Reviewing the Correlation of Fish Quality Alteration and In-Package Headspace Composition: Evidence From a pH Freshness Indicator Case Study
Abstract
Smart packaging is a continuously evolving sector that may provide solutions to several cold chain management challenges and shows great potential toward food waste reduction. Fish is a highly nutritious yet perishable commodity with increasing statistics of annual loss and waste. This study investigates the application of pH-sensitive indicators on fish products, aiming to analyze and highlight the importance of comprehensive monitoring of the physicochemical alterations and their interactions with the headspace composition during refrigerated storage of fresh fish. Insights into this process can contribute to developing more practical spoilage indicators and dynamic shelf life prediction methods. A brief case study is presented on the development and application of a smart indicator prototype. The freshness pH indicator was prepared by incorporating methyl red into a starch and cellulose matrix using the dip coating method and was tested for its sensitivity to pH, ammonia vapor, and the detection of gilthead sea bream spoilage. The deterioration in the quality of sea bream fillets, as indicated by the observed color change of the indicator, was confirmed through microbiological and chemical analyses of the fish flesh. The color response of the pH indicator (red–pink turned pale yellow) was found to correlate with fish alteration patterns and reflected the headspace gas composition thus enabling “real-time” monitoring of fish spoilage.
1. Introduction
The demand for aquatic foods has shown a remarkable increase (average annual rate of 3.0% since 1961), considering the 1.6% population growth rate. Fish and seafood are globally recognized for their crucial role in food security and human nutrition, given their high concentration in high-quality protein, essential omega-3 fatty acids, and micronutrients [1]. However, the intrinsic characteristics of fish (neutral pH, high water activity, and increased number of nonprotein nitrogen (NPN) molecules) favor bacterial growth resulting in a highly perishable food with limited shelf life compared to other food categories [2].
To date, numerous technologies have been developed and are currently applied to delay the spoilage of fish and extend product shelf life, mainly through processing and preservation technologies (e.g., smoking, superchilling, and high hydrostatic pressure) [3]. Many novel preservation technologies are being investigated, for example, cold plasma treatment, pulsed electromagnetic fields, and ozonation [4, 5]. Adequate quality monitoring based on continuous and accurate determination of the actual quality level and remaining shelf life of fish products would contribute to better management of the cold chain and reduction of the estimated 30%–35% of total fish production which is currently wasted [6].
A combination of autolytic, oxidative, and microbial activities during fish storage is responsible for the development of undesirable odors, as well as changes in texture and appearance, which are associated with loss of freshness [7]. For instance, Pseudomonas spp. secrete extracellular proteases that degrade proteins into peptides and free amino acids. These amino acids serve as a nitrogen and carbon source for bacteria, leading to the production of sulfur-containing compounds, such as hydrogen sulfide (H₂S) and methanethiol, which contribute to off-flavors. These quality alterations can be assessed based on various biochemical indices, including total volatile basic nitrogen (TVB-N), ammonia, and trimethylamine (TMA); physicochemical indices, such as pH; microbiological indices, such as total viable counts (TVCs); and sensory markers, for example, consumer overall acceptance [8].
Fish that have not been processed before commercialization and consumption are even more prone to deterioration and have a shorter shelf life than processed fish [9]. However, shelf life can be significantly improved by choosing an appropriate packaging system. Recent packaging systems are increasingly distinct from the traditional ones, offering excellent opportunities for protecting food quality and extending shelf life [10]. In recent years, the role of packaging is evolved from a passive to more active function, focusing on extending shelf life while retaining product quality [11]. Packaging not only provides an essential solution for retail purposes but also enhances the properties of the final product [12]. In this sense, conventional packaging materials are slowly but steadily replaced by alternatives with improved properties, in terms of environment sustainability (e.g., less plastic usage) and the package functionality (e.g., addition of bioactive compounds to enhance antioxidant and antimicrobial activity or addition of pigments to inform consumers about food spoilage) [13, 14].
Fish products have been generally traded unpackaged, exposing them primarily to atmospheric conditions. However, there is an increasing trend toward fish products in sealed packages [15]. The headspace composition inside the fish package is of great significance, since it remains in continuous contact with the fish tissue from the production stage until it is delivered to the consumer. Enclosing fish tissue in a sealed container with normal atmospheric conditions (rather than using modified atmospheres or vacuum packaging) gradually alters the in-package gaseous composition compared to the initial headspace, due to the volatile compounds generated during the deterioration of the fish. For instance, lipid oxidation, particularly in fatty fish, results in the formation of aldehydes (e.g., malondialdehyde and hexanal), which are key indices of oxidative rancidity. The interaction between oxygen and polyunsaturated fatty acids generates hydroperoxides, which subsequently decompose into volatile secondary oxidation products that can be detected in the headspace.
Changes in the surrounding atmosphere of the product directly affect the deterioration pathways [8]. In the initial fish spoilage levels (where % O2 concentrations are still high), Pseudomonas spp. are the dominant spoilage microorganisms. Sulfur-containing amino acids like cysteine and methionine are metabolized by Pseudomonas spp. through enzymatic reactions releasing H₂S, ammonia, and pyruvate. Inside a sealed fish package with an initial atmospheric composition (approximately 78% N2, 21% O2, 0.04% CO2), O2 decreases significantly, potentially below 5% at the end of storage period. Under low-oxygen conditions, Pseudomonas spp. utilize sulfur compounds as electron acceptors, further contributing to the accumulation of volatile sulfur compounds. Analyzing the chemical interactions between headspace gases and fish tissue components during storage enables the design of adequate smart packaging tools, which can provide reliable estimations of fish quality and remaining shelf life.
Storage conditions, for example, temperature and relative humidity, highly affect the in-package headspace evolution [16]. Higher storage temperatures accelerate microbial metabolism, resulting in rapid depletion of oxygen (O2) and increased concentration of carbon dioxide (CO2) and other spoilage gases. A study on cape hake fillets stored under passive modified atmosphere packaging (MAP) without absorbent pads at 0°C, 4°C, and 8°C reported that headspace O2 levels decreased more rapidly at higher temperatures, indicating enhanced microbial respiration [17]. Excessively high relative humidity can lead to moisture condensation, creating favorable conditions for microbial growth and spoilage [18].
Freshness indicators aim to monitor food alterations and communicate them to the end user through a colorimetric response [11]. pH freshness indicators provide information about changes that occur in the environment in which the food is conditioned, since they are not in direct contact with the food sample. As mentioned, changes inside the food package headspace are attributed to deterioration processes, [19], and, therefore, linking these gaseous modifications to the relative color change of the pH freshness indicator and interpreting them to the corresponding level of fish spoilage is valuable.
Up-to-date several literature reviews have been focusing on the development of smart indicators for food spoilage monitoring, mainly regarding their application and the fabrication processes, for example, pigment origin, combination of biopolymers, ways of immobilization of the pigment, and mechanical functionality of the final films [20–23]. There are also literature reviews regarding the headspace composition of packaged products that delve deeper into its connection with spoilage patterns; however, they are limited in number. For instance, Kim et al. [24] investigated how the composition of gases within the packaging of perishable foods evolved during chilled storage and how these changes related to microbial spoilage. They pointed out oxygen depletion and carbon dioxide promotion in respect to aerobic bacteria growth and suggested that headspace gas evolution could serve as indicator of microbial spoilage in perishable foods. Martin et al. [8], in their review about the evolution of volatiles (volatile organic compounds (VOCs)) during the storage of packaged fresh fish, highlighted the strong influence of packaging conditions and atmospheric composition changes on the type of microorganisms contaminating the fish, their growth, and their metabolic activity that was evident through VOC profiling.
