Pruritus and Its Association With Cancer and Mortality in Dermatomyositis and Polymyositis: A Nationwide Cohort Study in Taiwan From 2005 to 2022
Abstract
Background: Pruritus is the most common initial symptom reported by patients with dermatomyositis (DM) and polymyositis (PM). However, there is limited data regarding the impact of pruritus on cancer and mortality in patients with DM and PM. In this study, we aimed to investigate how pruritus is associated with cancer and mortality in patients with DM and PM.
Methods: This nationwide, population-based retrospective cohort study included adult DM and PM patients from Taiwan’s National Health Insurance Research Database between 2005 and 2022. Sex- and age-matched pruritic patients, identified by over 6 weeks of antipruritic medication use, and nonpruritic patients were analyzed. The primary outcome was cancer occurrence or all-cause mortality. The association between pruritus and these outcomes was estimated using Cox proportional hazards models.
Results: Among 919 matched pairs of pruritic and nonpruritic patients, cancer was observed in 19.96% in the long-term pruritic group (LPG), 14.63% in the short-term pruritic group (SPG), and 10.34% in the non-pruritic group (NPG) (p < 0.0001). All-cause mortality was documented as 30.37% in the LPG, 29.69% in the SPG, and 37.76% in the NPG (p < 0.0001). After adjusting for sex, age, and other comorbidities, pruritus was associated with an increased risk of cancer (hazard ratio (HR) 1.708, 95% confidence interval (CI) 1.229–2.374) and a lower risk of all-cause mortality (HR 0.483, 95% CI 0.409–0.569).
Conclusion: This population-based study revealed that pruritus appeared to be associated with increased risks of cancer and decreased all-cause mortality. Thus, pruritus may serve as a pragmatic factor for risk stratification and tailored treatment strategies in DM and PM. Cancer screening, particularly for nasopharyngeal and breast cancers in East Asian populations, is recommended for patients with DM or PM, especially those presenting with pruritus. Meanwhile, patients without pruritus may require vigilant management for potentially life-threatening complications and comorbidities.
1. Introduction
Dermatomyositis (DM) and polymyositis (PM) are rare but debilitating autoimmune diseases. In the United States, the annual incidence of DM is 9.63 cases per million people [1]. In Taiwan, DM and PM together occur at a frequency of 11.5 cases per million people [2]. Both conditions are characterized by progressive symmetrical proximal muscle weakness. DM also presents with distinct skin manifestations, such as pruritus, heliotrope rash, and Gottron’s papules. In addition, patients with DM and PM have increased risks of cancer, interstitial lung disease (ILD), stroke [3, 4], and ischemic heart disease [3, 4], and they have one of the poorest prognoses among connective tissue diseases.
Pruritus is the most common initial symptom reported by patients with DM, and it is associated with increased cutaneous severity. Up to 63%–90% of patients with DM experience pruritus during the course of the disease [1, 5, 6]. Furthermore, pruritus in DM is associated with poor mental health and has a more significant impact on quality of life than other pruritic dermatoses, such as psoriasis and atopic dermatitis [6–8].
Pruritus has been identified as a notable prognostic factor in several diseases. In conditions such as Hodgkin’s lymphoma [9, 10] and hemodialysis [11, 12], pruritus is associated with a reduced survival. Conversely, in polycythemia vera, pruritus is associated with a lower risk of arterial thrombosis and improved survival [13]. Furthermore, pruritus might serve as a marker of occult cancer, regardless of the type of underlying diseases [14]. Regarding DM and PM, previous studies have shown that pruritus was correlated with an elevated risk of cancers [15–17], while one study presented the contradictory evidence [18]. This discrepancy highlights the need for large-scale studies to investigate the association between pruritus and the complications in patients with DM and PM.
In this study, we investigated the potential association between pruritus and cancer risk in patients with DM and PM by using Taiwan’s national database and registry. We aimed to investigate the impact of pruritus on the risk of cancer in DM or PM, as well as the association between pruritus and overall survival in these patients. Through our investigation, we elucidated the clinical importance of pruritus, which may help stratify these patients into subgroups and guide management strategies in DM and PM.
2. Methods
2.1. Data Sources
All participant data were obtained from the Registry of Catastrophic Illness Database in Taiwan, a subset of the National Health Insurance Research Database (NHIRD). This database was established for public research purposes as part of Taiwan’s National Health Insurance Program, which was initiated in 1995 and covered more than 99% of Taiwan’s 23 million residents. The case definitions of DM and PM were strictly limited to the ones who underwent comprehensive clinical and laboratory assessments that met the diagnostic criteria by Bohan and Peter [19, 20]. Only patients with a diagnosis of probable or definite DM and PM were given the catastrophic illness certificate. The accuracy and validity regarding DM and PM in the Registry of Catastrophic Illness Database have been well-established in the prior studies [2, 21].
