Chlorogenic Acid Mitigates Ferroptosis by Activating the Nrf2/GPX4 Pathway through Keap1 Blockade in Vascular Dementia Rats
Abstract
Chlorogenic acid (CGA) is a dietary phenolic acid widely distributed in daily food and plants, but its role in vascular dementia (VaD) is still unclear. Hence, this study aimed to investigate whether CGA could rescue cognitive impairment in VaD, providing a new option for drug discovery. Novel object recognition and Morris water maze experiments revealed that CGA enhanced the learning and memory abilities in VaD rats. Nissl staining, Western blot, and transmission electron microscopy results demonstrated that CGA inhibited neuronal loss in the hippocampal CA1 region of VaD rats, increased the expression of synaptic-related markers SYP and PSD95, thickened the postsynaptic density, and suppressed mitochondrial ridge rupture in neurons. GSH detection, MDA detection, and Western blot experiments indicated that CGA alleviated hippocampal GSH reduction and MDA elevation and increased the protein levels of GPX4 and SLC7A11. Cell activity, ROS detection, lipid peroxidation detection, intracellular Fe2+ level detection, GSH detection, and Western blot revealed that CGA inhibited the increase of intracellular Fe2+, ROS, and lipid peroxidation induced by OGD in PC12 cells, mitigated GSH reduction, and increased the protein levels of GPX4, SLC7A11, SYP, and PSD95. Western blot and immunofluorescence staining showed that CGA increased the expression of pSer40-Nrf2 in the hippocampal tissue of VaD rats and PC12 cells, promoting Nrf2 nuclear translocation. Knockdown of Nrf2 prevented CGA from rescuing ferroptosis and synaptic damage induced by OGD in PC12 cells, as well as Nrf2 pathway activation. Molecular docking analysis suggested that CGA competitively bound to the Kelch domain of Keap1 with Nrf2 and then promoted Nrf2 release and activated downstream signaling pathways. In conclusion, our study suggests that CGA may activate the Nrf2 signaling pathway by disrupting the interaction between Nrf2 and Keap1 proteins, thereby inhibiting hippocampal neuronal ferroptosis and synaptic damage and ameliorating cognitive impairment in VaD. Practical Applications. Vascular dementia (VaD) stands as the second most prevalent type of dementia following Alzheimer’s disease. However, there is no effective clinical treatment drug available, and its pathogenic mechanisms remain elusive. This study proposed that CGA might activate the Nrf2-GPX4 signaling pathway by competitively binding to Keap1, thereby attenuating hippocampal neuronal ferroptosis and synaptic damage and consequently ameliorating cognitive impairment in VaD. Our study may provide a novel option for drug research and development for VaD.
1. Introduction
Vascular dementia (VaD) is a prevalent type of dementia, ranking second only to Alzheimer’s disease [1, 2]. It is characterized by neurodegeneration and cognitive impairment resulting from cerebrovascular diseases or vascular risk factors [2, 3]. The primary etiology of VaD lies in vascular abnormalities, encompassing atherosclerosis, cerebral small-vessel diseases, multiple cerebral infarctions, and hemorrhagic or ischemic strokes [4]. These conditions precipitate alterations in cerebral hemodynamics, leading to reduced cerebral blood flow, inadequate supply of oxygen and nutrients to the brain, deposition of harmful substances, heightened oxidative stress, disruption of the blood-brain barrier, white matter damage, and subsequent neuronal loss, ultimately culminating in severe cognitive impairment [4–6]. Despite accounting for at least 20–40% of all dementia diagnoses [4], effective therapeutic methods to halt VaD progression are currently lacking.
Ferroptosis, characterized by elevated intracellular Fe2+ levels, reactive oxygen species (ROS), and lipid peroxidation, plays a pivotal role in aging, neurodegenerative diseases, and cerebrovascular diseases [2, 7]. Oxidative stress induced by chronic cerebral hypoperfusion emerges as a major pathogenic mechanism underlying VaD-related cognitive deficits [4]. Cerebral hypoperfusion upregulates the expression of transferrin and its receptor, augmenting cellular iron uptake while diminishing ferritin levels and impeding iron efflux [2]. It has been evidenced elevated levels of lipid peroxidation and increased iron deposition in the hippocampus of VaD patients [4]. Ferrostatin-1, a ferroptosis inhibitor, has shown significant improvements in cognitive ability in VaD rats [8], suggesting that ferroptosis may represent a critical event in the onset and progression of VaD.
