Dissemination of antimicrobial-resistant isolates of Salmonella spp. in wild boars and its relationship with management practices
Abstract
Antimicrobial resistance (AMR) is a global concern and controlling its spread is critical for the effectiveness of antibiotics. Members of the genus Salmonella are broadly distributed, and wild boar may play an important role in its circulation between peri-urban areas and the environment, due to its frequent interactions both with livestock or human garbage. As the population of these animals is rising due to management on certain hunting estates or the absence of natural predators, the aim of the present work is to identify the mechanisms of AMR present and/or expressed in Salmonella spp. from wild boar populations and to determine the possible role of management-related factors applied to different game estates located in central Spain. The detection of Salmonella spp. was carried out in 121 dead wild boar from 24 game estates, and antimicrobial resistance traits were determined by antibiotic susceptibility testing and screening for their genetic determinants. The effects of feeding supplementation, the proximity of livestock, the existence of a surrounding fence and the density of wild boar on the AMR of the isolates were evaluated. The predominant subspecies and serovar found were S. enterica subsp. enterica (n = 69) and S. choleraesuis (n = 33), respectively. The other subspecies found were S. enterica subsp. diarizonae, S. enterica subsp. salamae and S. enterica subsp. houtenae. AMR was common among isolates (75.2%) and 15.7% showed multi drug resistance (MDR). Resistance to sulphonamides was the most frequent (85.7%), as well as sul1 which was the AMR determinant most commonly found. Plasmids appeared in 38.8% of the isolates, with IncHI1 being the replicon detected with the highest prevalence. The AMR of the isolates increased when the animals were raised with feeding supplementation and enclosed by fences around the estates.
1 INTRODUCTION
The spread of antimicrobial resistance (AMR) in Salmonella strains, especially multidrug-resistant (MDR) strains, has become a major health problem that involves the interaction between humans, livestock and wildlife (Karp et al., 2017; Torres et al., 2020). The study of wild species as environmental sentinels of AMR has acquired increasing consideration worldwide (Darwich et al., 2019; Furness et al., 2017; Hassell et al., 2019; Martín-Maldonado et al., 2020; Swift et al., 2019; Vittecoq et al., 2016). Several studies have shown that wildlife carries antimicrobial-resistant bacteria in a wide range of habitats linking AMR, hosts, bacterial species and their geographic locations (Botti et al., 2013; Dias et al., 2015; Gil Molino, Risco Perez, et al., 2019; Gonçalves et al., 2013; Radhouani et al., 2013; Zottola et al., 2013), a phenomenon that can impact human health through zoonotic diseases and/or emerging resistant pathogens (Radhouani et al., 2014).
The proximity of human activities to wildlife influences their microbiological populations, making them more prone to acquiring AMR (Allen et al., 2010; Navarro-Gonzalez et al., 2013; Navarro-Gonzalez et al., 2018). Additionally, feeding habits, climate and migration are also environmental factors contributing to the dissemination of resistant bacteria in wildlife (Vittecoq et al., 2016). Among this, the wild boar is highly adapted to the presence of humans, its population has increased considerably in recent decades throughout Europe, and it has become a regular visitor to communal garbage dumps as well as to livestock farms in search of food (Torres et al., 2020). The consumption of waste from these places may be linked to antimicrobial resistance carriage (Literak et al., 2010) and could represent a major epidemiological link between domestic animals, humans and wildlife (Navarro-Gonzalez et al., 2012; Vittecoq et al., 2016).
The rapid dissemination of resistance genes and their accumulation in MDR bacteria present in different hosts has been largely attributed to inter- and intraspecific DNA exchange, mainly through the horizontal transfer of integrons and/or plasmids carrying linked determinants against different families of antibiotics (Fluit & Schmitz, 2004).
Several studies have focused the phenotypic characterization of AMR in Salmonella spp. isolates recovered from wild boar (Dias et al., 2015; Methner et al., 2010; Navarro-Gonzalez et al., 2013; Navarro-Gonzalez et al., 2012; Razzuoli et al., 2021; Zottola et al., 2013). However, little is still known about the interactions between environmental and genetic determinants of AMR in bacteria from this animal species since available information is limited to a few screenings (Caleja et al., 2011b; Gil Molino, Risco Perez, et al., 2019; Literak et al., 2010).
Therefore, the aim of the present study was to identify the mechanisms of AMR present and/or expressed in Salmonella spp. from wild boar populations and to determine the possible role of management-related factors applied to different game estates located in central Spain.
