Continuous Introduction of H5 High Pathogenicity Avian Influenza Viruses in Hokkaido, Japan: Characterization of Viruses Isolated in Winter 2022–2023 and Early Winter 2023–2024
Abstract
High pathogenicity avian influenza (HPAI) has impacted poultry and wild birds globally. The number of H5 HPAI virus (HPAIV) infection cases in wild birds in Hokkaido (Northern Japan) was high in the last two seasons, contributing to virus spillover to resident birds and poultry. Therefore, H5 HPAIVs in birds and mammals in Hokkaido in winter 2022–2023 and 2023–2024 were monitored and viruses were phylogenetically, antigenically, and pathogenetically characterized. Thirty HPAIV isolates were subtyped and pathotyped by sequencing the hemagglutinin (HA) gene of viruses. Phylogenetic analysis of the HA gene revealed that all isolated HPAIVs were categorized into clade 2.3.4.4b and divided into three groups (G2b, G2c, and G2d). Most isolates belonging to subgroup G2d clustered with isolates in winter 2021–2022 in Hokkaido. The other isolates were categorized into two subgroups, G2b and G2c, mainly composed of isolates in Honshu Island in winter 2021–2022 and 2022–2023, respectively. Two H5 HPAIVs isolated in Eastern Russia in spring and autumn 2022 were genetically close to most Hokkaido isolates (G2d), and a virus isolated in Hokkaido in November 2023 was also grouped in subgroup G2d. Further analysis of all eight gene segments identified six types of gene constellations. Cross-hemagglutination inhibition test indicated that the antigenicity of H5 HPAIVs isolated in the last several seasons was similar within them but slightly different from that in the 2010s. Three chicken breeds were intranasally challenged with four representative isolates to assess their pathogenicity. All chickens except one broiler chicken were dead until 5-day postchallenge with different pathogenicity of these viruses. The pathogenicity of one HPAIV strain was significantly lower in broiler chickens than in layer chickens. The mixture of multiple characteristics of HPAIVs in Hokkaido was confirmed by bird migration routes. Thus, many HPAIVs can be brought and scattered anywhere on Earth.
1. Introduction
Avian influenza is a highly contagious disease in poultry caused by influenza type A virus infection [1]. Wild birds, especially migratory ducks, are the natural reservoir for avian influenza virus (AIV). AIVs belonging to genus Alphainfluenzavirus and family Orthomyxoviridae comprise eight single-stranded, negative-sense, segmented RNAs that can be categorized into 16 and 9 subtypes based on the antigenic characteristics of hemagglutinin (HA) and neuraminidase (NA) surface proteins, respectively [2, 3]. AIVs can naturally spread among wild aquatic birds and infect various bird species, resulting in varying clinical symptoms ranging from low to severe, sometimes leading to increased mortality. AIVs can classified into high pathogenicity (HPAIVs) or low pathogenicity (LPAIVs) according to pathogenicity in chickens [1]. HPAIVs are only restricted to H5 and H7 subtypes because of multiple basic amino acid residues in the cleavage site of the HA protein in natural settings, which can be cleaved by the ubiquitous protease in birds and lead to systemic infection [4, 5].
The worldwide circulation of H5 HPAIVs has contributed to a significant global economic loss due to outbreaks in the poultry industry across Asia, Europe, Africa, and, most recently, North and South America [6–9]. Most H5 HPAIVs are descendants of an isolate A/goose/Guangdong/1/1996 (H5N1) (Gs/Gd) lineage that has been circulating in wild birds for >25 years and has branched into numerous clades and subclades due to selective genetic mutations and reassortment with multiple influenza subtypes [10]. Among those clades, H5 HPAIVs in clade 2.3.4.4 have rapidly spread and constantly caused outbreaks worldwide since 2014 [11]. In particular, H5 HPAIVs in clade 2.3.4.4b have been consistently isolated in Europe and Asia since 2016 [12]. In late spring 2021, a new epidemiologic trend of H5N1 HPAIV was confirmed in Europe by reassortant viruses between clade 2.3.4.4b H5N8 HPAIVs and LPAIVs locally circulated and led to a large number of infections in wild birds and poultry in many European countries [13]. It was further introduced into the Far East, such as Japan, the Republic of Korea, Russia, and China, possibly by bird migrations [14–16].
In Japan, in winter 2021–2022, 25 high pathogenicity avian influenza (HPAI) outbreaks in poultry and 107 cases in wild birds were reported [17]. Four HPAI outbreaks in poultry, 70 cases in wild birds, and two cases in wild mammals from an Ezo red fox (Vulpes vulpes schrencki) and a raccoon dog (Nyctereutes procyonoides) were confirmed in Hokkaido Prefecture, located in the northernmost part of Japan and facing the Far East parts of Russia which usually have the nesting site of migratory birds [15, 18]. Based on the genetic analysis of HPAIVs isolated in Hokkaido in winter 2021–2022, most H5N1 HPAIVs were genetically close to H5 HPAIVs circulating in Europe and North America in the same winter, demonstrating that the wider dispersion of HPAIVs to both edges of the Eurasian continent in the same season could be originated from the lakes in Siberia, where migratory birds nest in summer [15].
In summer 2022, no H5 HPAIV cases were reported in Japan, differing from the situation in Europe, where H5 HPAIVs were sustained throughout summer 2022. However, HPAIVs were reintroduced back to Japan at the beginning of the new winter season 2022–2023. Subsequently, 242 HPAI cases and 84 outbreaks were reported in wild birds and poultry, respectively [19], implying that Japan should still be at high risk of HPAIV introduction. The continual introduction of H5N1 HPAIVs with various genetic features may display a different level of pathogenicity in chickens, with slight antigenic differences even within clade 2.3.4.4b H5 HPAIVs [9]. Thus, in this study, H5 HPAIVs isolated from wild birds, mammals such as foxes, and poultry in Hokkaido in winter 2022–2023 were genetically analyzed to obtain epidemiologic findings relating to HPAIV incursion to Hokkaido. Furthermore, the antigenic difference of isolates was analyzed to determine whether the antigenicity of H5 HPAIVs had changed during maintenance in birds, and the pathogenicity of four representative HPAIV strains was experimentally assessed in chickens.
2. Materials and Methods
2.1. Fecal and Swab Sample Collection, Virus Isolation, and Identification
Two hundred fourteen fecal samples of wild waterfowls were collected during the active survey at Lake Komuke (latitude: 44°25′47′′N; longitude: 143°50′25′′E) and Notsuke Peninsula (latitude: 43°36′12′′N; longitude: 145°17′35′′E) in Eastern Hokkaido in October 2022. The collected fecal samples were mixed with a virus transport medium consisting of minimum essential medium (Nissui, Tokyo, Japan) containing 10 mg/mL streptomycin (Meiji Seika Pharma, Tokyo, Japan), 10,000 U/mL penicillin G (Meiji Seika Pharma, Tokyo, Japan), 250 U/mL nystatin (Sigma–Aldrich, St. Louis, MO, USA), 0.3 mg/mL gentamicin (MSD, Tokyo, Japan), and 0.5% bovine serum albumin fraction V (Roche, Basel, Switzerland) and inoculated into 10-day-old embryonated eggs for virus isolation [20]. To identify the host species, DNA was extracted from the fecal sample using NucleoSpin® DNA Stool (Takara bio, Shiga, Japan) and 749 base pairs near the 5′-terminus of the cytochrome c oxidase (COI) gene of a host bird were amplified using the primers described previously [21]. The polymerase chain reaction (PCR) product was sequenced and compared to the sequence data of the COI gene obtained from Barcode of Life Data Systems (http://www.barcodinglife.com). For the passive survey of HPAIV infections in dead animals in Hokkaido, tracheal swabs collected from dead birds, organ homogenates from dead foxes, and lung homogenates of dead chickens in poultry farms with HPAI outbreaks were inoculated into 10-day-old embryonated eggs for virus isolation. Lung samples from the deceased wild fox (Ezo red fox) were homogenized using a Multi-Beads Shocker (Yasui Kikai, Osaka, Japan) to prepare 10% suspension in a virus transport medium. Lung homogenates of dead chickens from HPAI-affected poultry farms were kindly provided by the Ishikari livestock hygiene services in Hokkaido.