The present review case study aims to systematically and comprehensively present an overview of the current available and scattered knowledge regarding the phenomena that take place during the spoilage of fish stored in sealed package with an initial atmospheric composition. It also seeks to elucidate the chemical pathways through which these phenomena can be reflected in an appropriate pH-sensitive indicator. The novelty of this work lies in the integration of gas analysis (% CO2, % O2, and % N2) with microbial and chemical profiling in the process of developing an accurate and real-time freshness indicator for monitoring gilthead sea bream freshness during refrigerated storage.
2. Postmortem Changes in Fish Flesh
Fish and seafood product quality is a complex issue affected both by extrinsic and intrinsic factors. Extrinsic factors include geographical location, harvest season, type of harvest water (freshwater, saltwater, tropical or arctic water, etc.), depth of living, feeding conditions, and sexual maturity. The influence of these factors on the final product quality is high but their importance often varies with culture and markets [25]. Intrinsic factors mostly refer to chemical and physiological attributes of the fish meat that are inherited and end up affecting the protein, lipid, or moisture content of the fish. Intrinsic factors are highly associated with the alterations of fish flesh occurring postharvest [26]. Figure 1 summarizes the postmortem phenomena that take place during fish spoilage.
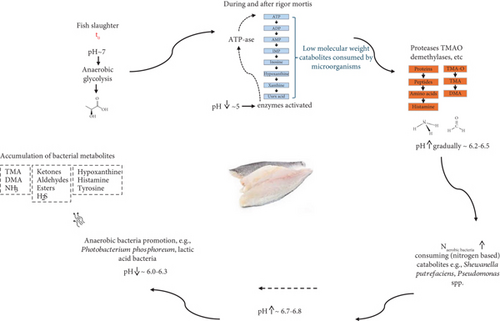
2.1. Autolytic Alterations
While aquatic animals are alive, enzymatic reactions take place in their living muscle cells as part of normal biological functions. Most of these enzymatic reactions persist even after the fish death, and hence cause modifications in the flesh due to degradation [27]. These initial alterations in fish flesh are primarily autolytic and not triggered by microbial activity [28].
The autolytic alterations are highly related to the activity of various enzymes during and after rigor mortis. Initially, adenosine 5 ′-triphosphate which is abundant in the fish muscle (prerigor phase) is degraded into adenosine diphosphate (ADP), adenosine monophosphate (AMP), and inosine monophosphate (IMP) by nucleotide degradation enzymes (during and after rigor mortis) [3]. In living cells, ATPase enzymes play a role in maintaining cellular homeostasis by regulating ion gradients and cellular energy balance. After death, the cessation of homeostatic mechanisms causes a disruption in ion gradients, particularly calcium ions, which can trigger the activation of ATPases. Since muscle cells do not receive adequate amounts of oxygen after fish die, anaerobic glycolysis is triggered, and lactic acid is produced from glycogen with the activation of glycolytic enzymes and this is associated with a rapid drop in pH from 7.0 to almost 5.0 [27]. These changes lead to the activation of other enzymes such as proteases, calpains, collagenases, and TMA oxide demethylases, which start to break down the connective tissue of the flesh and degrade high molecular weight compounds (e.g., lipids and proteins) into lower molecular weight compounds [29]. In this way, proteins are degraded into polypeptides and amino acids, eventually leading to the production of histamine (in fish with high initial levels of histidine in the flesh), while TMA oxide (TMAO) is converted to TMA [16]. At the same time, the number of microorganisms increases due to the excess of low molecular weight compounds. The formation of TMA and histamine, as well as the degradation of IMP, marks the onset of the spoilage phase when fishy-off odors become apparent [27].
2.2. Oxidative Alterations
Oxidative reactions that lead to alterations of fish flesh include thermal, enzymatic, photo, and auto-oxidation. The most common type of oxidation in fish is auto-oxidation of lipids due to their interaction with atmospheric oxygen [30]. The lipid content of fish is a determining factor for the degree of flesh oxidation. The majority of the saturated or unsaturated aldehydes, alcohols, and carbonyls in fish flesh come from the autoxidation of polyunsaturated fatty acids. Fish species with an intrinsically higher content of unsaturated lipids (e.g., mackerel and herring) tend to spoil faster than lean fish species (e.g., sea bream and sea bass) because of the reaction between fatty acid double bonds and oxygen [31, 32].
Through a series of nonenzymatic lipid oxidation stages that initiate with the formation of free radicals from fish fatty acids, hydroperoxides are formed. More auto-oxidation products are produced when lipid hydroperoxides are further degraded to lower molecular weight compounds such as malondialdehyde, 4-hydroxynonenal, hydrocarbons, and volatile organic acids [33, 34]. Extrinsic factors, such as temperature, pH, irradiation, and the presence of metal ions, contribute to the oxidation of lipids in fish flesh, leading to an increased spoilage rate [35, 36].
Generally, oxidative alterations in fish flesh lead to reduction in quality attributes, such as compromised texture and appearance; however, they predominantly affect the flavor of the fish and are responsible for off-odors due to the production of odor-active volatile compounds [37]. Duflos et al. [19] reported enzymatic oxidative pathways such as the lipoxygenase pathway, which causes oxidation of polyunsaturated fatty acids into lower molecular weight compounds, such as short-chain volatile carbonyls, unsaturated alcohols, and diene compounds. Two fatty acids commonly found in fish are arachidonic acid and eicosapentaenoic acid. The oxidation of arachidonic acid by the enzyme 12-lipoxygenase results in the production of the unsaturated aldehyde 2-nonenal and 1-octen-3-ol and 2-octene that generate a strong metallic odor. The same enzyme is also responsible for the oxidation of eicosapentaenoic acid and leads to the formation of 2,5-octadiene and 2,6-nonadienal [38]. Heptanal is a slightly volatile alkyl aldehyde that occurs in fish flesh when n-9 monounsaturated fatty acids and n-6 polyunsaturated fatty acids such as oleic acid and docosahexaenoic acid, respectively, are oxidized [39]. Alasalvar et al. [40] reported that the main compounds arising from oxidative alterations in sea bream flesh include alcohols (e.g., hexanol and heptanol) and aldehydes (e.g., hexanal, heptanal, octanal, and nonanal). Similar results were obtained in the study of Kritikos et al. [41], who reported hexanal and nonanal to be the most abundant compounds during MAP storage of sea bass and salmon slices at 2°C. Their findings are quite consistent, indicating that, irrespective of fish species, lipid oxidation follows the same biochemical pathway. However, while Duflos et al. [19] offered mechanistic insights, Alasalvar et al. [40] and Kritikos et al. [41] mainly recognized the oxidation products without directly connecting them to enzymatic pathways. It is worth mentioning that by comparing these studies, it helps to realize that assessing both the biochemical origins and storage conditions associated with lipid oxidation is important for the development of better preservation strategies for seafood.
2.3. Microbiological Alterations
Microorganisms are naturally present in fish capture. There are several parameters affecting the original microbial flora of fish with the most important being the harvest season and the type of harvest water (fresh or saltwater and tropical or arctic water) and the less important being the species [42]. For instance, the lowest populations of initial microflora are found in fish that grow in an environment with cold, clean water compared to warm water conditions [43]. As long as fish are still alive or newly caught, no microorganisms are present in their flesh, as their immune system serves as an effective barrier to impeding their entry and growth. After fish are caught, killed, and preserved, the immune system begins to collapse, allowing microorganisms to gradually spread. If there is an adequate nutrient supply, these microorganisms can proliferate freely. This indicates that the populations of existing microbiota in the digestive tract and on the outer surfaces of fish (e.g., skin and gills) increase gradually, invade the flesh by migrating between muscle fibers, and finally spread within the various fish tissues [44]. The proliferation of TVCs within fish flesh is typically observed around 48 h postmortem.
