Lysosome: The metabolic signaling hub
Abstract
For the past five decades, the lysosome has been characterized as an unglamorous cellular recycling center. This notion has undergone a radical shift in the last 10 years, with new research revealing that this organelle serves as a major hub for metabolic signaling pathways. The discovery that master growth regulators, including the protein kinase mTOR (mechanistic target of rapamycin), make their home at the lysosomal surface has generated intense interest in the lysosome's key role in nutrient sensing and cellular homeostasis. The transcriptional networks required for lysosomal maintenance and function are just being unraveled and their connection to lysosome-based signaling pathways revealed. The catabolic and anabolic pathways that converge on the lysosome connect this organelle with multiple facets of cellular function; when these pathways are deregulated they underlie multiple human diseases, and promote cellular and organismal aging. Thus, understanding how lysosome-based signaling pathways function will not only illuminate the fascinating biology of this organelle but will also be critical in unlocking its therapeutic potentials.
1 INTRODUCTION
As the degradative endpoint of the endosomal pathway, the lysosome has gained much notoriety as the “recycling center” of the cell. Its characterization as a major catabolic center traces its origins to its discovery in the early 1950s. While investigating the mechanism of action of insulin, Christian de Duve made the serendipitous discovery of “sac-like structures” that contained lytic activity.1, 2 Ultrastructural characterization of these compartments by Alex Novikoff3 led de Duve to rename them lysosomes. Following de Duve's work, Werner Strauss traced the fate of radiolabeled extracellular proteins and discovered that these proteins localized to the lysosome and were found fragmented rather than intact.4 These pioneering studies cemented the lysosome as the cell's degradative organelle.
The acidic environment of the lysosome maintained by the lysosomal v-ATPase,5 combined with a pantheon of luminal hydrolases, results in an organelle that is perfectly suited for the breakdown of major macromolecules, including lipids, polysaccharides and proteins.6 Once degraded, free fatty acids, monosaccharides and amino acids are transported to the cytoplasm by specific permeases, where they can be reused in anabolic processes.7 The degradative functions of the lysosome are key to maintaining cellular homeostasis, with perturbations in these processes leading to an array of human disorders collectively cataloged as lysosomal storage diseases.8 In addition to the basal-level degradative processes undertaken by the lysosome, environmental stressors trigger a massive upregulation of degradation through the self-catabolic pathways known as autophagy. These pathways are critical in restoring homeostasis during times of metabolic imbalance and not surprisingly are also deregulated in multiple human maladies.9
Our notion of the lysosome as a simple recycling center has undergone a dramatic revaluation over the last decade. With the discovery that the master regulator of cell growth, mechanistic target of rapamycin complex 1 (mTORC1), is localized to the lysosomal surface, we have now come to appreciate that the lysosome also functions as a platform for metabolic signaling. In this mini review, we focus on the lysosome as a metabolic signaling hub, which integrates different environmental signals to regulate core anabolic and catabolic pathways critical in the maintenance of cellular homeostasis. We also touch on how deregulation of these pathways leads to human pathologies.
2 METABOLIC SIGNALING AT THE LYSOSOMAL SURFACE
The mTOR is an evolutionarily conserved phosphatidylinositol-3-kinase (PI3K)-like serine/threonine protein kinase that is inhibited by rapamycin, a small molecule originally discovered in the soil of Easter Island.10 The history of rapamycin's development as a pharmaceutical is recounted in full elsewhere; but briefly, rapamycin's potential as pharmaceutical was immediately apparent due to its ability to slow or arrest cell proliferation. Soon after, it and several derivatives—“rapalogs”11—were developed as immunosuppressants and later trialed as anticancer agents.
The 1990s saw an intense search by several laboratories to discover the molecular mechanism by which rapamycin acts. Discoveries in yeast and in mammalian cells revealed that rapamycin acts by binding to the immunophilin FKBP12, forming a complex that then binds to and inhibits the mTOR protein kinase.12-14 Later work identified two distinct mTOR complexes,15 with shared as well as unique protein subunits and different substrates. mTORC1, which is acutely sensitive to rapamycin, is defined by the association of mTOR with the adaptor proteins Raptor and mLST816-18; while mTORC2, which is acutely rapamycin-resistant, is defined by the association of mTOR with the adaptor proteins Rictor, mLST8 and mSin1.19-22 The list of proteins that physically associate with mTORC1 or mTORC2 continues to grow, although it is likely that some of these proteins interact with mTORC1 or mTORC2 only in particular cell types or environmental conditions.
