Enhanced Recovery of Apple Pomace Phenolic Compounds: Optimizing Subcritical Water Extraction
Abstract
This study was aimed at optimizing the extraction process of phenolic compounds, particularly antioxidants and antimicrobial agents, from apple pomace using the subcritical water extraction method. The Box–Behnken experimental design was utilized to ascertain the optimal extraction conditions, focusing on maximizing yield, phenolic compound content, and antioxidant activity. Three independent variables were investigated: extraction temperature (120°C, 140°C, and 160°C), extraction time (20, 30, and 40 min), and water-to-apple pomace ratio (1:20, 1:40, and 1:60 g/mL). The optimized subcritical water extraction process yielded a significantly higher extraction efficiency (18.05%), total phenolic content (1341.11 mg/100 g), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging capacity (92.39%) compared to the conventional maceration method. The experimental results aligned well with the predicted values obtained from the Box–Behnken model. The antimicrobial properties of the optimized extract were assessed against Staphylococcus aureus, Escherichia coli, and Aspergillus niger through the serial dilution method. The extract at a concentration of 30 mg/100 mL exhibited superior antifungal and antibacterial properties compared to potassium sorbate (10 mg/100 mL). This research demonstrates the potential of subcritical water extraction as a sustainable and efficient method for extracting phenolic compounds from apple pomace, offering a promising alternative to conventional methods and contributing to the valorization of agricultural waste.
1. Introduction
Various additives play a crucial role in food production, as many products rely on them for successful manufacturing. Antioxidants and antimicrobial compounds are important for extending shelf life and reducing food waste. While numerous synthetic additives have been approved by regulatory bodies for use in food products, their application is often limited due to safety concerns [1].
In response to these concerns, there has been a growing interest in natural additives as potential replacements for synthetic counterparts. One such natural additive is apple pomace, which is generated in significant quantities—approximately four million metric tons (MMT) annually—comprising apple peels, leftover flesh, cores with seeds, and stems. Apple pomace is rich in valuable bioactive compounds, including phenolic acids, flavonols, quercetin glycosides, dihydrochalcones, and anthocyanins, which exhibit antimicrobial and antioxidant properties [2–4]. The extraction method influences the quantity and bioactivity of these compounds.
Conventional and novel extraction methods are utilized to isolate bioactive compounds. One notable approach is subcritical water, which differs from normal water’s properties and is a versatile solvent with adjustable polarity. This feature, along with its nontoxic nature, environmental compatibility, and affordable availability of water, makes subcritical water a favorable option for extracting active compounds [5, 6].
The extraction of various phenolic compounds—ranging from nonpolar tocopherols, carotenoids, sterols, and terpenoids to medium-polarity and polar phenolic compounds such as catechins, flavonoids, isoflavones, alkaloids, and phenolic acids—has been successfully achieved using subcritical water or mixtures of water and ethanol [7, 8]. Given the nature of the phenolic compounds in apple pomace, subcritical water extraction holds promise for yielding higher quantities of these valuable substances.
The effectiveness and properties of extracts produced via subcritical water extraction are affected by factors related to sample preparation (such as particle size and storage conditions) and extraction parameters, including temperature, solid-to-water ratio, extraction duration, and the use of specific additives. Consequently, optimizing these parameters is essential for enhancing extraction feasibility and efficiency while managing costs. The response surface methodology, comprising a set of mathematical and statistical techniques, is commonly employed to develop and optimize processes influenced by multiple variables and has been extensively applied to refine the extraction of bioactive compounds from various sources [9–13]. However, further research and developing refined models are necessary to determine optimal conditions for effectively utilizing subcritical water in extracting phenolic compounds from apple pomace.
Given the significance of optimizing agricultural and food industry waste, such as that generated by apple juice production, this research is aimed at improving the extraction of phenolic compounds using the subcritical water process. The study specifically focuses on the effects of temperature, time, and the ratio of dry matter to water while also investigating the antimicrobial activity (both antifungal and antibacterial properties) of the extracts obtained under optimal conditions.
2. Materials and Methods
2.1. Materials
The apple pomace (Malus domestica) was obtained from the Razavi factory in Mashhad, Razavi Khorasan Province, and it was promptly dried in an oven at 45°C until the moisture content fell below 14%. The dried samples were then ground using a laboratory mill, and the particle size was adjusted using a 40-micron sieve. The powdered samples were kept in a freezer at −18°C until the experiments were carried out. Other materials used in this research included 96% ethanol, hydrochloric acid, sodium chloride, magnesium nitrate, microbiological culture media, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and methanol, all sourced from Sigma and Merck. The bacteria and fungi studied included Staphylococcus aureus (PTCC1112), Escherichia coli (PTCC1399), and Aspergillus niger (PTCC5009), which were obtained as lyophilized cultures from the Research Institute of Iran.
