Reliability of water-based medium-expansion foam as a depopulation method for nursery pigs and cull sows
Andréia G. Arruda and Magnus R. Campler contributed equally for the work.
Abstract
Animal disease preparedness plans including depopulation guidelines are paramount to prevent the spread of emerging infectious diseases but difficult to implement for swine under field conditions. However, water-based foam (WBF) is currently an approved and successfully deployed depopulation methodology in poultry. Therefore, the reliability of WBF as a depopulation method and the effectiveness and irreversibility of consciousness and consequential mortality in pigs of different ages was assessed across two trials. Trial 1 investigated the time to loss of consciousness and cessation of cardiac activity in nursery pigs (n = 72) at six different foam immersion time points (2.5, 5, 7.5, 10, 12.5 and 15 min) when placed in a 1.47 m3 (1.2 × 1.2 × 1.02 m, length × width × height) plastic bulk container. One pig per replicate was implanted with an ECG bio-logger. Irreversible loss of consciousness was observed after a 5-min immersion. The average (SD) time to development of a fatal arrhythmia from the initiation of the foam application was 7.3 min (1.82 s). Trial 2 aimed to validate the findings from Trial 1 in 75 larger cull sows across three replicates (n = 25). Sows were loaded into a 41-m3 sealed trailer (12.2 × 1.5 × 2.24 m), immersed in WBF and left undisturbed for 5 min post foam-filling completion. Six pigs in each replicate were implanted with an ECG bio-logger. A 5-min dwell time resulted in irreversible loss of consciousness and subsequent mortality in all cull sows. The average time (SD) to cessation of movement and fatal arrhythmia post foam-filling completion was 2.2 min (34.8 s) and 8.7 min (138.0 s), respectively. While a 5-min immersion in WBF induced irreversible loss of consciousness and death in both trials, a 7.5-min dwell time followed by observation for confirmation of death post WBF removal would be advisable for pigs of all sizes.
1 INTRODUCTION
The increasing threat of novel pathogens and emerging diseases for the swine industry should not be underestimated. Infectious diseases are on the rise as the movement of people, goods and animals increases globally (Gaudreault et al., 2020). Disease outbreaks that occur far from the originating source are likely to be associated with increased travel and trade, causing additional concern in terms of spread prevention, source identification and local preparation and mitigation (Gaudreault et al., 2020; USDA – APHIS, 2022). For instance, African Swine Fever, not currently present on US soil, has recently been detected in the Dominican Republic and Haiti, and could have a significant negative impact on both swine welfare and the US swine economy if additional spread occurs (Brown et al., 2021; Paulino-Ramirez, 2021; USDA – APHIS, 2022). Therefore, access to readily available contingency plans for large-scale swine depopulation is paramount to prevent spread when outbreaks occur.
Despite existing guidelines for euthanasia and mass depopulation to prevent additional spread once disease is detected (Leary et al., 2019, 2020), applying these guidelines to large swine populations under field conditions is challenging. During a depopulation event, it is important to minimize pain and distress for the animals while ensuring the safety and mental health of the personnel carrying out the task (Baysinger & Kogan, 2022; Leary et al., 2019; Stikeleather et al., 2013; Whiting & Marion, 2011). Preferentially, under emergency depopulation scenarios, animals should be able to walk under their own power to a designated area designed for the task, as depopulation of pigs in situ and the consequential carcass removal from the facilities would be labour intensive.
Currently recommended depopulation methods include nonpenetrating and penetrating captive bolt, gunshot, electrocution, manual blunt force trauma, movement to slaughter and inhaled methods (carbon dioxide and anaesthetic overdose) (Leary et al., 2019). However, these methods come with various challenges. For example, blunt force trauma is only recommended for young animals and is labour intensive, as it is deployed on an individual basis and difficult to successfully implement over an extended period. Depopulation by gunshot presents similar challenges for large populations, adding concerns over ricochet, consistent placement in unrestrained animals, heating of guns after repeated application and the need for special firearms training for personnel (Leary et al., 2019). The efficacy and safety of electrocution has previously been questioned (Probst-Miller, 2010) but when applied correctly using commercial conveyorized restrainers, such as the permanent placed restrainers used in packing plants for stun and exsanguination, it is possible to enable mass depopulation of large populations (Grandin, 2021). Logistic obstacles and risks posed by moving large quantities of animals to a centralized facility during a disease outbreak are likely to outweigh any benefits compared to depopulation on-farm. However, the use of mobile electrocution units has previously been investigated (Douma et al., 2012; Lambooy & Van Voorst, 1986; Mote & Woiwode, 2020), but to our knowledge, none are commercially available in the US to date.