Chlorogenic acid (CGA) is a phenolic compound belonging to the hydroxycinnamic acid family, widely distributed in various plants as a dietary phenolic acid [9, 10]. CGA exhibits diverse effects, including scavenging free radicals, regulating glucose and lipid metabolism, and inhibiting inflammatory responses [10–12]. Its potent anti-inflammatory and antioxidant properties make it highly promising for various applications and possess significant research value [12]. However, its therapeutic role in VaD remains unclear. Recent studies have demonstrated that CGA mitigates chronic stress-induced duodenal ferroptosis in rats by inhibiting the IL-6/JAK2/STAT3 signaling pathway [13] and suppresses ferroptosis and cell apoptosis through modulating immunity and the AKT/mTOR signaling pathway [14]. Furthermore, CGA has been shown to effectively reduce cerebral infarction volume, promote neuronal axonal growth, and reverse the expression of ferroptosis-related proteins, thereby alleviating hypoxic-ischemic brain injury in neonatal mice [15]. These findings suggest that CGA may ameliorate cognitive impairment in VaD by inhibiting neuronal ferroptosis.
In this study, we established a VaD rat animal model by permanent bilateral occlusion of the carotid arteries (2-VO) and the oxygen-glucose deprivation (OGD) cell model by glucose-free culture medium with CoCl2 and assessed cognitive abilities in rats and the levels of ferroptosis-related markers through various methods to evaluate the effect of CGA on cognitive function and the pathological progression of VaD. In addition, we examined nuclear factor erythroid-2 related factor 2 (Nrf2) signaling pathway-related indicators and preliminarily determined, through molecular docking analysis, that CGA could bind to the Kelch-like ECH-associated protein 1 (Keap1) homodimer, thereby promoting Nrf2 release, phosphorylation, and nucleus translocation and initiating the expression of downstream antioxidant genes such as glutathione peroxidase 4 (GPX4). It potentially inhibited hippocampal neuronal ferroptosis, alleviated synaptic damage, and improved memory and cognitive function in VaD rats. Our study may provide a novel option for drug research and development for VaD.
2. Material and Methods
2.1. Animals
Male Sprague–Dawley (SD) rats weighing about 200 ± 20 g were purchased from the Animal Research Center of Nanjing University of China and were housed under standard laboratory conditions with a 12-hour light-dark cycle and a temperature of 21–23°C in a humidity-controlled vivarium. During the study period, the rats were granted ad libitum access to food and water. All experimental protocols involving animal subjects strictly adhered to the guidelines outlined by the Institutional Animal Care and Use Committee of the Experimental Animal Research Center of Nanjing University, consistent with the Institute of Laboratory Animal Resources guidelines [16, 17].
2.2. Establishment of a 2-VO Rat Model
After 7 days of adaptation, 2-VO surgery was performed to induce VaD in rats according to the procedures described before [18, 19]. Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneally) and placed in a supine position. A midline incision was made in the neck, and the bilateral common carotid arteries were carefully exposed and double-ligated with a 5-0 silk suture. The Sham group underwent the same surgical procedure without artery occlusion. After surgery, the rats were allowed to recover and were housed under standard conditions with free access to food and water.
2.3. Drug Administration
The rats were divided into four groups: Sham, Model, CGA-L (20 mg/kg), and CGA-H (50 mg/kg). CGA was dissolved in distilled water, and administration for the CGA-L and CGA-H groups commenced 2 h after surgery, with oral administration once daily for 4 weeks. Equivalent volumes of distilled water were administered to the Sham and Model groups. The detailed information on the reagents used is shown in Supplementary Table 2.
2.4. Cell Culture and Treatment
PC12 cells (Procell) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. The OGD cell model was induced by using glucose-free culture medium supplemented with 50 μM CoCl2 [20]. Following induction, the cells were treated with CGA dissolved in DMSO at concentrations of 10 μM and 100 μM or DMSO alone in equivalent volumes as a control. The transfection of siRNA was performed following the manufacturer’s protocols using a transfection reagent. The negative control siRNA (si-NC) consisted of empty vectors purchased from the OBiO company. The sense sequence of si-Nrf2 was 5′-GAGGAUGGGAAACCUUACUTT-3′, and the antisense sequence was 5′-AGUAAGGUUUCCCAUCCUCAT-3′. The detailed information on the reagents used is shown in Supplementary Table 2.
2.5. Novel Object Recognition (NOR)
The NOR test was conducted to assess recognition memory in rats [21]. Initially, rats were allowed to freely explore a square open field for 5 min. Following a 24 h retention interval, the rats were reintroduced to the same open field environment, where two identical objects were placed, and allowed to explore for another 5 min. Subsequently, after an additional 2 h interval, one of the objects was substituted with a different-shaped object that maintained the same color and size as the original. Throughout the 5 min test period, the exploration time of each object was meticulously recorded. In addition, the discrimination index, representing the ratio of time spent exploring the novel object to the total exploration time of old and new objects, was calculated as an indicator of recognition memory.