Virus propagation in the collected allantoic fluid was confirmed by the hemagglutination assay. The isolated viruses were subtyped by an hemagglutination inhibition (HI) test using chicken hyperimmune sera against the referenced influenza virus strain for subtyping [3]. Two H5N1 HPAIVs, A/crow/Khabarovsk/776-56/2022 (H5N1) and A/goose/Magadan/2272-5/2022 (H5N1), were isolated from crow in April 2022 and wild goose in October 2022, respectively, in Eastern Russia. To determine the pathogenicity of isolates, viral RNA was extracted from the allantoic fluid using TRIzol LS Reagent (Thermo Fisher Scientific, Waltham, MA, USA) or QIAamp viral RNA mini kit (Qiagen, Hilden, Germany) and sequenced by direct sequencing after PCR using a region-specific primer set to confirm multiple basic amino acid residues at the HA gene, the molecular marker of HPAIV [22]. The full length of the viral genome was amplified by reverse transcription-PCR using gene-specific primers to each of the eight viral gene segments for Sanger sequencing [23]. Each gene was directly sequenced using BigDye Terminator v3.1, Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA), and 3500 Genetic Analyzer (Thermo Fisher Scientific). For next-generation sequencing (NGS) of isolates in Japan, the primer sets described by Ip et al. [24] were used to amplify the whole genome of AIV for NGS analysis together with three primer sets that specifically improve the yield of PB2, PB1, and PA segments (Table S1). Oxford Nanopore libraries were prepared using the NEB Ultra II End Repair/dA-Tailing Module (New England Biolabs, Ipswich, MA, USA) and sequenced on Flongle using the Nanopore Direct cDNA sequencing kit or Ligation Sequencing kit V14 (Oxford Nanopore Technologies, Oxford, England). The obtained reads were mapped and assembled using FluGAS version 2 (World Fusion, Tokyo, Japan). For whole-genome sequencing of isolates in Russia, cDNA synthesis was performed using the cDNA Synthesis Kit (Roche, Basel, Switzerland). The libraries of genome-wide sequencing were prepared by using the commercial Nextera XT Library Prep Kit (Illumina, Hayward, CA, USA) and Nextera XT Index Kits (Illumina, Hayward, CA, USA) and sequenced on MiSeq genetic analyzer (Illumina, Hayward, CA, USA) following the manufacturer’s instructions for the resequencing of small genomes. The obtained reads were mapped in GS Reference Mapper v2.7 and de novo assembled in GS De Novo Assembler v2.7.
2.2. Genetic Analysis
All whole genomes of 30 HPAIVs isolated in Hokkaido and two HPAIVs from Russia were used in the genetic analysis (Table 1). The nucleotide sequences of the 32 isolates were phylogenetically analyzed based on the maximum likelihood method using the best-fit general time-reversible model of the nucleotide substitution with gamma-distribution rate variation among sites (with four rate categories, Γ) according to the Tamura and Nei model [25]. Bootstrap analysis under 1,000 replications was applied to construct the phylogenetic tree in MEGA 7 with default parameters [26]. Sequence data of the gene were compared to the reference nucleotide sequence of the representative H5Nx (with different NA subtypes) in clade 2.3.4.4 strains downloaded from the Global Initiative on Sharing All Influenza Data (GISAID) and GenBank. Basic Local Alignment Search Tool (BLAST) in the GISAID database was used to identify the most homologous nucleotide sequences of HPAIVs isolated in this study.
| ID | Virus | Subgroup | Genotype | Sample collection date | City/town | Latitude | Longitude | 1Accession number of strain |
|---|---|---|---|---|---|---|---|---|
| 1 | A/Eurasian wigeon/Hokkaido/Q71/2022 (H5N1) | G2b | V | 11/11/2022 | Betsukai | 43°36′12″N | 145°17′35″E | EPI_ISL_15576617 |
| 2 | A/Eurasian wigeon/Hokkaido/M184/2022 (H5N1) | G2b | V | 27/11/2022 | Monbetsu | 44°25′47″N | 143°50′25″E | EPI_ISL_15732766 |
| 3 | A/large-billed crow/Hokkaido/B003/2022 (H5N2) | G2d | III | 28/10/2022 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_15732741 |
| 4 | A/slaty-backed gull/Hokkaido/011M111/2022 (H5N1) | G2d | I | 06/11/2022 | Shari | 43°56′17″N | 144°42′43″E | EPI_ISL_16698579 |
| 5 | A/slaty-backed gull/Hokkaido/011M114/2022 (H5N1) | G2d | I | 16/11/2022 | Abashiri | 44°01′15″N | 144°16′24″E | EPI_ISL_16955798 |
| 6 | A/red-crowned crane/Hokkaido/20221120001/2022 (H5N1) | G2d | I | 20/11/2022 | Tobetsu | 43°13′47″N | 141°31′32″E | EPI_ISL_16698576 |
| 7 | A/white-tailed eagle/Hokkaido/20221123001/2022 (H5N1) | G2d | I | 23/11/2022 | Urahoro | 42°48′33″N | 143°39′30″E | EPI_ISL_16955829 |
| 8 | A/large-billed crow/Hokkaido/011E084/2022 (H5N1) | G2d | I | 24/11/2022 | Mukawa | 42°37′31″N | 142°00′32″E | EPI_ISL_16955748 |
| 9 | A/large-billed crow/Hokkaido/0112P083−2/2022 (H5N1) | G2d | I | 05/12/2022 | Kushiro | 42°57′14″N | 144°31′56″E | EPI_ISL_16955765 |
| 10 | A/large-billed crow/Hokkaido/B004/2022 (H5N1) | G2d | IV | 12/12/2022 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17267427 |
| 11 | A/large-billed crow/Hokkaido/B00B/2022 (H5N1) | G2d | IV | 18/12/2022 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17317372 |
| 12 | A/large-billed crow/Hokkaido/B00C/2022 (H5N1) | G2d | IV | 19/12/2022 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17317373 |
| 13 | A/large-billed crow/Hokkaido/B005/2022 (H5N1) | G2d | I | 21/12/2022 | Sapporo | 43°04′50″N | 141°20′26″E | EPI_ISL_17267428 |
| 14 | A/large-billed crow/Hokkaido/B006/2023 (H5N1) | G2d | IV | 04/02/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17316075 |
| 15 | A/large-billed crow/Hokkaido/B007/2023 (H5N1) | G2d | IV | 13/03/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17316489 |
| 16 | A/large-billed crow/Hokkaido/B008/2023 (H5N1) | G2d | IV | 13/03/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17316965 |
| 17 | A/large-billed crow/Hokkaido/0103E088/2023 (H5N1) | G2d | I | 24/03/2023 | Mukawa | 42°37′31″N | 142°00′32″E | EPI_ISL_17950087 |
| 18 | A/large-billed crow/Hokkaido/0103E089/2023 (H5N1) | G2d | I | 29/03/2023 | Mukawa | 42°37′31″N | 142°00′32″E | EPI_ISL_17950253 |
