Wildlife as a Sentinel for Pathogen Introduction in Nonendemic Areas: First Detection of Leishmania tropica in Wildlife in Spain
Abstract
Leishmaniasis is a chronic global arthropod-borne zoonotic disease produced by several species of Leishmania with cutaneous, mucocutaneous, and visceral clinical manifestations. In Spain, only Leishmania infantum has been reported so far, although other species of Leishmania, such as L. tropica and L. major, are present in surrounding countries. The aim of this work is to analyze the occurrence of Leishmania spp. infection in European wildcats (Felis silvestris) as sentinels, including their genotypic characterization. Necropsies of 18 road-killed wildcats were conducted. Samples of ear skin and spleen were taken for DNA isolation and PCR of the highly sensitive SSU-rDNA target. Subsequent PCR tests were performed using more specific targets for the determination of Leishmania species: hsp70 and ITS1. Positive samples were sequenced, and phylogenetic trees were constructed. Seven wildcats were found positive for Leishmania spp. Based on the hsp70 and ITS1 sequences, an animal was found to be infected only with L. tropica in ear skin samples, while two cats were found to be infected with L. infantum in both the ear skin and the spleen. In one animal, a clear sequence of L. infantum ITS1 and a sequence of L. tropica hsp70 were obtained from the ear skin. Since hsp70 and ITS1 sequencing was not possible in three cats, the species of Leishmania infecting them was not determined. This is the first report of autochthonous infection with L. tropica in the Iberian Peninsula. Health care professionals, including physicians, dermatologists, and veterinarians, must be aware of this for a correct diagnosis, treatment, and management of possible coinfections.
1. Introduction
Leishmaniasis is a global zoonotic disease transmitted by the bite of phlebotomine insects, with more than 1,000,000 new cases of the cutaneous form and 30,000 new cases of the visceral form estimated every year, in 92 endemic countries [1]. The clinical presentation in humans is variable and includes cutaneous (CL), mucocutaneous (MCL), visceral (VL), and post kala-azar dermal leishmaniasis (PKDL). The disease is caused by several species of Leishmania, with the L. donovani complex, the L. major complex, and the L. tropica complex being the most frequent in Africa, Asia, southern Europe, South America, and the Middle East, while the L. mexicana complex, the L. guyanensis complex, and the L. braziliensis complex are the most diagnosed in the America. The CL form of the disease is the most common and is responsible for the stigmatization and social isolation of thousands of people, particularly women, while VL is usually lethal without treatment and is caused by L. donovani in Africa and Europe, and L. infantum in southern Europe, west and central Asia, and the Americas. MCL is characterized by the destruction of mucous tissue of the nose, palate, and pharynx and can severely affect the daily life of thousands of people [2, 3]. Human leishmaniasis is more prevalent in low-income countries with displaced populations due to war, social, or economic conflicts and is classified by the WHO as a neglected tropical disease (NTD). This scenario makes early diagnosis and treatment of the disease, as well as the introduction of preventive measures, difficult [3].
In Europe, VL is caused by L. infantum, with humans and dogs as the target species. The infection is more prevalent in southern countries such as Portugal, Spain, Italy, and Greece. Dogs are heavily affected by this zoonotic species [4] and are considered the main reservoir. Human infections mainly affect immunocompromised patients (such as HIV+ or recipients of solid organ transplants) as well as occasional reports in elderly and children [5]. Several studies have stressed the paramount importance of wildlife in maintaining Leishmania infection [6, 7]. Wildcats (Felis silvestris) are susceptible to the same pathogens as domestic cats and therefore could be potential reservoirs for the infection. However, the studies conducted have been scarce [8, 9, 10] with a limited number of animals analyzed in each study (n = 4, n = 3, n = 4, respectively). The three studies identified L. infantum using a small fragment of kDNA or qPCR from the internal transcribed spacers-5.8 ribosomal DNA 2 (ITS2) regions.
Despite suitable bioclimatic conditions and vectors available for anthroponotic L. tropica (Phlebotomus sergenti) and L. major (P. papatasi) [11, 12], only L. infantum has been reported in the Iberian Peninsula [6, 12, 13]. However, L. major has recently been reported in a cat from Lisbon, Portugal [14]. Moreover, it is worth noting that L. tropica has been previously reported in Greece [15] and the three species (L. infantum, L. tropica, and L. major) are present in Morocco [16] as well as in other countries bordering Europe [17]. The availability of a larger number of wildcats allowed us to identify the presence of Leishmania in various tissues and genetically characterize the samples. The aim of this work is to analyze the occurrence of Leishmania spp. infection in wildcats as sentinels, including their genotypic characterization.