2.2. Extraction of Phenolic Compounds From Apple Pomace
2.2.1. Extraction by Maceration
Dried apple pomace powder was mixed with an aqueous-alcoholic solvent (70% ethanol and 30% water) at a ratio of 1:10 (w/v) and stirred for 24 h at room temperature in the dark using a magnetic stirrer (RCT Basic, IKA, Germany). The resulting solution was filtered under vacuum, and the extraction process was repeated on the residue for 24 h. The filtrates from both extraction stages were combined and concentrated under vacuum using a rotary evaporator (Laborota 4000 efficient, Germany) at 45°C until complete dehydration. To prevent foaming and achieve the appropriate temperature for freeze-drying, the concentrated solution was stored in a freezer at −70°C for 19 h before being transferred to a freeze-drier (Operon FDB-550, Korea).
Approximately 200 mL of each sample was freeze-dried (−55°C, 0.15 mmHg) for 20 h and kept in the dark at −18°C until analysis.
2.2.2. Extraction With Subcritical Water
A custom-designed device from the Laboratory of Modern Technologies at the Institute of Food Science and Industries )RIFST, Mashhad, Iran) was employed for the extraction process. The apparatus consisted of a deionized water tank, a pressure pump (comet type: MTP AX 2/70 m), a 140 mL extraction chamber, a heating coil, a pressure gauge, and a digital controller. The extraction conditions, including the apple pomace to water ratio, temperature, and time, were based on the Box–Behnken design, as detailed in Table 1. Deionized water served as the extraction solvent, and the appropriate temperature and pressure were determined using thermodynamic tables.
| Trial | Time (min) | Temperature (°C) | Apple pomace-to-water (g/mL) |
|---|---|---|---|
| 1 | 40(1) | 140(0) | 1:40(1) |
| 2 | 30(0) | 160(1) | 1:40(1) |
| 3 | 20(−1) | 140(0) | 1:40(1) |
| 4 | 30(0) | 140(0) | 1:30(0) |
| 5 | 20(−1) | 160(1) | 1:30(0) |
| 6 | 30(0) | 120(−1) | 1:40(1) |
| 7 | 40(1) | 160(1) | 1:30(0) |
| 8 | 40(1) | 140(0) | 1:20(−1) |
| 9 | 20(−1) | 140(0) | 1:20(−1) |
| 10 | 30(0) | 140(0) | 1:30(0) |
| 11 | 40(1) | 140(0) | 1:30(0) |
| 12 | 20(−1) | 120(−1) | 1:30(0) |
| 13 | 30(0) | 140(0) | 1:30(0) |
| 14 | 30(0) | 120(−1) | 1:20(−1) |
| 15 | 30(0) | 160(1) | 1:20(−1) |
The times required to reach temperatures of 120°C, 140°C, and 160°C (200, 360, and 600 kPa, respectively) were approximately 6, 7, and 8 min, respectively. Once the desired temperature and pressure were achieved, extraction was conducted for 20, 30, and 40 min. After extraction, the extract was cooled using a cold water flow system. The cooled extract was filtered under vacuum using Whatman No. 4 paper and freeze-dried (−55°C, 0.15 mmHg) for 20 h and kept in the dark at −18°C until analysis. The extracted samples were evaluated for their ability to scavenge DPPH radicals, quantify phenolic content, and determine extraction efficiency.
2.2.3. Chemical Properties of Apple Pomace
Chemical analyses followed established protocols distinct from [14]. Sample moisture was analyzed via a drying process in a controlled-temperature oven. Ash levels were determined by incinerating samples at 550°C in a muffle furnace. Protein concentration was calculated using the Kjeldahl method with a nitrogen-to-protein conversion factor of 6.25. Lipid content was extracted using a Soxhlet system, while total carbohydrate content was derived by subtracting moisture, protein, ash, and lipid percentages from the total composition.
2.2.4. Analysis of Phenolic Profiles in Apple Pomace
Phenolic compounds in apple pomace were quantified following the method outlined by Łata, Trampczynska, and Paczesna [15]. Separation was achieved using a high-performance liquid chromatography (HPLC) system (Cecil, United Kingdom), equipped with a dual pump (Cecil 4100) and a UV/Vis detector (Cecil 4201 UV/Vis). A C18 reversed-phase column (250 mm length, 4.5 mm internal diameter, and 5 μm particle size) was used for separation, complemented by a C18 guard column (5 mm length, 4 mm internal diameter, and 5 μm particle size).