2.6. Morris Water Maze (MWM)
The MWM test was employed to evaluate spatial memory in rats [22]. A circular pool filled with water was divided into four quadrants, and a hidden platform was placed within one quadrant. Rats were gently introduced into the water one by one from two marked starting points positioned along the perimeter of the pool. The time each rat took to locate and climb onto the hidden platform within a 2 min interval was recorded, representing the escape latency. If a rat failed to find the platform within this time frame, it was guided to the platform and allowed to remain there for 30 seconds, with the escape latency recorded as 2 min. This training protocol was repeated for 5 consecutive days. After 24 h following the final training session, the platform was removed from the pool. Subsequently, the rat was placed into the water from a position equidistant between the two designated starting points, and its behavior was observed for 2 minutes. The duration spent by the rat in the target quadrant where the platform was previously located and the number of times it crossed through the area where the platform was situated served as measures of spatial memory performance.
2.7. Nissl Staining
Nissl staining was conducted following the previously described protocols [23]. In summary, rat brain paraffin sections underwent staining with Nissl staining solution for 5 minutes after complete hydration. Subsequently, the sections were rinsed in water until colorless and dehydrated in a 65°C oven until dry. Finally, the sections were cleared in xylene and cover-slipped for observation. The detailed information on the reagents used is shown in Supplementary Table 2.
2.8. Transmission Electron Microscope (TEM)
Fresh rat hippocampal tissues were obtained and fixed in 2.5% glutaraldehyde, followed by postfixation in 1% osmium tetroxide. Subsequently, they were dehydrated and embedded in epoxy resin. Ultrathin sections were then prepared using an ultramicrotome (Leica EM UC7), followed by staining with uranyl acetate and lead citrate. Finally, the sections were observed under a TEM (Hitachi HT7700) to visualize ultrastructural morphology.
2.9. Protein Extraction and Quantification
Rat hippocampal tissues were rapidly dissected and immediately placed on ice. The tissues were then rinsed with ice-cold phosphate-buffered saline (PBS) to remove blood and other contaminants. After being minced into small pieces using fine scissors, the tissues were homogenized in 10 volumes of ice-cold RIPA lysis buffer supplemented with protease and phosphatase inhibitors. PC12 cells were cultured to 80%–90% confluence and then washed twice with ice-cold PBS. Cells were scraped and collected in ice-cold RIPA lysis buffer supplemented with the same protease and phosphatase inhibitors. The suspension of hippocampal tissues or PC12 cells was incubated on ice for 30 minutes to ensure complete lysis and then was centrifuged at 12,000 g for 15 min at 4°C. The supernatant, containing the soluble proteins, was carefully collected without disturbing the pellet and transferred to a new prechilled microcentrifuge tube. Protein concentrations were determined using the BCA protein assay kit according to the manufacturer’s protocol, with bovine serum albumin (BSA) as a standard. The absorbance readings of the BSA standards were used to generate a standard curve, with protein concentrations in the samples calculated by comparison to this curve. The detailed information on the reagents used is shown in Supplementary Table 2.
2.10. Western Blot
To evaluate the expression levels of target proteins, Western blot analysis was performed as in the previous study [24]. Briefly, the equal amounts of protein (20–30 μg) were mixed with 5X SDS-PAGE sample loading buffer and heated at 95°C for 5 min to denature the proteins. Then, the proteins were separated by SDS-PAGE on a 10–12% polyacrylamide gel, depending on the molecular weight of the target proteins. After electrophoresis, the separated proteins were transferred onto a PVDF membrane (Millipore, USA). To prevent nonspecific binding, the membrane was blocked in Tris-buffered saline with 0.1% Tween-20 (TBST) containing 5% skim milk for 1 h at room temperature. Next, the membrane was incubated overnight at 4°C with the primary antibody. After washing the membrane three times with TBST, it was incubated with the appropriate HRP-conjugated secondary antibody for 1 h at room temperature. The membrane was washed again three times with TBST, and the bands were visualized using an enhanced chemiluminescence substrate and detected with a ChemiDoc Imaging System (Bio-Rad, USA). The intensity of the bands was quantified using the ImageJ software (NIH, USA), with the target protein levels normalized to β-actin. The specific antibodies used, their sources, and dilutions are detailed in Supplementary Table 1.
2.11. Reduced Glutathione (GSH) and Oxidized Glutathione (GSSG) Detection
The GSH and GSSG levels in rat hippocampal tissues and PC12 cells were assessed utilizing the GSH/GSSG assay kit in accordance with the manufacturer’s protocols. Briefly, the assay relied on Ellman’s reagent DTNB, which reacted with GSH to produce a yellow-colored product. The rate of change in optical density, measured at 412 nm, was directly proportional to the concentration of glutathione in the sample. The detailed information on the reagents used is shown in Supplementary Table 2.
2.12. Malondialdehyde (MDA) Detection
Hippocampal lipid peroxidation was detected using the MDA assay kit, following the manufacturer’s instructions. In brief, MDA reacted with thiobarbituric acid to produce a colorimetric product, which could be measured at 532 nm using a microplate reader. The absorbance was proportional to the concentration of MDA present in the sample. The detailed information on the reagents used is shown in Supplementary Table 2.
