrate that used for culturing C. militaris may affect functional activity of the extract.
This study introduced innovative insights into the effects of C. militaris extracts on enteric pathogenic bacteria, examining its active compounds for their antioxidant, antibacterial, antiadhesion, and anti-invasion properties. Additionally, the anti-inflammatory effects of C. militaris extracts were investigated using the Caco-2 colon cell model. These findings highlight the potential of C. militaris as a multifaceted therapeutic agent, addressing the need for novel treatments against bacterial infections and inflammation.
2. Materials and Methods
2.1. C. militaris Cultivation
The C. militaris var. CMRU-04 strain was obtained from AAVA Group Co., Ltd., Bangkok, Thailand, in collaboration with Mushroom Research and Development center (MRDC), Chiang Mai, Thailand (mrdcthailand.com). C. militaris was cultured on potato dextrose agar (PDA) for 1 week. Subsequently, the mycelium was transferred to PDB and incubated in a shaking incubator (120 rpm) at 18°C–22°C with 70% humidity in a dark condition for 2 weeks. The C. militaris from the PDB medium was inoculated into substrate containing PDB mixed with barley and rice bran and further incubated in light condition at 18°C–22°C with 80% humidity. After 2 months culture, FBs and the substrate were harvested.
2.2. C. militaris Extraction
The dried C. militaris was separated in to two groups, FB and FBS for extraction (Figure S1). 95% ethanol (RCI Labscan, Bangkok, Thailand), methanol (RCI Labscan, Thailand), and distilled water were used as solvents for extraction at the ratio of 1:10 (w/v) dry sample to solvent. The 200 g of dried C. militaris was macerated with 2000 mL of solvents such as 95% ethanol or methanol and then incubated on shaking incubator at room temperature for 72 h. For the aqueous extraction, dried C. militaris was extracted with distilled water by incubation at 45°C for 3 h in a water bath [33]. The extraction process was repeated twice, and the extract was filtered using Whatman No. 1 filter paper. Then, the extracts were evaporated using a rotary evaporator with temperature control at 45°C and reduced pressure at 100 mbar for 16 h to preserve the active compounds in C. militaris and prevent degradation due to high temperatures. After that, the extracts were dried through lyophilization. The dried extracts were stored at −20°C until use.
2.3. Determination of Bioactive Compounds in C. militaris Extract
2.3.1. Cordycepin and Adenosine
The contents of cordycepin and adenosine were detected by high-performance liquid chromatography (HPLC) [34]. The extracts and standard compounds, cordycepin (Sigma-Aldrich, Darmstadt, Germany) and adenosine (Sigma-Aldrich, Germany), were filtered through a 0.45 μm microfilter, and 20 μL of the filtered sample was injected into the HPLC system (Agilent Technologies, Santa Clara, CA, USA). A 254 nm UV detector in the Inertsil ODS-3 column (4.6 × 150 mm, 5 μm, GL Sciences, Tokyo, Japan) was employed for further analysis. The HPLC system was controlled with a flow rate of 1.0 mL/min and 30 min running time. Cordycepin and adenosine in the C. militaris were separated by water and methanol (RCI Labscan, Thailand) at a ratio of 92:8 (v/v) elution solvents, and the standard compounds were used for comparison with the extract. The peak area of each compound was calculated by Agilent ChemStation level-5 program, and then, standard graphs of each compound were generated by plotting concentrations (12.5–200 μg/mL) against peak area. Thus, the content of cordycepin and adenosine in the extract was calculated from the standard graph of each compound.
2.3.2. Total Carotenoid Content
The total content of carotenoids was measured by UV–visible absorption spectra against a solvent blank using a UV–visible spectrophotometer (DYNEX Technologies, Chantilly, VA, USA). The extract solution (25 mg/mL) was prepared in 50% ethanol, and the UV–visible diffuse absorbance was recorded from 200–600 nm [35]. The peak of each extract was detected at a wavelength showing maximum absorbance (λmax) and compared to the absorption spectrum of β-carotene (Sigma-Aldrich, Germany) standard compound (λmax = 448). Moreover, the standard curve of β-carotene was generated with various concentrations and the absorbance of compounds. The total content of carotenoids was calculated by comparing the extract to the content of standard β-carotene and is expressed as mg β-carotene equivalent per gram extract (mg BCT/g extract).
2.3.3. Total Phenolic Compounds
The total phenolics content was determined by the Folin–Ciocalteu method [36]. C. militaris extract (250 μL) was mixed with 125 μL of 50% Folin–Ciocalteu reagent (Merck, Billerica, MA, USA) and 250 μL of 95% ethanol. The mixture was incubated in the dark at room temperature for 5 min. Subsequently, 250 μL of 5% sodium carbonate (RCI Labscan, Thailand) was added and then further incubated in the dark at room temperature for 1 h. The absorbance was detected at 725 nm using a spectrophotometer (Genesys 20, Thermo Scientific, Germany). The total phenolics content was calculated by comparing the extract to the content of standard gallic acid (Sigma-Aldrich, Germany) and is expressed as mg gallic acid equivalent per gram extract (mg GAE/g extract).
2.3.4. Total Flavonoids Content
The total flavonoids content was determined by the aluminum chloride colorimetric method [36]. C. militaris extract (500 μL) was mixed with 100 μL of 10% aluminum chloride (RCI Labscan, Thailand), 1.5 mL of methanol, 100 μL of 1 M potassium acetate (RCI Labscan, Thailand), and 2.80 mL of deionized water. The mixture was incubated in the dark at room temperature for 30 min, and then, the absorbance was detected at 415 nm using a spectrophotometer (Genesys 20, Thermo Scientific, Germany). The total flavonoids content was calculated by comparing the extract to the content of standard quercetin (Sigma-Aldrich, Germany) and is expressed as mg quercetin equivalent per gram extract (mg QUE/g extract).
2.4. Determination of Antioxidant Activity of C. militaris Extract
2.4.1. DPPH Radical Scavenging Assay
The antioxidant activity of the extract was assessed by comparing the samples to that of standard gallic acid and is expressed as mg gallic acid equivalent per gram extract (mg GAE/g extract).
2.4.2. ABTS Radical Cation Decolorization Assay
The antioxidant activity of the extract was assessed by comparing the samples to that of standard Trolox and is expressed as mg Trolox equivalent per gram extract (mg TEAC/g extract).