2. Materials and Methods
2.1. Animals, Samples, and Geographic Area
In this study, samples of 18 European wildcats (F. silvestris) that were killed on the road during 2022 and 2023 in the Spanish provinces of Ávila, Burgos, Ciudad Real, Guadalajara, Soria, and Valladolid, were analyzed. Samples from the skin and spleen were selected, since they are the preferred sites for CL and/or VL Leishmania amastigotes. All road-killed wildcats were included in the study period. Animals were transported to the wildlife recovery centers of Castilla y León (Burgos, Valladolid) and Castilla-La Mancha (Ciudad Real, Guadalajara), systematic necropsies were performed, and the presence/absence of lesions annotated. A portion of the ear skin and spleen was taken and frozen at −20°C for further processing. The dates, age, sex, and location (geographic coordinates where the dead animals were found) were included in the database.
2.2. Maps
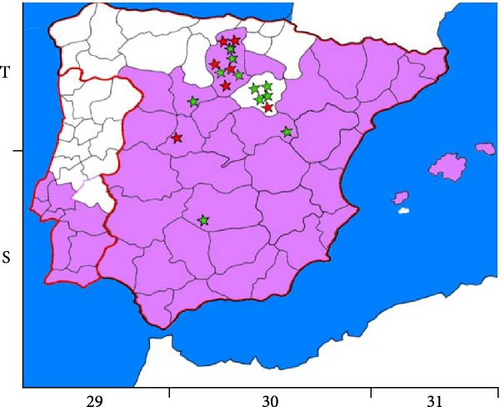
The map shown in Figure 1 was created taking as reference the European Centre for Disease Prevention and Control (ECDC) maps of distribution in Europe of P. sergenti, the main vector of L. tropica, in 2022 and 2023 [18] and the Universal Transverse Mercato (U.T.M.) geographic coordinates of the closest villages to where the wildcats analyzed in this study were found. The AutoCAD program (Autodesk® Inc., Landmark, San Francisco, CA, USA) was used to observe the overlaps between the two maps.
2.3. DNA Isolation
DNA was isolated from the spleen (10 mg) and the skin of the ears (20 mg) using the NZYTech Tissue gDNA isolation kit (NZYTech, Portugal) and following the manufacturer’s instructions. DNA elution was performed in 60 µL of elution buffer instead of 100 µL to increase DNA concentration. The positive and negative controls were used in each batch of the experiment. The positive control consisted of samples derived from L. major (clone V1: MHOM/IL/80/Friedlin) or L. braziliensis (MHOM/BR/75/M2904) promastigotes in culture (1 × 106 promastigotes), while the negative control consisted of water free of DNase. The DNA content was measured on a Multiskan GO microplate spectrophotometer with µDrop™ plates (Thermo Fisher Scientific, MA, USA). DNA from cultures of L. major and L. braziliensis was used as internal controls for sequencing also. These two isolates were chosen because they were absent from Spain.
2.4. PCR of Small Subunit Ribosomal RNA (SSU-rDNA), Internal Transcribed Spacers-5.8 Ribosomal DNA 1 (ITS1), and Heat Shock Protein 70 (hsp70) Targets
DNA samples from the skin of the ear and spleen of the 18 cats were used for Leishmania DNA detection. DNA from different species of Leishmania was also used as an internal control for PCR and sequencing: L. major (clone V1: MHOM/IL/80/Friedlin) and L. braziliensis (MHOM/BR/75/M2904). Only samples with good DNA quality (absorbance ratio at 260/280 nm > 1.7) were used for all PCRs. All PCR conditions have been previously described and adapted to the working conditions recommended by the enzyme manufacturer (Supreme NZYTaq II 2x Master Mix, NZYTech, Lisbon, Portugal).