2.13. Cell Activity
Cell viability was evaluated utilizing the Cell Counting Kit-8 (CCK8). PC12 cells were seeded in 96-well plates and exposed to different experimental conditions for 24 h. Then, 10 μL of CCK8 solution was added to each well, followed by incubation at 37°C in a cell culture incubator for 2 h. The absorbance was measured at 450 nm using a microplate reader. The detailed information on the reagents used is shown in Supplementary Table 2.
2.14. ROS Detection
The intracellular ROS levels were quantified using a ROS assay kit according to the manufacturer’s protocol. Briefly, PC12 cells were treated with or without CGA for 24 h and then incubated with 10 μM 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) probes at 37°C in a cell culture incubator for 30 min. The fluorescence intensity was measured using a microplate reader. The detailed information on the reagents used is shown in Supplementary Table 2.
2.15. Intracellular Lipid Peroxidation Detection
The C11 BODIPY 581/591 probe was utilized to detect lipid peroxidation in PC12 cells. According to the manufacturer’s protocol, PC12 cells were incubated with 10 μM probe for 1 h at 37°C in a cell culture incubator. After washing with PBS, the corresponding fluorescence signals were measured using a microplate reader. The detailed information on the reagents used is shown in Supplementary Table 2.
2.16. Intracellular Fe2+ Level Detection
The FeRhoNox-1 (Fe2+ indicator) probe was used to detect the intracellular Fe2+ level in PC12 cells. Following the manufacturer’s protocol, PC12 cells were incubated with 1 μM probe for 1 h at 37°C in a cell culture incubator. The fluorescence intensity was measured using a microplate reader. The detailed information on the reagents used is shown in Supplementary Table 2.
2.17. Immunofluorescence Staining
Immunofluorescence staining was conducted following the previously described protocols [25]. Initially, PC12 cells were fixed with 4% paraformaldehyde, permeabilized using Triton X-100, and blocked with goat serum. Subsequently, the cells were incubated overnight at 4°C with anti-Nrf2 antibody. Following this, they were exposed to Cy3-conjugated AffiniPure goat anti-rabbit IgG (H + L) for 1 h at room temperature in the dark. Nuclei were counterstained with DAPI, and images were acquired utilizing a fluorescence microscope. The information on relevant antibodies is shown in Supplementary Table 1. The detailed information on the reagents used is shown in Supplementary Table 2.
2.18. Molecular Docking Analysis
Molecular docking analysis was performed to investigate the potential interaction between CGA and Keap1 [26]. The three-dimensional structure of Keap1, obtained from the Protein Data Bank (PDB) (4ZY3), was processed by removing solvent molecules and incorporating hydrogen atoms. The CGA molecule, sourced from the PubChem website (Compound CID: 1794427), underwent structural optimization and partial charge assignment. Subsequently, docking analysis between Keap1 and CGA was conducted using AutoDockTools 1.5.6, and the docking results were visualized using PyMOL.
2.19. Statistical Analysis
All data were presented as mean ± SEM. Each experiment had at least three independent biological replicates. Statistical analysis was conducted using the GraphPad Prism 8.0 software. A two-tailed unpaired Student’s t-test was used for comparisons between two groups, while one-way or two-way analysis of variance (ANOVA) was utilized for analyses involving multiple groups. Statistical significance was defined as p < 0.05.
3. Results
3.1. CGA Markedly Enhanced Learning and Memory Functions in VaD Rats
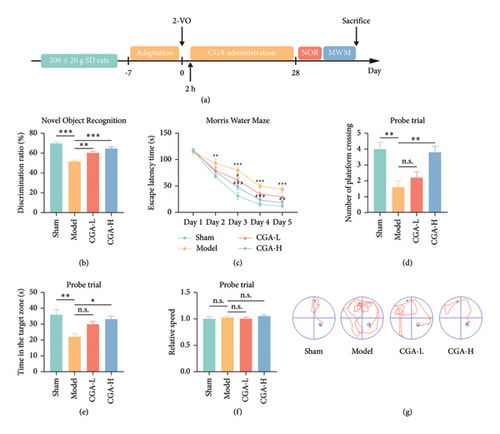
Cerebral hypoperfusion is recognized as a critical pathogenic factor in VaD [5]. Thus, 2-VO surgery was performed in SD rats to establish the VaD animal model. As illustrated in Figure 1(a), the first administration of CGA (20 mg/kg, 50 mg/kg, once daily, i.g.) was conducted 2 h after surgery, followed by a 28-day treatment course. The learning and memory functions of the rats were assessed using the NOR test and the MWM test. The NOR test result indicated a decline in the ability of VaD rats to discriminate between new and old objects. However, CGA treatment significantly enhanced the discrimination ability of VaD rats in a dose-dependent manner (Figure 1(b)). In the Morris water maze test, during the 5-day training period, the model group rats exhibited significantly longer times to find the platform compared to the Sham group, indicating impaired learning function in VaD rats. After CGA treatment, the escape latency was reduced, demonstrating an improvement in the learning abilities of the rats (Figure 1(c)). Additionally, following the removal of the platform, CGA treatment reversed the reduced platform crossings and the decreased time spent in the target quadrant observed in VaD rats (Figures 1(d) and 1(e)). Unfortunately, while there was a trend towards improved memory function in VaD rats treated with the low dose of CGA during the MWM test, this difference was not statistically significant when compared to the model group. Moreover, this study found that the swimming speeds of the animals in all groups were consistent (Figure 1(f)), indicating that the improvement in MWM-related parameters due to CGA was not attributable to enhanced motor function. The swimming trajectories of the rats in each group are shown in Figure 1(g). These findings suggested that CGA significantly improved cognitive function in VaD rats in a dose-dependent manner.