Microbial growth and activity in the flesh and skin are the main causes of fish spoilage [3]. As outlined above, the composition and chemical processes that lead to the degradation of fish provide an excellent substrate for microbial growth. The low carbohydrate content of fish muscle tissue (< 0.5%) results in low concentrations of lactic acid postmortem and thus higher pH (typically > 6) in their flesh. Under these conditions, pH-sensitive spoilage bacteria such as Shewanella putrefaciens are not inhibited and can readily grow. Furthermore, in addition to the protein fraction, there are large amounts of NPN in fish flesh consisting of low molecular weight water-soluble nitrogen compounds (e.g., free amino acids and nucleotides) that can be directly used by bacteria as a growth substrate. Lastly, the high concentrations of endogenous TMAO in fish flesh provides an ideal substrate for the growth of various spoilage bacteria such as Shewanella putrefaciens, Photobacterium phosphoreum, and Vibrionaceae which consume TMAO and produce TMA [26].
Microbial spoilage of fish becomes detectable when off-odors and off-flavors are generated as a result of metabolic breakdown processes. In fact, only a small fraction of microorganisms, referred to as specific spoilage organisms (SSOs), is responsible for generating these off-odors and off-flavors [26]. SSOs outgrow the rest of the microbiota depending on the surrounding conditions, resulting in a range of breakdown and metabolic compounds. Therefore, the total number of bacteria present in fish is not always informative about fish spoilage, since it is better correlated with the growth of SSOs [45].
The composition of the fish spoilage bacteria depends on the packaging conditions (air, vacuum, or modified atmosphere). There have been several studies focusing on packaging conditions for fish and their effect on shelf life. It is commonly reported that the SSO of refrigerated fish stored in atmospheric air is Pseudomonas spp. which are strictly aerobic. When in-package oxygen begins to decrease, the population of Pseudomonas spp. is limited [26]. Also, in aerobic storage conditions, Shewanella putrefaciens is primarily responsible for the generation of volatile compounds and thus the formation of H2S odors [46]. When fish are stored under a vacuum or in a modified atmosphere without oxygen, TMAO is used as the terminal electron acceptor in anaerobic respiration by SSOs such as Photobacterium phosphoreum, Shewanella putrefuciens, and Vibrionaceae and results in TMA accumulation. Additionally, under MAP conditions, Photobacterium phosphoreum and lactic acid bacteria (LAB) tend to grow faster in fish flesh, possibly due to their ability to tolerate CO2 [47]. Analyzing the key findings of these studies regarding seafood microbial alterations, it is evident that researchers suggest a shift toward a proactive approach by developing more accurate microbial detection tools [46], revealing previously undetected spoilage risks [45], and predicting microbial behavior under different storage conditions [47]. Limitations of traditional methods are pointed out, indicating that more advanced approaches are necessary for delaying fish spoilage.
3. Physicochemical Markers of Fish Spoilage
During the food deterioration process, multiple physicochemical markers can signal the changes occurring within the food matrix. The microbial activity and chemical reactions that take place in fish tissue during storage and until the end of its shelf life result in a series of intricate physicochemical transformations. Depending on parameters such as fish species, origin, and storage conditions (e.g., time–temperature conditions, headspace composition, water activity, and packaging type), each physicochemical marker can vary significantly [48].
A primary physicochemical marker of fish quality is pH, which changes immediately after fish is slaughtered. The typical pH of live fish muscle is around 7.0, but a few hours after death, fish muscle glycogen is metabolized into lactic acid resulting in a noticeable drop in pH [49]. During the early stages of refrigerated storage in air, the pH of fish remains relatively low, typically between 6.2 and 6.3. The pH of the fish can vary based on the storage temperature and the species of fish. As storage progresses, microbial activity produces metabolites that cause an increase in pH, which reaches values around 6.6–6.7, primarily due to the accumulation of volatile compounds and biogenic amines. After several days of storage, the growth of LAB is likely and leads to a decrease in pH [50, 51].
During fish storage, metabolites are primarily produced due to microbial growth, resulting in unpleasant organoleptic characteristics. Among these metabolites, VOCs are often used as indicators to assess fish freshness. These compounds are responsible for the characteristic smell of fish and seafood [52]. Key volatile organic compounds found in packaged, refrigerated fish as identified in the literature, include alcohols (e.g., 3-methyl-1-butanol, 2,3-butanediol, 1-penten-3-ol, 2-ethyl-1-hexanol, and ethanol), [53, 54], aldehydes (e.g., 2 or 3 methyl-butanal, hexanal, heptanal, and nonanal) [55, 56], acids (e.g., acetic acid) [57], ketones (e.g., acetoin) [58], amines (e.g., TMA and dimethylamine) [40, 56], esters (e.g., ethyl acetate) [59], and sulfur compounds (e.g., dimethyl disulfide, dimethyl trisulfide, and carbon disulfide) [60, 61]. The most common methods for detecting VOCs in fish are headspace/mass spectrometric (HS/MS) analysis and solid phase microextraction/gas chromatographic/mass spectrometry (SPME/GC/MS). Alcohols and aldehydes are also known to be flavoring compounds, typically originating from the oxidation or autoxidation of lipids, or from enzymatic reactions. Soto-Valdez et al. [35] found that the predominant volatile organic compounds identified by VOC analysis of refrigerated sea bass and salmon were primarily aldehydes, followed by alcohols, ketones, esters, and a single acid (acetic acid). By examining the relative concentrations of VOCs at different storage intervals (Days 2, 7, 11, and 14), they observed that some compounds fluctuated throughout the spoilage process (increased or decreased without having a clear upward or downward trend) while others remained relatively low during the storage experiment but increased rapidly just before the end of the fish shelf life. Furthermore, the variation in VOC levels and their fluctuation during storage was characteristic of different fish species [41]. A similar study conducted by Duflos et al. [19] in whiting, cod, and mackerel stored at 4°C for 10 days, reported that out of 20 compounds analyzed, only three, TMA, 3-methylbutanol, and 2,3-butanediol, could be confirmed since they had three main mass fragments that matched the discriminant ions. This highlights the difficulties of using VOCs for monitoring fish spoilage, due to their very low molecular mass and the lack of specificity of the generated mass fragments. Many of the detected volatile compounds during fish spoilage share numerous mass fragments, making it nearly impossible to identify individual volatile compounds involved in spoilage [19].
Among the produced metabolites, volatile amines (TVB-N) are the most prevalent compounds produced during the spoilage of seafood products and provide adequate quality markers [62, 63]. TMA is the major volatile base produced during fish spoilage from TMAO, and it is responsible for the generation of fishy off-odors [8]. Martin et al. [8] in their review concluded that the use of VOCs (apart from TMA) for shelf life monitoring remains limited, despite the wider availability of GC-MS and SPME instruments, and that packaging conditions and the atmosphere during storage significantly influence the dynamics and metabolism of contaminating microbiota and consequently, the volatiles emitted (volatilome). This study, in accordance with similar ones [19, 41], highlights the complication of developing universal and standardized VOC methods for freshness monitoring across different types of seafood. Recognizing the inherent complexities in VOCs could contribute to developing more accurate and adaptable freshness-monitoring systems tailored to specific seafood products.