The SSU-rDNA target was chosen as the first PCR because of its higher sensitivity compared to other PCR targets. Nested PCRs (nPCR) to amplify a 358 bp fragment of the SSU-rDNA target were performed using primers R221 (5′-GGTTCCTTTCCTGATTTACG-3′) and R332 (5′-GGCCGGTAAAGGCCGAATAG-3′) for the outer section and primers R223 (5′-TCCATCGCAACCTCGGTT-3′) and R333 (5′-AAGCGGGCGCGGTGCTG-3′) for the inner section [19], following previously described conditions [20].
Primers L5.8S (5′-ACACTCAGGTCTGTAAAC-3′) and LITSR (5′-CTGGATCATTTTCCGATG-3′) were used for the outer section of the nPCR for ITS1 region and primers SAC (5′-CATTTTCCGATGATTACACC-3′) and VAN2 (5′-CGTTCTTCAACGAAATAGG-3′) were used for the inner section to amplify a fragment of 280–330 bp, which allows species determination, as previously described [20, 21].
The nPCR used for hsp70 amplified a fragment of 741 bp and was based on the primers and methods previously described for phylogenetic purposes [14, 22]. Briefly, the external primers HSP70-F25 (5′-GGACGCCGGCACGATTKCT-3′) and HSP70-R1310 (5′-CCTGGTTGTTGTTCAGCCACTC-3′) were used at 0.8 µM each, and the internal primers HSPF (5′-GACAACCGCCTCGTCACGTTC-3′) and HSPR (5′-GTCGAACGTCACCTCGATCTGC-3′) were used at 0.4 µM each. In the first PCR, 40 cycles of 94°C for 40 s, 61°C for 60 s, and 72°C for 120 s were used, while in the second PCR, 40 cycles of 94°C for 40 s, 61°C for 60 s, and 72°C for 60 s were used.
All PCR reactions were performed in 25 µL using 12.5 µL of Supreme NZYTaq II 2x Master Mix. An initial step of 95°C for 5 min to activate the enzyme and a final elongation step of 72°C for 10 min to allow the elongation of the PCR products were used in all PCR reactions. In the first PCR, 5 µL of DNA was used, while in the second PCR reactions, 5 µL of a 1 : 50 dilution of each first PCR product were used for hsp70, while a 1 : 40 dilution of the first PCR product was used for SSU-rDNA and ITS1. The results of nPCR were visualized on 1% agarose gels stained with SYBR™ safe DNA gel staining (Invitrogen, Thermo Fisher Scientific, MA, USA) under UV light.
2.5. Sequencing and Alignment
Samples containing the amplicons of the expected size were sent to the facilities of Macrogen Spain (Madrid, Spain) for Sanger sequencing in forward and reverse directions. The resulting sequences were aligned using Molecular Evolutionary Genetics Analysis v.11 software (MEGA XI) [23] and FinchTV 1.4.0 software (Geospiza, Inc.; Seattle, WA, USA), manually checked, and subjected to BLAST analysis using the nucleotide Basic Local Alignment Search Tool (BLAST; National Library of Medicine, Rockville, MD, USA). Only sequences with more than 150 bp that were clear in both directions were sent to GenBank for accession numbers. PCR and sequencing were repeated at least twice for each sample to ensure results.