2.5. Antibacterial Activity of C. militaris Extract
The minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were determined by the broth dilution method [39]. Enteric pathogenic bacteria, including Escherichia coli, E. coli O157:H7 DMST 12743, Shigella dysenteriae DMST 1511, Salmonella Typhi DMST 22842, Vibrio cholerae, and Bacillus cereus DMST 6228, were cultured in Mueller–Hinton (MH) broth (Difco, USA) and incubated at 37°C for 18–24 h. The MH broth (0.1 mL) was dispensed into a sterile 96-well plate, and 500 mg/mL of the extract or compound (0.1 mL) was added into the first well. The sample was serially diluted into each well, and bacterial culture at 0.5. McFarland standard (1.5 × 108 CFU/mL) was then added to all wells and then incubated for 18–24 h. Gentamycin was used as an antibiotic control for the drug susceptibility test. The MIC value was determined from the lowest concentration of extract not showing any bacterial growth when compared to the control. The selected culture broth with the MIC value was streaked on an agar plate, and the presence of a bacterial colony was determined after incubation. The MBC value was found at the lowest concentration of extract inhibiting bacterial growth by 99.9%.
2.6. Adhesion and Invasion Assay
The cytotoxicity of C. militaris extract and compounds was determined on Caco-2 colorectal cancer cells (Caco-2 cells) by MTT assay [40]. The Caco-2 cells (102 cells/mL) were grown on cover slips in 6-well plates and incubated at 37°C in 5% CO2 for 24 h. C. militaris extract was added to the epithelial cells in nontoxic concentrations. The bacterial culture was adjusted to 0.5 McFarland standard (1.5 × 108 CFU/mL or OD600 = 0.08–0.1) and added to the wells. The culture was incubated at 37°C in 5% CO2 for 1 h. For the adhesion assay, the cultivation mixture was washed three times with phosphate buffer saline (PBS) to remove any nonadherent bacteria from the epithelial cells. The cell-adherent bacteria were fixed with 95% methanol for 5 min and stained with Giemsa (LABCHEM, Australia) solution (0.38% in 50% methanol and 50% glycerol) for 15 min. The adherent bacteria were counted on 100 epithelial cells under a compound microscope [41]. For the invasion assay, the number of internalized bacterial cells was counted using the total plate count technique after inhibiting nonadherent bacteria with 100 μg/mL gentamycin (BIO BASIC CANADA, Canada) and lysed with 0.05% trypsin (Caisson Labs, Smithfield, UT, USA) [42]. The inhibition of bacterial adhesion and invasion was calculated in percent and compared to the cell control.
2.7. Cytotoxicity of C. militaris Extract
The cytotoxicity of C. militaris extract was evaluated using MTT assay [40]. The colon cell model was employed using the human colorectal carcinoma (Caco-2) cell. The cell was cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (HyCloneTM, Pittsburgh, PA, USA), 100 Units/mL penicillin, and 100 μg/mL streptomycin (CAISSON, Smithfield, UT, USA). After incubation at 37°C in a 5% CO2 incubator (SHEL LAB, USA), the cells were washed twice with PBS (pH 7.4) and trypsinized with 0.05% (v/v) trypsin-EDTA solution (CAISSON, USA). The cells (1 × 105 cells/mL) were plated into 96-well plates and incubated at 37°C in a 5% CO2 incubator for 24 h. After incubation, various concentrations of C. militaris extracts and compounds were added and incubated at 37°C for 24 h. MTT (BIO BASIC CANADA, Canada) solution (2 mg/mL) was added to the cells and incubated for 4 h. Next, blue formazan crystals were dissolved with DMSO, and the absorbance of the solutions was recorded at 540 and 630 nm using a microplate reader (EZ Read 2000, Biochrom, Cambridge, UK). The percentage of cell viability was calculated and compared to the cell control.
2.8. Nitric Oxide (NO) Production Measurement
The NO production was measured after Caco-2 cells were induced with LPS and treated with C. militaris extract or compounds, by using collected culture media and the Griess Reagent system [43]. The Caco-2 cells (1 × 105 cells/mL) were seeded in a 96-well plate and incubated at 37°C in a 5% CO2 incubator for 24 h. After incubation, 10 μg/mL LPS (Sigma-Aldrich, St. Louis, MO, USA) were added and incubated for an additional 1 h. The C. militaris extracts or compounds were added into each well in nontoxic concentrations, and the solution was incubated for another 24 h. Following, 100 μL of cultured medium was transferred to a new 96-well plate before mixing with the same volume of Griess reagent. The reaction was incubated at room temperature for 10 min, and the absorbance was measured by a microplate reader at 540 nm. The NO production was calculated and compared to the LPS-treated group.
2.9. Determination of Inflammatory Gene Expression in Caco-2 Cells by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The mRNA of inflammatory gene expression, including iNos, Cox-2, NF-κB, TNF-α, AP-1, TLR-4, IL-1ß, and IL-6 genes, was evaluated after treatment with C. militaris extract and bioactive compounds by employing qRT-PCR. Caco-2 cells (2 × 105 cells/mL) were stimulated with 10 μg/mL LPS for 1 h and then treated with the samples for 24 h. After incubation, the total mRNA of Caco-2 cells was extracted using TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA). The RNA quality was accepted at A260/A280 ratios above 2.0 by the NanoDrop system (Thermo Scientific, Waltham, MA, US). The RNA (1000 ng) was reversed to cDNA using ReverTra Ace qPCR RT Master Mix (TOYOBO, Osaka, Japan). The master mix of the reaction contained 100 ng of cDNA, 2X SensiFAST SYBR No-ROX Mix (BIOLINE, London, UK), 400 nM of forward primer, and 400 nM of reverse primer. The negative control was contained the PCR reaction without the sample. The sample analysis was carried out through qTOWER3 Real-Time PCR (Analytik Jena, Jena, Germany). Achieved cycle thresholds (Ct) were analyzed with the ΔΔCT method to determine the differences in the gene expression. The Ct values of the apoptotic genes were calculated from the relative expression by normalizing the data with the expression of the internal control gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The analyzed data from three independent experiments were tested for statistical differences using Student’s t-test. Sequences of the forward and reverse primers for PCR were designed based on previous studies. Primer sequences for the amplification of inflammatory genes are shown in Table S1.