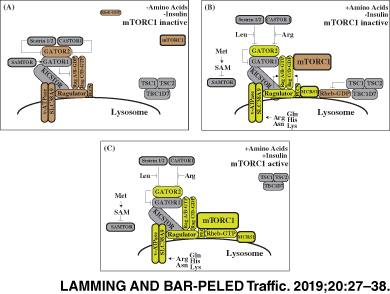
The two mTOR complexes have different cellular roles. mTORC1 functions as a key integrator of environmental and hormonal cues, sensing the availability of amino acids, glucose, cholesterol; cellular energy status; and hormones including insulin, insulin-like growth factor 1 (IGF-1), leptin and adiponectin.23-28 When these states combine to form a permissive environment for cell growth and anabolism, mTORC1 localizes to the lysosome and interacts with its obligate activator Rheb-GTP to phosphorylate substrates including S6K1, 4E-BP1 and ULK1 to promote ribosomal biogenesis, translation and lipogenesis while suppressing autophagy.19, 29-32 Conversely, if one or more of these environmental or hormonal cues is not permissive for growth, mTORC1 remains inactive and these anabolic activities are inhibited, while autophagy is activated.
In contrast, mTORC2 is primarily an effector of the insulin/IGF-1/PI3K signaling pathway. While the molecular mechanism by which PI3K regulates mTORC2 has proven difficult to pin down, it was recently shown that phosphatidylinositol (3,4,5)-trisphosphate (PIP3) directly activates mTORC2 by relieving an inhibitory interaction with mSIN1.33 mTORC2 activity is also responsive to fatty acids,34 and may require interaction with ribosomal protein subunits.35 When activated, mTORC2 phosphorylates numerous AGC kinases, including AKT, SGK and PKCα; these phosphorylations have typically been shown to promote both the activity and stability of the substrate proteins.36-38
Work done over the last decade, and in particular over the last 5 years, have demonstrated that the activation of mTORC1—requires the coordinated interaction of many proteins at a previously unsuspected location, the lysosome. While previously conceived of as simply the “recycling center” of the cell, it is now clear that this hitherto unglamorous organelle serves as a critical platform for coordinating environmental and hormonal cues with mTORC1 and mTORC2 activation. The logic of this placement can be understood through an evolutionary context39; in yeast, the TOR pathway is localized to the vacuole, the lysosomal ortholog,7 which functions as a major storage repository for nutrients. Recent technological advances40, 41 have determined that the mammalian lysosome is likewise selectively enriched in certain nutrients, including certain amino acids. Thus, the localization of mTORC1 to the lysosome allows mTORC1 to immediately gauge the nutrient status of the cell and rapidly shift its activity as required to maintain homeostasis.
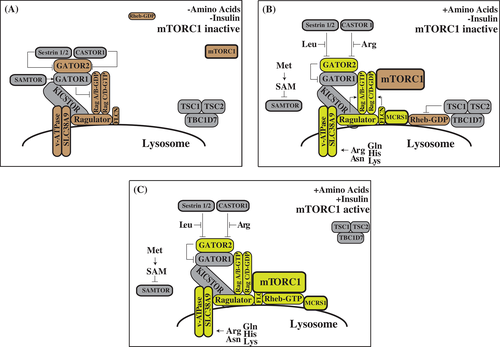
mTORC1 is particularly sensitive to cellular and lysosomal levels of amino acids. mTORC1 is recruited to the lysosome in response to amino acids by the Rag family of heterodimeric small GTPases, orthologs of the yeast small GTPases Gtr1 and Gtr2.42-45 When amino acid levels drop within a cell, RagA/B bind GDP and RagC/D bind GTP (RagAGDP/RagCGTP), a state that does not interact with mTORC1. Upon amino stimulation, Rags flip their nucleotide-bound state (RagAGTP/RagCGDP) and strongly interact with mTORC1 through its Raptor subunit, thus activating mTORC1.44, 45 Over the last 5 years, it has been shown that amino acids regulate mTORC1 activity by interacting with sensor proteins at the lysosomal surface that control the nucleotide loading state of the Rags (Figure 1).