2.6. Phylogenetic Trees
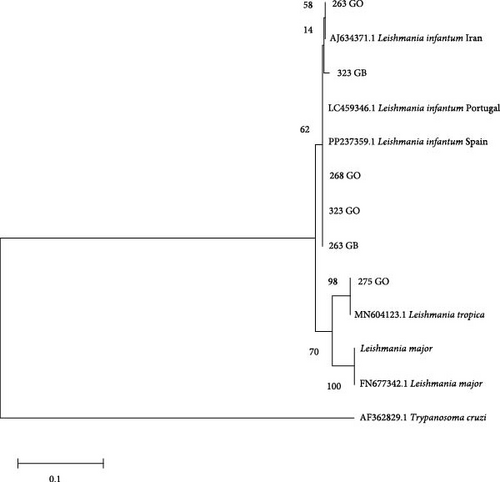
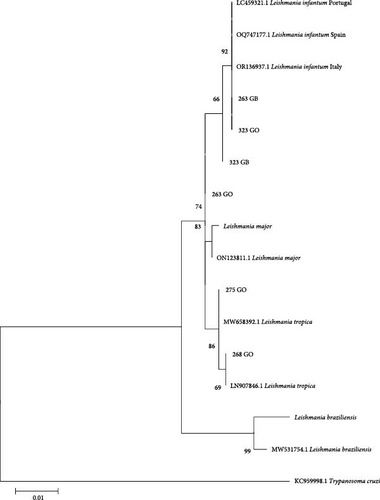
Phylogenetic trees were constructed using the sequences obtained in the present study and other sequences available from GenBank. Only the trees for hsp70 and ITS1 are shown because the SSU-rDNA target is a conserved region and only a difference of 1–2 bp was observed between the sequences obtained. In all trees, a sequence from Trypanosoma cruzi was included as an outgroup reference. Additionally, other sequences of Leishmania spp. from GenBank and sequences from L. infantum and the species of Leishmania mentioned above were included from cultures to verify similarity (L. major and L. braziliensis). The sequences had a minimum length of 292 nucleotides for the ITS1 region and 635 nucleotides for hsp70. Evolutionary history was inferred using the maximum likelihood method based on the Tamura–Nei model [24]. The tree with the highest logarithmic likelihood was displayed. The percentage of trees in which the associated taxa clustered together was shown next to the branches. Initial trees for the heuristic search were obtained automatically by applying the neighbor-joining and BioNJ algorithms to a pairwise distance matrix estimated using the maximum composite likelihood (MCL) approach and selecting the topology with the superior log-likelihood value. The trees were drawn to scale, and the lengths of the branches were measured on the basis of the number of substitutions per site. The analysis included 13–18 nucleotide sequences depending on the tree. The included codon positions were 1st + 2nd + 3rd + noncoding. All positions that contained gaps and missing data were eliminated. Evolutionary analyzes were conducted via MEGA XI [23]. A bootstrap of 2,000 replicates was used to assess the reliability of the trees.
2.7. Statistical Analysis
A descriptive statistical analysis with absolute (n) and relative frequency of infection (%) was carried out, with a confidence level of 95%, using the free online tool WinEpi (Working in Epidemiology) and considering that the diagnostic techniques used were perfect (nPCR of the SSUrDNA). Odds ratio analysis was used to analyze the risk factors “sex” and “Leishmania infection” [25].
3. Results
3.1. Detection and Identification of Leishmania spp. in Wildcats
In this study, samples of the ear skin and spleen of 18 wildcats were analyzed, except for the spleen of two animals that were in poor condition when the cats were found (Table 1). None of the animals showed lesions comparable to those produced by Leishmania infections. Taking into account all animals positive for Leishmania PCR, 38.89% (16.37%–61.41% and 95% confidence interval) were found to be infected. A nonsignificant higher proportion of infection was found in males (OR = 27.17, 95% CI: 0.33, 2213.63), since only males were found infected, but we must consider that we obtained a smaller number of samples from females (n = 3/18).
| Sample | Age | Sex | Date | U.T.M. geographic coordinates | Leishmania infection |
|---|---|---|---|---|---|
| 262 | Adult | M | 20-04-2022 | 30T 439302, 4683502 | + |
| 263 | Adult | M | 21-04-2022 | 30T 308223, 4488924 | + |
| 268 | Adult | M | 22-06-2022 | 30T 438633, 4729501 | + |
| 272 | Adult | M | 13-07-2022 | 30T 370885, 4584961 | − |
| 275 | Adult | M | 15-07-2022 | 30T 427252, 4615635 | + |
| 276 | Adult | M | 15-07-2022 | 30T 510221, 4613053 | − |
| 310 | Adult | M | 21-12-2022 | 30T 538981, 4592797 | − |
| 317 | Adult | M | 24-02-2023 | 30T 455150, 4702613 | − |
| 318 ∗ | Adult | M | 13-03-2023 | 30T 397133, 4707688 | + |
| 319 | Adult | F | 13-03-2023 | 30T 464371, 4722997 | − |
| 320 | Adult | M | 23-03-2023 | 30T 548263, 4605806 | − |
| 323 | Adult | M | 28-04-2023 | 30T 544058, 4577807 | + |
| 365 | Adult | M | 08-09-2023 | 30T 480564, 4725184 | + |
| 366 | Adult | M | 08-09-2023 | 30T 553424, 4621448 | − |
| 367 | Adult | F | 08-09-2023 | 30T 457990, 4653201 | − |
| 375 | Adult | M | 05-10-2023 | 30S 386031, 4251590 | − |
| 376 | Adult | F | 05-10-2023 | 30T 401098, 4678137 | − |
| 378 ∗ | Adult | nd | 05-10-2023 | 30T 592304, 4494125 | − |
- ∗Spleen not analyzed. M, male; F, female; nd, not determined.