2 MATERIAL AND METHODS
2.1 Game estates
The main characteristics of the 24 game estates included in this study are listed in Table 1. Briefly, all the estates were located in the south-west quadrant of the Iberian Peninsula and, depending on the degree of human intervention, were categorized as estates with or without management. The latter did not have any human intervention aimed at influencing the wild boar populations present on the estate and the estates with management included those on which the wild boar populations were controlled by census and certain management measures were applied occasionally (feeding supplementation, vaccination, grouping of animals etc.). The density of animals on each of the estates was categorized as either medium-low, high or very high (1–20, 21–40, >40 wild boars/100 ha, respectively) according to the most common population densities in central-south Spain, which are greater than those in the rest of Europe (Gonçalves Blanco, 2017). The estates were also categorised by the presence of a perimeter hunting fence preventing the free circulation of animals.
| Code | Density† | EMT‡ | Livestock§ | Supplemen-tation¶ | Fence# | No. isolates | No. subspecies/serotypes |
|---|---|---|---|---|---|---|---|
| E1 | Very high | HF | No | Yes | Yes | 10 | 2/2 |
| E2 | Very high | Yes | No | Yes | No | 2 | 2/2 |
| E3 | High | Yes | Yes | Yes | Yes | 5 | 2/4 |
| E4 | High | Yes | Yes | Yes | Yes | 22 | 3/15 |
| E5 | High | No | Yes | No | No | 4 | 2/3 |
| E6 | Very high | Yes | No | Yes | Yes | 3 | 1/3 |
| E7 | Very high | Yes | No | Yes | Yes | 5 | 1/3 |
| E8 | High | No | No | No | Yes | 2 | 1/2 |
| E9 | High | No | No | No | Yes | 6 | 3/5 |
| E10 | Medium-low | No | Yes | No | No | 7 | 2/2 |
| E12 | High | Yes | No | Yes | Yes | 13 | 2/8 |
| E13 | Very high | Yes | No | Yes | Yes | 1 | 1/1 |
| E14 | Very high | Yes | No | Yes | Yes | 3 | 2/3 |
| E15 | Very high | Yes | No | Yes | Yes | 3 | 1/2 |
| E17 | High | Yes | No | Yes | Yes | 3 | 2/3 |
| E20 | Medium-low | Yes | No | Yes | Yes | 5 | 1/4 |
| E22 | Medium-Low | No | No | No | No | 3 | 1/1 |
| E23 | Medium-low | No | Yes | No | No | 7 | 2/3 |
| E26 | Medium-low | No | Yes | No | No | 4 | 1/3 |
| E29 | High | No | No | No | No | 1 | 1/1 |
| E35 | Medium-low | Yes | No | Yes | Yes | 1 | 1/1 |
| E36 | Very high | Yes | Yes | Yes | Yes | 1 | 1/1 |
| E37 | Very high | HF | No | Yes | Yes | 7 | 2/3 |
| E38 | Medium-low | Yes | No | Yes | Yes | 3 | 1/3 |
- † Density categories (wild boars/100 ha): medium-low 1–20; High 21-40; very high > 40.
- ‡ Estate management type (EMT): no (without management); yes (with management); HF (hunting farms).
- § Presence of livestock in the same fields as the wild boars.
- ¶ Feeding supplementation to the wild boars.
- # Fenced estate.
2.2 Sampling and bacteria
A total of 121 isolates of Salmonella spp. were obtained from dead wild boars originating from 24 different game estates (Table 1) over a 5-year period (2010–2015). The wild boars were either killed by hunting parties or found dead by the estate gamekeepers (86 hunted/35 found dead). The ethical board of the University of Extremadura was consulted regarding the sampling strategy proposed in the present study. The authors were informed that no special authorisation was needed, as none of the animals were killed specifically for the purpose of the study.
Samples from these animals were obtained and sent to the Clinical Veterinary Hospital (CVH) of the University of Extremadura by a hunting management company (Ingulados S.L. Cáceres, Spain). Samples were split between different studies, including the present one (Gil Molino, García Sánchez, et al., 2019; Gil Molino, Risco Perez, et al., 2019), so the sampling procedure differed between the hunted specimens and the wild boars found dead on the estates. The latter included samples from the lungs, liver, spleen, kidneys, and intestinal content, while the other procedure included colon swabs, submandibular lymph nodes and tonsils. As no information about prevalence of Salmonella in the wild boar was intended in this study, the authors considered the inclusion of a larger number of samples beneficial, in order to increase the impact of the results.