3 REGULATORS OF THE RAG GTPases
The lysosomal platform upon which mTORC1 activation occurs is known as the Ragulator, a protein complex composed of the proteins MP1, p14, HBXIP1, C7ORF59 and p18 (encoded by MAPKSP1, ROBLD3, HBXIP, c7orf59 and c11orf59, respectively), and is anchored to the lysosome by p18.46-48 The Ragulator, which is orthologous to the yeast EGO complex,49, 50 tethers the Rag GTPases to the lysosomal surface and has guanine nucleotide exchange factor (GEF) activity for two of the Rag proteins, RagA and RagB,47, 51 with the G-domains of the Rag proteins projecting away from the Ragulator surface.51-54 Control of Ragulator activity is one mechanism by which amino acids regulate mTORC1. Amino acids control the Ragulator in part via the lysosomal vacuolar ATPase (v-ATPase), which interacts with Ragulator in a manner sensitive to the availability of lysosomal amino acids and v-ATPase activity.55
The importance of lysosomal metabolic signaling to human physiology has come to light by the identification of patients with partial defects in Ragulator function. Mutation in the 3′ UTR of the p14 component of Ragulator leads to an introduction of an aberrant splice site,56 which reduces both p14 transcript and protein levels. Children bearing this mutation are below the third percentile for height and suffer from a complex immune disorder that is marked by B-cell and cytoxic T-cell (CTL) deficiency, neutropenia and partial albinism.57 The short stature and immune disorders of these patients are characteristic of animal models where mTORC1 signaling is attenuated.58, 59 Correspondingly, patient fibroblasts have a marked reduction in amino acid-induced activation of mTORC1 signaling.46 The clinical phenotype associating albinism and immunodeficiency is similar to other disorders caused by defects in specialized secretory lysosomes such as melanomes and cytotoxic granules.60 Furthermore, in patients with reduced Ragulator activity late-endosome/lysosomal distribution was altered, with an increased distance from the nucleus. These results posit a lysosome-mTORC1 feedback loop, wherein inactivation of mTORC1 leads to altered distribution and function of the late-endosome/lysosomal compartment in multiple cell types including CTLs and melanocytes. Whether lysosomal function is compromised in other mTORC1-driven diseases or how mTORC1 activity is altered in lysosomal storage disorders (LSDs) represents an exciting area of research.
The nucleotide loading state of RagA and RagB are also controlled by the GATOR complexes.61, 62 GATOR1, which consists of the proteins DEPDC5, NPRL2 and NPRL3, functions as a GTPase activating protein (GAP) for RagA and RagB. In contrast, GATOR2, which consists of the proteins Mios, WDR24, WDR59, Seh1L and Sec13, acts to inhibit GATOR1 activity by unknown mechanisms. Both GATOR1 and GATOR2 are conserved in yeast (as SEACIT and SEACAT, respectively) as well as higher eukaryotes.49 The structure and regulation of the GATOR complexes is an area of active research; recent studies have taken a cryo-electron microscopy (EM) approach to shed light on how GATOR1 functions,63 and have identified a new protein complex dubbed KICSTOR (KPTN, ITFG2, C12ORF66 and SZT2), which recruits GATOR1 to the lysosomal surface and enables its interaction with the Rag proteins.64, 65 KICKSTOR is required for the negative regulation of mTORC1 by amino acid or glucose deprivation, and its loss leads to hyperactivity of mTORC1; however, it is unknown if the recruitment of GATOR1 by KICSTOR is an important mTORC1 regulatory mechanism.
While much effort has focused on understanding the regulation of RagA and RagB, the nucleotide loading state of RagC and RagD are essential for this complex's interaction with mTORC1.66 The FLCN complex, composed of FLCN, FNIP1 and FNIP2, has emerged as a positive regulator of RagC and RagD through its GAP activity for these GTPases, converting them from the GTP-bound state to the GDP.66 FLCN is required for amino acids to recruit mTORC1 to the lysosome, and is itself recruited to the lysosomal surface by amino acid depletion.67 FLCN is recruited to the lysosome by interaction with RagA/BGDP and requires GATOR1 GAP activity; FLCN recruitment to the lysosome is thus likely regulated by the same set of amino acid sensors upstream of GATOR1/2 that act to regulate mTORC1.68 One possible explanation for the localization of FLCN to the lysosome following amino acid depletion was recently discovered: FLCN sequesters lysosomal leucine when amino acids are limited by blocking PAT1, a lysosomal amino acid transporter, thus helping to preserve mTORC1 activity during nutrient limitation.69
Complicating our understanding of the role of FLCN is that it is mutated in a devastating disease, Birt-Hogg-Dube, that is characterized by large benign tumors which are the hallmark of other cancers driven by loss of negative regulators of the mTORC1 pathway. A possible explanation for this paradox may be that the function of FLCN as a tumor suppressor is context dependent. One recent study reports that FLCN is a ciliary protein that recruits LKB to activate AMPK—an inhibitor of mTORC1 signaling—in response to flow stress.70 Thus, cell type, local environmental conditions, and possibly, the subcellular compartment being investigated may impact the net effect of FLCN loss on mTORC1 activity.