The use of carbon dioxide (CO2) has been criticized as highly aversive and distressful to pigs (Raj & Gregory, 1996; Steiner et al., 2019), despite being an approved euthanasia and recommended depopulation method by the AVMA (Underwood & Anthony, 2020). Gas-based methods may also be impacted by limited gas supplies and gas delivery systems depending on season and geographical location. Insufficient data has been generated under field conditions to establish firm guidelines for emergency situations, but several mobile prototypes for depopulating larger cohorts of pigs using inhalants such as CO2 have been proposed (Kinsey et al., 2016; Meyer & Morrow, 2005; Pepin et al., 2022; Rice et al., 2014; Stikeleather et al., 2013). Lastly, ventilation shutdown with the addition of elevated temperature and humidity has been listed in the AVMA guidelines under ‘permitted in constrained circumstances’, but little peer-reviewed information on the method is available in the literature (Baysinger et al., 2021).
A depopulation alternative currently approved for poultry is water-based foam (WBF) (Alphin et al., 2010; Benson et al., 2018; Gurung et al., 2018). The benefits of WBF compared to gas inhalants include the relative ease of use and minimal risks to personnel in case of a leak or equipment malfunction. As depopulation using foam periodically occurs for poultry in emergency situations such as the occurrence of Highly Pathogenic Avian Influenza (HPAI) outbreaks (Gurung et al., 2018), supplies are readily available in the National Veterinary Stockpile and could easily be adapted for swine in case of an emergency. Despite limited research on foam as a depopulation method in pigs, aspirated foam may be a promising alternative to pure gas inhalants such as CO2 and nitrogen gas (N2) (Lindahl et al., 2020). In addition, WBF has been reported to be equally as effective as CO2 and N2 gas alone or in combination with WBF (Lorbach et al., 2021).
The objectives of the present study were (1) to assess the reliability of WBF in inducing irreversible loss of consciousness and cessation of cardiac activity in nursery pigs and (2) validate findings from Trial 1 in cull sows by investigating the minimum time needed to induce a nonrecoverable state.
2 MATERIAL AND METHODS
2.1 Ethics and institutional oversight
This trial was completed in accordance with animal use protocol 2020A00000036 approved by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University. The secondary euthanasia method available on site throughout the trials was penetrating captive bolt, which would be applied by an experienced user as needed. All nursery pigs and cull sows used in the studies were housed and handled according to the Guide for the Care and Use of Agricultural Animals in Research and Teaching.
2.2 Animal subjects and study design
Trial 1: Reliability, irreversibility and cessation of cardiac activity in nursery pigs
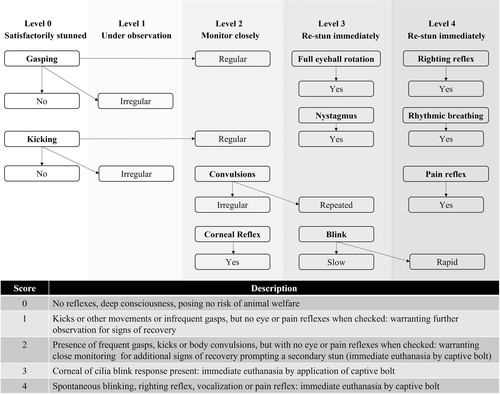
Seventy-two nursery pigs (mean weight ± SD, 19.9 ± 4.9 kg, minimum = 8.6 kg; maximum = 31.8 kg) of mixed sex was sourced locally from The Ohio State University Swine Center and housed on-farm until the start of the trial. Pigs were assigned into 12 replicates, six pigs in each replicate, balanced to maintain near uniform individual weight within a replicate (mean replicate weight = 19.3 ± 2.8 kg, minimum = 15.1 kg, maximum = 24.5 kg). The day prior to the study start, one pig from each replicate (mean weight 20.9 ± 4.6 kg, minimum = 14.5 kg, maximum = 30.4 kg), was selected by convenience for the implantation of a subcutaneous bio-logger for activity and heart rate monitoring (DST-Centi-HRT ACT, Star-Oddi, Gardabaer, Iceland) and given a minimum 24 h recovery time before trial start. Immediately prior to the trial start, pigs from each replicate had a uniquely coloured nylon rope attached (tied and taped) to the right rear leg. Each colour-coded rope corresponded to one of six WBF foam immersion time points (2.5, 5, 7.5, 10, 12.5 and 15 min), which started after foam-filling completion (detailed in the next sections). The ropes facilitated retrieval of each pig at the given time interval with minimal disturbance to other pigs. All pigs with bio-loggers were assigned to the 15-min time point group. After the ropes were fastened and secured, all pigs (n = 6) within each replicate were moved to an opaque, 1.47 m3 plastic bulk container (1.2 × 1.2 × 1.02 m, length × width × height). The container provided 0.24 m2 of available floor surface space per pig. Water-based medium-expansion foam was then pumped into the container using a single pump and one aspirated foam nozzle. Pigs remained immersed in the foam until their specific time allocation based on the colour-coded rope. Upon removal of individual pigs according to the time schedule, each pig was laid on a designated concrete surface and observed for level of consciousness using a consciousness assessment rubric (Figure 1) adapted from Atkinson et al. (2012). In brief, pigs scored at levels 2, 3 or 4 in relation to gasping, kicking or corneal reflexes immediately received secondary euthanasia using a penetrating captive bolt. All pigs were confirmed dead prior to carcass disposal. All replicates were conducted within the same day.
