Determination of Eight Catechins and Four Theaflavins in Qingzhuan Dark Tea of Different Years (1, 5, 9, and 13 Years)
Abstract
Catechins and theaflavins are the key polyphenolic compounds in Qingzhuan dark tea (QDT). They not only give QDT unique taste and color but also have significant health benefits such as antioxidant, anti-inflammatory, and cardiovascular protection, which are the core of the quality and healthcare value of QDT. However, the variation of its content is closely related to the vintage of the tea. The aim of this study was to develop a rapid, sensitive, and accurate high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) method for quantifying eight catechins and four theaflavins in QDT of different years (1, 5, 9, and 13 years). An orthogonal experimental design was employed to determine the optimal extraction conditions. Under these conditions, QDT samples from different years (1, 5, 9, and 13 years) were extracted, and eight catechins and four theaflavins were quantified using the established HPLC-MS/MS method. The results showed that the optimal extraction conditions for methanol were determined as follows: methanol concentration of 60%, temperature of 90°C, and extraction time of 15 min, while for water extraction, the optimal conditions were temperature of 90°C and extraction time of 30 min. Methodological validation of the eight catechins and four theaflavins was conducted, including specificity, linearity, lower limit of quantitation, precision, accuracy, stability, and reproducibility. Different extraction methods (methanol extraction and water extraction) significantly influenced the content of eight catechins and four theaflavins, with higher content observed with methanol extraction compared to water extraction. Significant differences were also observed in the content of eight catechins and four theaflavins among QDT samples of different years (1, 5, 9, and 13 years), with higher years associated with lower content. Conclusion: The method established in this study is reliable, accurate, and reproducible, and could be used to evaluate QDT of different years by quantifying the content of eight catechins and four theaflavins.
1. Introduction
Tea is one of the most popular beverages globally, with consumption levels comparable to coffee and cocoa. It possesses various pharmacological effects, such as anticancer, blood sugar reduction, and antiobesity properties [1–3]. Qingzhuan dark tea (QDT), as a precious heritage of Chinese tea culture, has consistently attracted the attention of tea researchers. QDT is distinguished by its distinctive production process and storage methods. In contrast to traditional loose-leaf tea or tea cakes, QDT is shaped into brick-like blocks. These blocks are generally square or rectangular, varying in thickness. The coloration of QDT ranges from deep green to dark green, and in some instances, it may appear coffee-colored. QDT has been associated with weight loss [4], cholesterol reduction [5, 6], antioxidant properties [7], and regulation of metabolism [8]. Catechins and theaflavins, belonging to the class of tea polyphenols, are major bioactive components in tea. These compounds not only impart unique flavors and aromas to tea but also offer numerous potential health benefits, making them useful for identifying trace amounts of tea [9]. Reported benefits of catechins and theaflavins to health include anti-inflammatory [10], antihypertensive [11], antiobesity [4], and anticancer effects [3]. Additionally, they have demonstrated antioxidant [12], antifungal [13], and antiviral [14] effects on cardiovascular health [15] and impact on microcirculation [16].
QDT belongs to the category of postfermented tea, which typically requires a period of storage and aging to achieve optimal flavor quality [17, 18]. However, during this process, chemical constituents in QDT may undergo changes [19, 20]. The value of QDT varies with different years, where older vintages command higher prices. However, research has demonstrated a decline in the nutrient content of tea as it ages, likely attributable to oxidative degradation processes. Furthermore, there is often a presence of fraudulent practices in the market, where lower-grade teas are passed off as higher-year vintage QDT to maximize profits or even counterfeit, posing negative impacts on consumers’ health. Therefore, it is essential to conduct quantitative analyses of eight catechins and four theaflavins in QDT from different years to not only reveal the evolution of its components over time but also aid in understanding its potential health benefits and facilitating the identification of different vintage QDT.
In summary, analyzing the content of eight catechins and four theaflavins in QDT is of significant value for a comprehensive understanding of its quality, flavor, storage methods, and potential health effects. Presently, analytical methods for these phenolic compounds in tea include high-performance liquid chromatography (HPLC) [21–23] and high-performance liquid chromatography–tandem mass spectrometry (LC-MS/MS) [24, 25]. In recent years, a number of quantitative research methods have been developed gradually to evaluate the content of compounds in QDT and even to identify the year of production. Some scholars use quantitative data alongside multivariate analysis to discriminate and predict the storage year of QDT [26]. Moreover, a rapid method was presented for the determination of 19 polyphenols in tea by ultra-HPLC coupled with quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF MS) [27]. In view of this, the purpose of this study is to establish a rapid, sensitive, and accurate HPLC-MS/MS method for determining the content of eight catechins and four theaflavins to evaluate and differentiate the vintage of QDT. Furthermore, the optimal conditions for both alcohol and water extraction methods were established through orthogonal experiments, enabling a comparative analysis of their respective extraction efficiencies. Subsequently, the content and change of eight catechins and four theaflavins in QDT from different production years were systematically examined, leading to the derivation of comprehensive conclusions.
2. Materials and Methods
2.1. Experimental Reagents and Instruments
QDT (1, 5, 9, and 13 years) were provided by Hubei Zhao Liqiao Tea Factory Co. Ltd, Hubei, China. Gallocatechin (GC), epigallocatechin gallate (EGC), catechin (C), epicatechin (EC), epigallocatechin gallate (EGCG), gallocatechin gallate (GCG), epicatechin gallate (ECG), and catechin gallate (CG), theaflavin (TF1), theaflavin-3-gallate (TF2A), theaflavin-3,3′-gallate (TF3), theaflavin-3′-gallate (TF2B), and acetaminophen (internal standard, IS) were supplied by Shanghai Yuanye Biochemical Technology Co. Ltd, Shanghai, China (purity > 98%). Methanol and formic acid were purchased from Thermo Fisher, Waltham, MA, USA. Ultrapure water was provided from Watsons (Hong Kong, China).