The isolation method for the colon swabs, intestinal content, tonsils and lymph nodes consisted of the incubation of the swab or 1 g of content/tissue in sterile sampling bags with 10 ml of buffered peptone water (1/10 dilution in BPW according to ISO 6579:2002) at 37°C for 24 h; thereafter, 0.1 ml was inoculated in enrichment broth (Rappaport-Vassiliadis) and kept at 42°C for 24 h. Then, 10 μl of broth was inoculated onto two plates of selective media, xylose lysine dioxycholate agar (XLD) and xylose lysine tergitol 4 (XLT4). The plates were incubated for 48 h at 37°C. In the case of the samples from the lungs, liver, spleen and kidneys, they were cultured on blood agar, McConkey agar and xylose lysine dioxycholate agar (XLD) in aerobic conditions for 24 h at 37°C.
Each bacterial strain was isolated from samples taken from animals by mixing 2–3 morphologically compatible colonies grown on Salmonella selective media. Before analysing their molecular characteristics, each strain was clonally established by selecting a single colony from the secondary culture of the mixture of the 2–3 primary morphologically compatible colonies. Bacterial identification was carried out using an automated procedure (Phoenix 100, Becton Dickinson) and confirmed by detection of the invA gen by PCR (Hoorfar et al., 2000). Isolates confirmed by PCR were sent to the National Reference Laboratory for Salmonella in Spain (Algete, Madrid, Spain) for Kauffman–White serotyping.
2.2.1 AMR, genetic determinants and plasmid replicons
Antibiotic susceptibility testing was performed on all 121 Salmonella isolates by disk diffusion in agar for 13 antibiotics whereas polymyxin (colistin) resistance was screened by inoculating Mueller Hinton II plates containing 2 mg/L colistin, its clinical breakpoint according to the Clinical and Laboratory Standards Institute (CLSI, 2020b). The following discs (Bio-Rad®) were used: ampicillin (10 μg), cefotaxime (30 μg), ceftiofur (30 μg), gentamicin (10 μg), neomycin (30 μg), streptomycin (10 μg), tetracycline (30 μg), doxycycline (30 μg), enrofloxacin (5 μg), nalidixic acid (30 μg), trimethoprim/sulphamethoxazole (23.75/1.25 μg), sulphonamide (200 μg) and chloramphenicol (30 μg), with Escherichia coli ATCC 25922 used as control strain. Isolates were classified as susceptible, intermediate or resistant according to the Clinical and Laboratory Standards Institute (CLSI, 2020, 2020a,b). Any isolate resistant to ≥4 antimicrobials was considered as MDR. Multiple Antibiotics Resistance index (MAR) was calculated as the ratio between the number of antibiotics to which the organism is resistant and the total number of compounds tested (Krumperman, 1983).
The presence of AMR-linked sequences was verified in all isolates by specific PCRs for blaOXA (Chen et al., 2004), blaTEM, blaSHV and blaCTXM (Monstein et al., 2007) tetA, tetB, strA, strB, aadA (Rahmani et al., 2013), aph2 (Aarestrup, Lertworapreecha, et al., 2003), sul1, sul2 and sul3 (Kozak et al., 2009), and the class 1 integrase gene Int1 and associated gene cassettes (Leverstein-van Hall et al., 2002). Quinolone resistance determinant regions (QRDRs) of gyrA and gyrB genes from isolates sharing low susceptibility to nalidixic acid were amplified by PCR (Randall et al., 2005). DNA fragments were sequenced (STABVIDA, Caparica, Portugal) and analysed to confirm their identity or, for topoisomerase encoding genes, to detect polymorphisms involved in AMR. In addition, the presence of any of the 18 plasmid replicons frequently found in Enterobacteriaceae was screened by using the three multiplex panels PCR described in previous research (Johnson et al., 2007), with positive controls kindly provided by Alessandra Carattoli (Istituto Superiore di Sanità, Rome, Italy).
2.3 Statistics
The effects on AMR of certain management measures (feeding supplementation, livestock and perimeter fence) and the wild boar density were evaluated by comparing the Phenotypic Resistance Intensity (PRI) and the Genetic Resistance Intensity (GRI) between groups subjected to different management conditions. PRI and GRI are artificial indexes obtained by the sum for a given isolate of the number of different traits involved in resistance, either phenotypes (PRI) or genotypes (GRI). Thus, 0 attributes give an index of 1, whereas isolates with 1, 2 or 3 resistance traits have an index of 2 and isolates with 4 or more have an index of 3. Differences between groups were evaluated by Mann–Whitney tests and p values smaller than .05 were considered significant. Two-tailed tests were always applied except when evaluating the effect of the presence of plasmids on the AMR of the isolates. As the only possible result was an increase in AMR or no change, a one-tailed test was applied (Motulsky, 2016).