Given the lack of information available in the literature with this specific method, sample size calculations for Trial 1 were based on detecting a difference of 50% in proportion of pigs achieving loss of consciousness between two time points using 80% power, and 95% confidence (Epitools, n.d.).
Trial 2: Validation of findings in cull sows
A total of 75 healthy cull sows (approximate purchase weight 200 to 275 kg) arrived 2 days before the trial started and were divided into three replicate groups with 25 sows in each replicate. A convenience sample of six sows from each replicate was selected for the implantation of a subcutaneous bio-logger (see Trial 1) for activity and heart-rate monitoring (DST-Centi-HRT ACT, Star-Oddi, Gardabaer, Iceland) immediately prior to the trial start. A 64.5-m3 (dimensions 12.2 × 2.36 × 2.24 m; length, width and height) rendering trailer was customized to allow animal loading via a ramp and carcass unloading post-depopulation through a hydraulic lift system through the back of the trailer. A combination (side to side, ½ width slide for loading; whole gate top-hinged for unloading) cut gate was located at mid-length, allowing for added compartmentalization within the trailer during loading and foaming (Figure 2). Flooring within the rendering trailer was coated with elastomeric polyurethane to improve traction and reduce slips, but trailer walls were smooth to limit animal escapes. For each replicate, all cull sows (n = 25) were directly loaded from the holding pens into the modified rendering trailer using a single file loading ramp. The trailer stocking density after loading was approximately 1.15 m2/sow, exceeding the 0.73 m2/sow transport stocking density recommendation for a 227 kg sow in Europe (Council of the European Union, 2004; Lammens et al., 2007) and in North America (Pereira et al., 2018; Pilcher et al., 2011). Shortly after completion of the loading process, foaming was initiated through the open canopy section at the top of the trailer and continued until the trailer was filled, and all pigs were immersed. Three individual foaming systems were used in tandem in all replicates to ensure a rapid fill of the trailer. Once filled, the trailer was not disturbed for an additional 5 min (following recommendation from Trial 1, which is described in detail under the results section). Thereafter, the trailer was hydraulically lifted for sows to be removed. Individual sows were immediately evaluated for stunning efficacy and level of consciousness by several trained research team members (Figure 1). Residual foam in the trailer was evacuated using a leaf blower in between replicates. Penetrating captive bolt guns were available as secondary euthanasia tools but were not used as no animals showed signs of return to consciousness or recovery. Due to data downloading and carcass disposal scheduling, Trial 2 was conducted over 2 days, with two replicates completed in the morning and afternoon of day 1 and the third during the morning on day 2.

2.3 Foam production and application
During both trials, foam was generated using gas-powered water pumps and a medium-expansion foam nozzle(s). Foam was generated during the trials using PHOS-CHEK WD881 Class A foam concentrate (Perimeter Solutions, Rancho Cucamonga, CA, USA) added to freshwater vessels (1,135 and 1,892 L, Trial 1 and Trial 2, respectively) creating a 1% foam-water solution. The foam-water solution was pumped from the foam-solution holding vessels by gasoline-powered water pumps (AMT Pump Company 2MP13HR, Royersford, PA, USA) via a 5.08-cm diameter suction hose connected to the pump inlet. The foam-water solution was delivered out of the pump in a 3.81-cm diameter firehose (15 m in length) to a medium expansion aspirated foam nozzle (KR-M4, ANSUL, Marinette, WI, USA). This setup enabled a 40–50:1 foam-to-water expansion ratio. One research team member operated the pump, while another operated the nozzle to fill the depopulation chamber. For Trial 2, three foam production setups with associated aspirated foam nozzles as previously described, were used to increase fill speed for the larger trailer. Foam was delivered until a visible overflow of either the bulk container or trailer occurred. Personnel involved in the operation wore personal protective equipment including hats, gloves, glasses, boots and appropriate waterproof clothing at all times. To prevent falling accidents off or into the trailer during the foaming procedure, staff wore fall protection harnesses equipped with snap hook lanyards attached to a railing on top of the trailer (Figure 3).