Liquid chromatography (LC) system was purchased from Shimadzu Corporation (Kyoto, Japan), consisting of CBM-20A system, a column oven (CTO-20 AC), an autosampler (SIL-20 AC), and LC-20 ADXR pumps.
2.2. HPLC-MS/MS Assay
The chromatographic separation was carried out on a Shim-pack ODS column (5.0 μm × 250 mm × 4.6 mm inner diameter (i.d.)) equipped with a guard column (4.6 μm, 5 mm × 2 mm i.d.) at a temperature of 35°C. The mobile phase consisted of A (0.5% formic acid water) and B (methanol). The flow rate was set to 0.2 mL/min. The gradient elution flow program was as follows: 0–10 min, 20% B⟶ 51% B; 10–18 min, 51% B.
The precursor ion, corresponding product ion, and collision-induced dissociation (CID) for each compound were optimized. The detecting condition was set in multiple reaction monitoring (MRM) mode. The tuning results are shown in Table 1. Other optimized mass spectrometry (MS) conditions were as follows: ion source gas, 3 L/min; curtain gas, 10 L/min; ion spray voltage, 4.5 kV; desolvation line (DL) temperature, 250°C; heating block temperature, 400°C.
| Analyte | Precursor ion (m/z) | Product ion (m/z) | Q1 | Q3 | CE (V) |
|---|---|---|---|---|---|
| GC | 307.1 | 151.0 | −16 | −29 | −12 |
| EGC | 307.1 | 289.0 | −16 | −19 | −10 |
| C | 291.1 | 165.1 | −15 | −16 | −13 |
| EC | 291.1 | 147.0 | −15 | −25 | −22 |
| EGCG | 459.1 | 153.1 | −14 | −15 | −29 |
| GCG | 459.1 | 153.1 | −14 | −27 | −30 |
| ECG | 443.1 | 273.1 | −13 | −18 | −12 |
| CG | 443.1 | 273.1 | −13 | −18 | −13 |
| TF1 | 565.1 | 139.0 | −28 | −26 | −33 |
| TF2A | 717.1 | 139.0 | −22 | −24 | −36 |
| TF2B | 717.1 | 139.1 | −20 | −24 | −35 |
| TF3 | 869.1 | 139.1 | −26 | −28 | −17 |
2.3. Methanol and Water Extraction Experiment
The experiment utilized 5 years QDT as a sample, with the evaluation standard being the total content of 12 compounds (eight catechins and four theaflavins) extracted. The optimal conditions for methanol extraction (including the concentration of methanol, temperature, and time) were determined through orthogonal experiments, as well as the optimal conditions for water extraction (including temperature and time). Based on these results, an optimal extraction protocol was developed for determining the content of the 12 compounds in QDT of different years. The experimental steps were as follows: dissolve 1 g of tea leaves in 50 mL of solvent and perform extraction under different conditions. After centrifugation at 12,000 rpm/min for 15 min at 4°C, the supernatant was obtained. Then, take 500 μL of the supernatant and filter it through a 0.22-μm filter, 10 μL of IS (1.0 μg/mL) was added to the filtrate and mixed well, and 10 μL was taken into HPLC-MS/MS for analysis.
2.3.1. Optimization of Methanol Extraction Method via Orthogonal Experiment
The experiment employed an L9 (33) design to investigate the effects of methanol concentration (40%, 60%, and 80%), extraction temperature (50°C, 70°C, and 90°C), and extraction time (15, 30, and 45 min) on the total content of the 12 compounds, aiming to determine the optimal extraction conditions. Detailed experimental conditions are shown in Table 2.
| Level | Factors | ||
|---|---|---|---|
| Methanol concentration (%) | Extraction temperature (°C) | Extraction time (min) | |
| 1 | 1 (40) | 1 (50) | 1 (15) |
| 2 | 1 | 2 (70) | 2 (30) |
| 3 | 1 | 3 (90) | 3 (45) |
| 4 | 2 (60) | 1 | 2 |
| 5 | 2 | 2 | 3 |
| 6 | 2 | 3 | 1 |
| 7 | 3 (80) | 1 | 3 |
| 8 | 3 | 2 | 1 |
| 9 | 3 | 3 | 2 |
2.3.2. Optimization of Water Extraction Method
The experiment examined the effects of extraction temperature (50°C, 70°C, and 90°C) and extraction time (15, 30, and 45 min) on the total content of the 12 compounds to establish the optimal extraction conditions. Detailed experimental conditions are shown in Table 3.
| Level | Factors | |
|---|---|---|
| Extraction temperature (°C) | Extraction time (min) | |
| 1 | 50 | 15 |
| 2 | 50 | 30 |
| 3 | 70 | 15 |
| 4 | 90 | 15 |
| 5 | 70 | 30 |
| 6 | 50 | 45 |
| 7 | 70 | 45 |
| 8 | 90 | 45 |
| 9 | 90 | 30 |
2.4. Method Validation
The experiment followed the Food and Drug Administration (FDA) guidelines, assessing selectivity, linearity, lower limit of quantification (LLOQ), accuracy, precision, and stability.
2.4.1. Specificity
Specificity was assessed by analyzing chromatograms of blank solution, blank solution spiked with 12 reference standards, and the extract solution of QDT.
2.4.2. Linearity
The mixed standard solution should be gradually diluted to form different concentrations of the standard solution, and the concentration was used as the horizontal coordinate (X), and the ratio of the peak area of each compound to the peak area of the IS was used as the vertical coordinate (Y) to plot the standard curve and obtain the linear regression equation.