Phenolics were identified at a wavelength of 280 nm, and their quantification was performed using an external standard calibration curve. Each sample was analyzed in triplicate to ensure the accuracy and reliability of the results. For compound identification, a dual-solvent system was employed, with water containing 2% acetic acid (Pump A) and methanol (Pump B). The gradient of acetic acid in methanol began at 10%, increased to 100% over 20 min, maintained this concentration from 20 to 25 min, and then reverted to the initial condition of 10% within 2 min, sustained until the 30-min mark. For the identification of chlorogenic acid and phlorizin dihydrate, a solvent system with 0.1% formic acid in water (Pump A) and methanol (Pump B) was employed, following a consistent gradient profile.
Polyphenolic compounds were identified by comparing the retention times of standard compounds with those of the apple pomace extract. Standard solutions were prepared with a concentration of 0.2 mg/mL in methanol. For analysis, 20 μL of extracts and standards were injected into the HPLC system. Quantification of phenolic compounds was performed by calculating the area under the curve and comparing it with that of the corresponding standards.
2.2.5. Determination of Total Phenolic Content
The Folin–Ciocalteu method, as described by Usenik, Fabčič, and Štampar [16], was applied to measure the total phenolic content. A 100 μL aliquot of the extract, diluted in methanol at a 1:10 (v/v) ratio, was mixed with 6 mL of double-distilled water and 500 μL of Folin–Ciocalteu reagent. After an incubation period of 8 min, 1.5 mL of 20% (w/v) sodium carbonate solution was added to the mixture. The reaction was allowed to proceed at 40°C for 30 min. Absorbance was subsequently measured at 765 nm using a UV-1600 spectrophotometer. Quantification was achieved by comparing sample absorbance to a standard calibration curve constructed using gallic acid solutions at concentrations ranging from 100 to 950 mg/L. The outcomes were reported in terms of gallic acid equivalents (GAE) (mg GAE/100 g).
2.2.6. Determination of Extraction Yield
2.2.7. Antioxidant Activity
2.2.8. Antimicrobial Activity
The minimum concentration of the optimally extracted sample required to inhibit microbial growth (minimum inhibitory concentration (MIC)) was evaluated through the tube dilution method. A series of 20 sterile test tubes were prepared for the experiment. Eighteen of these tubes were used to test various concentrations of the extract—specifically at 1, 2, 5, 10, 20, and 30 mg/100 mL—each combined with culture medium, normal saline, and a microbial suspension at a concentration of 1.5 × 106 CFU/mL. The negative control was only culture medium, total plate count, and normal saline. The positive control contains the microbial suspension, culture medium, and normal saline. Additionally, one tube included potassium sorbate (10 mg/100 mL) alongside the microbial suspension, culture medium, and normal saline. Separate tests were conducted for each microorganism, like bacterial and fungal strains.
After inoculation, the tubes containing Staphylococcus aureus and Escherichia coli were incubated at 37°C for 24 h. In contrast, the tubes with Aspergillus niger were incubated at 25°C for a duration of 3–5 days. Following the incubation periods, the tubes were examined visually for turbidity, which served as an indicator of microbial growth. The turbidity of the samples was quantitatively assessed using a spectrophotometer (UV-1600) at a wavelength of 600 nm [19].
2.2.9. Statistical Analysis
The effects of process variables in subcritical water extraction on the phenolic compound yield from apple pomace were investigated using a Box–Behnken design. This experimental design involved three variables at three levels, with three replications per treatment, as detailed in Table 1. Design Expert software (Design Expert 11) was applied for design experiments and analysis of results. Contour plots were constructed to depict the interactions between the independent variables and the outcome measures defined in the regression analysis. The adequacy of the fitted models was assessed through various statistical tests, including lack-of-fit, coefficient of variation, coefficient of determination (R2) values, adjusted R2, and model significance. The derived models reflected the behavior of variables concerning the characteristics of the extracted samples and were employed for optimization. The optimization process aimed to maximize the antioxidant activity of the extract. Under the identified optimal conditions, the properties of the extracts were subsequently measured. For the optimization and validation of the models, predicted results were compared with experimental outcomes using MStatC software, applying Duncan’s test at a significance level of 5% (p > 0.05).