2.4 Bio-logger placement
For Trial 1, pigs receiving DST-Centi-HRT-ACT bio-loggers were placed under general anaesthesia using a reconstituted mixture of Telazol (50 mg tiletamine, 50 mg zolazepam powder), 2.5 ml xylazine (250 mg total) and 2.5 ml ketamine (250 mg total) administered at 1 ml/27.2 kg of bodyweight intramuscularly in the rear leg. After confirmation of each pig achieving a surgical anaesthetic plane, they were placed in lateral recumbency and an area behind either the left or right triceps was blocked with 2% lidocaine, clipped and scrubbed aseptically with betadine soap and isopropyl alcohol. A 2.54-cm incision was made, and space created subcutaneously for the bio-logger via blunt dissection. After device placement, the wound was closed with 35 mm (35 W) surgical staples. For Trial 2, sows receiving DST-Centi-HRT-ACT bio-loggers were snared, and the site of implantation was prepared as indicated in Trial 1 with a lidocaine block (buffered with 8.4% sodium bicarbonate in a 1:9 ratio of bicarbonate to lidocaine) followed by bio-logger subcutaneous placement and closing of the wound using surgical staples.
2.5 Activity measurement and data processing
The DST-Centi-HRT-ACT subcutaneous data logger uses tri-axial acceleration technology to measure activity as external acceleration (EA) forces deviating from the normal force of gravity (g0, 9.8 m s−2) placed on the data logger in its resting state. Raw acceleration is measured on each of the three axes and later normalized to 1 g0 based on measurements for each axis during rotation. All data was downloaded from the bio-logger using the associated Mercury software v5.99 (Star Oddi, Gardabaer, Iceland). The software algorithm calculated the EA as vectorial sum of dynamic body acceleration in any plane (veDBA; reported by the software in mg0), allowing visualization of EA and axis plane force over time, indicating the force and direction of movement for each time stamp. The bio-loggers were pre-set to record EA every 15 s throughout the duration of the study, which was the minimum allowed by the device.
To differentiate activity from the constant effect of natural g-forces applied in the resting state, an activity threshold for each pig had to be established. The activity period of interest was established by truncating EA data collected for each individual pig from the start of foam fill to the end of the dwell period. The pig specific activity threshold (TEA) was then calculated as the third quartile (EAQ3) plus 1.5 times the inter-quartile range (EAQ3−EAQ1) from the EA obtained throughout the depopulation procedure (TEA = EAQ3 + 1.5 × (EAQ3–EAQ1)).
Time to cessation of movement was defined as the time stamp after the last observed activity above the individually calculated threshold (TEA) without any additional activity above the activity threshold, until the end of the 5-min dwell time. For example, if the last activity measurement above the set threshold was observed at 1-min immersion and no other activity above the activity threshold was observed thereafter, 1-min and 15 s was recorded as cessation of movement (COM). Currently, guidelines on best practices or ‘gold standards’ for establishing thresholds of true inactivity in swine using this specific bio-logger and EA measurements are lacking. Therefore, we opted for using a conservative threshold calculation due to the lack of visual confirmation during the application of the depopulation method.
2.6 Heart rate measurement and data processing
Electrocardiogram (ECG) tracings recovered from the subcutaneous bio-loggers were reviewed to determine the time of death as indicated by asystole or presence of a fatal arrhythmia (third degree atrioventricular block [AVB], atrial standstill or ventricular fibrillation [VF]). Persistent or pulseless electrical activity (PEA) or electromechanical dissociation was defined as presence of any rhythm at the time of physical confirmation of death (i.e. clinical asystole). ECG tracings were reviewed utilizing the associated Mercury software v5.99 and Pattern Finder software v1.17.0 (Star Oddi, Gardabaer, Iceland). The data logger was programmed to record 3 consecutive seconds (s) of ECG tracing every 15 s. Due to the timing of placement for bio-loggers, heart rate and rhythm collection used as ‘baseline’ were limited to a 5-min period prior to foam initiation. All heart rate data and ECG rhythm were collected up to 15 min post-foaming initiation for a total of 20 min.
2.7 Statistical analysis
For Trial 1, descriptive statistics including proportions and frequency distributions were used for irreversibility assessments and description of time to cessation of cardiac activity. Only time points producing 100% efficacy in inducing loss of consciousness were considered efficacious in our study. Time to cessation of cardiac activity for sows was descriptively presented as frequency distribution.