2.4.3. Accuracy and Precision
Precision and accuracy were evaluated by calculating the relative standard deviation (RSD) and recovery, respectively. The intraday and interday assays of accuracy and precision were evaluated by the determination of quality control (QC) samples [low QC (LQC), middle QC (MQC), and high QC (HQC)] following the same day and three consecutive days. The specific concentrations of HQC, MQC, and LQC are shown in Table 4.
| Analyte | LQC (μg/mL) | MQC (μg/mL) | HQC (μg/mL) |
|---|---|---|---|
| GC | 0.304 | 1.218 | 29.970 |
| EGC | 0.731 | 2.922 | 71.928 |
| C | 0.183 | 0.731 | 17.982 |
| EC | 0.365 | 1.461 | 35.964 |
| EGCG | 2.435 | 9.740 | 239.760 |
| GCG | 0.365 | 1.461 | 35.964 |
| ECG | 0.791 | 3.166 | 77.922 |
| CG | 0.122 | 0.487 | 11.988 |
| TF1 | 0.061 | 0.244 | 5.994 |
| TF2A | 0.122 | 0.487 | 11.988 |
| TF2B | 0.061 | 0.244 | 5.994 |
| TF3 | 0.061 | 0.244 | 5.994 |
2.4.4. Stability
The solutions of each of the 12 substances were diluted to three concentrations: high, medium, and low. To each solution, 10 μL of the IS was added to achieve a final volume of 500 μL, ensuring consistent dosing across samples. The analytes were prepared as LQC, MQC, and HQC samples. Each QC sample solution was prepared in six replicates and stored at room temperature (25°C) for 8 h to assess the stability of the analytes. The specific concentrations of HQC, MQC, and LQC are shown in Table 4.
2.4.5. Reproducibility
Six replicate extractions were performed and prepared from 5 years QDT extract. Each extract was separately injected in LC-MS/MS, and the RSD of the peak area ratios of 12 compounds to the IS peak area was calculated.
2.5. Determination of Eight Catechins and Four Theaflavins in QDT of Different Years
The content of eight catechins and four theaflavins in QDT from different years was determined. Based on the optimal extraction conditions obtained from the orthogonal experiment, the best conditions for methanol and water extraction were used to extract QDT from the 1, 5, 9, and 13 years. The experimental procedure was the same as described in Section 2.3.
3. Results and Discussion
3.1. Methodological Results
3.1.1. The Specificity
The results of specificity are shown in Figure 1. There was not any other interference peak between 12 components and IS under the chromatographic conditions.
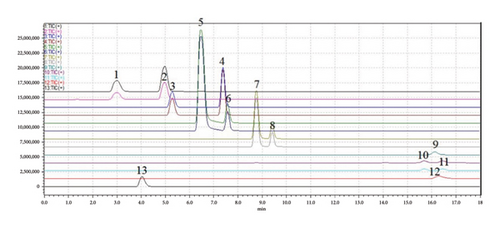
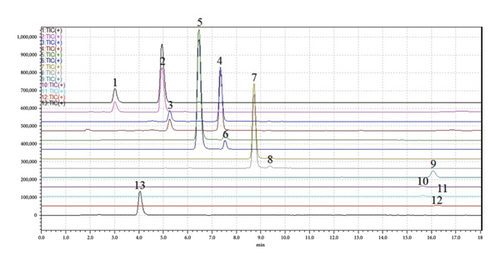
3.1.2. The Standard Curve and LLOQ
The linear equation, correlation coefficients, linear ranges, and LLOQ of the 12 compounds are shown in Table 5. The linear correlation coefficients of all the standard curves were greater than 0.9911, which indicated that the compounds had good linear relationships within the linear ranges.
| Analyte | Linear equation | R2 | Linear range (μg/mL) | LLOQ (μg/mL) |
|---|---|---|---|---|
| GC | y = 0.1186x + 0.0392 | 0.9997 | 0.152–38.961 | 0.152 |
| EGC | y = 0.0511x + 0.1993 | 0.9961 | 0.365–93.506 | 0.365 |
| C | y = 0.1333x + 0.0196 | 0.9976 | 0.091–23.377 | 0.091 |
| EC | y = 0.1817x + 0.2481 | 0.9911 | 0.183–46.753 | 0.183 |
| EGCG | y = 0.049x + 0.3653 | 0.9975 | 0.609–311.688 | 0.609 |
| GCG | y = 0.0298x + 0.0048 | 0.9959 | 0.183–46.753 | 0.183 |
| ECG | y = 0.118x + 0.3009 | 0.9967 | 0.396–101.299 | 0.396 |
| CG | y = 0.322x − 0.0015 | 1.0000 | 0.015–0.974 | 0.015 |
| TF1 | y = 0.2729x + 0.0064 | 0.9998 | 0.030–7.792 | 0.030 |
| TF2A | y = 0.035x + 0.0008 | 0.9998 | 0.030–15.584 | 0.030 |
| TF2B | y = 0.0925x − 0.0056 | 0.9998 | 0.030–7.792 | 0.030 |
| TF3 | y = 0.2193x + 0.0208 | 0.9998 | 0.030–7.792 | 0.030 |
3.1.3. Precision and Accuracy
The results in Table 6 show that the intra-day RSD and recovery rate were 0.168%–2.600% and 97.1%–108.3%, respectively. The interday RSD and recovery rate were 1.095%–2.979% and 93.9%–106.7%, respectively.