Variance analysis followed by the Dunn's test evaluated the differences in the number of AMR per isolate between subspecies and between serovars. The significance threshold used was p values less than .05. Correlation between the number of plasmids and the presence of AMR in the isolates was studied with the Pearson's r. Results were considered significant when p values were less than or equal to .05.
3 RESULTS
3.1 Serotype diversity of S. enterica from wild boar
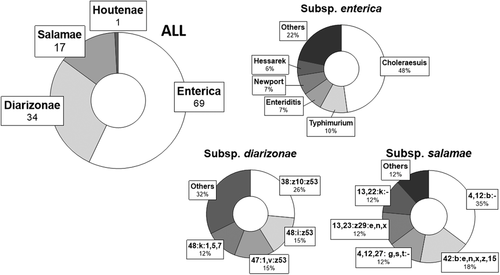
The 121 isolates from wild boar belong to four of the six subspecies of S. enterica and represent 44 serovars (Figure 1): S. enterica subsp. enterica (n = 69 strains, 57% of isolates), 22 serovars; S. enterica subsp. diarizonae (n = 34 strains, 28.1%), 14 serovars; S. enterica subsp. salamae (n = 17 strains, 14%), 7 serovars; and S. enterica subsp. houtenae (n = 1 strain, 0.8%). The two main serovars found within each subspecies were: enterica, S. choleraesuis (n = 33, 48%) and S. typhimurium (n = 7, 10%); diarizonae, 38:z10:z53 and 48:i:z53; and salamae, 4, 12:b:- and 42:b:e,n,x,z,15. The only isolate present in the subspecies houtenae was serotyped as 45:z4,z23:-.
3.2 AMR of S. enterica from wild boar
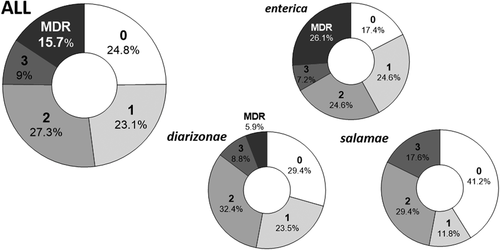
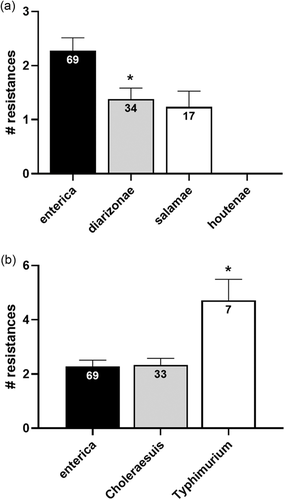
Antibiotic resistance was frequently found in the 121 strains analysed (Figure 2), since 91 (75.2%) of them showed resistance to at least one antimicrobial. Nineteen isolates (15.7%) presented a MDR phenotype and one unique isolate (Salmonella Bredeney 4.12:1,v:1.7) was resistant to the 9 antibiotics tested. In addition, 26 isolates (21.5%) shared a multiple antibiotic resistance index (MAR, the ratio between AMR and the number of antibiotics tested) higher than 0.2, which indicates that those isolates are likely to come from a source exposed to high use of antibiotics (Krumperman, 1983). Regarding the data for resistance by subspecies and serotypes, subspecies enterica presented both the highest rate of isolates carrying MDR or AMR (26.1% or 82.6%, respectively; Figure 2) as well as the highest number of resistances per isolate (2.28 ± 0.24. Figure 3a). Particularly, those isolates from the Typhimurium serotype of special relevance to human health (especially its monophasic variant; EFSA & ECDC, 2020; WHO, 2019), reached on average more than four resistances per isolate (Figure 3b).
The occurrence of AMR depends strongly on antibiotic nature (Table 2), with maximal resistance found to sulphonamide (85.7%) followed by streptomycin (46.2%), tetracycline (25.3%) and ampicillin (17.6%). In contrast, AMR rates to nalidixic acid, chloramphenicol, neomycin and cephalosporins (cefotaxime and ceftiofur) were lower than 10%. No strain was found to be resistant to polymyxin E (colistin) and the antibiograms for gentamicin and enrofloxacin showed only intermediate sensitivity. The high prevalence of intermediate sensitivity to streptomycin and neomycin (46.2% and 25.3%, respectively) is noteworthy, which is not counted as true AMR although it could compromise antibiotic efficacy (see below).