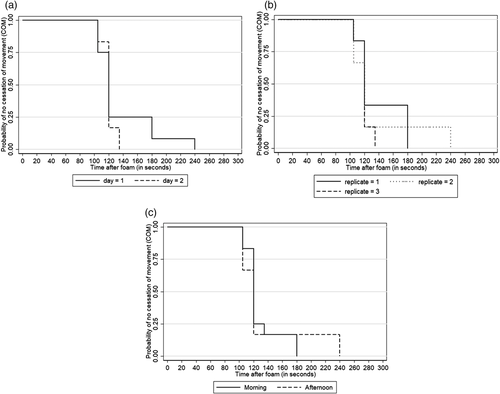
For Trial 2, descriptive statistics including estimation of mean, median, standard deviation, minimum and maximum were used to describe time to COM by replicate (1, 2 and 3), day (1 and 2) and time of day (morning or afternoon). Lastly, the effect of these variables on COM was tested in a univariate analysis fashion using the log-rank test of survival equality across strata, which is a nonparametric test recommended for skewed data. Finally, the probability of surviving to the outcome (cessation of movement) by strata was plotted using Kaplan-Meier curves for all significant predictors. Statistical significance was declared as p < .05, and all analysis and graphs were conducted using Stata 14 (College Station, Texas).
3 RESULTS
Trial 1: Reliability, irreversibility and cessation of cardiac activity in nursery pigs
The average time (mean ± SD) to fill the container with foam was 4.25 ± 1.1 s (minimum = 3.0 s, maximum = 7.0 s). At the 2.5-min time point, across all replicates, all 12 pigs displayed noticeable gasping events, whereof nine pigs displayed irregular gasping (level 1) and three pigs displayed regular gasping that required additional monitoring (level 2). In addition, all three pigs that showed irregular gasping also had a corneal reflex present (level 2). For the three pigs that displayed regular gasping, all also displayed slow blinking (level 3). No kicking or convulsions were observed. Thus, all pigs removed at the 2.5-min time point, showed a level of consciousness that could result in stunning reversibility and were thus immediately euthanized by penetrating captive bolt. For pigs removed at the 5-min time point, four out of 12 pigs showed signs of irregular gasping (level 1) that quickly ceased, and eight showed no signs of gasping at all. In addition, no corneal reflex, kicking nor convulsions were observed in any of the pigs at the 5-min time point. No pigs, at or after the 5-min dwell time, required euthanasia by penetrating captive bolt. No signs of consciousness or reversal into consciousness were observed for any pigs in each of the 7.5-, 10-, 12.5- or 15-min foaming intervals, and none of the pigs needed euthanasia by the secondary method. The data and observed single step euthanasia efficacy for each time point in Trial 1 were used to determine the 5-min dwell time parameter used in Trial 2.
The average nursery pig heart rate at loading (mean ± SD) across pigs was 149.8 ± 14.9 bpm. Due to amplified pig movement and muscle activity, ECG interpretation could not be successfully performed from the obtained bio-logger data for any of the pigs at the time of foam initiation and for seven out of 12 pigs at 1 min after foam initiation. Compared to loading time heart rates, average heart rates were reduced by 39.3 % at 2 min after foam application. The average heart rate increased and returned to loading time levels at 3 min after foam application (142.5 ± 14.0 bpm) before beginning a decline towards heart rates of 60–80 bpm at 5–8 min post foam fill as an increasing number of pigs with ceased cardiac activity (Figure 4).
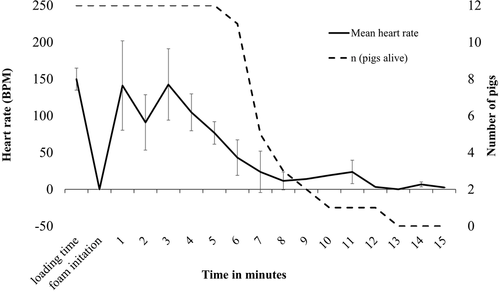
For cessation of cardiac activity, all pigs had some cardiac electrical activity up to 5 min post foam fill. At 7.5 min post fill, eight out of 12 pigs had no cardiac activity, and by 10 min one remaining pig had cardiac activity which lasted up until 12 min post-fill (Table 1). The average time to development of a fatal arrhythmia from the initiation of the foam application was 7.3 min ± 1.82 s (7 min 18 s ± 1 min 50 s) across the implanted pigs. Nine of the 12 pigs developed atrial standstill, one pig developed a severe third degree AVB with no ventricular response, and two pigs exhibited pulseless electrical activity (Table 1).