3.2. CGA Mitigated Hippocampal Neuronal Ferroptosis and Ameliorated Hippocampal Neuronal Loss and Synaptic Damage in VaD Rats
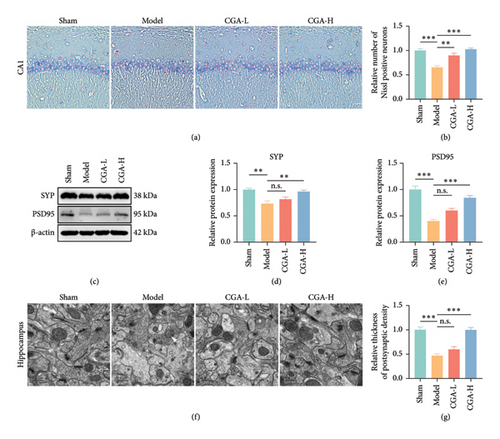
The hippocampus plays a pivotal role in learning and memory, with the CA1 region serving as a critical site for encoding and storing social cognitive memories [27]. It was hypothesized that cognitive impairment observed in VaD might be correlated with neuronal loss within the CA1 region. Thus, we examined the status of CA1 neurons in the hippocampus of VaD rats using Nissl staining. The results demonstrated a significant reduction in the number of Nissl-positive neurons in the hippocampal CA1 region of VaD rats. Notably, CGA treatment effectively reversed the hippocampal neuron loss induced by cerebral hypoperfusion (Figures 2(a) and 2(b)). Western blot analysis further revealed that the levels of the presynaptic marker SYP and the postsynaptic marker PSD95 were markedly reduced in the hippocampus of VaD rats, while CGA treatment significantly upregulated the expression of both proteins (Figures 2(c), 2(d), 2(e), Figure S1). These findings suggested synaptic damage in the hippocampal neurons of VaD rats, which was alleviated by CGA treatment. Additionally, we examined the ultrastructure of hippocampal synapses and mitochondria using a TEM. Consistent with the Western blot results, CGA treatment significantly increased the thickness of the postsynaptic density (Figures 2(f) and 2(g)), indicating that CGA exerted a protective effect on hippocampal neuron synapses. Moreover, TEM images revealed that VaD rats exhibited diminished mitochondrial volume, along with mitochondrial ridge and membrane rupture in hippocampal neurons (Figure 2(f), white arrows), suggesting the potential involvement of neuronal ferroptosis.
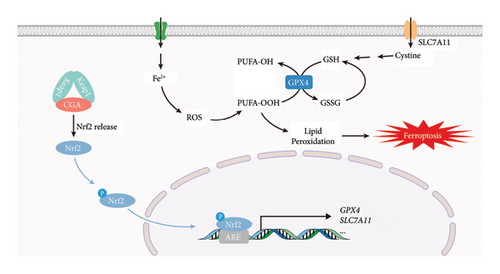
Ferroptosis is manifested by GSH depletion and increased lipid peroxidation [28]. Hence, we assessed hippocampal GSH, GSH/GSSG, and MDA levels. The results demonstrated that CGA effectively reversed the decrease in GSH and GSH/GSSG and elevation of MDA levels in the hippocampus of VaD rats (Figures 3(a), 3(b), 3(c)). GSH, as the primary antioxidant in the body, eliminates lipid peroxides through GPX4, thereby impeding cellular ferroptosis [29, 30]. Intracellular GSH synthesis necessitates the involvement of glutamate, cysteine, and glycine, with cysteine transport into cells and its combination with glutamate being a key rate-limiting step in GSH synthesis [31]. SLC7A11, a cysteine/glutamate transporter protein, is downregulated in ferroptosis, leading to impaired GSH synthesis [32]. Western blot revealed that CGA dose-dependently restored the significantly reduced protein expression levels of GPX4 and SLC7A11 in the hippocampus of VaD rats (Figures 3(d), 3(e), 3(f), Figure S1). These findings collectively suggested that CGA attenuated hippocampal ferroptosis and mitigated hippocampal neuronal loss and synaptic damage in VaD rats.