2.10. Statistical Analysis
All investigations were performed by way of three independent experiments on separate occasions. All data are presented as mean ± SD values of three independent experiments. A t-test and one-way ANOVA (Tukey’s post hoc test with 95% confidence interval) analysis were used to analyze the results of both the treatment and control groups. p value < 0.05 was considered statistically significant.
3. Results
3.1. C. militaris Extract Yields and Their Bioactive Compounds
The aqueous extracts of C. militaris FB and FBS showed percentage yields of 48.38% and 22.95%, respectively. The lower percentage yields of 9.98% and 5.79% were obtained from ethanolic extracts of C. militaris FB and FBS. Additionally, percentage yields of C. militaris methanolic extracts from FB and FBS were 25.64% and 7.24%, respectively. The HPLC assay was served for the identification of bioactive compounds of C. militaris extracts (Figure S1), including adenosine and cordycepin. The highest amount of adenosine and cordycepin was found in the aqueous fraction of C. militaris FB and FBS, with values of 2.156 ± 0.012 and 3.315 ± 0.007 mg/g extract (p < 0.05), respectively. Moreover, the ethanolic extract of C. militaris FB showed values of adenosine and cordycepin of 0.873 ± 0.024 and 1.763 ± 0.003 mg/g extract. In methanolic extract, the adenosine and cordycepin were 0.516 ± 0.061 and 2.030 ± 0.002 mg/g extract, respectively (Table 1 and Figure S2–S3). A high content of total carotenoids was found in the aqueous and methanolic extracts of C. militaris FBS with values of 8.593 ± 0.236 and 8.044 ± 0.116 mg BCT/g extract, respectively. The ethanolic extract of C. militaris FBS had the highest total phenolic content of 24.032 ± 3.470 mg/g extract (p < 0.05), followed by C. militaris FB aqueous extract of 22.318 ± 3.912 mg GAE/g extract. A high level of flavonoids was present in the ethanolic and methanolic extracts of C. militaris FB of 1.332 ± 0.089 (p < 0.05) and 0.918 ± 0.080 mg QUE/g extract, respectively. Therefore, the presence of these bioactive compounds may contribute to various antioxidant, antibacterial, and anti-inflammatory activities.
| C. militaris | Adenosine (mg/g extract) | Cordycepin (mg/g extract) | Carotenoids (mg BCT/g extract) | Phenolics (mg GAE/g extract) | Flavonoids (mg QUE/g extract) |
|---|---|---|---|---|---|
| Aqueous extracts | |||||
|
|
|
|
|
|
| Ethanolic extracts | |||||
|
|
|
|
|
|
| Methanolic extracts | |||||
|
|
|
|
|
|
- Note: The results are presented as mean value ± SD. The highest significant value (∗) in each bioactive compound is indicated and determined by Student’s t-test (p < 0.05) from independent experiments (n = 3).
- Abbreviations: FB, fruiting body; FBS, fruiting body with substrate.
3.2. Antioxidant Activity of C. militaris Extract
The radical scavenging activities of C. militaris extract were investigated against DPPH and ABTS free radicals using DPPH radical scavenging assay and ABTS radical cation decolorization assay, respectively. The results show that the aqueous extract of FBS had the highest antioxidant activity against DPPH and ABTS radicals of 5.105 ± 0.457 mg GAE/g extract and 18.090 ± 1.190 mg TEAC/g extract (p < 0.05), respectively (Table 2). Furthermore, the antioxidant activity in the FBS methanolic and FB ethanolic extracts could be scavenged for DPPH radicals at values of 3.318 ± 0.057 and 3.123 ± 0.190 mg GAE/g extract, respectively (Table 2). Moreover, the methanolic extract of FB and FBS showed the antioxidant capacity against ABTS radicals at the values of 15.252 ± 0.642 and 16.727 ± 0.854 mg TEAC/g extract, respectively (Table 2). Therefore, this study has confirmed that the active compounds, including adenosine, cordycepin, and carotenoids, found in the extracts have been shown the ability to inhibit DPPH and ABTS radicals. Carotenoids and cordycepin demonstrated the highest antioxidant activity by inhibiting DPPH and ABTS radicals, with values of 0.235 ± 0.011 mg GAE/g extract and 8.124 ± 2.047 mg TEAC/g extract, respectively (Table 2).
| C. militaris extracts | Antioxidant activity | |
|---|---|---|
| DPPH (mg GAE/g extract) | ABTS (mg TEAC/g extract) | |
| Aqueous extracts | ||
| FB | 2.153 ± 0.312 | 13.948 ± 0.966 |
| FBS | 5.105 ± 0.457∗ | 18.090 ± 1.190∗ |
| Ethanolic extracts | ||
| FB | 3.123 ± 0.190 | 8.459 ± 0.478 |
| FBS | 2.162 ± 0.777 | 7.099 ± 0.140 |
| Methanolic extracts | ||
| FB | 3.070 ± 0.225 | 15.252 ± 0.642 |
| FBS | 3.318 ± 0.057 | 16.727 ± 0.854 |
| Compounds | ||
| Adenosine | 0.011 ± 0.003 | 2.215 ± 0.139 |
| Cordycepin | 0.055 ± 0.006 | 8.124 ± 2.047# |
| Carotenoids | 0.235 ± 0.011# | 1.287 ± 0.176 |
- Note: The results are presented as mean value ± SD. The highest significant value (∗, #) in each extract/compound is indicated and determined by Student’s t-test (p < 0.05) from independent experiments (n = 3).
- Abbreviations: FB, fruiting body; FBS, fruiting body with substrate.