2.2. Study Design and Participants
Data on patients with DM or PM diagnosed between January 1, 2005, and December 31, 2022, were extracted from the Registry of Catastrophic Illness Database in Taiwan by using the International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) codes (Supporting Table S1). Only patients with a follow-up period of at least 1 year were included to allow for adequate assessment of prognosis and clinical outcomes. To minimize bias, patients with DM or PM diagnosed with concurrent conditions potentially associated with pruritus were excluded, such as dermatitis, urticaria, psoriasis, mycosis fungoides, Sézary syndrome, liver cancer, lymphoma, and polycythemia vera (as defined using ICD-9 and ICD-10 codes; Supporting Table S1). In addition, patients undergoing dialysis (as identified using expenditure codes; Supporting Table S1) were excluded. Given the high prevalence of dermatitis and urticaria, only patients with two consecutive diagnoses of these conditions were excluded.
2.3. Definition of Pruritus
The index date for a patient with DM or PM was defined as the date of the first medical record related to the diagnosis. Cases with chronic pruritus were defined based on the methodology previously outlined by Ting et al. [12]. Patients with DM or PM who were prescribed with antipruritic agents for 42 consecutive days or more after the index date were included in the pruritic group (PG) (as defined using anatomical therapeutic chemical codes, including systemic or topical antihistamines, menthol lotion, and combination ointments containing chlorpheniramine maleate, lidocaine hydrochloride, hexachlorophene, methyl salicylate, menthol, and camphor; Supporting Table S2). The PG was further divided into long-term pruritic group (LPG) for those treated for 84 days or more, and short-term pruritic group (SPG) for those treated for 42–83 days. Prescriptions separated by an interval shorter than 30 days were also considered consecutive. Patients without prescriptions for antipruritic agents, or those prescribed with such agents for less than 42 consecutive days, were classified into the nonpruritic group (NPG). To exclude other indications for prescribing antihistamines, prescriptions given under the diagnosis of allergic rhinitis (defined by ICD-9 and ICD-10 codes; Supporting Table S1) were removed from the prescription records.
2.4. Outcome Measurement
The primary outcomes of interest were cancer and ILD development, as well as mortality. Cancer development was defined as receiving a diagnosis corresponding to the International Classification of Diseases for Oncology, Third Edition codes C00–C97 in the Taiwan Cancer Registry between 2005 and 2022. Diagnoses related to nonmelanoma skin cancer and metastasis were excluded (defined by ICD-9 and ICD-10 codes; Supporting Table S3). Cancer cases were further categorized into two groups, based on registration date within 5 years prior to and 10 years after the index date. The mortality and cause of death were determined using the National Death Registry in Taiwan from 2005 to 2022.
To minimize the influence of potential confounding factors, additional factors including age at the index date, sex, and comorbidities known to be associated with both outcome risk and pruritus were evaluated. Comorbidities, including diabetes mellitus, hypertension, and other medical conditions were defined by ICD-9 and ICD-10 codes documented at least twice in the outpatient department visits or once during inpatient admissions before the index date (Supporting Table S1).
2.5. Statistical Analyses
The distributions of the demographic characteristics, and comorbidities of the patients with DM and PM, were presented as frequencies (percentages) for categorical variables, medians (with interquartile ranges) for continuous variables, and means ± standard deviations for approximately normally distributed continuous variables. Differences in distribution proportions of categorical variables were assessed using the Cochran–Mantel–Haenszel test, whereas the mean differences in continuous variables between the two groups were evaluated using the generalized estimating equation. Survival analysis was performed by the Kaplan–Meier method. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using multivariable Cox proportional hazard models to investigate the association between pruritus and the risk of newly diagnosed cancer and all-cause mortality. The individual risk of each confounding factor was assessed using simple Cox proportional hazard models, and confounders exhibiting significant outcome risks were subsequently included in multivariable Cox proportional hazard models for adjustment. All statistical analyses were conducted using SAS software (Version 9.4, SAS Institute, Cary, NC, USA), with a two-sided p value of < 0.05 considered significant.
2.6. Sensitivity Analyses
Sensitivity analyses were performed to evaluate the impact of varying pruritus definition thresholds and the exclusion of other potential pruritus-associated comorbidities (jaundice, hyperthyroidism, thyroid goiter, and hypothyroidism) on the outcome measures.
3. Results
3.1. Baseline Characteristics of the DM/PM Cohort
From 2005 to 2022, 2723 patients listed on the NHIRD were diagnosed with DM or PM (Figure 1). Among these patients, 122 were excluded for ages younger than 18 years, and 555 were excluded for concomitant pruritus-associated diseases (212 with dermatitis, 95 with urticaria, 122 with psoriasis, 4 with mycosis fungoides, 57 with liver cancer, 50 with lymphoma, 4 with polycythemia vera, and 90 undergoing dialysis). A total of 2046 patients with DM or PM remained. They were categorized into the PG (1123 patients) and the NPG (923 patients) based on their antipruritic medication prescriptions. After matching by the date of birth (±2 years) and sex at a ratio of 1:1, 891 patients of DM/PM with pruritus (PG) and 891 without (NPG) were included in the analysis.