3.3. CGA Significantly Inhibited Ferroptosis in PC12 Cells Induced by OGD
Cerebral hypoperfusion in VaD may induce ischemia and hypoxia in hippocampal neurons, leading to ferroptosis. It was hypothesized that CGA alleviated cognitive impairment in VaD rats, possibly by inhibiting ischemia/hypoxia-induced neuronal ferroptosis. To validate it, consistent with previous research [20], we induced OGD in PC12 cells using glucose-free culture medium and CoCl2 to explore the protective effects of CGA on ischemic and hypoxic neurons. First, we assessed the cytotoxicity of CGA. As shown in Figure 4(a), high concentrations of CGA did not reduce cell viability in normally cultured PC12 cells, confirming the safety of CGA treatment. Next, we optimized the conditions for constructing the OGD model in PC12 cells by evaluating cell viability. The results indicated that when PC12 cells were cultured in glucose-free medium with 50 μM CoCl2, cell viability was reduced by approximately 50% (Figure 4(b)). Therefore, in subsequent experiments, we used PC12 cells cultured under normal conditions as the Ctrl group and PC12 cells treated with glucose-free medium and 50 μM CoCl2 as the model group. The CGA treatment groups included a low concentration (CGA-L group, 10 μM) and a high concentration (CGA-H group, 100 μM).

As shown in Figure 4(c), both low and high concentrations of CGA significantly improved the viability of PC12 cells, indicating that CGA exerted a protective effect against OGD-induced injury. Fe2+ catalyzes ROS production via the Fenton reaction. Given that cell membranes and organelle membranes are rich in polyunsaturated fatty acids, they are susceptible to ROS attack, leading to lipid peroxide accumulation, membrane rupture, and ultimately culminating in cell death [33, 34]. Therefore, we utilized various cellular probes to measure intracellular ROS, lipid peroxidation, and Fe2+ levels. Our findings revealed that OGD led to a significant increase in ROS, lipid peroxidation, and intracellular Fe2+ levels, which were markedly attenuated by CGA treatment (Figures 4(d), 4(e), 4(f)). Furthermore, GSH assays and Western blot analysis demonstrated that CGA treatment significantly mitigated the OGD-induced reduction in GSH levels, the decrease in the GSH/GSSG ratio, and the downregulation of the ferroptosis markers GPX4 and SLC7A11 (Figures 4(g), 4(h), 4(i), 4(j), 4(k), Figure S1). In summary, these results suggested that OGD could trigger ferroptosis in PC12 cells, and CGA effectively suppressed this process.
3.4. The Nrf2-GPX4 Signaling Pathway Mediated the Protective Effect of CGA on Hippocampal Neuronal Ferroptosis, Synaptic Damage, and Cognitive Impairment in VaD
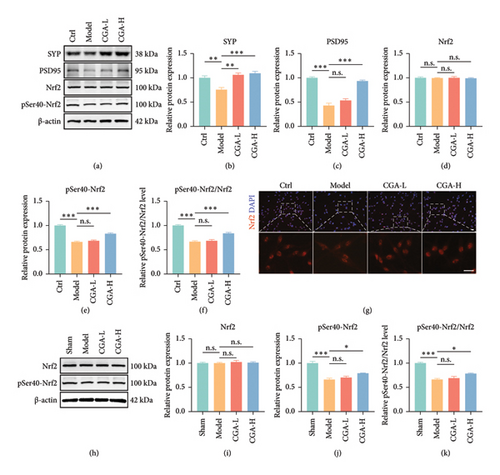
Nrf2 serves as a pivotal antioxidant pathway in combating ferroptosis [35–37]. After phosphorylation at Ser40, cytoplasmic Nrf2 translocates to the nucleus, where it binds to antioxidant response elements (ARE), thereby initiating the transcription of various antioxidant genes such as GPX4 and SLC7A11, increasing GSH levels, and enhancing resistance against ferroptosis (Figure 4(l)). Our findings demonstrated that CGA significantly attenuates ferroptosis in VaD rats and OGD-induced PC12 cells. Could the neuroprotective effects of CGA be associated with the Nrf2 pathway? The results of Western blot revealed a reduction in the expression levels of SYP and PSD95 in OGD-induced PC12 cells (Figures 5(a), 5(b), 5(c), Figure S1). Although the protein levels of Nrf2 remained relatively unchanged, there was a significant decrease in pSer40-Nrf2 levels, indicating inhibition of the Nrf2 pathway (Figures 5(a), 5(d), 5(e), 5(f), Figure S1). However, treatment with CGA elevated the protein levels of pSer40-Nrf2, facilitating Nrf2 nuclear translocation, which was also confirmed by immunofluorescence staining (Figures 5(a), 5(d), 5(e), 5(f), 5(g), Figure S1). Similar results were observed in the hippocampal tissue of VaD rats. The expression of pSer40-Nrf2 was reduced in the hippocampus of VaD rats, indicating inhibition of the Nrf2 signaling pathway. High-dose CGA treatment significantly increased the levels of pSer40-Nrf2, thereby activating the Nrf2 pathway (Figures 5(h), 5(i), 5(j), 5(k), Figure S1). These findings suggested that CGA exerted its antiferroptosis effect by activating the Nrf2 pathway.