3. Results and Discussion
3.1. Chemical Composition of Apple Pomace
The chemical characteristics of the dried apple pomace powder used in this study are presented in Table 2. The moisture content of crude apple pomace, initially at 67.90% ± 2.8%, was reduced by drying at 45°C to a moisture content of 12.74% ± 0.16%. Comparative studies report moisture levels of 4.4% [20], 10.5% [21], and 15% [22]. Apple pomace contains high levels of crude fiber and carbohydrates, while its fat and ash content is low. The phenolic compounds in apple pomace were quantified at 5.95 mg/g of dry weight. Reports indicate that the phenolic compound content obtained from various extraction methods ranges from 1.18 to 15.80 mg/g of dry weight of pomace, as measured by the Folin–Ciocalteu method [23]. Factors such as cultivar type, measurement method, juice preparation method, and drying technique significantly influence phenolic compound levels in pomace. Additionally, Table 2 indicates chlorogenic acid is in higher concentrations than other measured phenolic acids. Major phenolic acids in plants include derivatives of hydroxylated benzoic acids (such as 3,4-dihydroxybenzoic, ellagic, gallic, gentisic, protocatechuic, salicylic, and vanillic) and cinnamic acids (such as chlorogenic, caffeic, p-coumaric, ferulic, and sinapic), all of which exhibit antioxidant, antimicrobial, as well as potential anticancer and anti-inflammatory activities [24].
| Characteristics | Amount |
|---|---|
| Moisture (g/100 g) | 12.74 ± 0.16a |
| Ash (g/100 g) | 2.11 ± 0.09 |
| Crude fiber (g/100 g) | 37.23 ± 0.25 |
| Protein (g/100 g) | 4.11 ± 0.04 |
| Fat (g/100 g) | 3.04 ± 0.07 |
| Total carbohydrate (g/100 g) | 40.77 ± 0.22 |
| Total phenolic compounds (mg/g) | 5.95 ± 0.22 |
| Galic acid (mg/g) | 0.03 ± 0.001 |
| Ferulic acid (mg/g) | 0.07 ± 0.001 |
| Caffeic acid (mg/g) | ND |
| Chlorogenic acid (mg/g) | 1.02 ± 0.03 |
| Vanillic acid (mg/g) | ND |
| Phloridzin (mg/g) | 3.26 ± 0.09 |
| Rutin (mg/g) | 0.062 ± 0.001 |
| Catechin (mg/g) | 0.35 ± 0.07 |
- Abbreviation: ND, not determined.
- aMeans ± SD (standard deviation) of triplicate determinations from experiments.
Phlorizin, the most abundant polyphenolic compound identified in apple pomace, was quantified at 4.21 mg/g in the dry weight of powdered apple pomace, aligning with findings by Gumul et al. [25] and Kammerer et al. [26]. These studies confirmed phlorizin as the predominant polyphenolic compound found in apple pomace, which is absent in the pomace of other products.
3.2. Extraction of Phenolic Compounds From Apple Pomace Using Subcritical Water
3.2.1. Model Fitting
In order to establish a predictive model for the responses, various polynomial equations, such as linear, two-factorial (interactive), quadratic, and cubic forms, were applied to the dataset generated from response surface methodology. These models were subjected to statistical evaluation, ensuring their appropriateness through nonsignificant lack-of-fit tests and the highest values of the R2 and adjusted R2.
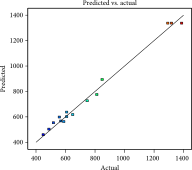
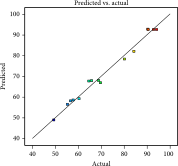
Analysis of variance (ANOVA) and regression were utilized to assess the adequacy of the proposed models and the statistical significance of the model variables. The regression coefficients of the empirical equations and their corresponding adjusted R2 values are presented in Table 3. The significance of models and equations was evaluated based on p values. In this study, the R2 for extraction yield, total phenolic content, and free radical scavenging capacity in the subcritical water process were found to be 0.98, 0.99, and 0.98, respectively. These results suggest that the offered models can effectively estimate optimal conditions for extracting phenolic compounds from apple pomace.