All Leishmania spp. infected animals were found in areas where P. sergenti, the main vector of L. tropica was identified, except for one animal in which L. infantum was determined in both ear skin and spleen (Figure 1).
Seven of the 18 animals were found to be positive by Leishmania PCR for SSU-rDNA. All were positive on the skin of the ears, and only two were also positive in the spleen samples (Table 2).
| Sample | Ear skin | Spleen | ||||
|---|---|---|---|---|---|---|
| SSU-rDNA | ITS1 | hsp70 | SSU-rDNA | ITS1 | hsp70 | |
| 262 | + | − | − | − | − | − |
| 263 | + | +2 | +4 | + | +2 | +4 |
| 268 | + | +2 | +3 | − | − | − |
| 275 | + | +1 | +3 | − | − | − |
| 318 | + | − | − | nd | nd | nd |
| 323 | + | +2 | +4 | + | +2 | +4 |
| 365 | + | − | − | − | − | − |
- Sequence identities are identified with super index numbers (1–4). nd, not determined; 1, ITS1 sequence 100% identity with L. tropica; 2, ITS1 sequence 100% identity with L. infantum; 3, hsp70 sequence 99.86%–100% identity with L. tropica; and 4, hsp70 sequence 99.24%–100% identity with L. infantum.
3.2. Sequences and GenBank Accession Numbers
The nucleotide sequences obtained in this study were deposited in the GenBank database under accession numbers PP389513-PP389521 for SSU-rDNA, PP177368-PP177373 for ITS1, and PP397157-PP397162 for hsp70. Sequences from L. major and L. braziliensis isolates were deposited in the GenBank database with accession numbers PP389522, PP389523, and PP389533.
The sequences obtained from SSU-rDNA had 295–327 bp and differ by only one or two nucleotide positions. Nine sequences were obtained from the animals analyzed, seven from the skin of the ears, and two from the spleen. All of them displayed 100% query coverage and 99.69%–100% identity with other Leishmania spp. sequences available at GenBank, including L. infantum and L. tropica, among other species (accession numbers MN757921and KF302745).
ITS1 sequences of 236–247 bp were obtained from the ear skin of four animals (263, 268, 275, and 323), two of which (263 and 323) also gave sequences when DNA from the spleen was used (Table 2). Three sequences displayed 100% query coverage and 100% identity with sequences from L. infantum (accession number MT416168, samples from ear skin of animals 263, 268, and 323, and samples from spleen of animals 263 and 323), while one sequence (from wildcat 275 ear skin) displayed 100% query coverage and 100% identity with sequences from L. tropica (accession number MH595857, isolate R24, Iran).
Sequences obtained from hsp70 amplification yielded four sequences from the ear skin and two sequences from the spleen with 635–711 bp. Of these, four sequences showed 100% coverage and 99.24%–100% identity with L. infantum (accession number OR136937, strain MHOM/IT/99/ISS1898). Sequences 275 from the ear skin showed 100% coverage and 100% identity with L. tropica (accession number MK335938, voucher ISS3183), while sequence obtained from the ear skin of 268 showed 100% coverage and 99.86 identity with L. tropica (acc. n LN907846, strain MHOM/IL/80/SINGER and others) and 99.72% identity with L. donovani (accession numbers MH202961 and JX021427; Table 2). Double peaks were observed at three and five positions of the sequences of the spleen of animal 323 and the skin of the ears of animal 263, respectively, indicating mixed sequences.
It is noteworthy to point out that Leishmania DNA was not detected by none of the three PCR methods in the spleens of cats with cutaneous infection with L. tropica. In one animal (275), the sequences from the ITS1 and hsp70 targets showed 100% identity with L. tropica. Two animals displayed sequences compatible with L. infantum on the skin of the ears and the spleen (263 and 323), and another wildcat was positive only on the skin of the ears with sequences compatible with L. infantum and L. tropica (268). Finally, in three animals (262, 318, and 365), the sequences could only be assigned to the Leishmania genus because they were only positive for SSU-rDNA (Table 2).