| Gene (+) isolates | |||||
|---|---|---|---|---|---|
| Antimicrobials | Resistant isolates† | Determinant found | G+/Ph+‡ | G+/Ph–§ | % G+/Ph+¶ |
| Sulphonamides | |||||
| Sulphonamide | 78 (85.7%) |
sul1 sul2 sul3 |
18 12 5 |
4 5 1 |
34.6% (27/78) |
|
Trimethoprim/ sulphamethoxazole |
29 (31.9%) | ||||
| Aminoglycosides | |||||
Neomycin Streptomycin |
2 (2.2%) 42 (46.2%) |
aadA1 strA strB |
5 18 17 |
4 1 1 |
47.7% (21/44) |
| Tetracyclines | |||||
|
Doxycycline Tetracycline |
19 (20.9%) 23 (25.3%) |
tetA tetB |
14 6 |
0 2 |
78.3% (18/23) |
| β-lactams | |||||
| Ampicillin | 16 (17.6%) | blaTEM | 11 | 0 | 75% (11/16) |
| Cefotaxime | 1 (1.1%) | ||||
| Ceftiofur | 1 (1.1%) | ||||
| Quinolones | |||||
| Nalidixic acid | 7 (6.6%) | gyrA | 5 | 0 | 71.4% (5/7) |
| Phenicols | |||||
| Chloramphenicol | 6 (6.6%) | int1# | 5 | 2 | 83.3% (5/6) |
- † Number of isolates resistant to a certain antibiotic and its percentage referred to the total number of resistant isolates in the study.
- ‡ Number of resistant isolates (Ph+) with a candidate gene identified (G+).
- § Number of susceptible isolates (Ph–) presenting a resistance determinant identified (G+).
- ¶ Percentage of isolates with any of the genes studied amongst the isolates resistant to any of the members of the antimicrobial family.
- # Int1 is frequently associated to the floR genes, which confer resistance to phenicols (Carattoli, 2001).
Among 26 AMR profiles found (Table 3), the most frequent was SUL (22%), followed by SUL-TRS (18.7%) and DOX-STR-SUL-TET (11%). No association was found between subspecies and specific AMR profiles.
| Phenotype profile | n | % | S. enterica | S. diarizonae | S. salamae | Estates(E) |
|---|---|---|---|---|---|---|
| SUL- | 20 | 22.0 | 14 | 5 | 1 | 1, 3, 4, 7, 12, 15, 17, 23, 26, 37 |
| SUL-TRS- | 17 | 18.7 | 5 | 8 | 4 | 4, 5, 12, 13, 15, 22, 23 |
| DOX-STR-SUL-TET- | 10 | 11.0 | 9 | 1 | – | 4, 9, 15, 36, 37 |
| STR-SUL- | 8 | 8.8 | 5 | 3 | – | 7, 9, 10, 14, 20 |
| STR-SUL-TRS- | 4 | 4.4 | – | 4 | – | 7, 10, 14, 17 |
| STR- | 4 | 4.4 | 1 | 3 | – | 1, 4, 38 |
| NAL-STR- | 3 | 3.3 | 3 | – | – | 1, 6, 29 |
| NAL- | 2 | 2.2 | 2 | – | – | 10 |
| AMP-DOX-STR-SUL-TET- | 2 | 2.2 | 2 | – | – | 3, 37 |
| AMP-DOX-STR-SUL-TET-TRS- | 2 | 2.2 | 2 | – | – | 6, 38 |
| STR-SUL-TET- | 2 | 2.2 | 2 | – | – | 4, 9 |
| AMP-SUL-TRS- | 2 | 2.2 | 1 | – | 1 | 4, 12 |
| AMP-CHL-DOX-STR-SUL-TET-TRS- | 2 | 2.2 | 2 | – | – | 14, 20 |
| DOX-TET- | 1 | 1.1 | 1 | – | – | 2 |
| NEO-SUL- | 1 | 1.1 | 1 | – | – | 1 |
| AMP-CHL-SUL- | 1 | 1.1 | 1 | – | – | 3 |
| AMP-CHL-CTA-CTF-DOX-STR-SUL-TET-TRS- | 1 | 1.1 | 1 | – | – | 3 |
| NAL-STR-SUL- | 1 | 1.1 | 1 | – | – | 26 |
| NEO- | 1 | 1.1 | 1 | – | – | 26 |
| AMP-NAL-TET- | 1 | 1.1 | – | – | 1 | 4 |
| AMP- | 1 | 1.1 | – | – | 1 | 4 |
| AMP-STR-SUL- | 1 | 1.1 | – | – | 1 | 4 |
| AMP-SUL- | 1 | 1.1 | – | – | 1 | 4 |
| AMP-CHL-DOX-SUL-TET- | 1 | 1.1 | 1 | – | – | 5 |
| CHL-STR-SUL- | 1 | 1.1 | 1 | – | – | 7 |
| AMP-STR-SUL-TET-TRS- | 1 | 1.1 | 1 | – | – | 38 |
- AMP, ampicillin; CHL, chloramphenicol; DOX, doxiciclin; STR, streptomycin; SUL, sulphonamide; TET, tetracycline; TRS, trimethoprim/sulphamethoxazole.