| Analyte | Concentration of HQC, MQC, and LQC (μg/mL) | Intraday | Interday | ||
|---|---|---|---|---|---|
| RSD (%) | Recovery rate (%) | RSD (%) | Recovery rate (%) | ||
| GC | 29.970 | 0.343 | 98.9 | 2.682 | 99.6 |
| 1.218 | 0.579 | 99.8 | 2.862 | 101.2 | |
| 0.304 | 1.650 | 99.8 | 2.517 | 96.1 | |
| EGC | 71.928 | 0.683 | 100.1 | 1.616 | 100.4 |
| 2.922 | 0.675 | 100.9 | 2.862 | 100.9 | |
| 0.731 | 0.490 | 99.9 | 2.437 | 99.9 | |
| C | 17.982 | 1.057 | 101.5 | 2.573 | 104.1 |
| 0.731 | 0.575 | 102.4 | 2.950 | 103.5 | |
| 0.183 | 1.434 | 102.4 | 2.711 | 99.4 | |
| EC | 35.964 | 0.682 | 101.5 | 2.346 | 104.2 |
| 1.461 | 0.687 | 102.1 | 2.034 | 103.0 | |
| 0.365 | 2.219 | 98.0 | 2.681 | 93.9 | |
| EGCG | 239.760 | 0.168 | 101.4 | 1.095 | 102.2 |
| 9.740 | 0.623 | 99.2 | 2.230 | 100.9 | |
| 2.435 | 0.274 | 99.2 | 2.174 | 96.6 | |
| GCG | 35.964 | 0.818 | 101.8 | 1.489 | 101.8 |
| 1.461 | 1.001 | 100.2 | 2.607 | 99.9 | |
| 0.365 | 1.087 | 97.2 | 2.932 | 97.1 | |
| ECG | 77.922 | 1.228 | 97.1 | 1.594 | 97.2 |
| 3.166 | 2.126 | 98.3 | 2.606 | 96.5 | |
| 0.791 | 2.600 | 98.7 | 2.766 | 100.6 | |
| CG | 11.988 | 2.009 | 100.8 | 2.813 | 102.7 |
| 0.487 | 1.491 | 99.1 | 2.974 | 106.7 | |
| 0.122 | 1.978 | 98.4 | 2.587 | 98.4 | |
| TF1 | 5.994 | 0.834 | 108.3 | 2.883 | 105.7 |
| 0.244 | 0.964 | 98.2 | 2.968 | 98.4 | |
| 0.061 | 1.181 | 101.4 | 2.713 | 100.6 | |
| TF2A | 11.988 | 0.812 | 102.2 | 2.816 | 99.6 |
| 0.487 | 1.333 | 98.2 | 2.937 | 98.6 | |
| 0.122 | 0.953 | 100.1 | 2.812 | 99.1 | |
| TF2B | 5.994 | 1.478 | 102.5 | 2.947 | 100.0 |
| 0.244 | 0.730 | 99.2 | 2.892 | 100.2 | |
| 0.061 | 1.950 | 99.4 | 2.939 | 94.7 | |
| TF3 | 5.994 | 0.679 | 101.7 | 2.568 | 100.3 |
| 0.244 | 0.899 | 98.1 | 2.735 | 99.3 | |
| 0.061 | 1.333 | 100.4 | 2.979 | 99.6 | |
3.1.4. Stability Tests
The HQC, MQC, and LQC were prepared and placed at room temperature (25°C) for 8 h and then injected into the samples to investigate the stability of eight catechins and four theaflavins; the results are shown in Table 7. It indicated that the analytes remained stable under the current conditions.
| Analyte | Concentration of HQC, MQC, and LQC (μg/mL) | Accuracy (%) | RSD (%) |
|---|---|---|---|
| GC | 29.970 | 93.21 | 0.14 |
| 1.218 | 104.21 | 0.70 | |
| 0.304 | 99.65 | 9.71 | |
| EGC | 71.928 | 95.68 | 0.79 |
| 2.922 | 99.10 | 1.18 | |
| 0.731 | 97.57 | 7.61 | |
| C | 17.982 | 93.21 | 0.11 |
| 0.731 | 103.32 | 0.84 | |
| 0.183 | 105.05 | 5.36 | |
| EC | 35.964 | 92.92 | 0.69 |
| 1.461 | 99.41 | 1.90 | |
| 0.365 | 103.56 | 9.73 | |
| EGCG | 239.760 | 95.37 | 1.29 |
| 9.740 | 100.94 | 9.06 | |
| 2.435 | 100.07 | 10.85 | |
| GCG | 35.964 | 94.10 | 0.06 |
| 1.461 | 95.69 | 0.42 | |
| 0.365 | 99.05 | 2.80 | |
| ECG | 77.922 | 91.90 | 1.13 |
| 3.166 | 92.31 | 2.35 | |
| 0.791 | 97.42 | 10.13 | |
| CG | 11.988 | 93.15 | 0.14 |
| 0.487 | 91.49 | 0.17 | |
| 0.122 | 95.76 | 10.28 | |
| TF1 | 5.994 | 94.61 | 0.08 |
| 0.244 | 99.39 | 0.18 | |
| 0.061 | 97.28 | 3.65 | |
| TF2A | 11.988 | 94.39 | 0.03 |
| 0.487 | 99.05 | 0.05 | |
| 0.122 | 92.81 | 0.81 | |
| TF2B | 5.994 | 93.00 | 0.04 |
| 0.244 | 100.12 | 0.01 | |
| 0.061 | 108.98 | 1.65 | |
| TF3 | 5.994 | 93.03 | 0.09 |
| 0.244 | 101.80 | 0.02 | |
| 0.061 | 96.31 | 5.00 | |
3.1.5. Reproducibility Experiments
QDT 2022 was utilized as the sample, and optimal methanol extraction conditions determined experimentally were applied to prepare the QDT extract following the procedures outlined in Section 2.3. The extract was subsequently divided evenly into six aliquots. Following HPLC-MS/MS analysis, the RSD of the peak area ratios of the 12 compounds to the IS peak area was calculated individually, with results presented in Table 8. All compounds exhibited RSD below 1%, meeting FDA standards and indicating excellent method reproducibility.
| Analyte | RSD (%) |
|---|---|
| GC | 0.574 |
| EGC | 0.635 |
| C | 0.307 |
| EC | 0.362 |
| EGCG | 0.170 |
| GCG | 0.206 |
| ECG | 0.243 |
| CG | 0.838 |
| TF1 | 0.411 |
| TF2A | 0.857 |
| TF2B | 0.366 |
| TF3 | 0.497 |
3.2. Orthogonal Test Results of 12 Compounds by Methanol Extraction
From the results of orthogonal test in Table 9, it can be seen that the larger R indicates the greater influence of its corresponding factor on the main index. Therefore, based on the magnitude of R, we can know the main order of influence on the extraction effect of the 12 substances as B (extraction temperature) > C (extraction time) > A (methanol concentration). The mean values for each group of factors indicate that the optimum in factor A is A2, the optimum in factor B is B3, and the optimum in factor C is C1. In conclusion, the optimal extraction conditions for the methanol extraction method were A2B3 C1, i.e., methanol concentration of 60%, extraction temperature of 90°C, and extraction time of 15 min. Literature indicates that the optimal methanol concentration for extraction lies between 50 and 80% [28–31]. A 60% methanol concentration is preferred as it ensures sufficient polarity to dissolve catechins and theaflavins while maintaining a water content that enhances the solubility of other less polar compounds. At an extraction temperature of 90°C, the kinetic energy of solvent molecules is increased, accelerating the rate of diffusion and thereby enhancing the extraction efficiency of the target compounds. Moreover, the solubility of catechins and theaflavins is significantly increased at this temperature. Although these compounds are temperature sensitive, short-term high-temperature treatments do not result in significant degradation or loss [32–34]. An extraction time of 15 min was deemed optimal as it balanced extraction efficiency and stability, thereby avoiding the degradation of compounds caused by excessively long extraction durations [27, 35].