4 AMINO ACID SENSORS, FROM HYPOTHESIS TO REALITY
The greatest recent advances in understanding the response of mTORC1 to amino acids have come from identifying specific proteins that bind to amino acids and regulate the function of the GATOR complexes. The existence of these “amino acid sensors” has long been hypothesized, but it has only been in the last several years that this hypothesis came to fruition with discovery of four sensing complexes. The Sestrin family of proteins (Sestrin1, Sestrin2, Sestrin3) are highly conserved and were recently found to function as negative regulators of mTORC1.71, 72 Originally, the Sestrins were proposed to function as guanine dissociation inhibitors (GDIs) for RagA/B, locking them in the GDP-bound state.73 However, the key residues required for GDI activity are found buried in the crystal structure of Sestrin2.74-76 Purification experiments and subsequent biochemical investigation have demonstrated that Sestrins bind to and inhibit the function of GATOR2. The interaction between Sestrin1/2 and GATOR2 is regulated by leucine; however, the interaction of Sestrin3 with GATOR is constitutive, suggesting another mode of regulation. in vitro binding assays demonstrate that Sestrin2 binds directly to leucine at an affinity similar to the concentrations sensed by mTORC1. Furthermore, the crystal structure of Sestrin2 prominently displays leucine bound to critical residues required for sensing, which when mutated blocks leucine sensing by mTORC1. Leucine binding relieves the inhibitory action of Sestrin2 upon GATOR2 and thus mTORC1.76, 77
The CASTOR proteins (CASTOR1 and its homolog CASTOR2) have recently been described as arginine sensors. The CASTOR proteins are found to bind to GATOR2 and inhibit its function during arginine withdrawal. in vitro binding assays confirmed that CASTOR1 can bind arginine at levels similar to media concentrations required for activation of mTORC1, and mutational studies demonstrated that CASTOR mutants that cannot bind arginine block arginine-induced activation of mTORC1, designating the CASTOR proteins as bona fide amino acid sensors.78, 79
A low-affinity lysosomal amino acid transporter, SLC38A9, interacts with both Ragulator and the v-ATPase, and acts as an amino acid sensor upstream of mTORC1 for asparagine, arginine, glutamine, histidine and lysine.80-82 The exact mechanism by which SLC38A9 regulates mTORC1 activity is not clear; while it was initially suggested that SLC38A9 might regulate the GEF activity of Ragulator, it was shown that in response to arginine, SLC38A9 regulates the lysosomal efflux of many amino acids that then stimulate mTORC1 from the cytoplasm.40 However, this model does not explain the significance of the physical interactions between SLC38A9, the Ragulator and the v-ATPase. Curiously, SLC38A9 also enables mTORC1 activation by cholesterol via recruitment of the Niemann-Pick C1 (NPC1) protein, which acts to inhibit mTORC1 in the absence of cholesterol.83 Understanding how the amino acid sensing and transport functions of SLC38A9 regulate mTORC1 activity and the sensitivity of SLC38A9 to other environmental cues is an important area for future research.
Finally, the SAMTOR protein was recently discovered as an evolutionarily conserved negative regulator of mTORC1 amino acid sensing that binds to GATOR1 directly to modulate its activity by an unknown mechanism. Instead of directly sensing amino acid levels, SAMTOR binds to the metabolite S-adenosylmethionine (SAM), which disrupts its interaction with GATOR1, positioning SAMTOR as an indirect sensor of methionine.84 Intriguingly, the discovery of SAMTOR provides the first molecular mechanism by which methionine restriction, a potent dietary intervention that improves metabolic health and extends rodent life span,85-87 may mediate mTORC1 activity.88-90 This study not only provides a key link between one carbon metabolism and the mTORC1 pathway but raises the question of whether additional intermediate metabolites in amino acid catabolism are also sensed by mTORC1. For example, mTORC1 has been shown to sense α-ketoglutarate, a product of glutaminolysis.91, 92
5 RHEB AND TUBEROUS SCLEROSIS COMPLEX
Recruitment of mTORC1 to the lysosome is a prerequisite for mTORC1 activation, because only at the lysosomal surface is mTORC1 able to interact with the Rheb-GTPase. A recent cryo-EM structure has revealed that Rheb-GTP binds to the mTOR protein kinase and allosterically realigns and activates kinase-site residues.93 Rheb-GTP is regulated by at least two mechanisms; the first is that lysosomal recruitment of Rheb is regulated by amino acids. Amino acids stimulate the binding of Rheb to microspherule protein 1 (MCRS1), which promotes its localization to the lysosome, although the molecular mechanisms that regulate this process remain uncertain.