3.3. Antibacterial Activity of C. militaris Extract and Bioactive Compounds
The antibacterial activity of C. militaris extract was evaluated by broth dilution method, and the bioactive compounds were also determined, including cordycepin and carotenoids. The results showed that the ethanolic and methanolic extracts of FB and FBS, as well as the bioactive compounds cordycepin and carotenoids, had the ability to inhibit all enteric pathogenic bacteria, including E. coli, E. coli O157:H7, S. dysenteriae, S. Typhi, V. cholerae and B. cereus. In contrast, the aqueous extracts of FB and FBS did not inhibit all tested bacteria. The ethanolic and methanolic extracts of FB and FBS exhibited the lowest MIC and MBC doses of inhibition against all tested bacteria at values of 31.25–250 mg/mL and 7.81–250 mg/mL, respectively (Table 3). In addition, the methanolic extract of FB exhibited the lowest MIC and MBC values of 7.81 mg/mL against B. cereus. Cordycepin and carotenoids were able to inhibit all bacteria with the lowest MIC and MBC of 0.5–1.0 and 0.625–5.0 mg/mL, respectively. Cordycepin showed the lowest MIC and MBC of 0.5 mg/mL against S. dysenteriae and V. cholerae. Carotenoids showed the greatest inhibition, demonstrated in B. cereus, with the lowest MIC and MBC of 0.625 mg/mL (Table 3).
| Extracts | MIC/MBC values (mg/mL) | |||||
|---|---|---|---|---|---|---|
| E. coli | E. coli O157:H7 | S. dysenteriae | S. Typhi | V. cholerae | B. cereus | |
| Ethanolic extracts | ||||||
| FB | 31.25/250 | 62.50/250 | 62.50/125 | 62.50/250 | 62.50/250 | 62.50/62.50 |
| FBS | 31.25/125 | 62.50/250 | 62.50/125 | 62.50/250 | 62.50/250 | 62.50/125 |
| Methanolic extracts | ||||||
| FB | 31.25/250 | 31.25/250 | 31.25/125 | 31.25/250 | 31.25/250 | 7.81/7.81 |
| FBS | 31.25/250 | 31.25/250 | 31.25/125 | 62.50/125 | 31.25/250 | 31.25/31.25 |
| Cordycepin | 0.5/1.0 | 1.0/1.0 | 0.5/0.5 | 0.5/1.0 | 0.5/0.5 | 0.5/1.0 |
| Carotenoids | 0.625/5.0 | 1.25/5.0 | 1.25/1.25 | 1.25/5.0 | 1.25/5.0 | 0.625/0.625 |
| Gentamycin (1 mg/mL) | 1.95/3.91 | 1.95/3.91 | 0.12/0.98 | 3.91/3.91 | 1.95/3.91 | 1.95/3.91 |
| DMSO (100%) | > 500/> 500 | > 500/> 500 | > 500/> 500 | > 500/> 500 | > 500/> 500 | > 500/> 500 |
- Note: The results are presented as mean ± SD values of triplicate independent experiments.
- Abbreviations: FB, fruiting body; FBS, fruiting body with substrate.
3.4. Effect of C. militaris Extract and Bioactive Compounds on Adhesion and Invasion of Bacteria in the Epithelial Cell
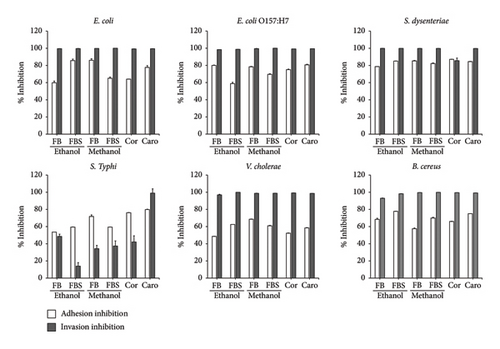
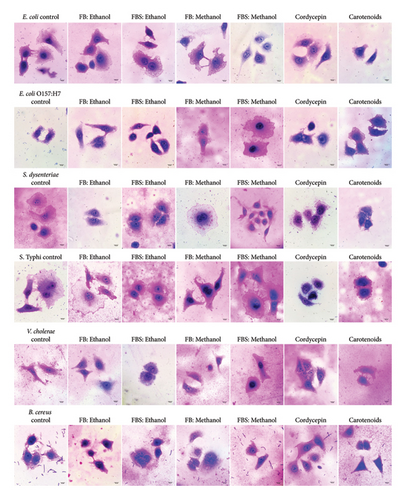
Nontoxic concentrations of the ethanolic and methanolic extracts of C. militaris (10 μg/mL) were selected to be tested against the adhesion and invasion of bacteria in the Caco-2 cell. Additionally, nontoxic concentrations of cordycepin and carotenoids at 25 and 40 μg/mL were used in this study. The methanolic extract of FB showed the greatest adhesion inhibition of 86.05% against E. coli adherent on Caco-2 cells (Figures 1 and 2). Subsequently, the ethanolic extracts of FBS could inhibit the adhesion of E. coli and S. dysenteriae on Caco-2 cells by values of 85.68% and 85.13%, respectively. The methanolic extract of FB inhibited the adherence of S. dysenteriae to Caco-2 cells by 85.30%. Moreover, the ethanolic extracts of FB and FBS had the ability to inhibit the adhesion of all tested bacteria to Caco-2 cells by 48.62%–85.68%. The methanolic extracts of FB and FBS had the ability to inhibit the adhesion of all tested bacteria on Caco-2 cells by 57.56%–86.05%. Moreover, FB and FBS extracts had the ability to block the invasion of bacteria in Caco-2 cells by 13.94%–99.96% (Figure 1). For their bioactive compounds, cordycepin and carotenoids were able to interfere with the attachment of bacteria on Caco-2 cells by 52.31%–87.17% for cordycepin and 58.47%–84.51% for carotenoids (Figures 1 and 2).