| 19 | A/large-billed crow/Hokkaido/B049/2023 (H5N1) | G2d | IV | 11/04/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17950254 |
| 20 | A/large-billed crow/Hokkaido/B052/2023 (H5N1) | G2d | I | 13/04/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_18007229 |
| 21 | A/large-billed crow/Hokkaido/B053/2023 (H5N1) | G2d | IV | 18/04/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_18007230 |
| 22 | A/large-billed crow/Hokkaido/B067/2023 (H5N1) | G2d | I | 23/11/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_18591747 |
| 23 | A/chicken/Hokkaido/HU-E001/2022 (H5N1) | G2d | II | 07/11/2022 | Atsuma | 42°43′26″N | 141°52′42″E | EPI_ISL_16698060 |
| 24 | A/chicken/Hokkaido/HU-E008/2022 (H5N1) | G2d | I | 11/11/2022 | Date | 42°30′44″N | 140°52′03″E | EPI_ISL_16698477 |
| 25 | A/chicken/Hokkaido/HU-E010/2022 (H5N1) | G2d | I | 11/11/2022 | Date | 42°30′44″N | 140°52′03″E | EPI_ISL_17267420 |
| 26 | A/chicken/Hokkaido/HU-B102/2023 (H5N1) | G2c | VI | 27/03/2023 | Chitose | 42°49′15″N | 141°39′05″E | EPI_ISL_17638141 |
| 27 | A/chicken/Hokkaido/HU-B202/2023 (H5N1) | G2c | VI | 02/04/2023 | Chitose | 42°49′15″N | 141°39′05″E | EPI_ISL_17638143 |
| 28 | A/chicken/Hokkaido/HU-B301/2023 (H5N1) | G2c | VI | 06/04/2023 | Chitose | 42°49′15″N | 141°39′05″E | EPI_ISL_17638448 |
| 29 | A/Ezo red fox/Hokkaido/3/2023 (H5N1) | G2d | IV | 20/02/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17857959 |
| 30 | A/Ezo red fox/Hokkaido/2/2023 (H5N1) | G2d | IV | 12/04/2023 | Sapporo | 43°03′50″N | 141°20′35″E | EPI_ISL_17766057 |
| 31 | A/crow/Khabarovsk/776-56/2022 (H5N1) | G2d | I | 15/04/2022 | Khabarovsk | 50°39′12″N | 136°57′16″E | EPI_ISL_18074199 |
| 32 | A/goose/Magadan/2272-5/2022 (H5N1) | G2d | I | 10/09/2022 | Magadan | 59°32′41″N | 150°53′08″E | EPI_ISL_18071580 |
- 1The Global Initiative on Sharing All Influenza Data accession numbers.
2.3. Antigenic Analysis
The antigenicity of HPAIVs isolated in winter 2022–2023 and an isolate in early winter 2023–2024 was compared to each other and to that in the past season in Japan using a cross-HI test (Table S3). Three representative HPAIV strains isolated in winter 2022–2023 A/chicken/Hokkaido/HU-E001/2022 (Ck/Hok/E001/22; H5N1) isolated from a dead chicken of an HPAI outbreak in a poultry farm in Hokkaido in October 2022, A/large-billed crow/Hokkaido/B003/2022 (Cr/Hok/B003/22; H5N2) isolated from a dead crow in an urban garden in the capital of Hokkaido Prefecture, and A/Eurasian wigeon/Hokkaido/Q71/2022 (Ew/Hok/Q71/22; H5N1) isolated from a fecal sample of wild waterfowl were selected according to the potential antigenic difference based on phylogenetic analysis. Hyperimmune serum against each of these three strains was prepared, as described by Kida and Yanagawa [3]. For antiserum production, 500 µg antigen was mixed with Freund’s complete or incomplete adjuvant at the first and second injections, respectively, and injected intramuscularly into the thigh muscle of a naïve chicken twice at 14-day intervals. Fourteen days after the second injection, whole blood was collected from the chickens to obtain the serum. Other antigens in the representative strains in clade 2.3.4.4, such as A/white-tailed eagle/Hokkaido/22-RU-WTE-2/2022 (WTE/Hok/R22/22; H5N1), A/northern pintail/Hokkaido/M13/2020 (H5N8) [27], A/Ezo red fox/Hokkaido/2/2023 (H5N1), A/large-billed crow/Hokkaido/B049/2023 (H5N1), A/chicken/Hokkaido/HU-B102/2023 (Ck/Hok/B102/23; H5N1), A/large-billed crow/Hokkaido/B067/2023 (H5N1), A/chicken/Kumamoto/1-7/2014 (Ck/Kum/1-7/14; H5N8) [28], A/black swan/Akita/1/2016 (Bs/Aki/1/16; H5N6) [29], and A/Muscovy duck/DR Congo/KAF1/2017 (Mdk/DRC/KAF1/17; H5N8) [30], were analyzed between and within clade 2.3.4.4 using antiserum; WTE/Hok/R22/22 (H5N1), Ck/Kum/1-7/14 (H5N8), Bs/Aki/1/16 (H5N6), and Mdk/DRC/KAF1/17 (H5N8) for cross-reactivity of hyperimmune antisera and their corresponding antigens. The Japanese stockpile vaccine strain for H5 HPAIV infection in Japan, A/duck/Hokkaido/Vac-1/2004 (Dk/Hok/Vac-1/04; H5N1), was also used for the cross-HI test [31]. Antigenic cartography was generated based on the HI test estimated using web-based software [32]. The x–y coordinates of each antiserum and antigen were obtained by loading the data of the titers of the cross-HI test.
2.4. Animal Experiments
Three chicken breeds free from the antibodies against H5 AIV were prepared to assess the pathogenicity of H5 HPAIVs. White Leghorn chickens were hatched from embryonated eggs and fed at Hokkaido University. Thirty-one-week-old Rhode Island Red chickens were kindly provided by the Field Science Center for Northern Biosphere, Hokkaido University (Hokkaido, Japan). Four-week-old Chunky chickens were kindly provided by Nippon White Farm Co., Ltd., (Hokkaido, Japan) and Prifoods Co., Ltd. (Aomori, Japan). Each of the four viruses, Ck/Hok/E001/22 (H5N1), Cr/Hok/B003/22 (H5N2), Ew/Hok/Q71/22 (H5N1), and Ck/Hok/B102/23 (H5N1), was challenged to four White Leghorn or Chunky chickens. Only three viruses, Ck/Hok/E001/22 (H5N1), Cr/Hok/B003/22 (H5N2), and Ew/Hok/Q71/22 (H5N1), were challenged to three Rhode Island Red chickens. All chickens were intranasally challenged with representative HPAIVs at 106.0 times of 50% egg infectious dose (EID50) and monitored daily for clinical manifestation for 14 days postchallenge (dpc). Tracheal and cloacal swabs were collected from each bird daily until 7 dpc or its death, and the swabs were suspended in a 2 mL of virus transport medium. The infectious titers of the swabs were expressed in 50% tissue culture infectious dose (TCID50), which was determined using Madin–Darby canine kidney cells by observing the cytopathic effect (CPE) of virus-infected cells. All chicken experiments were conducted at the Animal BSL-3 Facility, Faculty of Veterinary Medicine, Hokkaido University, which has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International since 2007 and were approved by the Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine, Hokkaido University with the approval numbers 18-0037, 23-0050, and 23-0053.