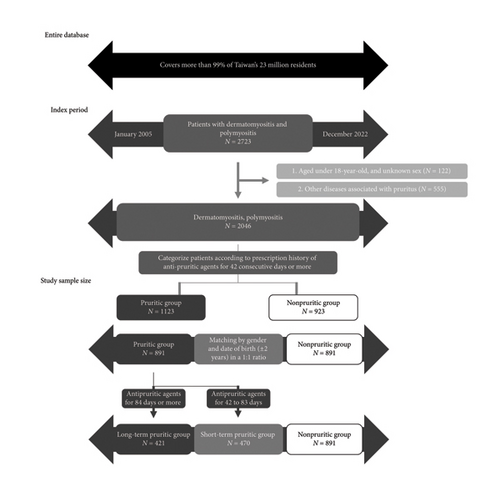
Demographic data of the overall cohort (patients with DM and PM) were examined. The average follow-up time was 6.8 years. Our cohort exhibited a female predominance, with a male-to-female ratio of approximately 1:2 (Table 1). The mean age at the index date was 52.1 years in the LPG, 51.3 years in the SPG, and 51.7 years in the NPG. No significant baseline difference was observed in comorbidities.
| Overall cohort | Dermatomyositis | Polymyositis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPG | SPG | NPG | p | LPG | SPG | NPG | p | LPG | SPG | NPG | p | |
| N = 421 | N = 470 | N = 891 | N = 260 | N = 248 | N = 415 | N = 161 | N = 222 | N = 476 | ||||
| Male, n (%) | 145 (34.44) | 166 (35.32) | 311 (34.90) | — | 95 (36.54) | 88 (35.48) | 142 (34.22) | — | 50 (31.06) | 78 (35.14) | 169 (35.50) | — |
| Age at index date, year, n (%) | ||||||||||||
| Mean (SD) | 52.1 (14.16) | 51.3 (15.02) | 51.7 (14.75) | 0.42 | 52.0 (13.88) | 51.4 (14.48) | 52.2 (14.49) | 0.15 | 52.1 (14.64) | 51.2 (15.63) | 51.3 (14.98) | 0.29 |
| Median (IQR) | 52 (42–62) | 52 (41–62) | 52 (41–63) | 52 (43–62) | 52 (41–62) | 51 (42–62) | 53 (41–63) | 52.5 (40–62) | 52 (41–63) | |||
| Min, max | 18, 88 | 18, 87 | 18, 89 | 18, 88 | 18, 86 | 18, 89 | 20, 84 | 19, 87 | 18, 87 | |||
| < 40 | 85 (20.19) | 100 (21.28) | 188 (21.10) | 0.92 | 50 (19.23) | 45 (18.15) | 79 (19.04) | 0.78 | 35 (21.74) | 55 (24.77) | 109 (22.90) | 0.32 |
| 40–65 | 261 (62.00) | 285 (60.64) | 537 (60.27) | 166 (63.85) | 161 (64.92) | 256 (61.69) | 95 (59.01) | 124 (55.86) | 281 (59.03) | |||
| > 65 | 75 (17.81) | 85 (18.09) | 166 (18.63) | 44 (16.92) | 42 (16.94) | 80 (19.28) | 31 (19.25) | 43 (19.37) | 86 (18.07) | |||
| Comorbidities, n (%) | ||||||||||||
| Diabetes mellitus | 51 (12.11) | 60 (12.77) | 124 (13.92) | 0.64 | 28 (10.77) | 29 (11.69) | 51 (12.29) | 0.37 | 23 (14.29) | 31 (13.96) | 73 (15.34) | 0.93 |
| Hypertension | 107 (25.42) | 121 (25.74) | 248 (27.83) | 0.27 | 58 (22.31) | 60 (24.19) | 108 (26.02) | 0.89 | 49 (30.43) | 61 (27.48) | 140 (29.41) | 0.65 |
| Vasculitis | 3 (0.71) | 3 (0.64) | 3 (0.34) | 0.56 | NA | NA | NA | 0.71 | NA | NA | NA | 0.32 |
| Arthritis | 20 (4.75) | 20 (4.26) | 43 (4.83) | 0.66 | 13 (5.00) | 10 (4.03) | 26 (6.27) | 0.58 | 7 (4.35) | 10 (4.50) | 17 (3.57) | 0.72 |
| Jaundice | NA | NA | 7 (0.79) | 1.00 | NA | NA | NA | 0.74 | NA | NA | NA | 0.32 |
| Hypothyroidism | 10 (2.38) | 14 (2.98) | 31 (3.48) | 0.49 | NA | NA | 11 (2.65) | 0.47 | NA | NA | 20 (4.20) | 0.87 |
| Thyroid goiter | 15 (3.56) | 21 (4.47) | 46 (5.16) | 0.41 | 9 (3.46) | 11 (4.44) | 23 (5.54) | 1.00 | 6 (3.73) | 10 (4.50) | 23 (4.83) | 0.80 |
| Hyperthyroidism | 9 (2.14) | 15 (3.19) | 31 (3.48) | 0.34 | 4 (1.54) | 5 (2.02) | 10 (2.41) | 0.11 | 5 (3.11) | 10 (4.50) | 21 (4.41) | 0.32 |
- Note: Overall cohort: patients with dermatomyositis and polymyositis. LPG, long-term pruritic group: receiving antipruritic agents for 84 consecutive days or more. SPG, short-term pruritic group: receiving antipruritic agents for 42 consecutive days or more but less than 84 days. NPG, nonpruritic group: receiving antipruritic agents for less than 42 consecutive days. NA, not available, because of number less than 3.