3.5. Effect of C. militaris Extract and Bioactive Compounds on Cell Viability of Caco-2 Cells
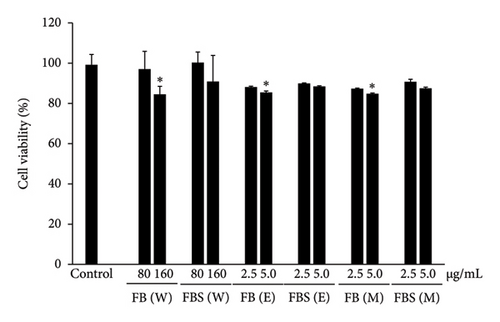
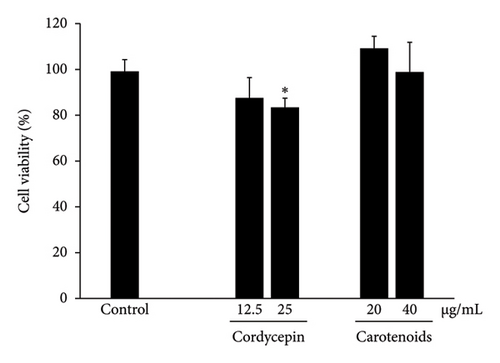
The optimal concentrations of C. militaris extract and bioactive compounds on viable Caco-2 cells were assessed by MTT assay, as shown in Figure 3. Caco-2 cells were treated with various concentrations of aqueous (40–5000 μg/mL), ethanolic (2.5–1250 μg/mL), and methanolic (2.5–1250 μg/mL) extracts of C. militaris, as well as the bioactive compounds cordycepin (6.25–100 μg/mL) and carotenoids (2.5–40 μg/mL) for 24 h. The maximum dose of extract and bioactive compounds did not show cytotoxicity on Caco-2 cells compared to the control group, which expressed over 80% cell viability after treatment. Doses of aqueous, ethanolic and methanolic extracts of 80–160 μg/mL of FB and 2.5–5.0 μg/mL of FBS did not have any toxic effect on the cells. In addition, doses of 12.5–25 μg/mL cordycepin and 20–40 μg/mL carotenoids were not toxic on the cells. Nontoxic doses of C. militaris extract and bioactive compounds were further investigated for anti-inflammatory activity.
3.6. Effects of C. militaris Extract and Bioactive Compounds on NO Production From LPS Stimulus in Caco-2 Cells
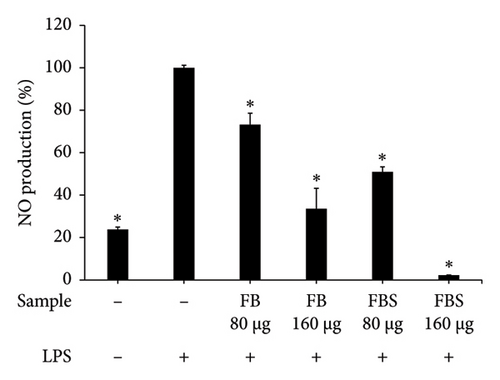
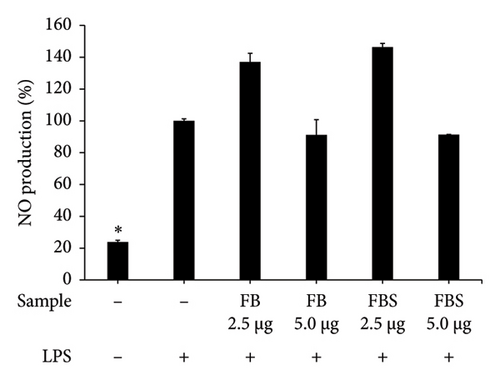
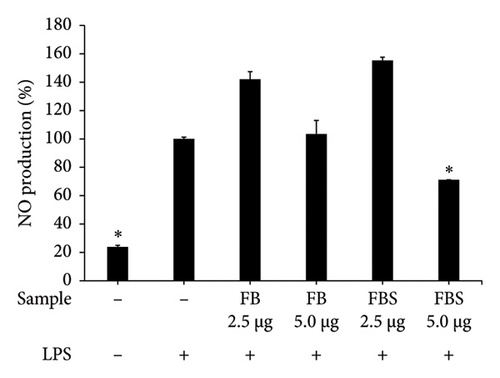
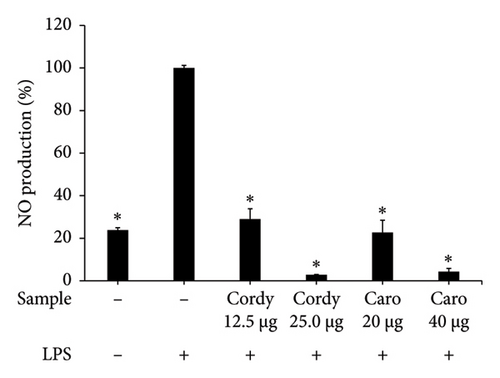
LPS-stimulated Caco-2 cells were treated with C. militaris extract and bioactive compounds for 24 h, and the NO production was determined using Griess reagent. The results showed that the aqueous extracts of FB and FBS at concentrations of 80–160 μg/mL had significant ability to attenuate NO production from the LPS-stimulated group (p < 0.05). The aqueous extracts of FB significantly decreased NO production by 33.58%–73.21%. Moreover, FBS significantly decreased NO production by 2.24%–50.96% (p < 0.05 for FBS; Figures 4(a), 4(b), and 4(c)). The methanolic extract of FBS (5.0 μg/mL) could significantly decrease the NO release from LPS-stimulated Caco-2 cells by 71.09% (p < 0.05 for FBS; Figure 4(c)). Cordycepin (12.5–5.0 μg/mL) and carotenoids (20–40 μg/mL) expressed significant inhibitory activity on NO from LPS-stimulated Caco-2 cells, with the percentage of NO production from 2.84% to 28.97% (p < 0.05 for all; Figure 4(d)).
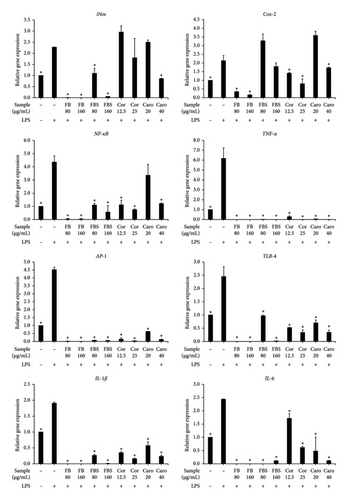
3.7. Effect of C. militaris Extract and Bioactive Compounds on Inflammatory Gene Expression in LPS-Stimulated Caco-2 Cells
The aqueous extract of C. militaris and bioactive compounds cordycepin and carotenoids significantly decreased NO production in LPS-stimulated Caco-2 cells. Thus, the expressions of inflammatory genes, including iNos, Cox-2, NF-κB, TNF-α, AP-1, TLR-4, IL-1ß, and IL-6, were also investigated by qRT-PCR. The results demonstrated that FB and FBS extracts (80–160 μg/mL) had significant ability to downregulate the inflammatory gene expressions by 1.8–6.2 and 1.2–6.2 folds (p < 0.05 for all; Figure 5), respectively. In contrast, FBS extract did not decrease the expression of the Cox-2 gene compared to the LPS-stimulated control (Figure 5). Cordycepin and carotenoids found in the aqueous extract of FB and FBS were able to support the downregulation of inflammatory genes. Cordycepin (12.5–25 μg/mL) showed significant inhibitory activity to downregulate the expressions of Cox-2, NF-κB, TNF-α, AP-1, TLR-4, IL-1ß, and IL-6 genes by 0.7-6.2 folds (p < 0.05 for Cor; Figure 5). Carotenoids (20–40 μg/mL) could significantly decrease the expressions of inflammatory genes, including TNF-α, AP-1, TLR-4, IL-1ß, and IL-6, by 1.3-6.2 folds (p < 0.05 for Caro; Figure 5). Moreover, carotenoids at a concentration of 40 μg/mL could significantly downregulate the expression of iNos, Cox-2, and NF-κB genes by 0.4-3.1 folds (p < 0.05 for Caro; Figure 5).