| Source | d.f. | Yield (%) | TPC (mg GAE/100 g) | DPPH SC (%) | |||
|---|---|---|---|---|---|---|---|
| Coefficient regression | Sum of squares | Coefficient regression | Sum of squares | Coefficient regression | Sum of squares | ||
| Model | 9 | −166.94*** | 134.58 | −21498.47 | 1379816.95 | −582.88 | 3239.32 |
| Time (A) | 1 | ns | ns | ns | 49.60 | ns | 1.76 |
| Temperature (B) | 1 | 1.67* | 2.87 | 226.08 | 21395.53 | 6.24 | 224.61 |
| Water to solid ratio(C) | 1 | ns | 0.15 | ns | 426.61 | ns | 0.03 |
| A2 | 1 | −0.05*** | 112.05 | −4.90 | 886010.5 | −0.26 | 2489.52 |
| B2 | 1 | −0.0006** | 18.30 | −0.79 | 365203.2 | −0.02 | 277.63 |
| C2 | 1 | −0.01*** | 6.94 | −2.64 | 25811.2 | −0.04 | 49.56 |
| AB | 1 | ns | 0.03 | ns | 1542.92 | 0.02 | 72.34 |
| AC | 1 | ns | 0.33 | ns | 2513.02 | 0.04 | 75.00 |
| BC | 1 | −0.005 | 4.52 | −0.38 | 23378.41 | 0.04 | 205.35 |
| Residues | 7 | 1.39 | 13927.91 | 48.49 | |||
| Lack of fit | 5 | ns | 1.13 | ns | 9221.90 | ns | 41.59 |
| Pure error | 2 | 0.26 | 470.01 | 6.90 | |||
| Total | 16 | 135.97 | 1393745 | 3287.81 | |||
| Std. dev. | 0.53 | 52.78 | 3.11 | ||||
| Average | 12.96 | 765.05 | 72.13 | ||||
| CV% | 4.07 | 6.90 | 4.32 | ||||
| R2 | 0.98 | 0.99 | 0.98 | ||||
| Adj R2 | 0.97 | 0.97 | 0.95 | ||||
| Pred R2 | 0.86 | 0.88 | 0.79 | ||||
| Predicted values vs. actual values | 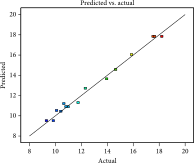 |
 |
 |
||||
- Note: ns: not significant (p > 0.05).
- Abbreviations: DPPHSC: scavenging activity of DPPH, TPC: total phenolic content.
- *Significant at p < 0.05.
- **Significant at p < 0.01.
- ***Significant at p < 0.001.
3.2.2. The Effect of Extraction Parameters on the Yield
The trend of changes in the extraction yield of phenolic compounds using the subcritical water extraction, considering extraction parameters, is illustrated in the response surface plot in Figure 1, with the resulting coefficient regression in Table 3. The results of this investigation highlight the important influences of extraction temperature, its squared value, extraction duration, and the ratio of water to pomace, along with the interaction between temperature and the mixing ratio (see Table 3). Analysis of the equation derived for extraction yield indicates a high R2 (R2 = 0.98) and an adjusted R2 (Adj R2 = 0.97), signifying robustness for predictive purposes. Additionally, the lack-of-fit test for the model is nonsignificant (p > 0.05), and the coefficient of variation is low (4.07%), suggesting the appropriateness of the proposed model.
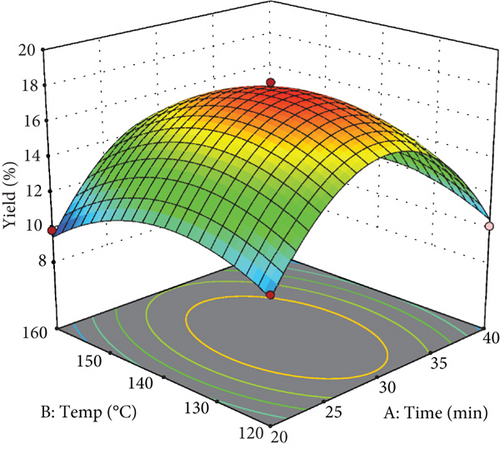


As illustrated in Figure 1, the extraction efficiency of phenolic compounds demonstrates a notable increase with temperature, peaking at 135°C, followed by a decline at elevated temperatures. Temperature is a pivotal parameter influencing both the efficiency and selectivity of the extraction process in subcritical water methods. The high temperature disrupts cohesive forces—such as hydrogen bonding, van der Waals forces, and dipole–dipole interactions—binding the particles within the pomace matrix. Concurrently, lowering solvent viscosity at higher temperatures enhances solvent penetration into the matrix particles, facilitating improved extraction outcomes [27].
Moreover, the literature indicates that increasing the temperature from 25°C to 200°C is due to a significant reduction of water’s dielectric constant from 75 to 35, approaching the dielectric constants of methanol and ethanol [28]. The observed decrease in extraction efficiency of phenolic compounds beyond 135°C is likely attributable to the thermal degradation of these compounds [29].
In addition, extraction efficiency increases with prolonged extraction time, reaching an optimal point at approximately 30 min before declining. It is considered that the extraction process is time-dependent, wherein phenolic compounds are gradually released from the apple pomace up to the 30-min mark. Based on Fick’s second law of diffusion, a specific time is required to achieve equilibrium between the solute concentration within the solid matrix and the surrounding solvent. Therefore, extending the extraction duration beyond this optimal period is not expected to significantly improve the extraction of bioactive compounds [30].
As depicted in Figure 1(c), the extraction of phenolic compounds rises as the water-to-apple pomace ratio increases from 20 to approximately 30 mL/g, after which it stabilizes. This behavior can be attributed to mass transfer principles; as the solvent-to-solid material ratio increases, the concentration gradient between the solid matrix and the solvent—the driving force for mass transfer—also intensifies [31].