2.5. Mutational Analysis
Mutational analysis was conducted using ClustalW in GENETYX version 16 (GENETYX Co., Tokyo, Japan) for sequence alignment of Hokkaido isolates for single nucleotide polymorphisms analysis and referring to other previously published mutation of concerns [33].
3. Results
3.1. Genetic Analysis
In winter 2022–2023, nine of 214 fecal samples of wild waterfowls, 56 swab samples of migratory or resident birds, 15 and 2 lung homogenate samples of dead chickens and wild foxes, respectively, in Hokkaido, were diagnosed as positive for H5 AIV infections by detection of H5 HA of AIV. No other HA subtypes of AIV were isolated from any other samples. All H5 viruses were subtyped as H5N1, except for one H5N2 strain isolated from a dead crow in the capital city of Hokkaido (Cr/Hok/B003/22; H5N2). The pathogenicity of all isolates in chickens was deduced as highly pathogenic by confirming the multiple basic amino acid motif at the HA1/HA2 proteolytic cleavage site on the HA gene. Twenty-nine selected isolates from 82 H5 HPAIVs were composed of six from poultry samples, two from fecal samples of migratory birds, two from fox carcasses, and the remaining 19 isolates from resident birds selected for genetic analysis. Two additional H5N1 HPAIVs isolated in Eastern Russia in spring and autumn 2022 were included for genetic analysis. During the early winter season 2023–2024, an HPAIV was additionally isolated from a resident crow in Hokkaido in November; the virus was also included in the genetic analysis. The whole sequences of all eight gene segments of these 32 HPAIVs were registered in the GISAID database (Table 1). In the COI gene database, the sequence of mitochondrial DNA extracted from fecal samples of two migratory birds showed 100% homology to the COI gene of Mareca penelope (Eurasian wigeon).
In the phylogenetic analysis of the H5 HA gene, all 32 isolates were categorized in clade 2.3.4.4b and classified into the Group 2 (G2) genotype, which is closely related to clade 2.3.4.4b H5N8 HPAIVs that shared the common ancestor with viruses detected in Europe in late 2020 [34] (Figure 1(a)). Based on the structure of the phylogenetic tree, the G2 genotype was further classified into multiple subgroups (G2a–e). Most isolates in 2022–2023 were entered into the same subgroup, which was tentatively designated as G2d, which contains HPAIVs isolated in Hokkaido in winter 2021–2022 and remarkably also clustered with A/crow/Khabarovsk/776-56/2022 (H5N1) and A/goose/Magadan/2272-5/2022 (H5N1) isolated in Eastern Russia in spring and autumn 2022, respectively. Interestingly, the isolate in Hokkaido in November 2023 was also grouped into subgroup of G2d. Ew/Hok/Q71/22 (H5N1) and A/Eurasian wigeon/Hokkaido/M184/2022 (Ew/Hok/M184/22; H5N1) isolated from fecal samples of wild waterfowls were categorized into a different subgroup of G2 clade (G2b), which was clustered with HPAIVs isolated in southern Japan in winter 2021–2022 [35]. Interestingly, one HPAIV A/chicken/Hokkaido/TU25-3/2022 (Ck/Hok/TU25-3/22; H5N1), which was isolated in the HPAI outbreak in a poultry farm in Hokkaido in May 2022, was also included in the G2b subgroup. At the end of March 2023, three H5 HPAI outbreaks were confirmed in poultry farms in Hokkaido. The causal agents of these outbreaks, Ck/Hok/B102/23 (H5N1), A/chicken/Hokkaido/HU-B202/2023 (H5N1), and A/chicken/Hokkaido/HU-B301/2023 (H5N1), were clustered into another new subgroup, which was tentatively designated as G2c. In this G2c subgroup, Hokkaido isolates were closely clustered with isolates predominantly prevalent in Southern Japan in 2022–2023.
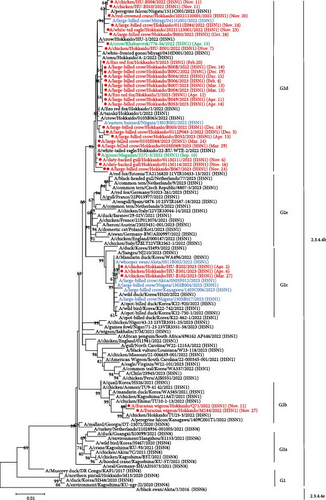
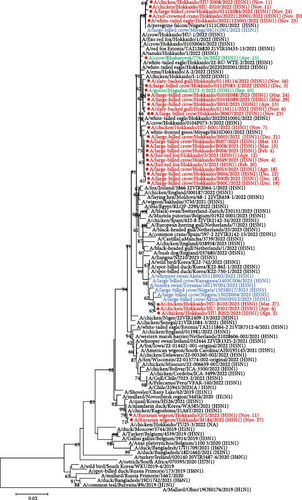
Based on the nucleotide sequence of the HA gene, HPAIVs isolated in Hokkaido were divided into three groups. Similar trends were also confirmed in phylogenetic trees of the N1 NA gene, except for one virus with H5N2 subtype, and other internal genes, such as polymerase basic protein 1 (PB1), nucleoprotein (NP), matrix (M), and nonstructural (NS) gene segments (Figure S1). However, there were different trends in the phylogenetic trees of polymerase basic protein 2 (PB2) and polymerase acidic (PA) genes. In phylogenetic trees of PB2 and PA genes, some isolates, such as Ck/Hok/E001/22 (H5N1), Cr/Hok/B003/22 (H5N2), and A/large-billed crow/Hokkaido/B004/2022 (H5N1), deviated from the major group of Hokkaido isolates in G2d subgroup, indicating that these isolates have undergone reassortment and acquired other wild bird lineages of PB2 and PA genes of LPAIVs (Table S2). Based on BLAST analysis, PB2 and PA genes were highly homologous to the corresponding gene segments of LPAIVs isolated in Far East Asia, including China, Mongolia, and Russia (Table S2). Phylogenetic analysis in all eight gene segments has identified six types of gene constellations of H5 HPAIVs in Hokkaido in 2022–2023 (Figure 2). The subgroup of G2d includes four different gene constellations; a major genotype among isolates in Hokkaido in 2022–2023 (genotype I) has the same gene constellation as the dominant genotype in 2021–2022 in Hokkaido and an isolate in the early winter 2023–2024 in Hokkaido, and two genotypes of two different gene constellations in PB2 and PA gene (genotypes II and IV) and one genotype of three different gene constellations in PB2, PA, and NA (genotype III) were identified as descendants of the dominant genotype in 2021–2022. Ew/Hok/Q71/22 (H5N1) and Ew/Hok/M184/22 (H5N1) in the subgroup of G2b formulated single gene constellation and were designated as genotype V, which also includes Ck/Hok/TU25-3/22 (H5N1). Meanwhile, all the H5 HPAIV isolates in subgroup G2c in Hokkaido also formed a unique gene constellation designated genotype VI.