- Abbreviations: IQR = Interquartile range, SD = Standard deviation.
3.2. Association Between Pruritus and Cancer
To investigate the association between pruritus and cancers in patients with DM or PM, we analyzed the prevalence of cancers, before and after the diagnosis of DM and PM, in the matched groups. For the overall cohort, a higher percentage of patients in the LPG and SPG developed cancer than in the NPG over the 5 years prior to and 10 years after the index date (Table 2; 18.05% for LPG, 15.53% for SPG, and 8.98% for NPG; p < 0.0001).
| LPG | SPG | NPG | p | |
|---|---|---|---|---|
| Overall cohort | N = 421 | N = 470 | N = 891 | |
| Cancer# | ||||
| Overall period | 76 (18.05) | 73 (15.53) | 80 (8.98) | < 0.0001 |
| Ever registered in 5 years prior to index date | 17 (4.04) | 20 (4.26) | 24 (2.69) | 0.05 |
| Ever registered in 10 years after index date | 62 (14.73) | 57 (12.13) | 59 (6.62) | < 0.0001 |
| All-cause mortality | 114 (27.08) | 146 (31.06) | 333 (37.37) | 0.0005 |
| Cancer-associated mortality | 37 (8.79) | 37 (7.45) | 44 (4.94) | 0.008 |
| Interstitial lung disease | 142 (33.73) | 123 (26.17) | 277 (31.09) | 0.23 |
| Dermatomyositis | N = 260 | N = 248 | N = 415 | |
| Cancer# | ||||
| Overall period | 61 (23.46) | 50 (20.16) | 48 (11.57) | 0.004 |
| Ever registered in 5 years prior to index date | 12 (4.62) | 17 (6.85) | 16 (3.86) | 0.53 |
| Ever registered in 10 years after index date | 50 (19.23) | 37 (14.92) | 35 (8.43) | 0.005 |
| All-cause mortality | 76 (29.23) | 86 (34.68) | 191 (46.02) | < 0.0001 |
| Cancer-associated mortality | 33 (12.69) | 27 (10.89) | 27 (6.51) | 0.17 |
| Interstitial lung disease | 82 (31.54) | 79 (31.85) | 165 (39.76) | 0.11 |
| Polymyositis | N = 161 | N = 222 | N = 476 | |
| Cancer# | ||||
| Overall period | 15 (9.32) | 23 (10.36) | 32 (6.72) | 0.92 |
| Ever registered in 5 years prior to index date | 5 (3.11) | 3 (1.35) | 8 (1.68) | 0.83 |
| Ever registered in 10 years after index date | 12 (7.45) | 20 (9.01) | 24 (5.04) | 1.00 |
| All-cause mortality | 38 (23.60) | 60 (27.03) | 142 (29.83) | 0.03 |
| Cancer-associated mortality | 4 (2.48) | 8 (3.60) | 17 (3.57) | 0.25 |
| Interstitial lung disease | 60 (37.27) | 44 (19.82) | 112 (23.53) | 0.67 |
- Note: Data are presented as n (%). Overall cohort: patients with dermatomyositis and polymyositis. LPG = Long-term pruritic group: receiving antipruritic agents for 84 consecutive days or more. SPG = Short-term pruritic group: receiving antipruritic agents for 42 consecutive days or more but less than 84 days. NPG = Nonpruritic group: receiving antipruritic agents for less than 42 consecutive days.
- #Patients had records available in the cancer registry between 5 years prior to and 10 years after the index date.