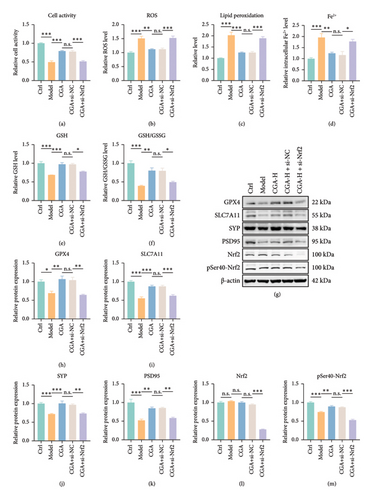
To further validate the critical role of Nrf2 in CGA-mediated protection against VaD, si-Nrf2 was introduced alongside CGA administration. The results demonstrated that si-Nrf2 nullified the protective effects of CGA, as evidenced by the failure to mitigate the decrease in cell viability, the increase in ROS production, lipid peroxide accumulation, intracellular Fe2+ level elevation, and depletion of GSH induced by OGD (Figures 6(a), 6(b), 6(c), 6(d), 6(e), 6(f)). Similarly, CGA was ineffective in restoring the protein levels of the synaptic markers SYP and PSD95, as well as the antioxidant-related proteins GPX4 and SLC7A11 (Figures 6(g), 6(i), 6(j), 6(k), Figure S2). Notably, the most prominent alteration was observed in GPX4, a crucial protein involved in GSH against lipid peroxidation. In addition, it was not surprising that the protein levels of Nrf2 and pSer40-Nrf2 also decreased after si-Nrf2 treatment (Figures 6(g), 6(l), and 6(m), Figure S2). These findings strongly suggested that the Nrf2-GPX4 signaling pathway played a central role in mediating the protective effects of CGA against hippocampal neuronal ferroptosis, synaptic damage, and cognitive impairment in VaD.
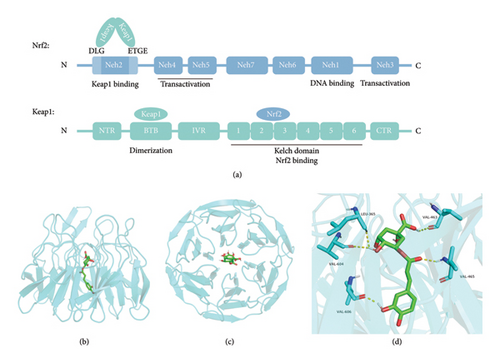
3.5. CGA Competitively Bound to Keap1 with Nrf2, Thereby Facilitating the Release and Nuclear Translocation of Nrf2
Under physiological conditions, the Neh2 domain of the Nrf2 protein contains two motifs, DLG and ETGE, which interact with the Kelch domain of the Keap1 homodimer to form a complex [38] (Figure 7(a)). Then, Nrf2 bound to Keap1 can be degraded via the ubiquitin-proteasome pathway to maintain basal levels [39, 40]. When oxidative stress occurs, Nrf2 dissociates from the Keap1 homodimer and translocates to the nucleus after phosphorylation at Ser40 to initiate transcription [39]. Could CGA promote the nuclear translocation of phosphorylated Nrf2 by competitively binding to Keap1? As shown in Figure 7(a), Keap1 consists of three main structural domains: BTB, IVR, and Kelch. The BTB domain participates in the homodimerization of Keap1. The IVR domain connects the BTB and Kelch domains. The Kelch domain mediates binding with the Neh2 domain of Nrf2 and serves as the binding site for inhibitors of the Nrf2-Keap1 interaction [38]. Thus, utilizing the Kelch domain as the binding site, we conducted docking analysis between it and the CGA molecule using AutoDockTools 1.5.6 and visualized the docking results using PyMOL. The docking analysis results indicated a binding energy of −9.1 kcal/mol between CGA and Keap1. The docking mode of CGA in Keap1 (PDB code 4ZY3) was depicted in the front view in Figure 7(b) and the top view in Figure 7(c). As shown in Figure 7(d), CGA exhibited five hydrogen bond interactions with amino acid residues in Keap1, namely Leu365, Val463, Val465, Val604, and Val606. These results suggested that CGA blocked the interaction between Nrf2 and Keap1 by binding to Keap1, thereby activating the Nrf2 signaling pathway and exerting neuroprotective effects.