4. Discussion
In this study, we investigated natural substances from edible, medicinal mushrooms, especially Cordyceps militaris, which contained bioactive compounds, antioxidants, antibacterial, and anti-inflammatory properties. The FB and FBS of C. militaris were extracted using water and organic solvents including 95% ethanol and methanol as polar solvent since they effectively extract moderately polar and polar phenolic molecules (e.g., flavonoid glycosides and phenolic acids). Moreover, they are low cost and less toxicity. The bioactive compounds including adenosine, cordycepin, carotenoids, phenolics, and flavonoids were also determined in the extracts. The results showed that the aqueous extract of FB contained the highest amount of adenosine, and the aqueous fraction of FBS contained the highest amount of cordycepin and carotenoids. The main active compounds were reported from previous studies including nucleosides, cordycepin, and adenosine, which were easily extracted by water [18]. Various bioactive compounds detected by LC-MS analysis are found in C. militaris such as adenine, adenosine, cordycepin, xylitol, fructose, mannitol, glucose, and trehalose, and the main active constituents are adenosine and cordycepin [44]. Cordycepin is usually detected in both FBs and the mycelium of C. militaris. However, adenosine is mainly found in the FBs of C. militaris [45]. Cordycepin exhibits a structural similarity to adenosine with differing only in the absence of a 3′-hydroxyl group [46]. This close structural resemblance positions in cordycepin make the cordycepin as a potent bioactive compound with significant implications for its nutraceutical applications. Numerous studies suggest that cordycepin competitively inhibits the synthesis and metabolism of DNA and RNA [47]. As a result, cordycepin is known for its diverse pharmacological actions, including antioxidant, immunomodulatory, hypolipidemic, anti-inflammatory, anticancer, antimicrobial, antiviral, and hypoglycemic properties [48]. The yellow-orange color in C. militaris has been confirmed as carotenoids, which are xanthophyll derivatives including β-carotene, lycopene, lutein, and zeaxanthin [49]. Moreover, high levels of both phenolic and flavonoid compounds were detected in the ethanolic extracts of FBS and FB. Previous reports have also discovered phenolic and flavonoid compounds in C. militaris [27]. In general, the level of flavonoids was lower than that of phenolic acid compounds. The study by Nguyen et al. detected specific flavonoid and phenolic compounds in ethanolic extracts of C. militaris, revealing that the flavonoid compound was quercetin, while the phenolic compounds were gallic acid, chlorogenic acid, caffeic acid, ferulic acid, and cinnamic acid [50]. The study by Reis et al. found phenolic acids such as p-hydroxybenzoic acid and cinnamic acid in C. militaris methanolic extracts [51]. However, specific flavonoid compounds in C. militaris methanolic extracts have not yet been reported.
The antioxidant activity of C. militaris extracts was determined by DPPH and ABTS radicals scavenging assays. The DPPH assay is stable and generates radicals that can be dissolved in organic solvent such as methanol. Therefore, it is applicable to detect hydrophobic compounds. However, the ABTS assay is more sensitive and based on the generation of a blue/green ABTS+, which is suitable to detect both hydrophilic and hydrophobic antioxidant compounds [52, 53]. Therefore, two different methods were used in this study to determine the antioxidant effects of hydrophobic and hydrophilic compounds in aqueous, ethanolic, and methanolic extracts of C. militaris. In this study, C. militaris extracts revealed the higher antioxidant activity by ABTS assay than DPPH assay since both hydrophilic and hydrophobic compounds can be detected by ABTS assay.
The aqueous extract of C. militaris FBS showed the greatest antioxidant capacity against DPPH and ABTS radicals that also related to a high cordycepin and carotenoids contents. Hence, aqueous extract of C. militaris exhibited the strongest antioxidant activity that was determined by DPPH and ABTS radical scavenging. The antioxidant C. militaris was also reported from previous studies [27, 54]. Moreover, cordycepin, adenosine, and carotenoids demonstrated the antioxidant activity against DPPH and ABTS radicals. Free radicals can damage biomolecules, leading to the progression of aging, cancer, inflammation, diabetes, metabolic disorders, atherosclerosis, and cardiovascular diseases [55]. Cordycepin is effective for restoring antioxidant status and decreasing lipid peroxidation in aged rats [56]. Carotenoids are efficient antioxidants scavenging singlet molecular oxygen and peroxyl radicals. In the human, carotenoids are part of the antioxidant defense system [57]. Therefore, adenosine isolated from C. militaris has benefits for the prevention of ROS-related diseases such as tissue damage. It also acts as a therapeutic agent against chronic heart failure and has anti-inflammation properties [58]. In addition, the methanolic extract of C. militaris demonstrated a greater ability to scavenge ABTS radicals when compared to the ethanolic extract, which was not correlated with phenolic and flavonoid contents. Methanol is more polar than ethanol, allowing it to extract a wider range of antioxidant compounds, including polar and semipolar ones, that may not be fully extracted by ethanol. While phenolics and flavonoids are known antioxidants, other bioactive compounds such as polysaccharides, nucleosides, and peptides, which also contribute to antioxidant activity, could be more efficiently extracted by methanol [59–61]. Furthermore, methanol may better preserve certain unstable antioxidant compounds that tend to degrade in ethanol, thereby retaining higher antioxidant capacity in the methanolic extract [62]. Therefore, C. militaris showed enzymatic antioxidant activity through xanthine oxidase (XOD) inhibition, which is crucial for applications in treating hyperuricemia and gout [63]. The polysaccharides from C. militaris were found to have effects on the activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and antihydroxyl radicals [64].