The second mechanism by which Rheb-GTP is regulated is the tuberous sclerosis complex (TSC), which is composed of the proteins TSC1, TSC2 and TBC1D7.94 The TSC complex acts as a GAP for Rheb, and acts as a “mini” signaling hub upstream of mTORC1.95 Many different kinases, including AKT, AMPK, ERK, GSK3 and IKKβ, phosphorylate distinct residues of TSC1 and TSC2, and thereby serve to regulate mTORC1 by altering the GAP activity of TSC toward Rheb.95-101 While it was originally thought that these posttranslational modifications directly altered the activity of TSC, it was recently shown that much like mTORC1 and Rheb, TSC is also regulated by localization. In the absence of insulin signaling, TSC is localized to the lysosome, where it can directly inhibit Rheb. However, when TSC is phosphorylated by AKT in response to insulin/PI3K signaling, TSC departs from the lysosome, removing its ability to inhibit Rheb, and permitting the activation of mTORC1.102 Another report suggests that TSC lysosomal localization is nutrient dependent, and may be recruited to the lysosome by the Rag GTPases in the absence of amino acids.103 The mechanism by which this process is mediated remains to be determined; also unknown is if relocalization of TSC is a generalized mechanism by which posttranslational modifications of TSC regulate the GTP loading of Rheb.
6 mTORC2
As noted above, mTORC2 is acutely resistant to rapamycin treatment, and this effect, along with the salt sensitivity of mTORC2, leads to a delay in its discovery.15, 20, 21 Even after its discovery, the lack of specific chemical inhibitors of mTORC2 has slowed our understanding of the importance of this complex to a diverse set of cellular processes. The subcellular localization of mTORC2 in particular has been something of a mystery; while mTORC2 is an effector of PI3K signaling and sensitive to PIP3, and thus one might suspect that it is localized to the plasma membrane, work published in 2011 demonstrated that mTORC2 is physically associated with the ribosome, an interaction that is stimulated by insulin.35 While this agrees well with a model in which mTORC2 phosphorylates some motifs of AKT co-translationally, the concept that mTORC2 is associated with the ribosome also received a later boost with determination that mTORC2 localizes to the mitochondria-associated endoplasmic reticulum, interacting with a specific mitochondrial tethering complex.104 Recent studies have also placed mTORC2 at the lysosome (see below) and in immune cells chronic mTORC2 activity inhibits lysosomal acidification.105 The effect of mTORC2 on lysosomal acidification in a more acute context, such as in response to physiological levels of PI3K signaling, is unknown.
7 LYSOSOMAL FUNCTIONS DOWNSTREAM OF mTOR COMPLEXES
While mTORC1 was first characterized as regulator of ribosomal biogenesis and protein translation, an ever-growing set of identified substrates, both of the mTOR protein kinase itself as well as effectors such as S6K1, has linked mTORC1 to a growing list of cellular processes. While we will not attempt to enumerate all of the processes downstream of the mTOR protein kinase, we will note broadly that these processes include the regulation of apoptosis, amino acid and ion homeostasis, metabolism and stress resistance downstream of mTORC2.19, 106-109 Downstream of mTORC1, the list has grown from ribosomal biogenesis and translation to include adipogenesis, amino acid transport, ketogenesis, lipogenesis and nucleotide synthesis.19, 110-114
In all of these processes, both mTORC1 and mTORC2 act in a common theme: to promote anabolic processes. However, both mTOR complexes also play a critical role in regulating catabolic processes. One recently discovered activity is that both mTORC2 and mTORC1 act to promote anaplerosis—the refilling of the citric acid cycle—by promoting the formation of α-ketoglutarate from glutamine.92 Here, we will focus on the lysosomal pathways that lie downstream of the mTOR complexes.
8 TRANSCRIPTIONAL CONTROL OF LYSOSOMAL FUNCTION
Exemplifying the interdependent role of lysosome as both a catabolic and anabolic signaling hub are recent discoveries linking the transcriptional control of lysosome function to mTORC1. In work published in 2009,115 the basic helix-loop-helix transcription factor, TFEB, was found to bind to genes containing the CLEAR element, which is enriched in the promoters of lysosomal genes.115, 116 TFEB is a member of the MiT/TFE transcription factor family,117 whose other members MiTF and TFE3 are now appreciated to have similar functions to TFEB. Activation of TFEB drives a major expansion of the lysosomal compartment and multiple steps in autophagy, denoting TFEB as the master transcriptional regulator of lysosomal function.118 The regulation of TFEB activity is nutrient dependent.119 During nutrient-replete conditions, mTORC1 binds to TFEB at the lysosomal surface and phosphorylates two key residues,119, 120 leading to 14-3-3 binding and cytoplasmic sequestration. Upon nutrient starvation, calcium is released by the lysosomal calcium channel MCOLN1, leading to the activation of a Ca2+-dependent phosphatase, calceniruin, which dephosphorylates TFEB,121 allowing its nuclear localization and activation of target genes. To suppress lysosomal gene expression, cells rely on the zinc-finger transcription factor, ZSCAN3, which sits at the promoters of multiple lysosomal genes and inhibits their expression during nutrient-replete conditions.122 Upon nutrient starvation or mTORC1 inhibition, ZSCAN3 is driven from the nucleus into the cytoplasm by an unknown mechanism. Other transcription pathways including FXR also sense cellular nutrient conditions (bile acids) and coordinate lysosomal gene expression.123, 124 A key challenge for future studies will be to provide an integrated portrait of how multiple nutrient-sensitive transcriptional pathways coordinate lysosomal gene expression in concert with anabolic signaling pathways.