| Level | Factors | 12 substance content (μg/mL) | ||
|---|---|---|---|---|
| Methanol concentration (%) | Extraction temperature (°C) | Extraction time (min) | ||
| A | B | C | ||
| 1 | 1 (40) | 1 (50) | 1 (15) | 37.779 ± 0.066 |
| 2 | 1 | 2 (70) | 2 (30) | 44.398 ± 0.783 |
| 3 | 1 | 3 (90) | 3 (45) | 45.235 ± 0.557 |
| 4 | 2 (60) | 1 | 2 | 38.376 ± 1.874 |
| 5 | 2 | 2 | 3 | 44.025 ± 1.182 |
| 6 | 2 | 3 | 1 | 52.309 ± 0.664 |
| 7 | 3 (80) | 1 | 3 | 41.466 ± 0.203 |
| 8 | 3 | 2 | 1 | 44.090 ± 0.393 |
| 9 | 3 | 3 | 2 | 38.750 ± 0.723 |
| K1 | 127.412 | 117.621 | 134.178 | |
| K2 | 134.711 | 132.513 | 121.524 | |
| K3 | 124.306 | 136.295 | 130.726 | |
| k1 | 42.471 | 39.207 | 44.726 | |
| k2 | 44.904 | 44.171 | 40.508 | |
| k3 | 41.435 | 45.432 | 43.575 | |
| Range (R) | 3.468 | 6.224 | 4.218 | |
| Order of priority | B > C > A | |||
| Optimum level | A2 | B3 | C1 | |
The K1 under factor A represents the sum of the results corresponding to 1 under factor A; the K1 under factor B represents the sum of the results corresponding to 1 under factor B; the K1 under factor C represents the sum of the results corresponding to 1 under factor C; K2, K3, and so forth follow this pattern. The k1 under factor A is the K1 under factor A divided by 3 (since we have 3 levels); the k1 under factor B is the K1 under factor B divided by 3; the k1 under factor C is the K1 under factor C divided by 3; and so on for k2, k3, and so forth. The optimal levels are determined based on k1, k2, and k3.
3.3. Test Results of 12 Substances Extracted by Water Extraction
The results of water extraction under different conditions are presented in Table 10. Across the nine different extraction conditions, with variations in extraction temperature and time, the content of the 12 compounds ranged from 16.655 to 22.661 μg/mL. The highest total content of compounds was obtained under the conditions of 90°C for 30 min. Compared to the alcohol extraction method, the optimal conditions for the water extraction method are 90°C and 30 min. The longer extraction time required for the aqueous method is attributed to the relatively weaker solubility and permeability of water, necessitating an extended duration to achieve high extraction efficiency.
| Level | Extraction temperature (°C) | Extraction time (min) | 12 substance content (μg/mL) |
|---|---|---|---|
| 1 | 50 | 15 | 16.655 ± 0.401 |
| 2 | 50 | 30 | 16.886 ± 0.142 |
| 3 | 70 | 15 | 21.134 ± 0.163 |
| 4 | 90 | 15 | 22.407 ± 0.909 |
| 5 | 70 | 30 | 18.309 ± 2.141 |
| 6 | 50 | 45 | 18.663 ± 0.169 |
| 7 | 70 | 45 | 19.856 ± 0.064 |
| 8 | 90 | 45 | 19.729 ± 0.246 |
| 9 | 90 | 30 | 22.661 ± 0.396 |
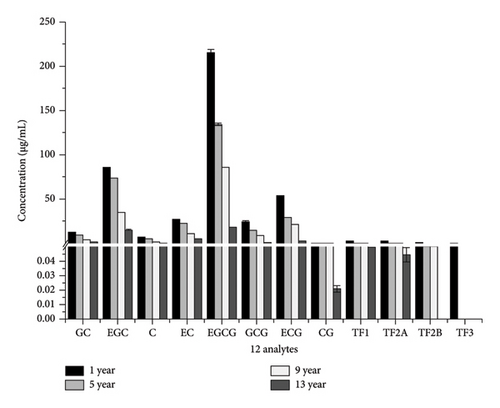
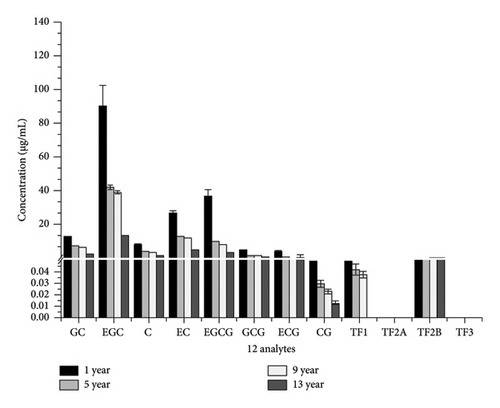
3.4. Comparison of the Content of 12 Substances in QDT (1, 5, 9, and 13 years) by Methanol Extraction
According to the orthogonal experiment results of methanol extraction mentioned above, under the optimal conditions, extraction of 12 compounds (eight catechins and four theaflavins) was conducted from QDT of different years (1, 5, 9, and 13 years), and the content of these 12 compounds in QDT of different years was compared. The results are presented in Table 11, Table 12, and Figure 2. The total content of the 12 compounds in QDT from the 1st year was the highest under methanol extraction conditions, followed by the 5th year, then the 9th year, and the least in the 13th year (1st year > 5th year > 9th year > 13th year). Specifically, for each of these 12 individual compounds, the content of each substance was highest in the 1st year, and the trend and characteristics were also 1st year > 5th year > 9th year > 13th year. Indeed, existing research suggests that prolonged storage time leads to a reduction in the polyphenol content, the primary bioactive compounds in tea. This phenomenon is likely attributed to the oxidation of polyphenols during extended storage periods [36]. Xu et al. [37] conducted a study demonstrating that while the biochemical characteristics of Ying tea exhibited minimal changes within the first 12 months, components such as water extracts and tea polyphenols continued to decline throughout the 6-year storage period. Tian et al. [38] examined the influence of storage duration (ranging from less than 1 month to 192 months) on the bacterial and fungal populations in Pu’er tea. Their findings indicated that the populations of fungi and bacteria, along with the polyphenol content decreased with storage time.