3.3. Phylogenetic Trees
Phylogenetic analysis of the sequences shows strong support for the clusters found with the two targets, in agreement with the percentage of identity of the sequences obtained. ITS1 sequences from this study were grouped with L. infantum (samples 263, 268, and 323 from the skin of the ears and samples 263 and 323 from the spleen) or L. tropica (sample 275; Figure 2).
In the phylogenetic tree generated with the hsp70 sequences obtained in the present study, two of them (samples 268 and 275 from the ear skin) clustered with L. tropica and three of them (samples 263 and 323 from the ear skin and 263 spleen) clustered with L. infantum sequences recovered from GenBank (Figure 3). One of the L. tropica sequences was obtained from the same animal (275) that rendered the L. tropica ITS1 sequence, while the other L. tropica hsp70 sequence was obtained from an animal (268) that rendered the L. infantum ITS1 sequence.
4. Discussion
In the present study, seven out of 18 wildcats (38.89%) were found to be positive for Leishmania infection, a value consistent with those found in previous studies [8, 10] although lower than the infection prevalence reported by Oleaga et al. [9] with all three wildcats tested being positive for Leishmania. L. infantum is endemic in the Iberian Peninsula [12, 13], but no infections of L. tropica in autochthonous species have been reported. Even considering the limitations of the study, including the low parasitic loads commonly found in wildlife and the number of animals, the information is of value, taking into account that the population of wildcats in Spain is considered relict and they are animals difficult to see unless they are sick or death, which is the case [26].
The risk of introduction of L. tropica in the Iberian Peninsula has been highlighted several times, considering the presence and distribution of the main vector, P. sergenti, and the identification in Spain of one genetic line of P. sergenti commonly found in Morocco [27, 28] where CL by L. tropica is endemic [21, 29, 30]. Large areas in Europe are considered suitable for L. tropica [11], and hot spots for foci of L. tropica have been suggested [27]. In addition, the possibility of L. tropica transmission by P. perniciosus has been pointed out [31, 32].
The most common targets used for the detection of Leishmania spp. infection are SSU-rDNA and kDNA with similar values of sensitivity [6]. However, other targets are useful for phylogenetic studies and determination of Leishmania spp., such as ITS1, cytB, g6pdh, and hsp70 [14, 20, 21, 22, 29, 33, 34]. In the present study, ITS1 and hsp70 have been used to confirm the finding of L. tropica in autochthonous fauna, and sequences of L. tropica with ITS1 and hsp70 targets are reported. L. tropica sequences were detected in at least two wildcats positive for PCR with targets hsp70 and/or ITS1, widely used for phylogenetic studies. Our finding extends the reported presence of the species in Greece [15] and extends its distribution to the Iberian Peninsula for the first time.
The isolation of alive Leishmania was not feasible considering the source of material, but it is also noteworthy that in our study the skin samples of the ears showed more positive results than the spleen samples (n = 7/18 vs. n = 2/16). This finding is consistent with the fact that L. tropica infections are primarily associated with clinical forms of skin in humans, where the persistence of the parasite is close to the site of natural infections transmitted by the sandfly, although the parasite can also be found less frequently associated with VL, mainly in immune suppressed people [35]. Imported CL cases from L. tropica have been sporadically reported in Spain after travelling to Morocco [36], but no previous autochthonous cases have been reported in Spain or Portugal. Apparently, L. tropica is expanding its distribution, and the main risk factor for the appearance of the disease is the presence of the vector [37]. It should be noted that some of the wildcats came from latitudes over 42° N (e.g., animal 365, geographic coordinates U.T.M. 30T 480564, 4725184) and during the last decade, P. sergenti has expanded to the northern areas of the Iberian Peninsula, according to ECDC maps of distribution [18].