9 AUTOPHAGY
In response to nutrient limitation and damage to proteins or organelles, eukaryotic cells activate a highly regulated set of pathways collectively known as autophagy. Because generation of energy and metabolic intermediates under starvation and the clearance of toxic proteins and damaged organelles is a prerequisite for any cell, defects in autophagy pathways underlie human diseases ranging from neurodegenerative disorders to cancer.125
At the heart of autophagy lies the lysosome—serving as the degradative endpoint for these pathways by providing key enzymes and a harsh environment necessary for the breakdown of toxic protein aggregates, damaged lipids or complex carbohydrates. Studies initiated in yeast have provided a solid genetic foundation for autophagy pathways with the discovery of ATG (autophagy-related genes)126 and their mammalian homologs. In mammalian cells two autophagy pathways exist: macroautophagy and chaperone-mediated autophagy (CMA). Macroautophagy (herein referred to as autophagy) is the degradation of cytosolic components through a double-membrane vesicle known as the autophagosome. CMA is characterized by the degradation of specific proteins marked with a charged recognition motif. Both forms of autophagy are highly regulated processes that sense general cell stress and are sensitive to nutrient deprivation. While autophagy functions as a rapid response to starvation and is induced within minutes of nutrient withdrawal, CMA reaches its maximal activation after 1.5 days. In addition to autophagy and CMA, specialized forms of selective autophagy that target organelles (mitophagy)127 or complexes (ribophagy)128-130 have recently emerged.
Initiation of autophagy occurs within minutes of nutrient deprivation and central to its regulation are mTORC1 and the energy-sensing AMPK pathway. During nutrient-replete conditions, lysosomal mTORC1 phosphorylates sites on key components of the master autophagy regulator ULK complex, composed of ULK1/2, ATG13, FIP200 and ATG101. mTORC1 phosphorylation of the serine/threonine kinase ULK1/2 at S75730 and ATG13 at S258131 inactivates the ULK complex to block autophagy induction. Upon starvation, mTORC1 is inactivated, in part due to a lack of free amino acids, and AMPK phosphorylates different sites on ULK1/2, necessary for its kinase activity.30, 132 Active ULK translocates to the ER133 and phosphorylates the class III phosphatidylinositol-3 phosphate (PI3P) kinase, VPS34,134 a component of VPS34 complex I (BECN1, VPS34, ATG14L and VPS15). Once activated, VPS34 deposits PI3P at the omegasome, a subdomain of the ER believed to give rise to the isolation membrane (IM).
The increase in PI3P levels recruits additional ATG proteins,135 leading to the nucleation of the IM at the omegasome. IMs initially form a cup-shaped double membrane structure that is readily resolved by EM and signal autophagy induction. IM expansion is driven by vesicular traffic from different compartments (Golgi,136 mitochondria137 and plasma membrane138), which provide a source of membranes to the growing structures. As IMs continue to expand, the ATG16L1 complex (ATG16L, ATG12 and ATG5), attaches the molecule LC3 to the IM. LC3 conjugation with phosphatidylethanolamine is a critical for downstream effector protein recognition and autophagasome closure139 and is often used as a molecular marker for autophagy induction. Eventually, the IM pinches off from the omegasome and as it closes to form the autophagasome it swallows cytosolic components.