In addition, we found that the association between pruritus and cancer was more pronounced in patients with DM, with cancer occurring in 23.46% of patients in the LPG, 20.16% in the SPG, and 11.57% in the NPG (p = 0.004). In contrast, no significant association was observed in patients with PM, where cancer was noted in 9.32% of the LPG, 10.36% of the SPG, and 6.72% of the NPG (p = 0.92).
We next investigated the association between specific cancers and pruritus among patients with DM or PM. In our cohort, nasopharyngeal cancer, breast cancer, colorectal cancer, and lung cancer were the most prevalent cancer types, representing 23.1%, 18.8%, 17.5%, and 15.7% of cancers, respectively. Increased incidence was noted in the PG for nasopharyngeal cancer (5.16% vs. 0.79%, p < 0.0001) and breast cancer (3.14% vs. 1.68%, p = 0.04), compared to those in the NPG.
3.3. Association Between Pruritus and Mortality
In contrast to the association observed between cancer and pruritus, we identified a significantly lower all-cause mortality rate in the LPG and the SPG, than in the NPG (Table 2). Among the overall cohort during the 18-year follow-up period, the all-cause mortality rate was 27.08% in the LPG, 31.06% in the SPG, and 37.37% in the NPG (p = 0.0005).
Kaplan–Meier survival curves for the overall cohort (Figure 2) showed a significant difference between the PG and the NPG (p < 0.0001). A higher proportion of deaths in the NPG occurred within the first year after DM or PM diagnosis, compared to deaths in the PG. Among the overall cohort, the 1-year survival rate was 95% in the PG, compared to only 77% in the NPG. This disparity was particularly pronounced among patients with DM. The 1-year survival rate was 93% in the PG, compared to 68% in the NPG.
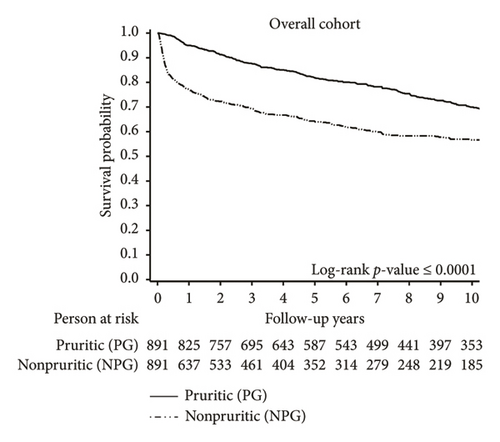
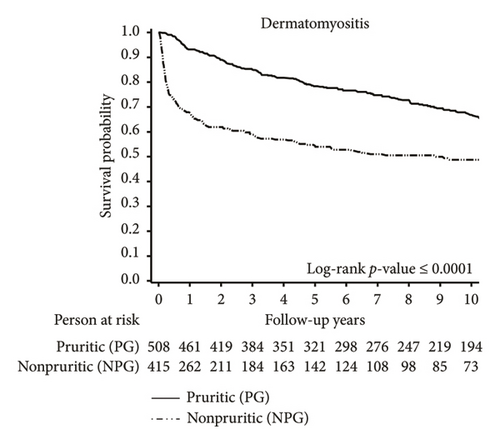
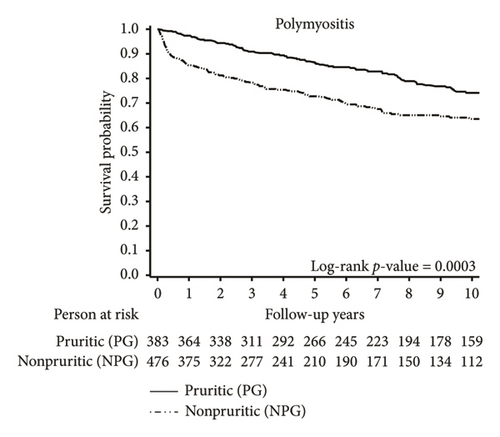
Further analysis of the causes of death in the study population revealed an elevated risk of cancer-related mortality in the LPG and SPG among patients with DM and PM. For the overall cohort, our findings revealed that 8.79% of patients in the LPG and 7.45% of patients in the SPG died from various types of cancers, whereas only 4.94% died in the NPG (p = 0.008). In contrast, when examining cancer-related deaths within the first year after diagnosis of DM and PM, a higher proportion was observed in the NPG compared to the PG (1.01% in PG vs. 2.36% in NPG; p = 0.03; Supporting Figure S1).
3.4. HR Analysis of Pruritus in DM and PM
To investigate the variables for patients’ survival, simple Cox proportional hazard models were utilized. The results supported our previous data that the presence of pruritus during the disease course was associated with a higher risk of newly diagnosed cancers for the overall cohort (Supporting Table S5; HR 1.633, 95% CI 1.196–2.231). Subgroup analysis revealed that long-term pruritus was significantly associated with cancer (HR 1.877, 95% CI 1.309–2.692), while short-term pruritus showed no significant association with cancer. Furthermore, older age of onset, including ages between 40 and 65 years, > 65 years, male sex, index year, diabetes mellitus, hypertension, vasculitis, and jaundice were significantly associated with newly diagnosed cancers.