| Analyte | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| GC | 19.297 ± 0.204 | 20.775 ± 0.551 | 25.296 ± 0.34 | 15.464 ± 1.641 | 16.778 ± 0.482 | 31.779 ± 0.871 | 16.309 ± 0.188 | 23.276 ± 0.275 | 17.121 ± 0.488 |
| EGC | 119.015 ± 0.833 | 130.53 ± 3.398 | 144.188 ± 3.603 | 128.321 ± 8.622 | 141.708 ± 21.62 | 189.897 ± 2.004 | 121.537 ± 0.586 | 140.889 ± 0.545 | 127.472 ± 2.96 |
| C | 8.575 ± 0.007 | 8.963 ± 0.203 | 11.19 ± 0.217 | 7.156 ± 0.57 | 7.726 ± 0.159 | 14.514 ± 0.935 | 7.078 ± 0.057 | 10.411 ± 0.139 | 7.659 ± 0.219 |
| EC | 34.553 ± 0.714 | 36.601 ± 0.675 | 40.688 ± 0.638 | 33.556 ± 2.338 | 34.953 ± 0.454 | 58.595 ± 1.218 | 32.477 ± 0.369 | 41.781 ± 1.144 | 35.156 ± 1.091 |
| EGCG | 252.329 ± 1.138 | 260.962 ± 8.642 | 312.14 ± 4.258 | 277.789 ± 13.093 | 299.25 ± 11.101 | 352.472 ± 3.406 | 283.288 ± 1.642 | 291.081 ± 3.155 | 258.462 ± 7.357 |
| GCG | 18.944 ± 0.608 | 18.564 ± 0.546 | 33.819 ± 0.71 | 20.056 ± 1.352 | 25.436 ± 0.869 | 26.856 ± 3.509 | 24.746 ± 1.22 | 22.563 ± 1.015 | 20.295 ± 0.466 |
| ECG | 63.706 ± 0.514 | 65.6 ± 1.493 | 81.129 ± 1.026 | 67.064 ± 4.539 | 77.836 ± 1.208 | 87.02 ± 1.573 | 78.035 ± 0.691 | 74.149 ± 0.716 | 65.872 ± 1.254 |
| CG | 0.511 ± 0.032 | 0.515 ± 0.021 | 0.988 ± 0.041 | 0.518 ± 0.04 | 0.659 ± 0.017 | 0.698 ± 0.035 | 0.641 ± 0.006 | 0.575 ± 0.03 | 0.474 ± 0.014 |
| TF1 | 0.656 ± 0.011 | 0.663 ± 0.012 | 0.892 ± 0.017 | 1.072 ± 0.054 | 1.329 ± 0.028 | 1.412 ± 0.135 | 1.326 ± 0.013 | 1.274 ± 0.019 | 1.327 ± 0.034 |
| TF2A | 0.219 ± 0.059 | 0.221 ± 0.038 | 0.442 ± 0.037 | 0.531 ± 0.06 | 0.794 ± 0.026 | 0.669 ± 0.046 | 0.911 ± 0.043 | 0.722 ± 0.018 | 0.753 ± 0.044 |
| TF2B | N.D. | N.D. | 0.044 ± 0.009 | 0.074 ± 0.011 | 0.183 ± 0.021 | 0.071 ± 0.022 | 0.299 ± 0.01 | 0.086 ± 0.014 | 0.148 ± 0.011 |
| TF3 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
- Note: N.D. indicates that the concentration is below the minimum detectable limit and could not be detected.
| Analyte | 1 yr (μg/mL) | 5 yr (μg/mL) | 9 yr (μg/mL) | 13 yr (μg/mL) |
|---|---|---|---|---|
| GC | 11.871 ± 0.109 | 9.469 ± 0.022 | 4.477 ± 0.023 | 1.606 ± 0.088 |
| EGC | 84.818 ± 0.620 | 74.301 ± 0.112 | 35.616 ± 0.235 | 15.261 ± 0.720 |
| C | 7.227 ± 0.153 | 5.604 ± 0.032 | 2.474 ± 0.011 | 0.898 ± 0.024 |
| EC | 26.552 ± 0.353 | 22.517 ± 0.330 | 10.595 ± 0.080 | 4.566 ± 0.086 |
| EGCG | 214.982 ± 3.387 | 134.570 ± 1.452 | 85.962 ± 0.430 | 17.751 ± 0.392 |
| GCG | 24.106 ± 1.830 | 15.224 ± 0.181 | 9.346 ± 0.374 | 0.835 ± 0.029 |
| ECG | 53.871 ± 0.714 | 29.616 ± 0.474 | 21.647 ± 0.245 | 2.793 ± 0.158 |
| CG | 0.579 ± 0.038 | 0.308 ± 0.003 | 0.226 ± 0.022 | 0.021 ± 0.002 |
| TF1 | 3.300 ± 0.033 | 0.991 ± 0.011 | 0.641 ± 0.022 | 0.087 ± 0.005 |
| TF2A | 2.585 ± 0.030 | 0.679 ± 0.006 | 0.571 ± 0.011 | 0.044 ± 0.005 |
| TF2B | 0.698 ± 0.012 | 0.085 ± 0.002 | 0.084 ± 0.004 | N.D. |
| TF3 | 0.168 ± 0.048 | N.D. | N.D. | N.D. |
| Total | 430.757 | 293.364 | 171.649 | 103.799 |
3.5. Comparison of the Content of 12 Substances in QDT (1, 5, 9, and 13 Years) by Water Extraction
According to the above water extraction test results, the optimal conditions were selected for different years (1, 5, 9, and 13 years) of QDT, and the contents of 12 compounds (eight catechins and four theaflavins) in QDT of different years were compared. The results of 12 substances content in different years of QDT are shown in Table 13, Table 14, and Figure 3. It could be seen that the 12 substances content of 1st year QDT was the highest under the water extraction condition. Similar to the results of methanol extraction, whether it is the total content of the 12 substances or the individual comparison of the content of the 12 substances, the trend remains consistent: 1st year > 5th year > 9th year > 13th year.