3.3 AMR determinants: genotype–phenotype relationships of S. enterica from wild boar
Detection of AMR determinants revealed that 49 out of the 91 resistant isolates (53.8%) contained at least one genetic marker and that, among them, 17 isolates (18.7%) carried four or more (Table 4). The most frequent genotype profile was sul1, followed by gyrA and strA-strB-sul2-tetA (Table 4), although not all isolates with resistance genes developed phenotypic resistance, since 12 isolates carried AMR determinants that were not expressed (Table 2 and Supplementary Table). The case of the aadA1 gene is particularly interesting, detected in 5 isolates but conferring resistance to streptomycin in only one of them (Table 2). Phenicols and tetracyclines were the antimicrobial groups which showed higher expression of resistances, respectively, 83.3% and 78.3% of resistant isolates among those carrying AMR determinants, followed by β-Lactams and quinolones, 75% and 71.4%, whereas resistance to sulphonamides was only expressed in 34.6% of the isolates carrying sul genes (Table 2).
| Genotype profile | n | % | S. enterica | S. diarizonae | S. salamae | Estates(E) |
|---|---|---|---|---|---|---|
| sul1- | 9 | 18.4 | 9 | – | – | 1, 4, 5, 7, 37, 38 |
| gyrA- | 4 | 8.2 | 4 | – | – | 3, 10, 26 |
| strA-strB-sul2-tetA- | 4 | 8.2 | 4 | – | – | 4, 37 |
| blaTEM-strA-strB-sul2-tetB- | 3 | 6.1 | 3 | – | – | 3, 6, 37 |
| sul2- | 3 | 6.1 | – | 2 | – | 9, 12, 22 |
| blaTEM- | 3 | 6.1 | – | – | 3 | 4 |
| strA-strB-sul1-sul2-tetA-tetB- | 2 | 4.1 | 2 | – | – | 4, 9 |
| strA-strB-tetA- | 2 | 4.1 | 1 | 1 | – | 9, 37 |
| aadA1-blaTEM-dhfrA1-sul1-sul3- | 2 | 4.1 | 2 | – | – | 14, 20 |
| strA-strB- | 1 | 2.0 | 1 | – | – | 1 |
| aadA1-blaPSE-sul1- | 1 | 2.0 | 1 | – | – | 3 |
| aadA1-strA-strB-sul1-sul2- | 1 | 2.0 | 1 | – | – | 3 |
| gyrA-sul1- | 1 | 2.0 | 1 | – | – | 6 |
| sul3- | 1 | 2.0 | 1 | – | – | 26 |
| tetB- | 1 | 2.0 | – | 1 | – | 12 |
| aadA1-strA-strB-sul1-sul2-tetA- | 1 | 2.0 | 1 | – | – | 4 |
| strA- | 1 | 2.0 | – | 1 | – | 4 |
| blaTEM-strA-strB-sul1-sul2-sul3-tetA- | 1 | 2.0 | – | – | 1 | 4 |
| aadA1-blaTEM-tetB- | 1 | 2.0 | – | – | 1 | 4 |
| sul1-sul2- | 1 | 2.0 | 1 | – | – | 23 |
| aadA1- | 1 | 2.0 | 1 | – | – | 23 |
| aadA1-sul3-tetA- | 1 | 2.0 | 1 | – | – | 7 |
| strA-strB-tetB- | 1 | 2.0 | 1 | – | – | 38 |
| aadA1-blaTEM-sul1-sul3-tetA- | 1 | 2.0 | 1 | – | – | 38 |
| strA-strB-sul1-tetA- | 1 | 2.0 | 1 | – | – | 37 |
| strA-strB-sul1-sul2-tetA- | 1 | 2.0 | 1 | – | – | 37 |
When screening the QRDR of genes encoding topoisomerase II, where mutations that confer nalidixic acid resistance are frequently located, single missense polymorphisms producing gyrA mutations D87N or D87Y were found in two S. enteritidis and three S. choleraesuis isolates, among the seven isolates conferring resistance to nalidixic acid and intermediate sensitivity to enrofloxacin (Table 2, Supplementary Table), while gyrB mutations were not observed.