Several biogenic amines, such as hypoxanthine, histamine, putrescine, and cadaverine, are produced during fish spoilage. However, these compounds are not generated in substantial quantities in nonscombroid fish [59, 64]. Other physicochemical markers reflecting fish quality include thiobarbituric acid reactive substances (TBARSs), which reflect lipid oxidation, as well as the K value, which indicates adenosine triphosphate (ATP) depletion. K value is defined as the ratio of the sum of inosine and hypoxanthine concentrations to the total concentration of ATP metabolites. However, this marker has limitations, as ATP degradation products resulting from autolytic changes affect sensory qualities only in the early stages of shelf life and not consistently throughout storage [65]. Ultimately, the determination of fresh fish shelf life is influenced more by the accumulation of microbial metabolites than by ATP degradation alone [66].
4. Headspace Gas Composition and Smart Packaging
Freshness of fish products is highly determined by the packaging conditions [10]. The atmosphere (gas composition) in which the fish product is stored and traded is critical for microbial growth and physicochemical alterations, and thus its shelf life [67]. Recent research findings indicate that compounds in the package headspace that originate from microbial breakdown and deterioration pathways are more likely to be reliable [8, 19, 41]. Headspace gas composition of packaged fish products is significant since the volatile profile of seafood changes during storage, and these variations can be utilized to assess its freshness over time [68].
The atmospheric conditions into which fish products are packaged refer to the concentration of oxygen (O2), carbon dioxide (CO2) and nitrogen (N2) gases. Apart from conventional packaging under aerobic conditions, fish is often packaged in a vacuum (VP) or in a modified atmosphere (MAP) to extend product shelf life [69]. Numerous researchers have reported that VP and MAP achieve shelf life extension of refrigerated fish of up to 2 weeks [70, 71]. The basic principle of MAP is to promote an increased CO2 percentage (usually between 40-60%) in the package headspace, which is proposed to delay aerobic bacterial growth by prolonging the lag phase and thus extend fish shelf life [58]. During storage, the headspace composition evolves at a slower or faster pace with time depending on the storage conditions (e.g., temperature), but in a way that is not yet totally predictable. There are various parameters that affect the evolution of headspace gas composition, such as the solubility of the gases O2 and CO2 in the food matrix, the volume ratio of headspace to food, the permeability coefficients of the packaging materials to O2 and CO2 (thick trays provide a more appropriate barrier to gas transport than the thinner covering material), the dimensions of the packaging (larger surfaces cause higher gas exchange), and the storage conditions (external temperature and pressure) [72, 73].
It is well known that during the fish shelf life irrespective of packaging conditions (air, VP or MAP) O2 concentration is expected to decrease, and CO2 is expected to increase due to aerobic microbial respiration (bacterial populations consume O2 and produce CO2) [74]. Following the growth of bacterial populations, the production of volatile metabolic compounds significantly affects the in-package headspace gas composition. However, despite this clear trend in the headspace gas composition at the beginning and end of the fish product shelf life, further exploration in the intermediate phases of fish spoilage is of interest, particularly in understanding how they modify the in-package gas composition at numerous time intervals.
As stated by many researchers, spoilage characteristic of fish (e.g., VOCs concentrations during shelf life) may fluctuate significantly between species and packaging conditions, which explains the difficulty in defining common markers for fish spoilage, thus highlighting the importance of smart, real-time, low-cost assessment of fish quality and safety [19, 41, 75]. Colorimetric indicators are smart packaging systems that base their functionality on the intrinsic attribute of a chemical compound (dye) to change its structure and characteristic wavelength of absorbance based on the pH of the surrounding environment. They are comprised of two parts; the pH responsive dye (visible color change to the human eye) and the dye carrier (usually a polymer matrix). The basic principle behind pH freshness indicators is the modification of the in-package environment due to chemical interactions that take place as fish spoils. The generation of ions within the package alters the internal headspace. The ions come into contact with the surface of the indicator, disrupting the ion balance and triggering acid-base interactions with the enclosed pH indicator. The structure of the immobilized pigment/dye changes, hence a color change of the indicator becomes visible [76, 77]. The primary goal of the pH indicator strategy is not only to detect the end of shelf life but also to monitor the intermediate stages of fish quality by the gradual color change of the indicator, thereby providing an index of the remaining shelf life.
5. Freshness Indicators and (Bio)Sensors for Fish Shelf Life Monitoring
Food safety and security requires consistent progress in research and development of technologies that will provide smart, easily applicable and cost-effective solutions to the major ongoing challenge of food waste. To date there have been remarkable efforts to design and commercialize direct quality tools for fish quality monitoring, based on the different characteristics of the fish spoilage process [20]. Such quality monitoring tools may encompass freshness indicators, (bio)sensors and radio frequency identification tags.
Freshness indicators are real-time quality monitoring tools that have as a working principle the detection of alterations in product condition (e.g., change in pH, gas production, chemical substances accumulation, etc.) and are typically placed into the internal part of the packaging in the form of stickers or labels [78]. These indicators provide a visual alert about changes in product condition, by providing an easily observable color change or by displaying a distinguishing signal, e.g., visual change in morphology. Freshness indicators serve as live date markers, by directly providing information about the quality level of the packed food products.
Time-temperature indicators focus on the effect of temperature over time on the fish quality parameters and track the exposure of the product to different time-temperature conditions [79]. During this exposure, a sensitive chemical, enzymatic or bacterial reaction takes place and provides a visible, irreversible response to the end user. TTIs are categorized in terms of the operation principle, which includes mechanical, chemical, electrochemical, enzymatic, microbiological or photochemical reactions [80]. Various types of TTIs have been developed for fish freshness monitoring and have been tried industrially [10, 81]. Ye [82] designed a time-temperature indicator based on the Maillard reaction to reflect the freshness of chilled seafood. This TTI showed a color change correlated with the TVB-N levels of mackerel stored under various temperature conditions, suggesting its potential for monitoring freshness in the fish supply chain. Another study focused on a photochromic TTI, which changed color on exposure to light and temperature. This indicator was tested in simulated fresh fish supply chains, showing satisfactory performance in providing a real-time indication of temperature abuse. The gradual color change indicated cumulative exposure to elevated temperatures, helping to assess how fresh the fish remained during transportation [83]. A UV activated photochemical TTI was also developed and modelled to test its ability to reliably predict the shelf life of seabream fillets during variable storage temperatures [84]. Based on the growth and metabolic activity of microbial strains Ellouze [85] developed the currently commercially available microbial TTI.