In endemic areas, such as Israel, domestic cats appear to be more susceptible to infection with L. tropica than dogs [38]. A study of stray cats in Turkey showed that all samples with ITS1 amplification were infected with L. tropica, while only one was coinfected with L. infantum [39]. Similarly, in the present study, while one animal showed infection by L. tropica only, another wildcat showed hsp70 and ITS1 sequences consistent with coinfection by L. infantum and L. tropica or by a hybrid of L. infantum and L. tropica. Mixed infections with more than one Leishmania species have also been reported in other endemic areas, hosts, and Leishmania species, such as in Brazil (L. infantum and L. braziliensis) in one dog [40] and 8/30 dogs [41], rodents (10% of L. infantum and L. braziliensis) [42], hedgehogs (L. major and L. infantum) in 8/12 animals and another coinfected with L. tropica [43], and human clinical cases [44, 45] of the new world. The possibility of hybridization between Leishmania species cannot be ruled out, as L. tropica shows a higher rate of natural hybrid formation than other species [46]. Indeed, at least one natural hybrid of L. donovani and L. aethiopica has been described in Ethiopia employing ITS1, hsp70, and cysteine proteinase B (cpb) targets [47]. The authors suggested that hybridization could take place in a vector permissive to both species, as it has been described in P. pernicious for L. infantum and L. tropica [31, 32]. One hybrid of L. infantum and L. major was also described in a natural infection in Portugal [48]. However, to further investigate the existence of hybrids, isolates from infected animals are necessary [49, 50], and in our study, they could not be obtained [51].
5. Conclusions
This is the first report of L. tropica infection in autochthonous wildlife in the Iberian Peninsula, extending the presence of the species in continental Europe. The main vector of L. tropica has been identified in Spain during the last decade, and its distribution has increased northward each year. There are no conclusive data on the zoonotic transmission of L. tropica, and the actual spread of the infection to other species is not yet known and should be investigated. Our results support the sentinel value of wild species in the detection of previously unreported infections. In addition, the presence of L. tropica in Spain should be communicated to health professionals, including physicians, dermatologists, and veterinarians, considering that L. tropica can produce both CL and VL in humans and pets. Awareness of the possibility of L. tropica infections and L. infantum/L. tropica coinfections is necessary to ensure the appropriate diagnosis and management of clinical cases.
Ethical Approval
The use of samples for parasitological investigations was approved by regional authorisation of the Junta de Castilla y León, reference: “AB/is”, File “AUES/CYL/001/2021,” and of Castilla-La Mancha, reference: DGPFEN/SEN/avp_21_103_bis.
Disclosure
A preprint version of the manuscript with doi https://www.biorxiv.org/content/10.1101/2024.03.16.585353v2 has been published on bioRxiv [51].
Conflicts of Interest
The authors declare that there is no conflicts of interest with respect to the publication of this article.
Authors’ Contributions
Marta Mateo Mateo Barrientos, Iris Azami-Conesa, and María Teresa Gómez-Muñoz contributed to conceptualization. Iris Azami-Conesa, Paula Pérez-Moreno, and María Teresa Gómez-Muñoz contributed to methodology and formal analysis. Marta Mateo Barrientos, Pablo Matas Méndez, and Javier Carrión contributed to collection of data and biological samples. Iris Azami-Conesa and María Teresa Gómez-Muñoz contributed to writing the original draft. Pablo Matas Méndez, Javier Carrión, Iris Azami-Conesa, Marta Mateo Barrientos, María Teresa Gómez-Muñoz, and José María Alunda contributed to interpretation of data. Marta Mateo Barrientos and María Teresa Gómez-Muñoz contributed to supervision. José María Alunda, Marta Mateo Barrientos, and María Teresa Gómez-Muñoz contributed to resources. All authors critically reviewed the manuscript, intellectually contributed to the content, and approved the final version. Marta Mateo Barrientos and María Teresa Gómez-Muñoz contributed equally to this work and share last authorship.
Acknowledgments
We deeply thank the cooperation of Junta de Castilla y León, Consejería de Fomento y Medio Ambiente, Dirección General de Patrimonio Natural y Política, and Castilla-La Mancha, Consejería de Desarrollo Sostenible, Dirección General de Medio Natural y Biodiversidad for permits to take samples of dead wildlife of the area. The research was partially funded by the ICPVet research group of UCM (grant GRFN17/21).
Open Research
Data Availability
Sequences obtained in the present study have been deposited in the GenBank database under accession numbers: PP389513-PP389521 for SSU-rDNA, PP177368-PP177373 for ITS1, and PP397157-PP397162 for hsp70. The sequences of the isolates of L. major and L. braziliensis are PP389522, PP389523, and PP389533.