| Foam fill time | Clinical death | ||||
|---|---|---|---|---|---|
| Replicate | Start | End | Death | Time to death (hh:mm:ss) | Cause of death |
| 1 | 6:22:30 | 6:22:34 | 6:30:20 | 0:07:50 | Asystole |
| 2 | 6:44:09 | 6:44:13 | 6:50:10 | 0:06:01 | Asystole |
| 3 | 7:37:52 | 7:37:55 | 7:44:46 | 0:06:54 | Third degree AVB |
| 4 | 7:59:53 | 7:59:56 | 8:06:56 | 0:07:03 | Asystole |
| 5 | 8:21:11 | 8:21:14 | 8:29:20 | 0:08:09 | Asystole |
| 6 | 8:40:03 | 8:40:08 | 8:52:12 | 0:12:09 | Sinus bradycardia |
| 7 | 8:58:28 | 8:58:32 | 9:04:34 | 0:06:06 | Asystole |
| 8 | 9:16:47 | 9:16:54 | 9:22:46 | 0:05:59 | Asystole |
| 9 | 9:34:16 | 9:34:20 | 9:40:16 | 0:06:00 | Asystole |
| 10 | 9:51:45 | 9:51:49 | 9:58:14 | 0:06:29 | Asystole |
| 11 | 10:08:41 | 10:08:46 | 10:17:50 | 0:09:09 | Sinus bradycardia |
| 12 | 10:25:20 | 10:25:24 | 10:31:36 | 0:06:16 | Asystole |
- Note: Times are presented in 24-h clock format
Trial 2: Validation in cull sows
The average time (mean ± SD) to fill the enclosed trailer with foam was 83.0 ± 12.0 s (minimum = 67.0, maximum = 96.0). The average time to COM across all three replicates was 130.8 ± 34.8 s (minimum = 105, maximum = 240). No difference in time to COM between replicates was observed (137.5 ± 30.5 vs. 135 ± 47.4 vs. 120 ± 8.7 s, replicate 1, 2 and 3, respectively, p = .78). No difference in time to COM between days 1 and 2 was observed (136 ± 39.9 vs. 120 ± 8.7 s, p = .88), and no difference in time to COM between morning and afternoon was observed (p = .94). Kaplan-Meier curves for these are presented in Figure 5.
The average heart rate (mean ± SD) of sows at loading was 129.0 ± 21.0 bpm. Due to amplified pig movement and muscle activity, heart rate data was corrupted and unrecoverable during the foam filling procedure. Electrocardiogram interpretation could not be successfully performed for nine out of 18 sows during the foam filling procedure, and for 13 out of 18 sows and for 5 out 18 sows at the 1-min and 3-min time points postfoaming, respectively. The average heart rate increased during the foam initiation but decreased rapidly during the next two successive minutes. After a temporary increase in heart rate at 3- and 4 min post foam initiation, heart rates decreased slowly for pigs still showing signs of cardiac activity (n = 4) up to 11 min postfoaming (Figure 6).
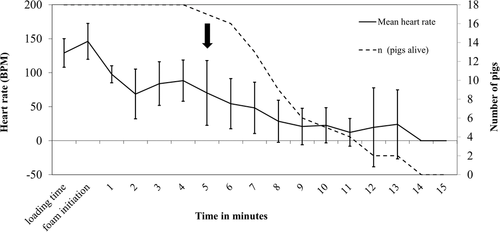
The average time to development of a fatal arrhythmia from the initiation of the foam application was 8.7 ± 2.3 min (8 min 41 s ± 2 min 20 s) across the bio-logger-implanted sows. Ten of the 18 sows developed atrial standstill, four sows developed ventricular fibrillation, three sows developed a severe third degree AVB with no ventricular response, and one sow developed pulseless electrical activity (Table 2). No significant foam breakdown from pig activity was observed, as fill levels remained stable throughout the test period and no additional top-off was needed.