The presence of pruritus, in contrast, was negatively associated with the mortality for the overall cohort (Supporting Table S6; HR 0.553, 95% CI 0.480–0.639). Subgroup analysis identified that both long-term and short-term pruritus were significantly associated with mortality (HR 0.534, 95% CI 0.441–0.647; HR 0.570, 95% CI 0.477–0.680, respectively). However, older age of onset, including an age between 40 and 65 years, > 65 years, male sex, diabetes mellitus, hypertension, ILD, and cancer were positively associated with mortality.
In the multivariable analysis for newly diagnosed cancer, adjustment was performed for significant confounding factors, including sex, age, index year, and comorbidities. In addition, ILD and cancer were considered in the analysis of mortality risk. The multivariate analysis (Table 3) indicated that pruritus was associated with an increased risk of developing cancer (HR 1.708, 95% CI 1.229–2.374), with a more pronounced effect for long-term pruritus (HR 1.867, 95% CI 1.284–2.714). Conversely, pruritus was associated with a lower risk of all-cause mortality (HR 0.483, 95% CI 0.409–0.569).
| Adjusted hazard ratio (95% confidence interval) | ||
|---|---|---|
| Newly diagnosed cancer | All-cause mortality | |
| Overall | ||
| Pruritus (yes vs. no) | 1.708 (1.229–2.374) | 0.483 (0.409–0.569) |
| Pruritus (vs. no) | ||
| SPG | 1.556 (1.059–2.286) | 0.498 (0.409–0.607) |
| LPG | 1.867 (1.284–2.714) | 0.465 (0.379–0.569) |
| Dermatomyositis | ||
| Pruritus (yes vs. no) | 1.888 (1.227–2.906) | 0.418 (0.335–0.523) |
| Pruritus (vs. no) | ||
| SPG | 1.587 (0.959–2.628) | 0.427 (0.327–0.558) |
| LPG | 2.154 (1.351–3.435) | 0.409 (0.315–0.531) |
| Polymyositis | ||
| Pruritus (yes vs. no) | 1.111 (0.632–1.953) | 0.497 (0.384–0.644) |
| Pruritus (vs. no) | ||
| SPG | 1.331 (0.702–2.525) | 0.548 (0.405–0.741) |
| LPG | 0.847 (0.396–1.809) | 0.432 (0.305–0.613) |
- Note: Data are presented as hazard ratio (95% confidence interval). Overall cohort: patients with dermatomyositis and polymyositis. LPG = Long-term pruritic group: receiving antipruritic agents for 84 consecutive days or more. SPG = Short-term pruritic group: receiving antipruritic agents for 42 consecutive days or more but less than 84 days. NPG = Nonpruritic group: receiving antipruritic agents for less than 42 consecutive days. Cancer model of patients adjusted for sex, age, index year, diabetes, hypertension, vasculitis, jaundice. Mortality model adjusted for sex, age, index year, diabetes, hypertension, interstitial lung disease, arthritis, and cancer. The bold values represent the results with statistical significance.
Sensitivity analyses confirmed the robustness of the results (Supporting Table S7). We consistently observed associations between pruritus and cancer, as well as pruritus and mortality, across various pruritus duration thresholds. Moreover, these associations remained significant even after excluding patients with conditions potenitally associated with pruritus, including jaundice, hypothyroidism, thyroid goiter, and hyperthyroidism.
4. Discussion
This study demonstrated the association between pruritus in patients with DM and PM, and their comorbidities and prognostic outcomes at a national level. Patients with DM and PM experiencing pruritus were found to have a higher risk of developing cancers but a lower risk of all-cause mortality, compared to nonpruritic patients. More specifically, the association between pruritus and cancer was evident in the DM subgroup but not in the PM subgroup. In contrast, the association between pruritus and increased mortality was observed in both DM and PM subgroups. The finding was further supported by the consistency of results across sensitivity analyses, including variations in pruritus duration thresholds and the exclusion of pruritus-associated comorbidities. More than half of our cohort experienced chronic pruritus, highlighting the need for ongoing and comprehensive screening for cancers in this high-risk patient population.
Assessing antihistamine usage may serve as a surrogate indicator for a patient’s pruritus in our study as previously reported [12]. In clinical practice, sedating antihistamines are commonly prescribed as first-line treatment for pruritus [22]. They alleviate pruritus by blocking H1 receptors on C-fiber nerve terminals [23], preventing mast cell degranulation [24], and providing a soporific effect. While not all pruritic diseases could be effectively controlled by antihistamines, most patients with chronic pruritus, regardless of its cause, will receive H1 antihistamines at some point to alleviate the itch [25, 26]. Antihistamines are favored for their proven efficacy in other pruritic dermatosis, such as chronic idiopathic urticaria [27, 28], atopic dermatitis, and psoriasis [29], as well as their affordability, accessibility, and minimal side effects. The cut-off point for antihistamine usage was set at 6 weeks in our study, as chronic pruritus is defined by the International Forum for the Study of Itch for itching that persists for over 6 weeks [30]. Short-term antihistamine uses for other conditions such as allergic reactions were excluded.