The antibacterial activity of C. militaris extract and bioactive compounds, especially cordycepin and carotenoids, on enteric pathogenic bacteria was also investigated. This study showed the ethanolic and methanolic extracts of FB and FBS to have inhibitory activity on all enteric pathogenic bacteria, including E. coli, E. coli O157:H7, S. dysenteriae, S. Typhi, V. cholerae, and B. cereus. Similarly, the methanolic extract of C. militaris has the potential to inhibit pathogenic bacteria: Staphylococcus aureus, B. cereus, Micrococcus flavus, Pseudomonas aeruginosa, Salmonella typhimurium, E. coli, and Enterobacter cloacae, as well as pathogenic fungi such as Aspergillus fumigatus, A. versicolor, A. niger, Trichoderma viride, Penicillium funiculosum, and P. verrucosum [51]. Additionally, C. militaris was revealed to be a rich source of bioactive compounds, including cordycepin and carotenoids, which were the antibacterial compounds of FB and FBS extract. Cordycepin demonstrated broad-spectrum inhibitory activity against Gram-positive and Gram-negative bacteria, as well as fungi [65, 66]. Cordycepin demonstrated inhibitory activity against DNA ligase of bacteria, similar to adenosine analog [51]. Additionally, carotenoids have been reported to inhibit porin proteins, located in the outer membrane of bacteria. Carotenoids bind to porin to form a polymer structure, thereby damaging porin and affecting the transport system of the cells [67].
The attachment of pathogenic bacteria to host surfaces is a critical step in infection, leading to invasion through direct engagement of host cell receptors or the translocation of bacterial proteins to facilitate entry into target cells for replication and dissemination within the host [12, 68]. This study revealed a new report that the ethanolic and methanolic extracts of FB and FBS exhibited the greatest inhibition of bacterial adhesion and invasion on Caco-2 colon cells. As for their bioactive compounds, cordycepin and carotenoids also interfered with the attachment and invasion of bacteria on Caco-2 cells. Moreover, the study by Chen et al. demonstrated that C. militaris polysaccharides significantly decreased the adhesion and invasion of Vibrio parahaemolyticus, Vibrio cholerae, Salmonella enterica, Enterobacter aerogenes, Proteus mirabilis, and enterotoxigenic E. coli (ETEC) on pig intestinal epithelial cells (IPEC-J2) that related to downregulate the expression of fimH type 1 fimbriae (fimH) gene [69]. Currently, antiinvasive and antibiofilm activities of carotenoids were demonstrated on Pseudomonas aeruginosa PAO1 [70]. Cordycepin has also been proven to inhibit the biofilm formation of Candida albicans [71]. Therefore, C. militaris should be considered as an antiadhesive agent to prevent bacterial infection in cells.
During enteric bacterial infection in the gastrointestinal tract, bacteria can damage the epithelial cells and produce endotoxins that provoke intestinal inflammation. Moreover, LPS of bacteria are the main cause of inflammation. In this study, C. militaris extract and its bioactive compounds cordycepin and carotenoids exhibited anti-inflammatory activity in the LPS-stimulated Caco-2 cell model. Interestingly, the aqueous extracts of FB, FBS, and the bioactive compounds had the ability to attenuate NO production in the LPS-stimulated group, better than the ethanolic and methanolic extracts. In addition, C. militaris extracted by water had a lower toxicity on Caco-2 cells than the ethanolic and methanolic extracts. In a model of LPS-induced inflammation in Caco-2 cells, C. militaris aqueous extract, cordycepin, and carotenoids reduced the expression of inflammatory genes including iNos, Cox-2, NF-κB, TNF-α, AP-1, TLR-4, IL-1ß, and IL-6. The stimulation of TLR-4 by LPS induces the release of crucial proinflammatory cytokines required to trigger potent immune responses. NO and prostaglandin E2 (PGE2) are critical mediators of inflammation. NO plays a pivotal role in various bodily functions but can also lead to cytotoxicity, inflammation, and autoimmune disorders [72]. The iNOS gene is one of the key enzymes responsible for generating NO production. Moreover, PGE2 is encoded by the Cox-2 gene and is produced by the inducible enzyme COX-2. The PGE2 has also been implicated as a crucial mediator in the development of numerous chronic inflammatory diseases. Furthermore, the production of TNF-α, IL-1β, and IL-6 is required for the synergistic induction of NO and PGE2 production [73]. Our study revealed that C. militaris extract, cordycepin, and carotenoids significantly inhibited the TLR4 gene receptor when stimulated with LPS, resulting in the suppression of iNos and Cox-2 genes in Caco-2 cells. This suppression was associated with a decrease in the gene expression of TNF-α, IL-1β, and IL-6.
Likewise, C. militaris has been confirmed to decrease the production of proinflammatory mediators, such as NO, TNF-α, and IL-6, which were studied using the LPS-stimulated macrophage model [74]. Cordycepin and ergosterol fractionated from C. militaris is able to inhibit the release of inflammatory mediators, including NO, TNF-α and IL-12, which can, in turn, inhibit the proliferation of colon tumor cells [25]. The presence of butanoic acid, 2-methyl, in the C. militaris FBS aqueous extract was noteworthy as this compound has been demonstrated to possess potent immunoregulatory and anti-inflammatory properties. Notably, butanoic acid has shown the ability to reduce concentrations of proinflammatory cytokines, including IL-8 and TNF-α [75]. Additionally, the reported ease of absorption of butanoic acid in colon epithelial cells contributed to its potential effectiveness in treating irritable bowel syndrome [76]. In particular, recent evidence shows that C. militaris extract, cordycepin and carotenoids are strongly linked to various intracellular signaling pathways, specifically the NF-κB and MAPK cascades, through the suppression of NF-κB and AP-1 genes. Cordycepin is also capable of suppressing NF-κB activity and the phosphorylation of mitogen-activated protein kinases (MAPKs) [77]. Moreover, carotenoids can act as antioxidants and anti-inflammatory agents, demonstrating anti-inflammatory potential through the MAPKs and NF-κB pathways [78]. Among these, NF-κB is important for LPS-stimulated inflammation, which regulates a number of inflammatory genes, including iNOS, COX-2, TNF-α, IL-1β, and IL-6. The MAPK family, consisting of p38 MAPK, ERK, and JNK, comprises a group of serine/threonine kinases that are activated in response to diverse extracellular stimuli. They regulate cellular signal transduction from the cell surface to the nucleus [79, 80]. A key target of the MAPKs is the activator protein 1 (AP-1) transcription factor [80].