The importance of pH freshness indicators as novel packaging technologies lies in their ability to provide distinct, condition-dependent and real-time freshness information in a non-destructive manner. Therefore, they could provide a dynamic alternative to static expiration dates, which may not reflect actual food conditions [86]. The rationale behind such indicators includes colorimetric changes caused by a shift in the in-package environment acidity or alkalinity. In particular, when packaged food begins to spoil, the corresponding food microflora releases volatile metabolic compounds (e.g., lactic acid or ammonia) that come in direct contact with the pH sensitive compound of the indicator and react (removal of H+ from the acid and addition to the base). During this reaction, the chemical structure of the pH sensitive compound (usually weak acid or base) is modified resulting in differentiated light absorption and thus visible color [87]. A schematic representation of this reaction is presented in Figure 2. In general, pH freshness indicators are most widely used for fish or meat shelf life monitoring and provide different colorimetric responses depending on the pH. Halochromic pigments are used as pH sensitive chemical compounds and may be synthetic or natural. The most common synthetic dyes include bromocresol green [88, 89], bromothymol blue [90, 91], methyl red [92, 93], methyl orange and neutral red [94]. The natural pigments include mostly anthocyanins [63, 93, 94], betalains [95], and carotenoids extracted from various plant systems such as curcumin [96], etc. Compared to natural pigments, synthetic pigments offer the benefits of ease of synthesis, low cost, enhanced stability, and high sensitivity to pH change. The carrier of pH sensitive dyes is usually a (bio)polymer, which should be chemically compatible with the dye and form a smart film with satisfactory properties (mechanical, optical, etc.). A wide range of polymers have been examined as possible carriers for embedding a diversity of agents (antimicrobials, dyes, antioxidants) in food packaging [95–97]. The most commonly tested materials include biopolymers such as starch [98], agarose [88], pectin [99], chitosan [98, 99], methylcellulose/chitosan [100], poly-vinyl-alcohol [100, 101], chitosan/fish gelatin [102], bacterial cellulose nanofibers [103], sodium alginate/gelatin [104]. Also, various systems for improving sensitivity were tested such as metal organic frameworks (MOF) incorporated to gelatin for developing freshness indicators with increased NH3 adsorption [105].

Gas indicators are quality indicators that rely on the detection of specific gases emitted when fish spoil, such as CO2, ammonia, H₂S. When the indicator reacts with the accumulated gases it produces a visible, usually colorimetric signal to inform the end user about the changes in headspace composition and the color change is correlated with the quality and remaining shelf life of the packed food [106]. A simple example of a gas freshness indicator is the label developed by [107], which contains bromothymol blue-phenol red as the pH-sensitive dye and was used to sense ammonia accumulation during skate (Raja kenojei) spoilage. Gradual color changes of the gas indicator correlated with the quality of fish and the pH during storage, implying that the indicator was not only visually functional but also aligned with established quality control metrics. The study also highlighted the ability of the gas sensor to perform as a real-time indicator at the fluctuated temperatures of the cold chain in a simple and non-destructive way.
Under the wide category of sensors, there are devices that operate by recognizing a signal associated with the food spoilage (i.e., a chemical reaction, the accumulation of volatile organic compounds) and create a response, which is usually physical or chemical, such as changes in pH, conductivity, absorption or emission of light. This type of quality monitoring tool can be divided into categories based on the type of chemical compound (analyte) they recognize in the spoilt food product (e.g., CO2, NH3, H2S, organic acids, biogenic amines, etc.) [108]. Duan [109] developed a complex chemical sensor capable of detecting simultaneously the biogenic amines histamine and tyramine produced when fish is spoilt, based on the principle of fluorescence resonance energy transfer using specific aptamers as recognition elements for histamine and tyramine. When the amines bind to their respective aptamers, conformational changes occur, leading to fluorescence recovery, which allows for their simultaneous detection. A similar approach for histamine detection and quantification was presented by Munzi [110] who developed a fast and direct colorimetric and fluorometric sensor for biogenic amines by means of a dinuclear Zn (II) Schiff-base complex. In another application of Bhadra [111] a wireless basic volatile sensor was developed to detect TVB-N compounds during fish spoilage, which showed a satisfactory correlation with the measured ammonia gas concentration. A differentiated perspective of an electrochemical sensor was presented by Itoh [112] who designed a microfluidic setup to determine fish freshness via adenosine 5-triphosphate concentrations (K value) and verified the device with HPLC results for jack mackerel, yellow tail, and sea bream. Collectively, these studies presented the development of nondestructive smart packaging solutions of high sensitivity and selectivity, underscoring the importance of detecting the spoilage markers at an early stage of spoilage, before destruction of organoleptic characteristics. The diversity in the sensor approaches points out the importance of tailored systems in smart packaging.
A common analyte detected during fish deterioration, being the base of many chemical sensors, are the gases produced during the spoilage process. The principle by which these sensors function is changes in conductivity in the sensing films triggered by the adsorption of gases and subsequent surface reactions caused by the food spoilage process [113]. More specifically, organic volatile substances produced by fish (e.g., TMA) are oxidized (oxidization requires high temperatures, > 200°C) and lead to increased electrical resistance. These sensors are usually made of metal oxide semiconductors and conduct organic polymers, or piezoelectric crystals [114]. One drawback of gas sensors is their sensitivity to changes in environmental conditions such as moisture and temperature [115].
Similar devices, such as the electronic nose, have been thoroughly studied as chemical–electrical sensors [116]. They rely on the interaction of one element with a particular chemical substance that will create an electrical signal, producing a unique “fingerprint”. Kim et al. [117] developed a hand-held sensor for rapid seafood freshness assessment that combines an array-based chemical sensor, custom electronics, and a microcontroller. The e-nose signal, generated by the contribution of, that is, changes of resistance from different sensor elements, was collected by a microprocessor, containing a simple algorithm that related the e-nose signals with seafood freshness. The sensor was successfully tested on different fish samples, establishing a linear relationship between microbiological count and the e-nose signal. By using such a sensor array in order to detect volatile organic compounds, this study provided an alternative, in addition, to the biogenic amine-based methods employed by Duan et al. [109] and Munzi et al. [110]. The wide focus upon a number of spoilage markers (volatile compounds) and the employment of pattern recognition techniques renders Kim et al.’s [117] approach rather flexible. Anisimov et al. [118] developed a portable electronic nose based on organic field-effect transistors (OFET), which were able to detect spoilage-related gases in the first hours of fish spoilage (104 CFU/g bacterial population). The e-nose functioned similarly to other sensors (e.g., Bhadra et al. [111] and Kim et al. [117]), but with higher affordability and portability, promoting industrial applicability and adding a new critical dimension to sensors. Li et al. [119] in their study confirmed that the data obtained using an electronic nose that collected odor signals was well correlated with results by chemometric methods for TVB-N and acid values (AV) measured in fish meal samples. Their findings underscored the sensitivity of the e-nose as spoilage detection solution and confirmed its efficacy as real-time freshness detector.
In recent years, biosensors have been explored as an alternative to chemical sensors. These sensors usually detect biological or chemical compounds (targets) produced during seafood spoilage by using biological molecules (i.e., enzymes and DNA) to bind or react with them. The signal produced from spoilage reactions is an electrochemical or optical response and can be measured [120]. Recent novel biosensors are based on alternative and often renewable raw materials [121]. Wang et al. [122] developed a paper-based enzyme biosensor which could detect hypoxanthine, a compound that accumulates during storage of fish. The two enzymes used, namely, xanthine oxidase and horseradish peroxidase, were immobilized on nitrocellulose membranes with 3,3 ′,5,5 ′-tetramethylbenzidine to produce a color signal. The dual-enzyme system was carried by chitosan oligosaccharide lactate-modified nitrocellulose membranes and exhibited excellent microfluidic aggregation effects. The developed enzyme biosensor produced a linear response across a concentration range (0.01–0.16 mmolL-1) and had a detection limit of 8.22 μmolL-1. Other researchers have also taken a similar approach for biosensors development for hypoxanthine detection [121, 122] and xanthine quantification [123] during fish spoilage.