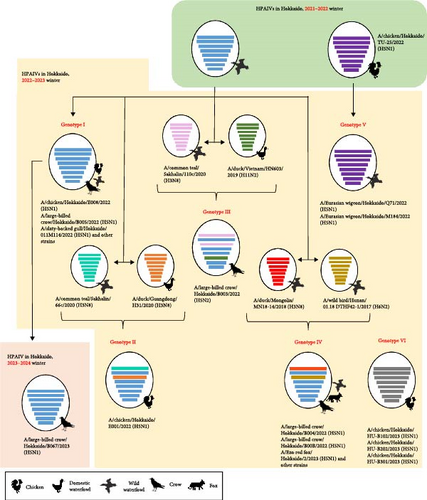
3.2. Antigenic Analysis
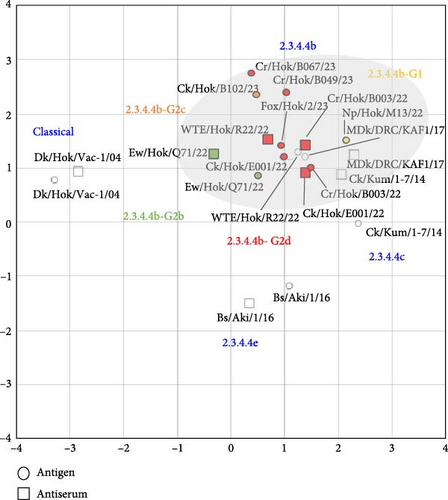
Three viruses, Ck/Hok/E001/22 (H5N1) in subgroup G2d, Cr/Hok/B003/22 (H5N2) in subgroup G2d, and Ew/Hok/Q71/22 (H5N1) in subgroup G2b, were selected for antigenic analysis using the cross-HI test. The antiserum against Ck/Hok/E001/22 (H5N1) showed homologous reaction (same HI titers) with Cr/Hok/B003/22 (H5N2) and Ew/Hok/Q71/22 (H5N1) antigens (Table S3). Meanwhile, the antiserum against Cr/Hok/B003/22 (H5N2) was closely reacted (twofold HI titer differences) with Ck/Hok/E001/22 (H5N1) antigens and Ew/Hok/Q71/22 (H5N1) antigens. The same applies to the antiserum against Ew/Hok/Q71/22 (H5N1), which also displayed close reactivity to the Ck/Hok/E001/22 (H5N1) (twofold HI titer differences) and Cr/Hok/B003/22 (H5N2) (fourfold HI titer differences). Antigenic cartography demonstrated that antigenicity among groups of HPAIVs isolated in winter 2022–2023 and an isolate in November 2023 was almost identical and similar to those of the previously isolated clade 2.3.4.4b (Figure 3). The antigenicity of clade 2.3.4.4b viruses was slightly different from Ck/Kum/1-7/14 (H5N8) in clade 2.3.4.4c. Viruses in clade 2.3.4.4b exhibited more than two antigenic units distant from the vaccine strain: Dk/Hok/Vac-1/04 (H5N1) in the classical clade and Bs/Aki/1/16 (H5N6) in clade 2.3.4.4e.
3.3. Pathogenicity Analysis of Four Representative HPAIV Strains in Chickens
All 4-week-old White Leghorn and Chunky chickens were intranasally challenged with 106.0 EID50 of each of the four viruses, and all 31-week-old Rhode Island Red chickens were intranasally challenged with same titer of three viruses. All chickens challenged with Ck/Hok/E001/22 (H5N1) died at 2 dpc, whereas chickens challenged with Cr/Hok/B003/22 (H5N2) died between 2 and 4 dpc (Figure 4). Chickens challenged with Ew/Hok/Q71/22 (H5N1) died between 3 and 5 dpc. Meanwhile, chickens challenged with Ck/Hok/B102/23 (H5N1) started to die between 2 and 4 dpc. Remarkably, one of the chickens inoculated with Ck/Hok/B102/23 (H5N1) survived until 14 dpc, but with no detectable antiserum collected on the last day of the experiment against the virus (Figure 4). Furthermore, no viruses were recovered from the swab samples of the survived chicken throughout 7 days after challenge.
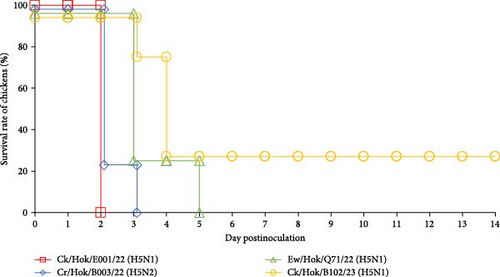

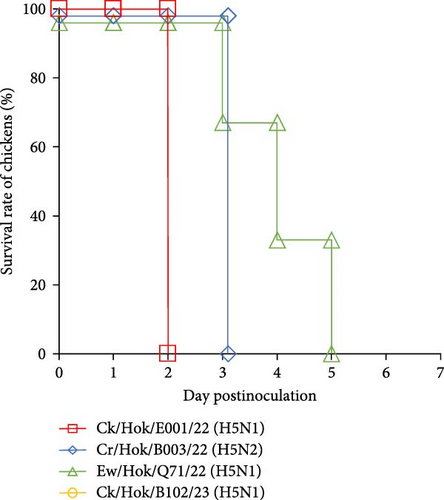
Virus titers in tracheal swabs collected from chickens challenged with Ck/Hok/E001/22 (H5N1) rapidly peaked at 1–2 dpc (Tables 2 and 3 and Table S4). In contrast, virus titers in tracheal swabs of chickens challenged with Cr/Hok/B003/22 (H5N2) at 1 dpc were low (<2.5 log10TCID50/mL) but rapidly increased at 2–3 dpc (>4.0 log10TCID50/mL) (Tables 2 and 3 and Table S4). Virus recovery was not confirmed from tracheal swabs of chickens challenged with Ew/Hok/Q71/22 (H5N1) on 1 dpc but confirmed with a steady increase in virus titer from 2 to 5 dpc and the virus titers recovered at the peak of infection was much lower (<4.0 log10TCID50/mL) when compared to ones after challenge of Ck/Hok/E001/22 (H5N1) and Cr/Hok/B003/22 (H5N2) (Tables 2 and 3 and Table S4). For the virus recovery from the tracheal swabs of chicken challenged with Ck/Hok/B102/23 (H5N1), virus growth was not recognized at 1 dpc (<0.5 log10TCID50/mL) but rapidly peaked at 2 and 3 dpc (>3.5 log10TCID50/mL). Interestingly, the virus recovery from the tracheal swabs of chunky chickens challenged with Ck/Hok/B102/23 (H5N1) showed a steady increase in the virus titers from 2 to 4 dpc (Tables 2 and 3). Virus recoveries from cloacal swabs also demonstrated a similar viral load observed in tracheal swabs in all three chicken breeds. The pathogenicity and viral titers observed in swab samples were varied in challenged strains. Although there was variation in the viral loads observed in tracheal and cloacal swabs among the three chicken breeds based on the challenged strains, the growth ability of each challenged virus exhibited among the three chicken breeds remained constant regardless of species and age.