After closure, autophagasomes merge with lysosomes to form autolysosomes, wherein the luminal contents of autophagasomes are degraded in the lysosomal lumen. While the molecular mechanisms governing autolysosome formation are not as well characterized as autophagasome initiation, it is now clear that autolysosome formation shares many components with from the lysosome-endosome fusion pathway.140 One of the best characterized components in autophagasome-lysosome fusion is the SNARE Stx17.141, 142 SNARE proteins are required for membrane fusion, with a SNARE (Q-SNARE) on a vesicle forming a coiled-coil structure with a SNARE (R-SNARE) on a target membrane generating a trans-SNARE complex and driving fusion of the vesicle with the target membrane. In the context of autophagy, Stx17 is recruited to the closed autophagasome from the ER by a poorly defined mechanism and binds to another Q-SNARE, SNAP29. The Stx17/SNAP29 complex is further stabilized by ATG14142 and interacts with the lysosomal R-SNARE, VAMP8, leading to membrane fusion and autolysosome formation.141 Additional components required for autolysosome formation are the small GTPase Rab7,143 required for late endosome/lysosome fusion events its effector the homotypic fusion and vacuolar sorting (HOPS)144 and its GEF the Mon1-Ccz1 complex.145 In addition to the molecular recognition between Stx17 and Vamp8, PLEKHM1 serves as a tether between lysosomal HOPS and LC3, ensuring correct autophagasome-lysosome fusion.146
Following the degradation of its contents, the lysosome needs to reform from the autolysosome to support additional rounds of autophagy through a process known as autophagic lysosome reformation (ALR).147, 148 ALR initiates with the formation of tubules in the autolysosome driven by clathrin, PI(4,5)P2 and the motor protein KIF5B.149, 150 Sorting of lysosomal proteins is thought to be mediated by the lipid kinase PI4KIIIß151 in a poorly understood manner. Curiously, ALR is regulated by mTORC1, as treatment with rapamycin inhibits this process, suggesting a model in which ALR functions as a stop-gap for autophagy once autophagy-generated nutrient levels are sufficient to reactivate mTORC1.152
10 CHAPERONE-MEDIATED AUTOPHAGY
In contrast to the degradation of cytosolic components under autophagy, CMA leads to the lysosomal degradation of only a subset of proteins that contain CMA motifs. CMA is a highly orchestrated process involving multiple steps: substrate recognition and lysosomal targeting, substrate binding and unfolding, substrate translocation and degradation in the lysosomal lumen. Key to CMA is the recognition of substrates by the cytosolic chaperone and heat shock protein HSC70.153 HSC70 binds to protein substrates with a consensus pentapeptide motif KFERQ154 that is recognized based on its charge. Additionally, incomplete motifs may be complemented through posttranslational modification of adjacent residues by phosphorylation or acetylation.155 These PTMs thus provide the necessary charge recognition by HSC70.156
Once bound by HSC70, the protein substrate is targeted to the lysosomal surface where it interacts with the cytoplasmic tail of LAMP-2A. LAMP-2A functions as the receptor for CMA substrates157 and multimerizers upon contact with the substrate to facilitate lysosomal entry.158 To enter the lysosomal lumen the substrate must unfold in a process mediated by HSC70 and its co-chaperones at the lysosomal membrane. Translocation of the substrate further requires a resident lysosomal luminal chaperone lys-HSC70.159 lys-HSC70 facilitates substrate entry by directly pulling on the incoming substrate or by actively blocking its departure from the lysosomal lumen. After substrate translocation, the LAMP-2A multimers are dissembled so the CMA cycle can begin again.
One regulatory point in CMA is the assembly/disassembly of LAMP-2A multimers. Stabilization of LAMP-2A multimers is enhanced by the protein GFAP in its unphosphorylated state. Recent work from the Cuervo lab has demonstrated that AKT phosphorylates GFAP, leading to the destabilization of LAMP2a multimers. This study connects CMA to a balance of AKT activation at the lysosomal surface and inhibition by mTORC2 and a protein phosphatase, PHLPP1.160 While conceivably mTORC2 at other cellular locations could also be involved in this process, Cuervo and coworkers found that mTORC2 associated with a subset of lysosomes; the mTORC2 component Rictor was found only in the subset of lysosomes engaged in CMA. In contrast, the mTORC1 subunit Raptor was enriched in lysosomes not engaged in CMA.160 In addition to being regulated by mTORC2, CMA is also regulated transcriptionally through the expression of LAMP-2A, which can be dynamically modulated during hypoxia, oxidative stress, genotoxic stress or prolonged starvation. This process is mediated in part by TFEB pathway.161
11 THE LYSOSOME IN AGING AND DISEASE
Lysosomal function is essential for the healthy functioning of both individual cells and for an entire organism. Aging represents the end result of accumulating deficits at the molecular, cellular and organismal level, and aberrant lysosomal signaling during aging contributes to the degradation of these processes. Loss of proteostasis has been proposed as one of the nine hallmarks of aging; while many different processes contribute to the maintenance of proteostasis, an important part of this process involves the degradation of proteins by the lysosome during both CMA and macroautophagy. Proteostasis declines with aging in most organisms, with the possible exception of the exceptionally long-lived naked mole rat.162, 163
Just a decade ago, it was demonstrated that rapamycin extends the life span of mice, a result that has been widely reproduced.164, 165 Indeed, even intermittent or transient treatment with rapamycin can extend the life span of mice and rejuvenate specific tissues, including the hematopoietic system, heart, immune system and kidney.166-172 In part, this may be due to the ability of rapamycin to block age-associated increases in mTOR signaling in certain tissues.173, 174 However, the specific molecular and physiological mechanisms by which rapamycin extends life span are difficult to examine in mammals; while it is clear that inhibition of S6K1 signaling extends life span, and that inhibition of translation downstream of 4E-BP1 is beneficial for metabolic health, the contribution of autophagy to the effects of rapamycin on mammalian longevity has not been examined in detail.175-177 However, there is reason to believe that many of the beneficial effects of rapamycin on aging may be driven in part by increased autophagy.