4. Discussion
This study performed 2-VO surgery in SD rats to establish an animal model of VaD, while a cell model of VaD was induced by OGD in PC12 cells. Behavioral experiments revealed that CGA significantly enhanced the learning and memory functions of VaD rats. Multiple biochemical indicators indicated that CGA exhibited a neuroprotective effect by mitigating ferroptosis of hippocampal neurons and synaptic damage in VaD rats through the activation of the Nrf2-GPX4 pathway. These findings were further corroborated in the OGD-induced PC12 cells, where the protective efficacy of CGA was compromised upon Nrf2 knockdown, failing to reverse neuronal ferroptosis and synaptic damage. Furthermore, molecular docking analysis was conducted to investigate the potential interaction between CGA and Keap1, which binds to cytoplasmic Nrf2. Utilizing AutoDockTools 1.5.6, the docking results revealed that CGA formed five hydrogen bond interactions with specific amino acid residues in Keap1, namely Leu365, Val463, Val465, Val604, and Val606. This suggested that CGA might activate the Nrf2 signaling pathway by competitively binding to Keap1, thereby disrupting the interaction between Nrf2 and Keap1 proteins and ultimately exerting neuroprotective effects.
With advancing age, there is a progressive accumulation of iron in the brain, leading to an increase in Fe2+ levels that trigger the generation of ROS via Fenton reaction [41]. The presence of polyunsaturated fatty acids in cell membranes and organelles renders them vulnerable to ROS attack, resulting in the formation of lipid peroxides, eventual cell membrane rupture, and cell demise [42]. This process, distinct from apoptosis, necrosis, or pyroptosis, is termed iron-dependent cell death, commonly known as ferroptosis. Prior research has underscored the pivotal role of ferroptosis in neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and VaD [7, 37, 43], with the ferroptosis inhibitor ferrostatin-1 demonstrating significant cognitive improvement in VaD rodent models [8]. Consistent with these findings, our study also revealed elevated levels of MDA, reduced GSH levels, and diminished expression of GPX4 and SLC7A11 in the hippocampal region of VaD rats, indicative of neuronal ferroptosis.
CGA, a naturally occurring compound ubiquitous in plants, modulates various signaling pathways including oxidative stress, lipid metabolism, and inflammation, rendering it a valuable candidate for research in neurodegenerative, cardiovascular, metabolic, and inflammatory diseases [12]. Li LY [15] and Zhao Y [13] have demonstrated that CGA can ameliorate neuronal damage induced by OGD, promote neuronal survival, diminish blood ROS levels, elevate GPX4 and GSH levels, and mitigate duodenal ferroptosis. Consistent with these findings, our study results also suggested that CGA attenuates neuronal demise and synaptic damage triggered by OGD, while concurrently boosting GSH levels and augmenting the expression of antiferroptosis proteins.
As shown in Figure 8, this study elucidated that CGA might facilitate the release of Nrf2 by competitively binding to Keap1 homodimers. Subsequently, upon phosphorylation, Nrf2 translocated into the nucleus and engaged with ARE, thereby fostering the transcription of downstream antioxidant genes such as GPX4 and SLC7A11, culminating in the elevation of the intracellular GSH level, the reduction of lipid peroxidation, and the mitigation of neuronal ferroptosis. The Nrf2 signaling pathway serves as a pivotal antioxidant cascade, and the potentiating effect of CGA on it has been extensively corroborated. It has been shown that CGA can ameliorate endometritis in murine models [44], mitigate hepatotoxicity induced by acetaminophen and thioacetamide [45, 46], and alleviate oxidative stress associated with intestinal inflammation [47] through the Nrf2 pathway. In consonance with these studies, CGA was found to activate the Nrf2 pathway not only in the hippocampus of VaD rats but also in PC12 cells induced by OGD. This activation of the Nrf2 pathway led to the upregulation of antioxidant genes, thereby mitigating cellular damage induced by oxidative stress. Moreover, consistent with studies by Liang N [48] and Adelusi TI [49], there appeared to be a potential interaction between CGA and Keap1 at the site where Nrf2 bound to it, highlighting the plausible mechanism by which CGA activated the Nrf2 pathway.
This study solely analyzed the potential interaction between CGA and Keap1 through molecular docking, without experimental validation. Subsequent experiments will be conducted to substantiate the binding of CGA to Keap1, such as the cellular thermal shift assay or isothermal titration calorimetry. In addition, gene editing techniques will be utilized to further authenticate the mechanism of CGA exerting neuroprotective effects in VaD rats.
5. Conclusion
In conclusion, this study proposed that CGA might activate the Nrf2-GPX4 signaling pathway by competitively binding to Keap1, thereby attenuating hippocampal neuronal ferroptosis and synaptic damage and consequently ameliorating cognitive impairment in VaD.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
KD conceived and designed the study. WS and GL performed the experiments and drafted the paper. CD and YW helped with the data analysis and manuscript revising. All authors have read and approved the final manuscript. Wen Shan and Guodong Liu should be recognized as co-first authors for this publication. Wen Shan and Guodong Liu contributed equally to this work and shared the first authorship.
Acknowledgments
We would like to express our gratitude to the Medical School of Nanjing University for providing the experimental platform for this study.
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.