| Viruses | Days on dead | Virus titers in swab sample (log10 TCID50/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |||||||||
| Tracheal | Cloacal | Tracheal | Cloacal | Tracheal | Cloacal | Tracheal | Cloacal | Tracheal | Cloacal | Tracheal | Cloacal | Tracheal | Cloacal | ||
| Ck/Hok/E001/22 (H5N1) | 2 | 1.4 | 2.7 | 5.5 | 3.7 | —1 | — | — | — | — | — | — | — | — | — |
| 2 | 2.5 | 3.5 | 4.7 | 5.0 | — | — | — | — | — | — | — | — | — | — | |
| 2 | <0.52 | <0.5 | 5.0 | 3.7 | — | — | — | — | — | — | — | — | — | — | |
| 2 | 2.0 | 2.2 | 4.7 | 4.3 | — | — | — | — | — | — | — | — | — | — | |
| Cr/Hok/B003/22 (H5N2) | 2 | <0.5 | <0.5 | 5.5 | 4.7 | — | — | — | — | — | — | — | — | — | — |
| 2 | <0.5 | <0.5 | 6.0 | 5.0 | — | — | — | — | — | — | — | — | — | — | |
| 2 | <0.5 | <0.5 | 5.5 | 3.5 | — | — | — | — | — | — | — | — | — | — | |
| 3 | <0.5 | <0.5 | 1.5 | <0.5 | 5.7 | 3.5 | — | — | — | — | — | — | — | — | |
| Ew/Hok/Q71/22 (H5N1) | 5 | <0.5 | <0.5 | <0.5 | <0.5 | 2.5 | <0.5 | 3.7 | 2.3 | 2.7 | 1.7 | — | — | — | — |
| 3 | <0.5 | <0.5 | 3.5 | 1.5 | 3.3 | 3.5 | — | — | — | — | — | — | — | — | |
| 3 | <0.5 | <0.5 | 3.5 | 2.0 | 3.5 | 2.5 | — | — | — | — | — | — | — | — | |
| 3 | <0.5 | <0.5 | 4.3 | 2.0 | 4.0 | 2.0 | — | — | — | — | — | — | — | — | |
| Ck/Hok/B102/23 (H5N1) | 4 | <0.5 | <0.5 | 1.8 | <0.5 | 3.0 | 2.7 | 4.5 | 2.3 | — | — | — | — | — | — |
| 4 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 4.5 | 4.7 | — | — | — | — | — | — | |
| 3 | <0.5 | <0.5 | 4.3 | 4.3 | 4.3 | 4.0 | — | — | — | — | — | — | — | — | |
| 14 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | |
- 1Indicate no samples were collected. 2<0.5 log10 TCID50/mL is the lowest detection limit of the virus titers.
| Viruses | Days on dead | Virus titers in swab sample (log10 TCID50/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |||||||
| Tracheal | Cloacal | Tracheal | Cloacal | Tracheal | Cloacal | Tracheal | Cloacal | Tracheal | Cloacal | ||
| Ck/Hok/E001/22 (H5N1) | 2 | 1.5 | 2.0 | 3.7 | 2.3 | —1 | — | — | — | — | — |
| 2 | 2.0 | 2.7 | 3.7 | 1.7 | — | — | — | — | — | — | |
| 2 | 4.0 | 1.3 | 4.7 | 3.0 | — | — | — | — | — | — | |
| 2 | 2.5 | <0.52 | 4.3 | 3.7 | — | — | — | — | — | — | |
| Cr/Hok/B003/22 (H5N2) | 2 | 2.5 | <0.5 | 6.0 | 3.5 | — | — | — | — | — | — |
| 2 | <0.5 | <0.5 | 4.0 | 3.0 | — | — | — | — | — | — | |
| 2 | 2.3 | 2.3 | 4.0 | 3.5 | — | — | — | — | — | — | |
| 4 | <0.5 | <0.5 | 1.7 | <0.5 | 3.7 | 2.7 | — | — | — | — | |
| Ew/Hok/Q71/22 (H5N1) | 5 | <0.5 | <0.5 | <0.5 | <0.5 | 1.0 | <0.5 | 2.7 | <0.5 | 2.7 | 1.5 |
| 5 | <0.5 | <0.5 | 1.0 | <0.5 | 1.7 | <0.5 | 2.0 | <0.5 | 2.7 | 1.8 | |
| 5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 3.5 | 1.7 | 2.7 | 2.0 | |
| 5 | <0.5 | <0.5 | <0.5 | 1.0 | 1.3 | 1.5 | 3.5 | 2.0 | 3.5 | 1.7 | |
| Ck/Hok/B102/23 (H5N1) | 2 | <0.5 | <0.5 | 3.7 | 3.0 | — | — | — | — | — | — |
| 2 | <0.5 | <0.5 | 4.0 | 2.7 | — | — | — | — | — | — | |
| 2 | <0.5 | <0.5 | 4.5 | 2.7 | — | — | — | — | — | — | |
| 4 | <0.5 | <0.5 | <0.5 | <0.5 | 2.7 | 2.7 | 2.3 | 2.3 | — | — | |
- 1Indicate no samples were collected. 2<0.5 log10 TCID50/mL is the lowest detection limit of the virus titers.
3.4. Mutational Analysis
Detailed mutational analysis was performed with 30 isolates in Hokkaido with whole-genome sequences to identify amino acid substitutions that could contribute to virulence, transmissibility, or host adaptation. Thirty-eight variable sites were identified and specifically linked to phenotypes, such as virulence, transmission, or host adaptation, as reported previously [33]. Of these 38 variable sites, eight were identified in PB2, three in PB1, one in PB1-F2 [36], five in PA, eight in HA based on H5 numbering, three in NP, three in M1, and seven in NS1 (Table S5). Due to the introduction of multiple HPAIV genotypes in Hokkaido in 2022–2023, different variables have been found across some genotypes, such as L292V in PB2, S224P in PA, and N319K in NP, previously reported to have contributed to enhancing polymerase activity and virulence in mice [37–39]. Some mutations, such as K207R in PB1 and Q400P in PA, known to decrease polymerase activity in mice, have also been identified in some genotypes [40, 41]. The essential results of the mutation analysis, which possess amino acid variation among the 30 isolates, are summarized in Table 4. Interestingly, one mutation, such as M105V in NP, was exclusively found in all isolates, except for Ew/Hok/Q71/22 (H5N1) and Ew/Hok/M184/22 (H5N1), as this mutation is known to increase pathogenicity in chickens [42]. In addition, the signature of mammalian adaptation gene markers, such as lysine and asparagine at positions 627 and 701 in PB2, was not found in all isolates investigated and deceased Ezo red foxes.
| Segment | Mutation | Amino acid in the following number of virus1 | Associated phenotypes | References | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | ||||
| PB2 | I292V | I | •2 | • | • | V | V | V | • | V | V | V | • | • | V | • | V | • | • | V | • | V | • | • | • | • | • | • | • | V | V | Enhanced polymerase activity, increased virulence in mice | [37] |
| PB1 | K207R | K | • | R | • | • | R | R | R | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | R | R | • | • | • | • | • | Decreased polymerase activity in mammalian cell line | [41] |
| PB1-F2 | N66S | N | • | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | Enhanced polymerase activity, virulence and antiviral response in mice | [36] |
| PA | S224P | S | • | • | P | P | • | • | P | • | • | • | • | • | • | • | • | • | • | • | P | • | • | • | • | • | • | • | • | • | • | Enhanced polymerase activity and virulence in mice and duck | [38] |
| Q400P | Q | • | • | P | P | P | P | P | P | • | • | • | P | • | • | • | P | P | • | P | • | P | P | P | P | P | P | P | • | • | Decreased virulence in mice | [38] | |
| NP | M105V | M | • | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | Increased virulence in chickens | [42] |
| N319K | N | • | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | K | • | • | • | K | K | Increased polymerase activity and replication in mammalian | [39] | |
- 1The number of the virus is corresponding to ID in Table 1. 2Dot indicates same amino acid in virus number 1.