Using multivariable Cox proportional hazard models, we found that DM and PM patients experiencing pruritus exhibited a significantly higher risk of newly diagnosed cancer (Table 3; adjusted HR 1.708, 95% CI 1.229–2.374). Previous studies showed that DM patients with pruritus might be predisposed to internal malignancies [15–17], with one contradictory study of 63 patients in Germany [18]. However, because of limited sample sizes in earlier studies, their findings regarding the association between pruritus and cancer appeared inconclusive or underpowered. Our findings, in contrast, provided robust evidence supporting the notion that DM and PM patients with pruritus exhibited an elevated risk of cancer.
The multivariable Cox proportional hazard models also identified pruritus as a protective factor against all-cause mortality in patients with DM and PM (Table 3; adjusted HR 0.483, 95% CI 0.409–0.569). Prior studies showed cancer, ILD, infection, and cardiac involvement as predominant causes of death in DM and PM patients [31–35]. The most common cancer associated with DM in East Asia is nasopharyngeal carcinoma; however, it is less associated with immediate patient mortality. In contrast, ILD, particularly rapidly progressive ILD, is associated with high mortality rates within the first year of diagnosis [36, 37], thus frequently necessitating aggressive immunosuppressive therapy [38–40]. In fact, further analysis in the causes of death revealed a higher incidence of deaths from infection in the NPG within the first year of DM or PM diagnosis. This supports the concept that nonpruritic patients may require higher doses of steroids and immunosuppressants to manage their disease.
The survival curve indicated a marked decline in survival during the first year in the NPG, a pattern not observed in the PG. Previous studies illustrated that lung complications were the major causes of death within the first 12 months, following DM and PM diagnosis [32, 41]. This disparity in survival between the PG and NPG was even more pronounced among DM patients. It is well-documented that DM is associated with a poorer prognosis compared to PM, particularly concerning ILD [42] and cancer-related mortality [41, 43]. Although no significant difference in ILD prevalence was observed between the PG and NPG, the clinical course of ILD may vary considerably. Rapidly progressive ILD is associated with significantly poorer prognosis, compared to chronic ILD. This form of ILD is more prevalent in DM than in other connective tissue diseases [37] and has been extensively reported in Asia, including Japan, Hong Kong, and Taiwan [34, 38, 39].
Several hypotheses may explain the paradoxical finding of higher cancer risk but lower all-cause mortality rates in the PG. First, disease severity in the NPG might be higher than the PG and require a higher dosage of immunosuppressants. We observed that patients in the PG received a significantly lower average dose of corticosteroids during the treatment course compared to those in the NPG (6.2 vs. 12.7 mg/day of prednisolone equivalent; p < 0.0001; Supporting Table S8). In addition, a greater number of patients in the NPG died from infection within the first year following the diagnosis of DM or PM (11 in PG vs. 32 in NPG; p = 0.0008). Secondly, the NPG may include a higher proportion of patients with overlap syndrome. Previous studies have classified DM into two subtypes: classic/pure DM and overlap myositis with DM features (OMDM). Pure DM is typically associated with an elevated risk of malignancy but demonstrates an excellent 15-year survival rate of approximately 92%. In contrast, patients with OMDM often develop myositis preceding the appearance of cutaneous manifestations and have a reduced 15-year survival rate of about 65% [44]. Thirdly, there may be a potential lead-time bias, whereby patients experiencing pruritus are more likely to seek medical attention earlier than those without pruritus. Consistent with this, patients in the PG were more frequently diagnosed with cancer at an earlier stage compared to those in the NPG (stage 0–2: 46.22% in PG vs. 32.76% in NPG; stage 3-4: 47.06% in PG vs. 53.45% in NPG; missing data: 6.72% vs. 13.79%, respectively; p = 0.04). However, given that cancer cases constituted only a small proportion of the overall cohort, this factor alone is unlikely to account for the observed difference in mortality between the two groups. Lastly, distinct myositis-specific and myositis-associated autoantibodies might be involved in the pathogenesis of pruritic and nonpruritic patients. Several autoantibodies may be the candidates for pruritic and nonpruritic conditions. Anti-transcriptional intermediary factor 1 γ (anti-TIF1γ) antibodies and anti-small ubiquitin-like modifier-1 activating enzyme (anti-SAE) antibodies are often associated with extensive skin involvement and a higher risk of cancer [45–49]. Conversely, patients with anti-melanoma differentiation-associated protein 5 (anti-MDA5) antibodies and anti-signal recognition particle (anti-SRP) antibodies typically present with less pruritic skin lesions, and a poor prognosis, despite having a lower risk of cancer [45, 50, 51]. Further investigation is warranted to prove our hypotheses.