First, both CMA178 and autophagy naturally decline with aging.179 Evidence from model organisms suggests that this is detrimental. Overexpression of chaperones extends the life span of worms and flies.180, 181 Worms and flies with defective autophagy have reduced life span, while conversely overexpression of autophagy genes such as Atg8a extends life span.182, 183 Finally, experiments in both worms and flies have found that an inhibition of autophagy genes blunts the effects of reduced mTOR signaling on life span.184, 185 In mammals, mice with genetic impairment of CMA have reduced life span, with an increase in age-related pathology and accelerated cellular senescence.186 While it is not yet known if upregulation of CMA can extend mammalian life span, an increase in hepatic CMA can rejuvenate the aging mouse liver.178 However, overexpression of Atg5, which enhances autophagy in mice, increases resistance to oxidative stress and increases life span.187
Discovering safe and effective ways to regulate lysosomal function and to boost autophagy is likely to be essential to promote healthy aging and to treat a variety of age-associated diseases. Many neurodegenerative diseases, including Alzheimer's disease, is thought to be driven in part by misfolded proteins; promoting proteostasis, either by increasing lysosomal function directly or by boosting autophagy through other means is therefore a potential mechanism to delay or treat the disease.188-190 In Alzheimer's disease, which is thought to be partly precipitated by amyloid aggregation, the hope is that stimulating autophagy will clear these protein aggregates.191 Conversely, autophagy is believed to be beneficial for tumorigenesis and for the survival of many types of cancer cells that are under nutrient stress,192 and inhibiting this process to disrupt proteostasis has emerged as a potential therapeutic option for some malignancies, with recent preclinical studies targeting core autophagy proteins showing promise.193, 194 Thus, autophagy has been catapulted to the forefront as a therapeutic avenue for multiple disease states; however, caution must be applied as targeting any metabolic signaling pathway must be considered in a context-dependent manner.
12 SUMMARY
As a newly described center for the integration of major catabolic and anabolic pathways, the lysosome is positioned as a key sensor of cellular nutrient levels. With a growing appreciation for its diverse functions within the cell, many questions still loom large. At the biochemical level, understanding whether additional nutrients such as nucleotides and lipids are sensed at the lysosome and their corresponding sensors will be critical in deciphering the inner workings of metabolic signaling at this organelle. At the cellular level, providing a systems level overview of nutrient signaling at the lysosome and exploring the crosstalk between the lysosome and other organelles will allow us to predict how different cellular stressors change nutrient flux and signaling. At the organismal level, the future lies at understanding how lysosome-based metabolic signaling translates to different tissues and organ systems. Underlying the desire to study metabolic signaling at the lysosome is the immediate realization that disruption of these pathways leads to major human pathologies. While the molecular mechanisms of lysosomal signaling in cancer, aging and immune disorders remain at their infancy, the last decade has seen the development of a numerous inhibitors targeting multiple parts of this pathway.195 While the utility of rapamycin in a handful disorders is clear, its overall success in preventing cancer growth remains poor. Whether this is due to an incomplete understanding of mTORC1's requirement for rapid cell proliferation, the ability of cancers to acquire rapamycin resistance,196 the reactivation of PI3K signaling due to systemic glucose-insulin feedback on most diets197 or its mechanism of action as an allosteric inhibitor that blocks the phosphorylation of only some of the mTORC1 substrates, remains to be seen. However, there is a palpable excitement that the new generation of therapeutic agents targeting metabolic signaling at the lysosome will have substantial benefits for human health.
ACKNOWLEDGMENTS
We thank Drs Lynne Chantranupong and Anne Strohecker for critical reading of the manuscript. The work is supported in part by the NIH (AG050135, AG051974, AG056771 to D.W.L.) and (CA215249 to L.B.-P.) and by a pilot grant to D.W.L. from the Diabetes Research Center at Washington University, grant no. 2 P30 DK020579. The Lamming laboratory is supported by the U.S. Department of Veterans Affairs (I01-BX004031). D.W.L. is an American Federation for Aging Research (AFAR) grant recipient. L.B.-P. is the Lallage Feazel Wall Fellow of the Damon Runyon Cancer Research Foundation (DRG-2178-14). This work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
CONFLICTS OF INTEREST
D.W.L. has received funding from, and is a scientific advisory board member of, Aeonian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases.
Editorial Process File
The Editorial Process File is available in the online version of this article.