4. Discussion
The global spread of clade 2.3.4.4b H5 HPAIVs has caused a large-scale outbreak, significantly impacting the poultry industry and wildlife [32]. The Far East, such as Japan and other countries, has been recognized as a hotspot due to the continuous introduction of HPAIVs. In winter 2021–2022, high numbers of dead wild birds and outbreaks in the poultry farm were affected in Hokkaido due to the introduction of 2.3.4.4b H5N1 HPAIVs [15]. The continuous circulation of H5 HPAIVs in 2023 by migratory birds has disseminated viruses into rarely affected countries in the South American region, such as Peru, Ecuador, and Venezuela [6]. Hokkaido was no exception to the continual invasion of clade 2.3.4.4b H5 HPAIVs in winter 2022–2023. In this study, 30 HPAIVs isolated in Hokkaido were genetically analyzed in all eight gene segments. Based on the phylogenetic analysis of the HA gene segment, all Hokkaido isolates still belonged to clade 2.3.4.4b and further formed three phylogenetic clusters of G2b, G2c, and G2d (Figure 1). HPAIVs in the G2d subgroup share common ancestors closely related to Hokkaido isolates in winter 2021–2022, together with A/crow/Khabarovsk/776-56/2022 (H5N1) and A/goose/Magadan/2272-5/2022 (H5N1) isolated in Eastern Russia in spring and autumn 2022. This might indicate that Hokkaido isolates in winter 2021–2022 in the G2d subgroup had been carried over outside of Hokkaido, including Eastern Russia, by spring migration and could have been maintained in the nested lake in northern Russia during summer 2022. An isolate in Hokkaido in November 2023 was also grouped into the subgroup G2d. The continuous introduction of HPAIVs in the subgroup G2d during the three consecutive seasons could reveal that the viruses in this subgroup should be sustained in the Russian Far East and carried back and forth from Hokkaido, even with three different subgroups (G2b, G2c, and G2d) of H5 HPAIVs been introduced into Hokkaido in winter 2022–2023. This finding can also be supported by the results of gene constellation analysis that genotype I, which is a dominant genotype in Hokkaido in winter 2022–2023, had dominantly caused outbreaks for a certain period [15], was also a majority in winter 2021–2022 and isolated at the beginning of winter 2023–2024. A similar assumption was also proposed for HPAIV isolates in the G2b subgroup. Based on BLAST analysis, some Hokkaido isolates in winter 2022–2023, such as Ew/Hok/Q71/22 (H5N1) and Ew/Hok/M184/22 (H5N1) in the G2b subgroup, showed high sequence identity to Ck/Hok/TU25-3/22 (H5N1) isolated in the poultry outbreak at the end of spring 2022 in Hokkaido. The nucleotide sequences of those isolates were highly homologous to H5 HPAIVs isolated in Southern Japan in winter 2021–2022. These observations also suggested that HPAIV isolates in the G2b subgroup were likely to be brought to Hokkaido by bird migration in spring 2022 and maintained in Northern and Eastern Russia during summer 2022. These HPAIVs were reintroduced into the Far East in the following autumn migration [15]. Multiple HPAI outbreaks caused by H5 HPAIVs in Hokkaido poultry were also reported in early April 2023. One of the isolates from the poultry outbreak, Ck/Hok/B102/23 (H5N1), clustered in G2c subgroup, showed high genetic similarity to isolates from Southern Japan in winter 2022–2023. Those isolates were also genetically close to those circulating in the Republic of Korea and China [16], and invasion of HPAIVs in Southern Japan to Hokkaido was speculated once again in winter 2022–2023 following the spring migration.
During the winter 2022–2023, multiple genotypes of HPAIVs could have been sporadically introduced into Hokkaido. Extensive reassortment events in 2.3.4.4b H5N1 HPAIVs have been frequently reported, such as the recent cases in the Republic of Korea and China, where genetic reassortment was observed between clade 2.3.4.4b H5 HPAIVs with Eurasian LPAIVs [14, 16, 34]. Based on phylogenetic trees of other internal gene segments in this study, it was confirmed that multiple genotypes, genotypes II and III, were reassorted (Figure 2). Reassorted HPAIVs were produced between clade 2.3.4.4b H5 HPAIVs, genotype I, circulating predominantly in Hokkaido in winter 2021–2022, and LPAIVs originated from wild birds in the Far East. It was speculated that dominant H5N1 HPAIVs in European or Asian countries could undergo genetic reassortment with other LPAIVs during migration or at the breeding sites of migratory birds. Thus, further genetic analysis should provide the role of wild birds in contributing to the reshuffle of genetic properties of AIVs, which allows the formation of a temporary genome constellation of viruses that might enhance the transmission and pathogenicity in wild birds [43]. However, there were limitations in this study, as many HPAIV cases in wild birds were reported through the passive survey for H5 HPAIV in Japan from the suspected dead birds. This may not represent the true ecology of the geographical distribution of three H5 HPAIV subgroups (G2b, G2c, and G2d) in Japan.
The unusually high number of dead crows positive for H5 HPAIVs in Hokkaido was also reported in winter 2022–2023, similar to winter 2021–2022 [15]. Interestingly, multiple H5 HPAIV genotypes, genotypes I, III, and IV (Figure 2), were identified among dead crows discovered in the city center of Hokkaido, in winter 2022–2023, indicating that crows could play a role in virus transmission by introducing various genotypes of HPAIVs from the outside to inside of the poultry farm. Many HPAI cases in crows may have led to virus spillover to foxes, as HPAIVs isolated from crows and foxes were genetically highly homologous to one another. The increase in spillover of HPAIVs from birds to mammals has also caused great concern for public health regarding the adaptation of HPAIVs in mammals.
The antigenicity of isolates in winter 2022–2023 and an isolate in November 2023 was close to those of clade 2.3.4.4b H5 HPAIVs isolated in Japan or the Democratic Republic of the Congo of Africa in the past few years and still close with H5 HPAIVs in clade 2.3.4.4c (Figure 3). In contrast, moderate differences in antigenicity between clades 2.3.4.4b and 2.3.4.4e H5 HPAIVs were observed, as supported by the previous study investigating clade 2.3.4.4b H5 HPAIVs in Japan and Africa [27, 30]. In addition, slight differences in antigenicity among isolates of three subgroups, G2b, G2c, and G2d, were observed, corresponding to an observation in a study among subgroups G2b and G2d of 2.3.4.4b H5 HPAIV isolates in Japan in winter 2021–2022 [9]. Since autumn 2021, the Eurasian lineage of H5 HPAIVs in clade 2.3.4.4b has been spreading globally and eventually introduced into countries such as the South American region, which are rarely affected by H5 HPAIVs in this lineage. The constant widespread of H5 HPAIV might generate HPAIVs with different characteristics, including antigenicity. However, the antigenicity of the isolates in Hokkaido has demonstrated minor antigenicity changes in the past several seasons, indicating preservation of H5 HPAIVs in clade 2.3.4.4b under less selective pressure for major antigenicity changes.