The Int1 element was rarely detected (7/121, 5.8%) and the int1-associated gene cassette (GC) (4/7, 57.1% of Int1+) even less frequently among S. enterica isolates from wild boar (Table 2). The dfrA1-aadA1 containing GC was found in two isolates, S. typhimurium and 48:i:z53, whereas GC containing single genes were linked to int1 in two isolates, an S. Bredeney carrying aadA1 and an S. typhimurium 1,4,5,12:i:1,2 that presented aadA1 and blaPSE in two separate elements. In addition, five out of the six isolates expressing resistance to chloramphenicol carried int1 (Table 2), presumably because this mobilization element is usually linked to the Salmonella genomic island I (SGII), a plastic region of the chromosome with which the floR gene conferring phenicol resistance is associated (Carattoli, 2001).
Replicon typing revealed that 47 out of the 121 isolates (38.8%) presented PCR signatures from between one to five plasmids (Supplementary Table), which were HI1, found in 34 isolates; FIIA, in 15; FIA, in 6; I1, in 5; B/O, in 4; K/B, in 4; HI2, in 4; FIB, in 3; FIC, in 2; W, in 2; Y, in 2; A/C, in 1; N, in 1, whereas P, T, Frep, X and L/M replicons were not found in any isolate. Interestingly, only those isolates carrying the FIIA incompatibility group presented more AMR and resistance genes per isolate than the average (AMR per isolate: 2.60 ± 0.40 vs. 1.85 ± 0.16; Res. genes per isolate: 2.33±0.55 vs. 1.10±0.16; p < .05 Mann–Whitney test). Although there was no clear association between replicons and the presence of certain resistance genes, it is noteworthy that more than 40% of the isolates with strA, strB or tetA resistance genes also presented HI1 or FIIA plasmids. The number of plasmids and AMR per isolate was not positively correlated, nor was any association detected between replicons, subspecies, or particular serotypes.
3.4 Management conditions and AMR of S. enterica from wild boar
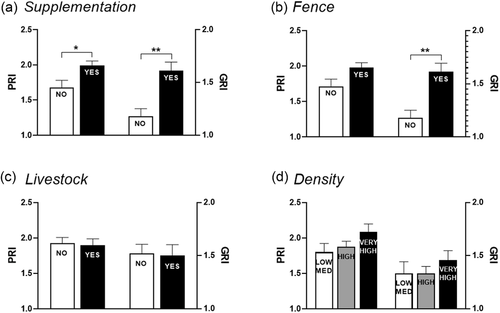
AMR of S. enterica isolates from wild boar increased when screened in animals reared with feeding supplementation and enclosed by perimeter fences around the estates. The intensities of resistance, both genotypic and phenotypic (see Section 2) were higher in fenced estates and in those where feeding supplementation was applied (Figure 4a and b). In contrast, neither the density of animals nor their proximity to livestock were significantly related to AMR (Figure 4c and d).
4 DISCUSSION
This study contributes to knowledge on the spread of AMR by Salmonella spp. isolated from wild boar in central Spain, contrasting phenotype expression from genotype traits found in 121 bacterial strains. The number of isolates studied make this the most extensive study regarding AMR and its genetic determinants in Salmonella spp. performed in European wild boar (Caleja et al., 2011a; Vieira-Pinto et al., 2011a). More than half of these 121 strains were classified as S. enterica subsp. enterica, which is in accordance with previous studies in this species (Chiari et al., 2013; Mentaberre et al., 2013; Navarro-Gonzalez et al., 2012; Vieira-Pinto et al., 2011b) and also reported by the EFSA (European Food Safety, European Centre for Disease, & Control, 2019).
Our results evidence that AMR is a major characteristic in Salmonella isolates from wild boar, (75%) in line with previous studies in Portugal (Caleja et al., 2011a; Pinto et al., 2010) and in Italy (Bonardi et al., 2019; Zottola et al., 2013), although much lower levels have been reported in other regions of Spain (Navarro-Gonzalez et al., 2013; Navarro-Gonzalez et al., 2012). Common environmental factors like climate, ecology and/or animal management practices could explain the similar prevalence observed between AMR of bacteria isolated from central-western Spain and that reported in studies carried out in Portugal. The western part of Spain is much closer to Portugal than to the north-eastern part of the country, where the other Spanish studies were conducted. In addition, the prevalence of Salmonella found in the wild boar in these studies was very low, which does not favour significant results regarding AMR in Salmonella in the wild boar population being obtained.