3.2.3. Effect of Extraction Parameters on the Phenolic Compounds
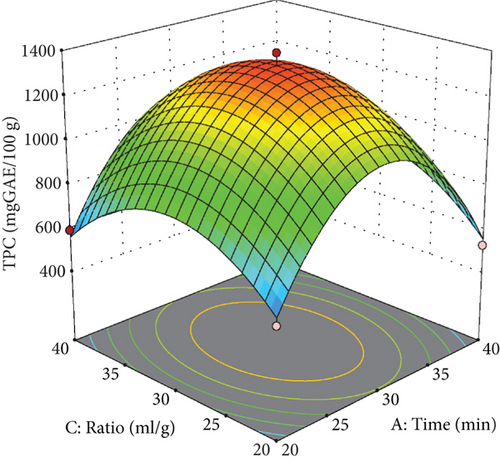
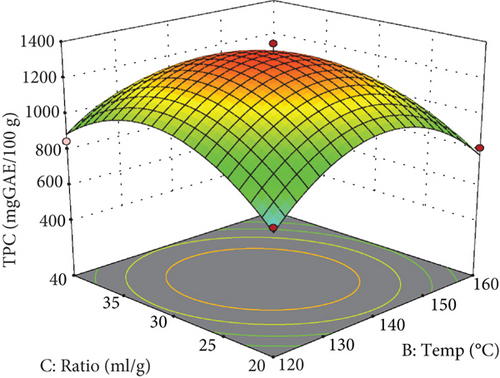
As detailed in Table 3, several parameters significantly influenced the extraction of phenolic compounds, including extraction temperature, the squared effect of extraction temperature, extraction time, and the water-to-pomace ratio. Additionally, the interactive effect of temperature and the mixing ratio was significant. The predictive model developed for estimating the amount of phenolic compounds demonstrated a high R2 (R2 = 0.99) and an adjusted R2 (Adj R2 = 0.97), as presented in Table 3. The coefficient of variation was acceptable at 6.90%, and the lack of fit test yielded nonsignificant results (p > 0.05), confirming the model’s appropriateness.
As evidenced by the quadratic term for extraction temperature in Table 3 and depicted in the response surface plot (Figure 2), raising the temperature to around 135°C improves phenolic compound extraction, after which a gradual decrease is observed. At subcritical temperatures, water’s reduced polarity enhances its ability to dissolve compounds of lower polarity.
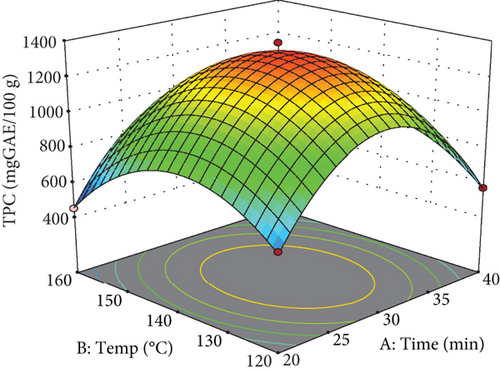
Consequently, the enhancement in the extraction of phenolic compounds from apple pomace at 135°C may be attributed to the semipolar characteristics of these compounds. Additionally, higher water temperatures lower viscosity and surface tension, which facilitates diffusion and enhances mass transfer rates during the extraction process [9]. Higher temperatures also facilitate the softening of plant tissues and disrupt the bonds between phenolic compounds and polysaccharides or proteins, thereby increasing the penetration and extraction rates of these compounds [32]. Similar trends have been reported in the literature regarding the influence of temperature on the extraction of polyphenolic compounds from various plant sources using subcritical water extraction methods [33, 34]. The observed decline in phenolic compound extraction at temperatures above 145°C in this study can likely be attributed to the thermal decomposition and degradation of these compounds [5].
Moreover, as illustrated in Figure 2, an increase in extraction time (up to approximately 35 min) initially enhanced the yield of phenolic compounds, likely due to the time-dependent nature of the extraction process, which facilitates the gradual release of phenolic compounds from the apple pomace until the 30-min mark. However, the increases in time resulted in a decrease in extraction yield. This decline can be associated with the effects of extraction temperature, which may lead to increased degradation of phenolic compounds at extended extraction times [6].
As illustrated in Figures 2(b) and 2(c), the concentration of phenolic compounds increases as the water-to-apple pomace ratio rises from 20 to approximately 35 mL/g, followed by a slight decrease. At higher water-to-apple pomace ratios, solvent diffusivity improved, allowing the solvent to become saturated with a greater amount of extracted material [35, 36].