A low morbidity rate but a sudden increase in lethality rates in broiler flocks has been described during an outbreak of clade 2.3.4.4 b H5N1 HPAIV in Italy in winter 2021–2022 [44]. Thus, to understand the pathogenicity of H5 HPAIVs in different chicken breeds, four representative HPAIVs with different gene constellations were selected for pathogenicity study in three chicken breeds. In this study, it was highlighted that Chunky (broiler) chickens also demonstrated similar mortality rates and clinical signs to the ones found in White Leghorn (layer) and Rhode Island Red (layer) chickens except for one strain. The pathogenicity of Ck/Hok/B102/23 (H5N1) in Chunky chicken was significantly lower than in White Leghorn chicken, corresponding to the observation reported in Italy broiler flocks even though the virus load recovered from the swab samples among Chunky and White Leghorn chickens were similar (Tables 2 and 3). The difference in the pathogenicity displayed in different chicken breeds can be due to the existence of some avian genes such as PLAU, VCAM1, TNFRSF1A, and PGF gene, which were downregulated during inflammatory response in the lungs of the chicken breed, which can affect disease tolerance; the commercial broiler in Spain was reported to be resistant toward the clade 2.3.4.4b H5N8 HPAIV infection [45]. In this study, it is estimated that 50% of chicken lethal dose of Ck/Hok/B102/23 (H5N1) in Chunky chicken should be 105.7 EID50, which is higher than the ones previously reported in White Leghorn chicken [9]. Furthermore, in the pathogenicity assessment of representative HPAIVs in all three chicken breeds, the pathogenicity of Ck/Hok/E001/22 (H5N1) was generally highest, followed by Cr/Hok/B003/22 (H5N2), lastly Ew/Hok/Q71/22 (H5N1) and Ck/Hok/B102/22 (H5N1). A significant difference was observed in the mean death time between Ck/Hok/E001/22 (H5N1) and Ew/Hok/Q71/22 (H5N1), corresponding to a slower virus growth kinetics in swab samples of Ew/Hok/Q71/22 (H5N1) (Tables 2 and 3 and Table S4). The slower growth kinetics of Ew/Hok/Q71/22 (H5N1) indicated the inability of the virus to replicate well in respiratory and intestinal organs compared to Ck/Hok/E001/22 (H5N1) and Cr/Hok/B003/22 (H5N2). This demonstrated that different genotypes of HPAIVs can contribute to different characteristics in pathogenicity even within the same clade 2.3.4.4b H5 HPAIVs, as lower pathogenicity was observed in chickens challenged with H5N8 HPAIV compared to H5N1 HPAIV that was circulating at the same time [9]. As different pathogenicity characteristics were observed among the representative viruses, the difference in the amino acid in each of the representative HPAIV strains was compared according to pathogenicity markers in previously reported AIVs [33]. All identified polymorphic sites in isolates have been linked to several associated phenotypes, such as altering the activities of the polymerase, replication efficacy in mammalian cells, and virulence in mammalian or avian hosts [33]. Interestingly, the relatively lower pathogenicity observed in chickens infected with Ew/Hok/Q71/22 (H5N1) might be due to the preservation of the amino acid (105M) in NP, as all isolates in this season, except for Ew/Hok/Q71/22 (H5N1) and Ew/Hok/M184/22 (H5N1) with the mutation (M105V) in NP, which could contribute to the increase in the pathogenicity of chickens [33, 42]. Though molecular determinants in contributing to different pathogenicity observed in four HPAIVs were unknown yet, it was speculated that viral segments originating from LPAIVs might play a role in contributing to switch virus pathogenicity, as some studies have demonstrated that reassortant viruses with PB2, PB1, PA, and NP genes could contribute to higher pathogenicity in ferrets [46]. As strain-specific mutations were observed across the substitution of polymerase units of the four representative viruses, the role of those unique amino acid residues observed across the investigated viruses might need to be clarified in future studies. Nevertheless, as a potential public health concern, the receptor binding sites of viral HA of all 30 isolates still indicate the preference to bind to α2,3-sialic acid linkage (237G, 238Q, and 240G; H5 numbering). Any associated markers contributing to high virulence in mammals were not found in Hokkaido isolates, demonstrating that threat of these viruses on public health should be very low [33]. However, cases among mink-to-mink transmission in Spain should heighten the concern about HPAIV adaptation in mammals if viruses continue circulating among wild birds and mammals globally [47].
Overall, H5 HPAIVs isolated in Hokkaido in winter 2022–2023 were phylogenetically, antigenically, and pathogenetically characterized. These were genetically close to Eurasian viruses isolated in winter 2021–2022 and consisted of multiple genotypes identified across wild birds and poultry, indicating the possibility of introducing HPAIVs with different genotypes by migratory birds to Japan. Furthermore, the same genotype of H5 HPAIV was also reintroduced to Hokkaido in early winter 2023–2024. Thus, the invasion of multiple viruses with distinct characteristics, especially transmissibility, host range, and pathogenicity in multiple animals, may perplex the understanding of the epidemiology and ecology of pathogens in the field to reduce their risk. Therefore, continuous monitoring and information sharing of the latest HPAIVs must be essential at the global level to understand dominant or critical HPAIV strains to establish effective control actions.
Conflicts of Interest
The current work was supported by four public research grants. None of the authors was employed by any of these funds or affiliated with an organization with the interests, and none other than the authors had an interest in the outcome of the current work. All the processes in this work, including conception, planning, research design, analysis, and preparing the manuscript were decided by the authors, independently from any of interests. Thus, the authors have no conflicts of interest to declare regarding the publication of this article.
Acknowledgments
We thank the Ministry of Environment for organizing the diagnoses of wild bird samples for AIV infection and further investigations. We also thank the Hokkaido Prefectural Office and Ishikari Livestock Hygiene Center for sharing the diagnosed poultry samples for further investigations. This work was partially supported by the World-Leading Innovative and Smart Education Program (1801) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We like to thank Nippon White Farm Co., Ltd. (Hokkaido, Japan) and Prifoods Co., Ltd. (Aomori, Japan) for providing the broiler chickens to be used in the animal experiments. This work was mainly supported by the Japan Initiative for World-Leading Vaccine Research and Development Centers under the Japan Agency for Medical Research and Development (AMED) (grant no. JP233fa627005). This work was partially funded within the framework of the Science and Technology Research Partnership for Sustainable Development under the Japan International Cooperation Agency (grant no. JP23jm0110019). This study was also partly supported by the Doctoral Program for World-Leading Innovative & Smart Education, powered by MEXT. The support was also provided by the WISE Grand-in-Aid for Graduate Students, Program for One Health Frontier of the Graduate School of Excellence, Hokkaido University (grant no. PH36210001). The research of the Russian Scientific Group (FGBI “ARRIAH”) was funded by the Ministry of Education and Science of Russia (grant no. 075-15-2021-1054).
Open Research
Data Availability
The data presented in this study are openly shared in the Global Initiative on Sharing All Influenza Data. All the accession numbers of the virus sequence data are described in Table 1.