| Analyte | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| GC | 11.781 ± 0.305 | 10.65 ± 0.635 | 12.921 ± 0.043 | 12.565 ± 0.288 | 10.934 ± 0.093 | 12.58 ± 0.08 | 13.341 ± 0.067 | 15.061 ± 0.299 | 17.15 ± 0.24 |
| EGC | 85.039 ± 2.736 | 84.035 ± 0.818 | 90.275 ± 1.154 | 89.591 ± 4.34 | 82.278 ± 1.217 | 95.817 ± 2.532 | 93.158 ± 0.853 | 83.284 ± 1.728 | 93.42 ± 0.96 |
| C | 5.471 ± 0.114 | 5.355 ± 0.039 | 6.065 ± 0.083 | 6.151 ± 0.205 | 5.075 ± 0.14 | 5.672 ± 0.005 | 6.095 ± 0.117 | 6.877 ± 0.175 | 8.01 ± 0.34 |
| EC | 24.933 ± 0.5 | 24.451 ± 0.243 | 26.066 ± 0.408 | 25.277 ± 1.26 | 24.11 ± 0.735 | 27.833 ± 0.215 | 27.547 ± 0.13 | 25.053 ± 0.606 | 28.66 ± 1.12 |
| EGCG | 64.022 ± 3.733 | 70.539 ± 0.92 | 110.505 ± 1.539 | 129.463 ± 9.499 | 74.93 ± 2.61 | 77.149 ± 0.841 | 91.479 ± 0.619 | 93.264 ± 1.091 | 109.85 ± 2.73 |
| GCG | 2.593 ± 0.104 | 2.845 ± 0.024 | 5.962 ± 0.548 | 13.611 ± 0.911 | 48.995 ± 78.261 | 3.033 ± 0.059 | 5.339 ± 0.14 | 11.935 ± 0.219 | 14.43 ± 0.17 |
| ECG | 10.346 ± 0.317 | 11.931 ± 0.11 | 21.85 ± 0.11 | 24.184 ± 1.515 | 11.743 ± 0.252 | 11.6 ± 0.232 | 15.331 ± 0.294 | 17.01 ± 0.232 | 20.78 ± 0.33 |
| CG | 0.039 ± 0.006 | 0.052 ± 0.004 | 0.135 ± 0.008 | 0.268 ± 0.009 | 0.061 ± 0.003 | 0.045 ± 0.012 | 0.1 ± 0.023 | 0.22 ± 0.005 | 0.29 ± 0.02 |
| TF1 | 0.074 ± 0.001 | 0.077 ± 0.001 | 0.164 ± 0.001 | 0.19 ± 0.009 | 0.105 ± 0.003 | 0.081 ± 0.005 | 0.103 ± 0.005 | 0.108 ± 0.001 | 0.12 ± 0.000 |
| TF2A | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| TF2B | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| TF3 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Analyte | 1 yr (μg/mL) | 5 yr (μg/mL) | 9 yr (μg/mL) | 13 yr (μg/mL) |
|---|---|---|---|---|
| GC | 12.533 ± 0.231 | 6.877 ± 0.062 | 6.523 ± 0.061 | 2.350 ± 0.050 |
| EGC | 90.022 ± 12.749 | 42.049 ± 0.987 | 38.830 ± 0.947 | 13.331 ± 0.365 |
| C | 7.810 ± 0.375 | 3.789 ± 0.047 | 3.541 ± 0.011 | 1.296 ± 0.034 |
| EC | 26.053 ± 1.788 | 12.850 ± 0.153 | 12.097 ± 0.094 | 4.616 ± 0.122 |
| EGCG | 36.763 ± 3.703 | 9.293 ± 0.199 | 7.490 ± 0.148 | 2.872 ± 0.227 |
| GCG | 4.274 ± 0.300 | 1.294 ± 0.017 | 1.220 ± 0.017 | 0.620 ± 0.035 |
| ECG | 3.758 ± 0.440 | 0.254 ± 0.035 | 0.056 ± 0.007 | 0.309 ± 1.331 |
| CG | 0.066 ± 0.006 | 0.030 ± 0.003 | 0.023 ± 0.002 | 0.013 ± 0.002 |
| TF1 | 0.084 ± 0.003 | 0.042 ± 0.005 | 0.037 ± 0.003 | N.D. |
| TF2A | N.D. | N.D. | N.D. | N.D. |
| TF2B | 0.064 ± 0.000 | 0.067 ± 0.002 | 0.066 ± 0.002 | 0.061 ± 0.000 |
| TF3 | N.D. | N.D. | N.D. | N.D. |
| Total | 181.429 | 76.560 | 69.896 | 25.468 |
3.6. Summary and Analysis of the Results
According to the tea polyphenol extraction process outlined in the literature [34], efficiency is higher at 80°C compared to 50°C. Numerous studies have used water at typical tea brewing temperatures for extraction, leading to the use of freshly boiled distilled water (90°C) as an experimental water extraction method [33]. Consequently, this study sets three temperature levels: 50°C, 70°C, and 90°C. Regarding phenol content, the Soxhlet extraction method with a 50% methanol mixture showed optimal results [28]. Rafique et al. evaluated the bioactivity of ethanol and methanol extracts from Camellia and other plants at different concentrations (30%, 50%, and 80%), finding that 80% methanol thyme extract exhibited the highest antibacterial activity [29]. Based on these findings, this study designed experiments with methanol concentrations of 40%, 60%, and 80%. In terms of extraction time, Li et al. conducted an experimental comparison of extraction times (10, 20, 30, 40, and 50 min) and found that polyphenol extraction efficiency increased significantly up to 30 min, after which the growth rate slowed. Therefore, this study sets extraction times at 15, 30, and 45 min to verify changes post-30 min [27].