3.2.4. The Effect of Extraction Parameters on the Free Radical Scavenging Capacity
The regression equation derived for the free radical scavenging percentage indicates a high R2 (R2 = 0.98) and an adjusted R2 (Adj R2 = 0.95), confirming its suitability for predicting free radical scavenging capacity. The lack-of-fit test yielded nonsignificant results (p > 0.05), while the coefficient of variation was low at 4.32%, further validating the proposed model.
As illustrated in the response surface plot (Figure 3(a), extraction temperature significantly influences the scavenging percentage, peaking at approximately 145°C.
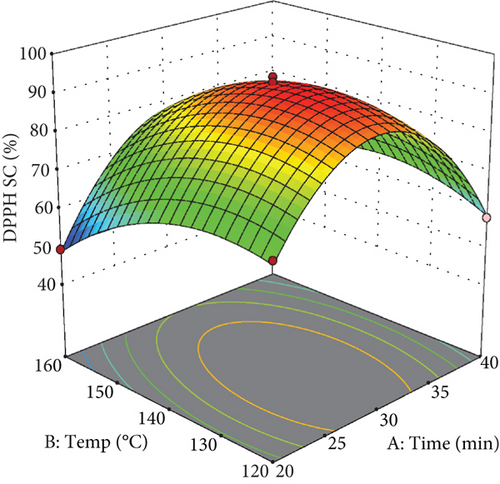

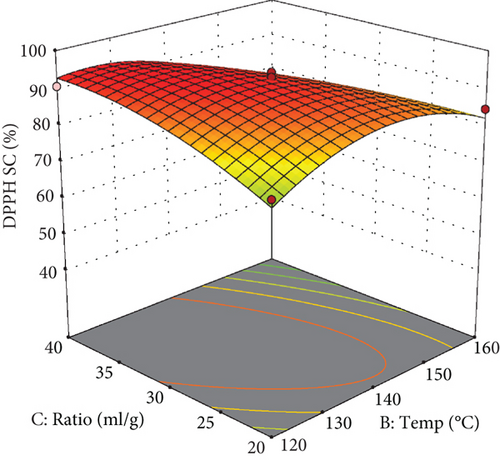
The elevated antioxidant activity observed in extracts obtained at this temperature is likely due to the reactions of various plant-derived compounds occurring at temperatures exceeding those used in the subcritical water method, leading to the formation of antioxidant or reducing compounds, such as those derived from Maillard reactions [37]. However, the reduction in antioxidant capacity at higher temperatures can be attributed to the thermal degradation of heat-sensitive polyphenols due to prolonged exposure to heat.
Figure 3 also illustrates the quadratic effect of extraction time on the percentage of free radical inhibition. Initially, extraction time significantly increased the inhibition percentage up to around 30 min, beyond which further increases resulted in a decline. This trend is likely due to the degradation of heat-sensitive antioxidant compounds subjected to prolonged heating.
According to the response surface plot (Figure 3(c)), increasing the water-to-apple pomace ratio up to approximately 30 mL/g enhanced the free radical inhibition percentage of the extract. At higher ratios, the inhibition percentage plateaued. Similarly, total phenolic content was highest at this ratio, suggesting that the increase in inhibition percentage can be attributed to greater extraction of these compounds at elevated water-to-solid ratios.
3.2.5. Optimizing the Subcritical Water Extraction Method and Verification
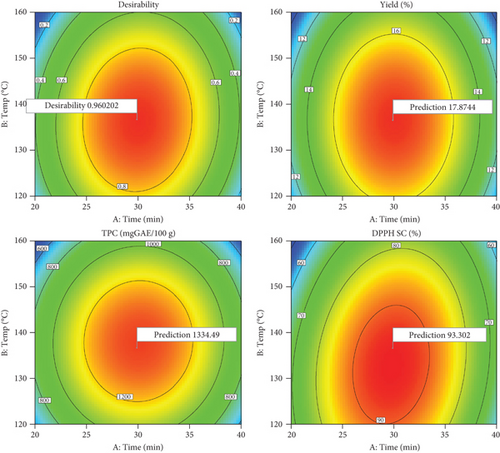
The optimal conditions for extracting apple pomace via subcritical water extraction were identified through both numerical and graphical optimization using statistical software. The primary goals were to maximize extraction yield, total phenolic content, and antioxidant activity based on free radical scavenging capacity. The optimal conditions identified through the response surface method and desirability function were an extraction temperature of 137°C, an extraction time of 29.91 min, a water-to-pomace ratio of 31 mL/g, and a desirability of 0.96. These conditions yielded the extract with the highest yield and antioxidant activity (Table 4 and Figure 4).
| Results | Yield (%) | TPC (mg/100 g) | DPPH SC (%) |
|---|---|---|---|
| Predicted values* | 17.84a** | 1334.46a | 93.30a |
| Experimental values | 18.05 ± 0.63a | 1341.11 ± 15.04a | 92.39 ± 0.56a |
| Solvent extraction values (maceration and control) | 7.9 ± 0.1b | 595.36 ± 12.69b | 53.83 ± 1.01b |
- *Predicted using response surface quadratic model.