Moreover, the impact of C. militaris extract, cordycepin, and carotenoids on inflammatory gene expression correlates with the downregulation of cytokines and protein expression. The study by Kwon et al. demonstrated that C. militaris effectively inhibited proinflammatory cytokines, indicating its anti-inflammatory properties through NF-κB inhibition. It also downregulated iNOS, COX-2, MAPKs, and AKT cascades in LPS-mediated inflammation in RAW264.7 macrophages [81]. Repression of receptor activator of NF-κB ligand (RANKL) was also associated with events that could be vital targets for controlling inflammation-related conditions such as cancer and colitis. Additionally, cordycepin significantly suppressed RANKL-activated NF-κB and p38 phosphorylation [82]. Targeting NF-κB could be an effective approach for treating inflammatory diseases, as many anti-inflammatory agents exert their effects by suppressing NF-κB signaling [77]. Cordycepin has previously been reported to significantly inhibit the LPS-induced release of TNF-α and IL-1β in RAW 264.7 cells. This inhibitory effect may be attributed to the suppression of TNF-α and IL-1β transcription, resulting in reduced protein expression [83, 84]. Furthermore, carotenoids significantly decreased TLR4 protein levels and were involved in the inhibition of the NF-κB and MAPK signaling pathways [85, 86]. In agreement with previous observations, the anti-inflammatory effects of C. militaris, cordycepin, and carotenoids showed the downregulation of NF-κB gene expression that appeared to involve inhibition of NF-κB activation by blocking LPS-stimulated IκBα degradation and translocation of the NF-κB/p65 protein to the nucleus [87, 88].
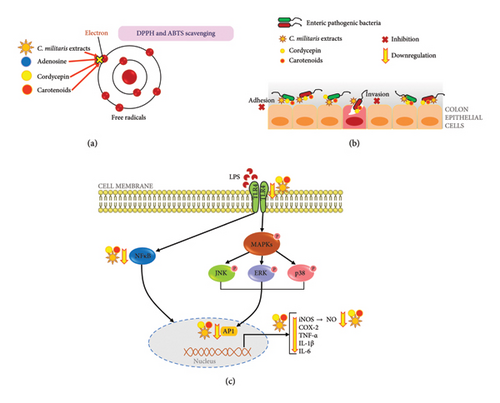
Therefore, this study reveals a new report that the inhibitory effects of C. militaris extract and bioactive compounds also impact the adhesion and invasion of bacteria interacting with colon cells. Moreover, this finding also demonstrates the anti-inflammatory activities of C. militaris aqueous extract and bioactive compounds on the LPS-stimulated Caco-2 cell model, that has the capability to suppress inflammatory mediators, including iNos, Cox-2, NF-κB, TNF-α, AP-1, TLR-4, IL-1ß, and IL-6. The study was conducted in vitro, and the observed effects may not fully translate to in vivo conditions. Further studies involving animal models and human trials are necessary to confirm the efficacy and safety of C. militaris extracts.
5. Conclusions
In conclusion, exploring natural, therapeutic agents derived from medicinal mushrooms, especially C. militaris, is emphasized to benefit from the bioactive compounds and the antioxidant, antibacterial, and anti-inflammatory properties. C. militaris extracted by water, ethanol, and methanol was found to have various bioactive compounds including adenosine, cordycepin, and carotenoids using HPLC. The aqueous extract of FB and FBS, and active compounds (adenosine, cordycepin, and carotenoids) showed the highest antioxidant activity against DPPH and ABTS radicals. Furthermore, the ethanolic and methanolic extracts of C. militaris and the bioactive compounds cordycepin and carotenoids demonstrated strong inhibitory effects on the growth of enteric pathogenic bacteria; E. coli, E. coli O157:H7, S. dysenteriae, S. Typhi, V. cholerae, and B. cereus. These substances are revealed as new agents for antiadhesion and anti-invasion that can interfere with infection of enteric pathogenic bacteria on the Caco-2 colon cells (Figure 6). This finding also demonstrates the anti-inflammatory activities of C. militaris aqueous extract and bioactive compounds on the LPS-stimulated Caco-2 cell model that has the capability to suppress inflammatory mediators, including iNos, Cox-2, NF-κB, TNF-α, AP-1, TLR-4, IL-1ß, and IL-6 (Figure 6). This study emphasizes the beneficial activity of C. militaris extract as an alternative food supplement. Moreover, the extract demonstrated therapeutic agent against enteric pathogenic bacterial infections and considerably promoted its antioxidant and anti-inflammatory agents for the benefit of health. A preprint has previously been published [89].
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
All authors contributed to this work significantly. Thida Kaewkod, Pornpimon Ngamsaoad, Kanok-orn Mayer, Nitsanat Cheepchirasuk, Itthayakorn Promputtha, and Yingmanee Tragoolpua designed the studies and performed the experiments. Thida Kaewkod and Yingmanee Tragoolpua wrote the paper and generated the figures. Thida Kaewkod and Yingmanee Tragoolpua contributed to the discussion of the results and to the revision of the manuscript. All authors read and approved the final manuscript.
Funding
This research study was supported by Fundamental Fund 2022, Chiang Mai University (grant number FF65/077).
Acknowledgments
This research study was partially supported by Natural Extracts and Innovative Products for Alternative Healthcare Research Group, and Division of Microbiology, Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand. We also would like to express our thanks to Julia Akins for language editing.
Supporting Information
Table S1: Oligonucleotide primers used for qRT-PCR. Figure S1: The C. militaris extraction processes and the extracts at 500 mg/mL in parts of fruiting body and fruiting body with substrate after extraction by water, ethanol, and methanol. Figure S2: HPLC chromatogram of standard compounds of adenosine and cordycepin. Figure S3: Adenosine and cordycepin compounds found in C. militaris extracts by HPLC assay.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