Radiofrequency identification is another technology that has been heavily explored for the development of fish quality sensors [123, 124]. This technology utilizes wireless sensors to reveal targets and communicate data. The identification signals are stored in tags and card readers. Huang et al. [125] developed a passive radiofrequency pH-sensing tag for fish freshness monitoring, based on producing an electrochemical potential in response to the pH level change. The sensor consisted of flexible iridium oxide electrodes connected to a passive transponder, which wirelessly transmits data by modulating the frequency of a relaxation oscillator based on the detected potential. The system operated without a battery, relying on inductive coupling with a reader to power the tag and transmit pH data in real time. In contrast to other similar sensors detecting chemical compounds (i.e., VOCs or amines), this monitors pH that remains a general marker of spoilage. VOCs and biogenic amines, in conjunction, may furnish more specific understanding into the food spoilage type and stage. Nevertheless, a combination of these approaches could be complementary, as pH levels typically change alongside the production of VOCs and amines throughout spoilage. However, RFID technology can be more cost demanding as a quality monitoring tool due to its higher complexity [126].
Integrating the technology of freshness indicators and sensors with blockchain technology would enhance transparency and traceability in the cold supply chain of perishable products, such as fish and seafood. The data obtained through such freshness monitoring tools could be utilized as immutable records in the blockchain technology and traceability as well as in spoilage detection, and it could contribute to origin tracking, consumer transparency and smart contract purposes [127]. Table 1 summarizes the physicochemical indices connected to fish quality degradation and presents their impact on headspace gas composition as well as the freshness indicators used for freshness monitoring.
| Physicochemical markers | Mechanism | Headspace gas composition | Freshness indicator types | Indicator’s mechanism | References |
|---|---|---|---|---|---|
| pH | Microbial activity and protein breakdown cause pH rise | ↑CO2 | pH-sensitive indicators | Acid–base reaction with pH-sensitive dye ➔ structure change ➔ color change | Ezati et al. [128], Moradi et al. [103], Yong et al. [21] |
| Volatile organic compounds (VOCs) | Produced by bacterial metabolism | ↑N2, CO2 | Gas sensors, electronic nose (e-nose) | Recognize changes in electrical conductivity or resistance | Kim et al. [117], Lee et al. [107], Semeano et al. [129] |
| Total volatile basic nitrogen (TVB-N) | Ammonia and volatile amines produced by microbial metabolism and protein breakdown | ↑TMA, DMA, NH3, N2 | pH-sensitive indicators, sensors | Acid–base reaction with pH-sensitive dye ➔ structure change ➔ color change | Bhadra et al. [111], Gasti et al. [100], Hu et al. [102], Mohseni-Shahri et al. [105], Mohseni-Shahri et al. [130], Zheng et al. [104], |
| Biogenic amines (histamine, hypoxanthine, putrescine, and cadaverine) | Produced by bacterial decarboxylation of amino acids | ↑NH3, N2 | Biosensors | Detect biological or chemical compounds by using biological molecules (i.e., enzymes and DNA) to bind or react with them | Duan et al. [109], Munzi et al. [110], Mustafa et al. [131], Wang et al. [122] |
| Thiobarbituric acid reactive substances (TBARS) | Produced by lipid oxidation | ↑Aldehydes, CO2 | Biosensors, gas sensors, electronic nose | React with the accumulated gas➔ produce a visible, usually colorimetric signal | Anisimov et al. [118], Kim et al. [117], Li et al. [119] |
| K-value | Measures ATP depletion caused by microbial activity | ↑Hypoxanthine NH3, N2 | Electrochemical biosensors, metal oxide semiconductors (MOS) | Biological or chemical compounds are oxidized ➔ electrical resistance ➔ conductivity | Huang et al. [132], Itoh et al. [112], Wang et al., [122] |
Consumers are steadily moving towards using natural and minimally processed food products, promoting the need for smart packaging [133]. Understanding consumer perception and expectations over smart packaging solutions is the main step for wider consumer adoption. Several researchers have been focusing on consumer attitude, such as Barska and Wyrwa [134] who investigated consumer awareness about active and intelligent food packaging in a sample of 372 participants. They reported that despite some widely available intelligent packaging systems consumers’ knowledge about these technologies was insufficient. In some cases, smart indicators have been questioned among consumers regarding their precision in real-world applications, especially in complex systems like food samples. The researchers highlighted the importance of enhancing education towards these technologies so consumer acceptance will steadily increase.
Pennanen et al. [135] investigated the consumer perception of TTIs in four European countries (Finland, Greece, France, and Germany). They concluded that consumers overall comprehend and appreciate TTI technology; however, work needs to be done towards wider acceptance of TTI applications in food packages. Among others, main concerns include mistrust about final quality and potential food price increase caused by the implementation of TTIs.
Liu et al. [136] reported that consumers are increasingly worrying about food safety and quality and prefer packaging options that involve natural compounds to monitor freshness. Doubts have been reported about synthetic indicators due to potential toxicity associated with health risks and environmental impact, including chemical and environmental pollution [137]. In this context, a growing consumer interest in natural freshness indicators is observed.
Since freshness indicators are incorporated inside the food packages and migration of substances is possible, specific safety considerations must be taken into account. These mainly involve the design process, material transparency, and proper consumer education [133]. FDA (Food and Drug Administration) has provided regulatory standards for the various types of substances incorporated in food packages. The nontoxicity (specific migration limits) and stability of the chosen materials is obligatory. Only food-safe and nontoxic (GRAS, “generally recognized as safe”) materials can be used as pH-sensitive substances and dye carriers, avoiding chemical compounds whose properties and functionality are not totally clarified [138]. Furthermore, the materials should be tested in extreme conditions (i.e., increased temperature) for stability and durability confirmation that could compromise the indicators’ efficacy. In respect to environmental threat, biodegradable materials are considered a better choice than synthetic; however, detailed risk assessment, including cytotoxicity tests, should be performed for both environmental and health risks [139]. Compliance with safety regulations and standards combined with consumer awareness could control potential safety risks and enhance commercial adoption of freshness indicators.
6. Evidence From a Case Study
Reviewing and interpreting the physicochemical markers of food alteration and their interaction is an important step for building capacity to monitor shelf life through a smart indicator technology without compromising the integrity of the packaging. Up to date, there have been numerous research attempts focusing on applying smart indicators to detect fish spoilage. However, there is limited research reported in the literature focusing on the systematic analysis of the actual in-package alteration phenomena of fish flesh and their connection with the headspace composition (evolution of O2, CO2, and N2 gases). The aim of this case study was to enlighten the dynamic in-package gas composition during fish refrigerated storage and reflect it through pH-sensitive indicators.
7. Materials and Methods
7.1. Smart Indicator Preparation
In the current case study, methyl red was chosen as the colorimetric pigment due to its lower pKa (5.1) than other synthetic dyes, which makes it a stronger acid, and enables it to react with lower concentrations of amines [94]. When a pH indicator dye is placed in a basic environment where deprotonation is prevalent, a shift in absorption spectrum wavelength maximum (λmax) is observed. MR is reported to show peaks at λ = 520 ± 15 nm and λ = 435 ± 20 nm, which are assigned to acidic and basic forms of methyl red, respectively. MR has a quinonoid (red) structure in the acid medium and a benzenoid (yellow) structure in the base medium [140].
Smart indicators were fabricated with the dip coating method using two biopolymers: starch and cellulose. In particular, starch powder (4% w/v) obtained from Merck (Darmstadt, Germany) and glycerol (≥ 99.5%) (30% w/w of dry matter) obtained from Sigma-Aldrich (St. Louis, United States) were dissolved by stirring in distilled water for 30 min/90°C and then methyl red (1% w/v) obtained from Sigma-Aldrich (St. Louis, United States) was added and stirred until complete dissolution. Cellulose filter paper was cut into 2 × 2 cm2 and immersed into the starch-MR solution for 1 min. The smart indicators were then removed and left to dry at room temperature in the dark.