| Foam fill time | Clinical death | |||||
|---|---|---|---|---|---|---|
| Replicate | SowID | Start | End | Death | Time to death (hh:mm:ss) | Cause of death |
| 1 | 1 | 11:10:40 | 11:11:47 | 11:19:37 | 0:07:50 | Asystole |
| 1 | 2 | 11:10:40 | 11:11:47 | 11:20:46 | 0:08:59 | Third degree AVB |
| 1 | 3 | 11:10:40 | 11:11:47 | 11:18:40 | 0:06:53 | Asystole |
| 1 | 4 | 11:10:40 | 11:11:47 | 11:21:42 | 0:09:55 | Third degree AVB |
| 1 | 5 | 11:10:40 | 11:11:47 | 11:19:36 | 0:07:49 | Asystole |
| 1 | 6 | 11:10:40 | 11:11:47 | 11:18:40 | 0:06:53 | Asystole |
| 2 | 7 | 14:04:12 | 14:05:28 | 14:18:18 | 0:12:50 | Asystole |
| 2 | 8 | 14:04:12 | 14:05:28 | 14:12:14 | 0:06:46 | Asystole |
| 2 | 9 | 14:04:12 | 14:05:28 | 14:14:06 | 0:08:38 | Third degree AVB |
| 2 | 10 | 14:04:12 | 14:05:28 | 14:18:18 | 0:12:50 | Asystole |
| 2 | 11 | 14:04:12 | 14:05:28 | 14:11:18 | 0:05:50 | Asystole |
| 2 | 12 | 14:04:12 | 14:05:28 | 14:16:12 | 0:10:44 | VB |
| 3 | 13 | 11:16:44 | 11:18:20 | 11:23:20 | 0:05:00 | Asystole |
| 3 | 14 | 11:16:44 | 11:18:20 | 11:29:24 | 0:11:04 | VB |
| 3 | 15 | 11:16:44 | 11:18:20 | 11:25:26 | 0:07:06 | VB |
| 3 | 16 | 11:16:44 | 11:18:20 | 11:27:18 | 0:08:58 | VB |
| 3 | 17 | 11:16:44 | 11:18:20 | 11:29:24 | 0:11:04 | Asystole |
| 3 | 18 | 11:16:44 | 11:18:20 | 11:25:26 | 0:07:06 | Third degree AVB |
- Note: Times are presented in 24-h clock format.
4 DISCUSSION
This study aimed to assess the reliability of WBF as a method to depopulate swine, and our results indicated that WBF causes irreversible loss of consciousness within 5 min of foam immersion for all nursery pigs and sows. Even though they were nonrecoverable, 25% of the Trial 1 pigs removed from the WBF at the 5-min mark displayed irregular gasping. This irregular gasping would complicate the best practice of observing animals to confirm death following application of any depopulation method. Therefore, we suggest a minimum dwell time of 7.5 min for pigs of all sizes with an additional observation period post WBF removal to ensure irreversibility.
On average, cardiac arrest occurred at 2.3 min after the onset of unconsciousness (Trial 1) and 3.7 min after an observed cessation of movement (Trial 2). However, individual pigs showed cardiac activity up until 8 min post unconsciousness or post cessation of movement in Trials 1 and 2, respectively. This met our expectation, as electrical cardiac activities and heart rates could remain detectable up to 12 min after stunning with gas (Lindahl et al., 2020) and 10 min after blunt force during humane euthanasia (Kramer et al., 2021). For example, Kramer et al. (2021) studied the effectiveness of different captive bolt technology and application locations in causing death in large, heavy weight boars and sows and measured heart rates with a pulse oximeter along with respiratory arrest by visual assessment. Similar to the present study, the authors noted that heartbeat could be detected for more than 10 min after stunning despite physical trauma to the brain tissue.
The ECG tracings were of sufficient quality for valid interpretation in all 30 implanted swine (12 nursery pigs and 18 sows combined). During limited parts of the trial, portions of some ECG tracings were unreadable due to abrupt and highly intensive pig movements, muscle activities or fasciculations, a finding also observed in another trial using heart rate bio-loggers (Terlouw et al., 2021). Fatal arrhythmias, such as third-degree AVB, atrial standstill and ventricular fibrillation, were identified in 27 out of 30 cases while the remaining three pigs developed PEA. Acidosis is a known factor that exacerbates ventricular mechanical failure and impairs the cardiovascular system due to its negative inotropic and vasodilatory effects (Desbiens, 2008). Together, the failing mechanical activity of the myocardium and vasodilation worsens the acidosis, which results in myocardial electromechanical dissociation, unconsciousness, and death. When compared across trials, average heart rates displayed a similar pattern. In both trials, an increase in average heart rate was noted during loading, at foaming initiation, and between 2 min and 3-min following foam application. These responses are indicative with activity and stress during loading, the reaction to the novelty of foam exposure and physiological reactions to a hypoxic environment (Lindahl et al., 2020). In Trial 1, the pigs were nonresponsive and clinically deceased on average at 7.5 min, indicating any ongoing rhythm to be pulseless electrical activity. In both trials, atrial standstill was the most frequent fatal arrhythmia identified. The delay in development of fatal arrhythmias for sows in Trial 2 when compared to pigs in Trial 1 is hypothesized to be contributed to the difference in body size in conjunction with differences in volume size of the chambers used in the present study. The chamber size difference led to increased time required to fill the larger trailer unit when compared to the smaller container used in Trial 1 which may have slightly delayed the effect of a hypoxic environment. To establish a better timeline between loading, unconsciousness and death, valuable data could be gained using additional techniques such as electroencephalogram to establish a more detailed timeline of brain functions during WBF.