Based on the optimization of extraction conditions for 12 compounds, the optimal parameters for methanol extraction were determined to be a methanol concentration of 60%, an extraction temperature of 90°C, and an extraction time of 15 min. The orthogonal experiments identified extraction temperature as the most significant factor influencing extraction efficiency, consistent with previous studies that higher temperatures enhance solvent permeability and solubility, thereby improving extraction efficiency. The choice of 60% methanol concentration balances high polarity for dissolving catechins and theaflavins with sufficient water content to dissolve less polar compounds, while also minimizing impurity extraction [30]. Although pure methanol is more effective in dissolving target compounds, it also extracts more impurities. Meanwhile, a 60% methanol concentration can effectively extract catechins and theaflavins, while reducing the extraction of nontarget substances, whereas 60% methanol efficiently extracts catechins and theaflavins with reduced impurity coextraction (B. J. [31]). The 90°C extraction temperature increases solvent molecular kinetic energy, accelerating diffusion into tea cells and enhancing compound solubility and extraction efficiency without causing significant degradation of catechins and theaflavins [32]. The 15-min extraction time optimizes efficiency and stability, achieving high extraction yields while preventing compound degradation from prolonged extraction. Similarly, for water extraction, the optimal conditions were determined to be 90°C for 30 min [35]. Water extraction requires a longer duration to achieve high efficiency, likely due to the lower solubility and permeability of water compared to methanol.
Regarding the impact of tea age on extract content, the study demonstrated that the extract content decreased with the increase of tea age, irrespective of the extraction method used methanol or water. The concentration of 12 substances in one-year QDT was significantly higher compared to other years, with a noticeable change trend from one to five years, which then tended to stabilize. This phenomenon could be attributed to the oxidation, polymerization, and degradation of the tea’s internal chemical components during long-term storage. Polyphenolic compounds such as catechins and theaflavins are particularly prone to oxidation, especially during the initial years of storage, a large amount of these substances oxidized, leading to a significant reduction in extract content [39].
The findings indicated that in the same year, the content of 12 substances extracted using the methanol extraction method was considerably higher than that obtained using the water extraction method. This discrepancy may be related to the solubility of these compounds, as they may dissolve more readily at a certain concentration of methanol [40, 41], or it may be due to their increased stability in the methanol solvent after extraction [42, 43]. Conversely, the water extraction method, due to its high polarity, exhibits lower extraction efficiency for certain nonpolar or weakly polar compounds. Additionally, in the water extraction method, polyphenols are more susceptible to hydrolysis, resulting in lower extract content.
4. Conclusion
This study established a rapid, accurate, and sensitive HPLC-MS/MS method for simultaneous determination of eight catechins and four theaflavins in QDT. The methodologies of the content determination of 12 compounds were validated, including specificity, linearity, limit of quantification, precision, accuracy, stability, and reproducibility. This method can not only be applied to quantify catechins and theaflavins in QDT but is also suitable for the analysis of catechins and theaflavins in black tea and various other types of tea, assessing the beneficial effects of tea, and conducting QC.
In the experiment, methanol and water were used for extraction of QDT, with the optimal conditions being methanol concentration of 60%, temperature of 90°C and time of 15 min, and temperature of 90°C and time of 30 min for methanol and water extraction, respectively. This extraction method provides a basis and reference for subsequent researchers to extract QDT. Additionally, we found that the content of these 12 compounds was higher with methanol extraction than with water extraction. In summary, these two different extraction methods will provide references for the preparation of QDT and even for the preparation of eight catechins and four theaflavins in tea leaves in future studies.
Most importantly, we discovered that the lower the vintage, the higher the content of catechins and theaflavins. This reminds us that when purchasing QDT, a higher price does not necessarily indicate better quality. If you wish to consume more catechins and theaflavins, lower vintage QDT may be more suitable. Since low-vintage QDT is often sold as high-vintage QDT on the market to profit, it is difficult for consumers to distinguish between them. However, our method of comparing catechin and theaflavin content revealed that lower-content QDT was actually of a higher vintage, which can be used to identify QDT vintage. In conclusion, the results of this study can provide reference for the extraction, identification, and content determination of eight catechins and four theaflavins in QDT, as well as for distinguishing different vintages of QDT, thereby safeguarding people’s health.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Xiaolei Miao, Yiye Zhao, and Ping Luo contributed equally to this work and both are co-first authors.
Funding
This work was supported by the Key Scientific Instrument Special Project of China’s National Natural Science Foundation (Project No. 81727805), Xianning Key Special Project for Scientific and Technological Research and Development (2021SFYF003), Hubei University of Science and Technology Research innovation team (Project No. 2023T10), and The Doctoral Research Fund of Hubei University of Science and Technology (Project No. Q201810).
Acknowledgments
This work was supported by the Key Scientific Instrument Special Project of China’s National Natural Science Foundation (Project No. 81727805), Xianning Key Special Project for Scientific and Technological Research and Development (2021SFYF003), Hubei University of Science and Technology Research innovation team project (2023T10), and The Doctoral Research Fund of Hubei University of Science and Technology (Project No. Q201810).
Open Research
Data Availability Statement
The underlying data supporting the findings of this paper can be found in the manuscript.