7.2. Smart Indicator Characterization
7.3. Monitoring Fish Freshness With Smart Indicators
The smart indicators were applied into packages (sealed pouches) containing gilthead sea bream (Sparus aurata) fillets. Specifically, two replicates of the indicators were placed inside the headspace of sterilized sealed packaging pouches (polyethylene/polyamide low-permeability pouches, 70-μm thick, and 15 × 20 cm2 in size) containing 50 g fish fillets. The samples were then stored isothermally at 4°C and measurements were carried out at appropriate time intervals during fish storage. At each sampling point, the in-package gas composition was measured with a gas analyzer (Danseonsor, CheckPoint 3). Afterwards, the color of the smart indicators was measured to determine the color change (ΔΕ), as described above as well as the change in a value from red to orange–yellow (Δa). Chemical indices, such as pH and TVB-N, and microbiological growth (TVC) of fish were determined during storage.
7.4. Data Analysis
The data obtained during the experimental processes were fitted using a Baranyi growth model [143] and curve fitting was enabled through DMfit 3.5 software (IFR, Institute of Food Research, Reading, UK) (available at http://www.combase.cc/index.php/en/, accessed on August 20, 2024). Significant differences for the parameters studied were determined using Duncan’s multiple range test at a significance level of 95% (a = 0.05).
8. Results and Discussion
8.1. Sensitivity of the Smart Indicators to pH Change
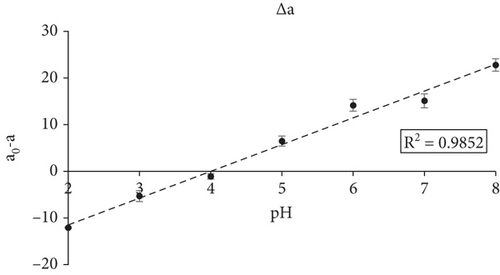
The color parameters (L, a, and b) of the smart films were evaluated before and after their exposure to different pH buffer solutions. During the pH sensitivity test, films became slightly brighter as the pH value of the buffer solutions increased from 2 to 8. The a value of the smart films decreased remarkably with the increase of pH, indicating a significant reduction of the red hue while the b value of the films increased, indicating a visible color change from red to yellow. The results are presented in Table 2.
| PH | L | a | b | Film photograph |
|---|---|---|---|---|
| Control | 83.99 ± 0.07e | 19.58 ± 0.12d | 10.98 ± 0.18e |  |
| 2 | 62.53 ± 1.46a | 31.69 ± 0.99g | −8.05 ± 0.57a |  |
| 3 | 65.77 ± 0.83b | 24.90 ± 0.44f | −6.57 ± 0.21b |  |
| 4 | 66.87 ± 1.23b | 20.68 ± 0.51e | −4.47 ± 0.57c |  |
| 5 | 70.40 ± 0.64c | 13.10 ± 0.72c | 8.06 ± 1.15d |  |
| 6 | 72.97 ± 0.46d | 5.43 ± 0.43b | 22.14 ± 1.10f |  |
| 7 | 72.51 ± 0.71d | 4.47 ± 1.09b | 54.70 ± 3.02g |  |
| 8 | 73.19 ± 1.32d | −3.23 ± 0.43a | 57.96 ± 2.96g |  |
- Note: Values are expressed as mean ± standard deviation. Values with different superscripts in the same column (a, b, c, d, e, f, and g) were significantly different as shown by Duncan’s multiple range test.
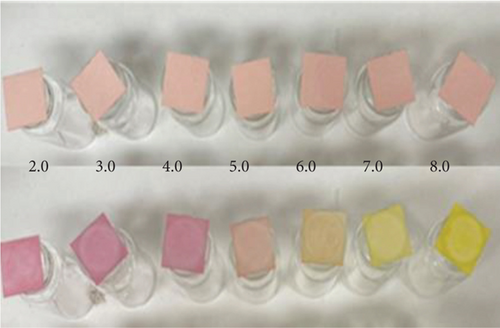
Figure 3a,b presents the color of the smart film when exposed to various pH buffer solutions. The color change of the smart films was clearly visible to the human eye, as well as the difference in color parameter a− (Δa) detected with a colour spectrophotometer, which represents the depletion of the red hue as the pH increased from 2 to 8, implying that the fabricated smart films could sense and visually reflect pH changes.
8.2. Sensitivity of the Smart Indicators to Ammonia Vapor
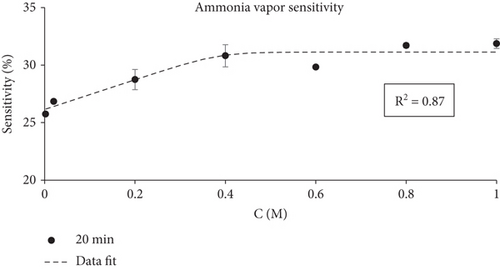
The smart indicators were tested for their ability to change color when exposed to various ammonia concentrations (0.002–1 M). The films changed color in all the tested conditions, as shown in Figure 4.

The sensitivity test indicated that the fabricated smart films could sense progressively increasing ammonia concentrations and reflect it through an increased visible color change. This alteration occurs due to the reaction between ammonia gas and water vapor, ultimately leading to the formation of ammonium hydroxide [130]. The color change of smart films was clearly visible from the first minutes of the experiment, and the ammonia sensitivity of smart films increased with time (Figure 5). At the end of the sensitivity test (20 min) the highest sensitivity score was observed with smart films exposed to 0.8 M and 1 M ammonia vapor (SRBG = 31.70% and SRBG = 31.86%, respectively, not a statistically significant difference). However, it is important to note that even the lowest ammonia concentration (0.002 M) caused a visible color change and led to a relatively high SRBG value (SRBG = 25.75%).
8.3. Fish Freshness Monitoring
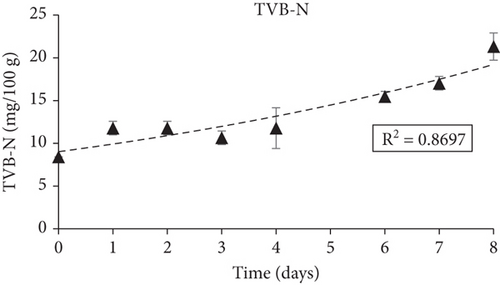
The quality parameters related to the isothermal storage (4°C) of fish in sealed bags with the smart indicators attached inside the headspace of the bag are presented and discussed below. The initial microbial population of the fish samples determined by TVCs were 3.6 ± 0.1 logcfu/g and reached 8.88 ± 0.3 logcfu/g on storage Day 8. According to the Baranyi model used to fit the TVC growth curves (Figure 6a) and to determine the growth kinetics, the growth rate, k, was 0.68 (d-1), the coefficient R2 was 0.97 and no lag phase was observed. Considering the legal limits for acceptable microbial contamination of 7 logcfu/g for TVC [144], the shelf life of sea bream fillets stored at 4°C was calculated as 6–7 days. TVB-N was initially (Day 0) calculated at 8.4 mg/100 g and increased gradually during storage reaching 21.31 mg/100 g by Day 6–7 without surpassing the limit of 25 mg/100 g fish. TVB-N values are presented in Figure 6b.