The appeal factor of certain mass-depopulation methods to the general public should not be underestimated in a time when animal welfare is ranked high in terms of importance for animal production (Fraser, 2008). As such, robust confirmation that a rapid onset of unconsciousness occurs far sooner than death with minimized suffering as a result, is important for gaining the trust and approval of the general public and other stakeholders responsible for approval and future implementation. In addition, it is important to disseminate to an unfamiliar audience that various number of possible physiological or behavioural occurrences may be observed during stunning or after loss of consciousness and should not be interpreted as acute suffering. For instance, loss of movement coordination, involuntary righting reflex, convulsions and involuntary movements such as muscle contractions and paddling are not uncommon during for instance commercial slaughter or depopulation using CO2 (Meyer & Morrow, 2005; Sadler et al., 2014; Terlouw et al., 2021).
The main strength of the present study is that nursery trial was conducted on-farm under simulated transport stocking density in containment that were appropriate for rapid full coverage of foam. In addition, the sow trial was conducted under field conditions, loading larger cohorts of commercial sows into a sealed containment vessel that controlled animal, waste and water effluents. While stocking density allowance was greater than transportation guidelines, the authors are confident that loading at transportation guidelines would not adversely influence time to death in pig populations. The field condition aspect is important when testing a methodology that will be applied during nonstandardized conditions and under time constraints. The development of a depopulation methodology designed to be applied outside of the animal housing areas for all pig sizes enables our concept to be more generalizable to the swine industry. In addition, this depopulation methodology can be applied to a wide range of commercial farms that may need to undergo depopulation without consideration of specific housing conditions, production type or population size. Another strength is the mobility of the trailer unit, the availability of the associated equipment and the ease of setup, breakdown and storage, enabling the depopulation trailer unit to be quickly moved to other locations if needed.
The present study also had limitations. As a function of the opaque nature of WBF, pigs were obscured from visual observation during the depopulation process; therefore, a threshold was needed to establish ‘cessation of movement’, as no visible confirmation could be performed to validate any activity output from the bio-logger. The bio-loggers used in this study had not previously been validated for activity in swine, but validation of the external acceleration as a good activity measurement have been conducted in other species such as salmon and sheep (Fuchs et al., 2019; Zrini & Gamperl, 2021) and used successfully in a recent swine depopulation study using CO2 (Pepin et al., 2022). Ideally, a true activity baseline should have been collected over the previous days to the field trials for increased sensitivity for activity threshold development, but this approach was not logistically achievable at the time of the study. Moreover, any housing of pigs on site would have had to be extended across multiple days to exclude the initial 48 h post regrouping where sows would be active and subjected to agonistic interactions and thus yield elevated activity levels (Hoy & Bauer, 2005). However, as the endpoint of the sows used ultimately was an irreversible state of unconsciousness and later death, focus was on the cessation of movement and irreversibility rather than bio-logger validation during WBF conditions. As no guidelines are available to define cessation of movements in swine for the bio-loggers used in this study, a conservative threshold had to be created, as no visible confirmation was possible due to the obscuring foam. Thus, we utilized a threshold based on the calculation of outliers for skewed data (Carling, 2000) and used activity data for the whole depopulation given this period contained moments of high activity during the initial phase of the foaming process.
In conclusion, results from this study indicates loss of consciousness occurs between 2.5 min and 5 min post foam immersion and that prolonged immersion in the foam past this time point guarantees irreversibility and subsequent death. Furthermore, the recommended immersion time during the application of WBF must be based on time from complete coverage of the animals, independent of time to fill containment, as container size will vary across applications and time to animal coverage will vary by animal size. Based on the measurements from the present study, the recommended immersion time for inducing an irreversible state of unconsciousness would be a minimum of 7.5 min, followed by visual observation of the animals post WBF removal to confirm death.
Future studies should implement EEG for increased time-line accuracy of brain reactions and time of induced unconsciousness during foam immersion. In addition, an assessment of depopulation field personnel and animal health official's perceptions of emergency depopulation euthanasia options, including currently approved methodologies and WBF as a candidate for approval in emergency conditions, is urgently needed.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Bailey Ward, Taylor Williams, Mike Sword, and the other The Ohio State University's students and staff that helped in the field execution of both trials. Funding was provided by the National Pork Board (Grant number 21–070).
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