- **Means ± SD (standard deviation) within a column with the same superscript letters are not significantly different at p > 0.05.
To confirm the optimized conditions, experiments were performed using the determined optimal parameters, with findings displayed in Table 4. The lack of significant discrepancies between the model predictions and the experimental results (p > 0.05) indicates the models’ validity.
3.3. Antimicrobial Activities
The MIC results for the apple pomace extract subcritical water extraction under optimized conditions are presented in Table 5. The MIC for the apple pomace extract at a concentration of 5 mg/100 mL was 35% and 44% for Staphylococcus aureus and Escherichia coli, respectively, and 30% and 62% for Aspergillus niger at concentrations of 10 and 20 mg/100 mL, respectively. Additionally, the MIC for potassium sorbate at a concentration of 10 mg/100 mL was 26.8%, 35.4%, and 59% for Staphylococcus aureus, Escherichia coli, and Aspergillus niger, respectively.
| Microorganism | Apple pomace SWE optimized extract (mg/100 mL) | Potassium sorbate (mg/100 mL) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 10 | 20 | 30 | 10 | |
| Escherichia coli (PTCC1399) | — | — | 35 | 52 | 81 | 100 | 35.4 |
| Staphylococcus aureus (PTCC1112) | — | 28 | 44 | 73 | 92 | 100 | 26.8 |
| Aspergillus niger (PTCC5009) | — | — | — | 30 | 62 | 100 | 59 |
Potassium sorbate is an effective preservative against a wide range of molds and yeasts, functioning by inhibiting dehydrogenase enzymes involved in fatty acid oxidation, disrupting sulfhydryl-containing enzymes, uncoupling oxidative phosphorylation, inhibiting catalase, and increasing hydrogen peroxide levels within cells. However, its antimicrobial efficacy against bacteria is comparatively lower [38].
The antimicrobial properties of the apple pomace extract can be attributed to its phenolic compounds, such as phloridzin and proanthocyanidins, which are soluble in polar solvents. The antimicrobial effect of these phenolic compounds arises from their ability to form complexes with the outer membranes of bacteria and soluble proteins associated with these membranes. Additionally, these compounds can penetrate cell membranes, leading to cellular disruption and subsequent antimicrobial activity [39].
As shown in Table 5, the antimicrobial efficacy of the apple pomace extract against Staphylococcus aureus is greater than that against Escherichia coli. This increased sensitivity of Gram-positive bacteria to the extract may be due to their simpler, single-layer cell wall structure, in contrast to the multilayered cell wall of Gram-negative bacteria, which includes an outer membrane and periplasmic space. The outer membrane of Gram-negative bacteria serves as a barrier, impeding the penetration of external substances, while the periplasmic space contains enzymes capable of degrading external molecules that penetrate the surrounding environment [39].
Based on the findings from this research, it can be concluded that the apple pomace extract exhibits significant antibacterial and antifungal activity in vitro against the studied strains, with concentrations of 20 and 30 mg/100 mL demonstrating significantly stronger antibacterial and antifungal properties compared to potassium sorbate.
4. Conclusion
The results confirmed the effectiveness of the selected statistical method, the Box–Behnken design, for modeling the extraction conditions of phenolic compounds from apple pomace. The models exhibited high R2 values (0.98–0.99) and adjusted R2 values (0.95–0.97), along with insignificant lack-of-fit tests, demonstrating their reliability in predicting the evaluated parameters. Under optimal subcritical water extraction conditions (an extraction temperature of 137°C, an extraction time of 29.91 min, and a water-to-pomace ratio of 31 mL/g), the extract showed significantly higher antioxidant activity compared to the conventional maceration method. These models not only facilitate the adjustment of extraction conditions but also enable the prediction and modification of desired characteristics based on varying extraction parameters.
Furthermore, the evaluation of antimicrobial activity for the extract obtained under optimal conditions revealed that the apple pomace extract at a concentration of 30 mg/100 mL exhibited significantly stronger antifungal properties than potassium sorbate at a concentration of 10 mg/100 mL. This research underscores the potential application of optimized apple pomace extracts in the development of natural preservatives and functional food ingredients.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding authors. The data are not publicly available due to privacy or ethical restrictions.