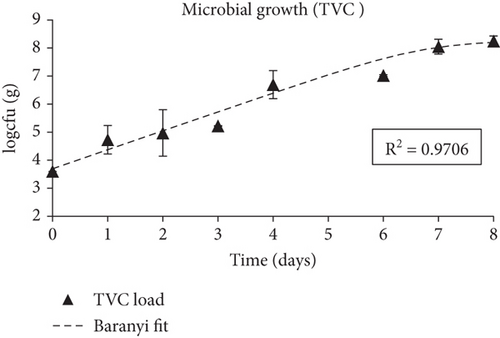
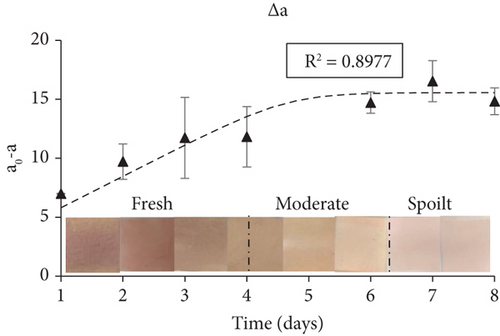
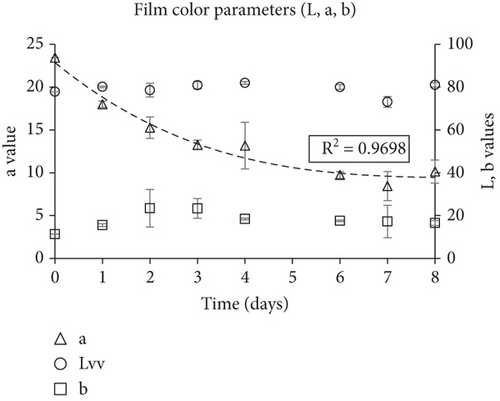
The smart indicators had a significant change in color during fish storage, starting from red–pink (Day 0), turning yellow and finally pale yellow–white (Day 8). The color change of the smart films was expressed through Δa (a value difference), and was always higher than 3, which is the lowest threshold detected by the human eye (Figure 6c). Δa had an increasing trend during fish storage and reached 16.5 by Day 7 and 14.8 by Day 8. The color parameter a, which reflected the red hue, began to decrease from Day 1 at an increasing rate as the fish freshness decreased while color parameter b (reflecting the yellow hue) increased (Figure 6d). The lightness of the films remained unchanged (no statistically significant difference) during the first days of storage and then increased slightly.
Fish flesh pH presented a characteristic drop between storage Day 1–2 (6.4) possibly due to autolytic reactions within the fish flesh and then increased progressively as the fish spoiled up to 6.6 on storage Day 7. It is interesting to note that the highest pH value (6.7) was observed on Day 6, which was classified as the last day before complete spoilage of the stored fish. On Day 8, a pH drop was observed and was attributed to the promotion of in-package CO2, which on reaction with water molecules (in-package moisture increases during fish storage) produces carbonic acid (H2CO3) and the potential growth of LAB [145]. Although the color parameters a and b changed slightly from Day 7 to 8, the color modification was not visible to the human eye. Table 3 presents the mean and standard deviation of fish flesh pH as well as the observed color change of the smart indicators during fish storage.
| Time (d) | 0 | 1 | 2 | 3 | 4 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| PH | 6.7 ± 0a | 6.7 ± 0a | 6.4 ± 0.1b | 6.5 ± 0.1c | 6.6 ± 0.1d | 6.7 ± 0a | 6.6 ± 0.1d | 6.5 ± 0c |
| Film photograph |  |
 |
 |
 |
 |
 |
 |
 |
- Note: Values are expressed as the mean ± standard deviation. Values with different superscripts in the same column (a, b, c, and d) were significantly different as shown by Duncan’s multiple range test.
The in-package headspace composition was continuously changing during fish spoilage. O2 concentrations dropped from 20.95% on storage Day 0 to 0.55% on storage Day 8, followed by a characteristic increase in CO2 concentrations which rose from 0.9% on Day 0 and reached 10.7% on Day 8. The rise in CO2 was attributed to microbial metabolic activity. As shown in Figure 7, CO2 started to increase at a higher rate after Day 4 when microbial growth was already advanced (> 6 logcfu/g).
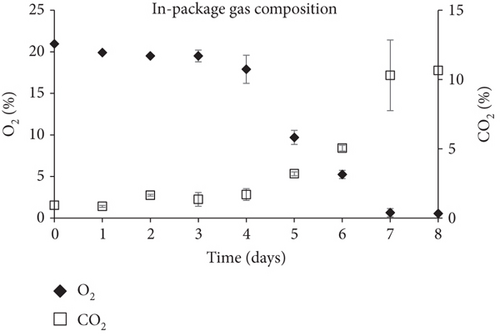
Overall, the fabricated smart films displayed satisfactory performance in monitoring the freshness of sea bream fillets stored at 4°C. TVB-N, associated with volatile amines produced during fish spoilage such as TMA, dimethylamine, and ammonia, progressed during fish storage experiments in a pattern similar to the smart films’ color change (Δa). Additionally, TVC growth and in-package % CO2 concentrations grew proportionally with the latter following the progress rate of the former. The curve fitting of both TVC and Δa data (obtained through the DMFit program) revealed a good correlation between fish spoilage and smart film colour response (R2 = 0.90). Moreover, the pH-sensitive smart films that were attached inside the package of fish fillets presented a color change (Δa) that was proportional to the microbial growth (TVC), suggesting a high correlation between these indexes.
9. Conclusions
The pH-sensitive smart indicators constitute a promising area for development of novel smart food packaging, which creates significant opportunities for food waste reduction. Thorough investigation of the deterioration mechanisms in packaged fish, combined with an understanding of the evolving in-package conditions will enhance our comprehension of the food ecosystem and its alterations. This, in turn, is anticipated to significantly improve the sensitivity and practicality of future freshness indicators. Also, it would contribute to the transformation of qualitative freshness indicators into quantitative indicators. In this review case study, the importance of the packaging headspace composition and its dynamic interaction with the packaged food was highlighted and presented through the development and application of a simple pH-sensitive indicator. The smart indicator exhibited sufficient color response to the dynamics of fish deterioration and correlated well with the continuous headspace composition change. Future research will focus on replacing synthetic compounds with natural ones, as well as conducting comprehensive selectivity studies to evaluate the indicators’ performance in the presence of other volatile organic compounds. The operating principle of the developed indicator could potentially be incorporated into biosensors or combined with biotechnological assays that could be integrated into smart devices within packaging or at quality control. Limitations such as scalability, cost-effectiveness, and consumers’ limited awareness of smart indicators should be thoroughly explored. The provided insights from the present study are expected to allow researchers to develop hypotheses that can be further explored and will open up new directions for future experimental research.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
The study was designed by E.B. and T.T. The experiments were carried out by E.B. and S.E.V., and they were supervised by M.S., E.F., F.B., D.P., and T.T. The data analysis was carried out by E.B. and S.E.V., and M.S., E.F., F.B., D.P., and T.T. supervised the research. T.T., E.F., M.S., F.B., and D.P. conceptualized the goals and aims of the research. E.B. wrote the main manuscript that was edited, completed, and reviewed by S.E.V., M.S., E.F., F.B., D.P., and T.T.
Funding
The research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the 5th Call for HFRI PhD Fellowships (Fellowship Number: 20599). This research has also received partial funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie Grant Agreement no. 872217 (ICHTHYS) https://www.ichthys-eu.org/about).
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