This study has several limitations. First, because our analysis relied on data from an administrative database, some potential ascertainment problems were unavoidable, including coding errors and mortality misclassifications. However, differences in coding error between the PG and NPG are unlikely. In addition, our study benefited from the stringent regulation of Taiwan’s Registry of Catastrophic Illness Database, which requires DM or PM cases to meet the criteria set by Bohan and Peter [19, 20]. Secondly, although assessing pruritus through prescription medication is an objective and quantitative approach, it inevitably underestimates patients with mild pruritus who did not require a prescription, those who discontinued antipruritic medication because of nonresponsiveness, and those who used over-the-counter medications or traditional Chinese herbal treatments for pruritus. Thirdly, we were unable to analyze clinical parameters such as cutaneous manifestations other than pruritus, laboratory parameters, and myositis-associated or specific antibodies because of the inherent limitations of the NHIRD. Fourthly, while overlap syndromes with other autoimmune diseases are known to complicate DM/PM, we were unable to analyze them thoroughly using the Registry of Catastrophic Illness Database. This limitation arose because only the DM/PM criteria were strictly followed while criteria for other autoimmune diseases may not have been as accurately applied. Finally, given that the population data were derived from a single country (Taiwan), further research is required to assess the generalizability and reproducibility of these findings in other populations.
5. Conclusion
In conclusion, this study employed nationwide insurance administration data and revealed a significant association between pruritus and cancer, as well as cancer-related mortality, in patients with DM and PM. Thus, cancer screening, particularly for nasopharyngeal and breast cancers in East Asian populations, is recommended for patients with DM or PM, especially those presenting with pruritus. Notably, nonpruritic patients were associated with a higher risk of all-cause mortality, especially during the first year after diagnosis. Therefore, patients without pruritus may require vigilant management for potentially life-threatening complications and comorbidities.
Nomenclature
-
- DM:
-
- Dermatomyositis
-
- PM:
-
- Polymyositis
-
- ILD:
-
- Interstitial lung disease
-
- NHIRD:
-
- National Health Insurance Research Database
-
- ICD-9:
-
- International Classification of Diseases, ninth revision
-
- ICD-10:
-
- International Classification of Diseases, tenth revision
-
- HR:
-
- Hazard ratio
-
- CI:
-
- Confidence interval
-
- PG:
-
- Pruritic group
-
- LPG:
-
- Long-term pruritic group
-
- SPG:
-
- Short-term pruritic group
-
- NPG:
-
- Nonpruritic group.
Ethics Statement
The study was approved by the Joint Institutional Review Board of Taipei Medical University (N202403054). The requirement for informed consent was waived by the Institutional Review Board because the dataset was deidentified.
Consent
Please see the Ethics Statement.
Disclosure
The abstract of this study was presented at EADV Congress 2024 at Amsterdam on 26 September 2024. A preprint of this study is available at doi.org/10.1101/2024.09.26.24314441.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Der-Jr Huang conceptualized the study, contributed to the study design and interpretation of the results, and drafted the manuscript. Yu-Hsuan Joni Shao contributed to the study design, data extraction and analysis, and reviewed the manuscript. Yi-Hsien Shih and Woan-Ruoh Lee contributed to the study design and reviewed the manuscript. Ling-Ya Huang contributed to the study design, data analysis, interpretation of the results, and reviewed the manuscript. Yu-Min Kuo and Quoc Thao Trang Pham contributed to the interpretation of the results and reviewed the manuscript. Hao-Jui Weng conceptualized the study, contributed to the study design and interpretation of the results, and reviewed the manuscript. All authors had access to the final study results and accepted responsibility for submitting them for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Hao-Jui Weng is the guarantor.
Funding
This work was supported by Taipei Medical University-Shuang Ho Hospital, Ministry of Health and Welfare (112YSR-02) and National Science and Technology Council, Taiwan (110-2314-B-038-028-MY3).
Acknowledgments
We followed the methods of Huang et al. [52]. We acknowledge the statistical support of the Health Data Analytics and Statistics Center, Office of Data Science, Taipei Medical University, Taiwan. We also acknowledge the support from the Health and Welfare Data Science Center (HWDC).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Data are available from the Health and Welfare Data Center published by the Ministry of Health and Welfare. Because of legal restrictions imposed by the Government of Taiwan in relation to the Personal Information Protection Act, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the Health and Welfare Data Center Administration (https://dep.mohw.gov.tw/DOS/cp-5119-59201-113.html).