The association of AMR traits in the same cell giving rise to MDR strains is a matter of serious concern (EFSA & ECDC, 2020) and given the rapid expansion of the wild boar across Europe and their increasing interactions with humans, monitoring of the carriage of MDR bacteria by this animal species in order to prevent future sanitary problems has been recommended (Torres et al., 2020). Following this, we detected MDR Salmonella from wild boar, with an intermediate rate (15.7%) compared to that reported for Portugal (68.2%; (Caleja et al., 2011a) and Italy (9.6%−5.5%; (Cilia et al., 2021; Zottola et al., 2013). On the other hand, considering wild boar as sentinel species for AMR and particularly MDR in their environment, our results could indicate a better health status of these settings when compared with Salmonella isolates from humans or pigs, which average 28.5% and 51.3% in the EU (EFSA & ECDC, 2020). Indeed, contact with humans and livestock has been pointed out as a risk factor for the spread of AMR in the environment (Navarro-Gonzalez et al., 2018; Skurnik et al., 2006). These percentages of MDR should be compared carefully, as some cross-resistances could appear when including more than one agent from the same antimicrobial family. Once this was taken into account, the percentage of MDR isolates in the present study was 7.4%.
Apart from contact with humans and livestock, prevalence of Salmonella in wild boar could be related to factors like animal age, animal density, geographical zone, season or sampling strategy (Bonardi et al., 2019; Gil Molino, García Sánchez, et al., 2019; Magnino et al., 2011; Navarro-Gonzalez et al., 2012; Sannö et al., 2018). However, very few studies analyse the influence of such factors over the presence of AMR in Salmonella in wild boar. This study presents evidence indicating that cohabitation with livestock did not modify AMR of Salmonella isolates from wild boar, in agreement with other studies also performed in Spain (Navarro-Gonzalez et al., 2012). It has been suggested that additional factors might contribute to the spread of bacteria between distant livestock and wild boar, like small rodents or birds, which could also be attracted to livestock feed and could be eaten by wild boars or transmit bacteria to them via faeces due to their omnivorous behaviour and rooting habits (Navarro-Gonzalez et al., 2012; Schley & Roper, 2003). Animals living in urban areas show higher levels of antimicrobial resistance than those living in remote areas, due to the possibility of contact with resistant bacteria and selective agents (Radhouani et al., 2014).
This study, besides the proximity to livestock, integrates management data from the game estates where the wild boar were hunted or captured. In Spain, especially in the central and southern regions, the application of management measures to control wild boar populations, their sanitary status and the quality of the hunting trophies are common practice (Cano-Terriza et al., 2018). According to data presented in this study, the temporary concentration of animals is a key factor for the spread of AMR Salmonella, which were more prevalent in animals raised in fenced estates and/or receiving supplementary feeding, following the trend of Salmonella prevalence in this species that has been explained by the contamination of the same frequented area favoured by the restriction of the animals’ movement (Ortega et al., 2020).
Regarding the serotypes found in the samples from the present study, the high percentage of resistant isolates in S. typhimurium is remarkable, more specifically in its monophasic variant, which represents 3 out of the 7 isolates of S. typhimurium. This serotype, and especially its monophasic variant, has been reported by the EFSA as one of the serotypes with the highest proportion of resistance and MDR isolates (EFSA & ECDC, 2020). Such high resistance rates have previously been described in wild boar (Razzuoli et al., 2021; Zottola et al., 2013); however, the presence of S. choleraesuis is an unusual finding only mentioned previously in one study with just one isolate of this serotype (Zottola et al., 2013). The frequent appearance of S. choleraesuis among the isolates of the present study is in line with the proportion of wild boars analysed with septicaemic processes, something uncommon in previous studies in this species. The high proportion of AMR observed in this serotype is in accordance with previous reports in this species (Donazzolo et al., 2017; Gil-Molino et al., 2020), although some studies did not find AMR in S. choleraesuis from European wild boars (Leekitcharoenphon et al., 2019). The frequency of appearance of AMR in the other subspecies from this study was lower than in S. enterica subsp. enterica, but it should be noted that some of the isolates from subspecies salamae or houtenae were MDR, as recently reported by other authors (Razzuoli et al., 2021).
The AMR most frequently observed in this study were in accordance with those reported in Salmonella isolates from swine in Spain (Antunes et al., 2011; Arguello et al., 2013; Astorga et al., 2007; García-Feliz et al., 2008; Gomez-Laguna et al., 2011; Mejia et al., 2006) and other locations (Bolton et al., 2013; Bonardi et al., 2013; Frye et al., 2011). Resistances against compounds such as sulphonamides, streptomycin and tetracyclines are generally attributed to their misuse as growth promoters in the past (Aragaw et al., 2007; Threlfall et al., 2003).
The predominant resistant patterns detected in this study were SUL and TRS-SUL, similarly to those previously reported for bacteria from wild boars, where a great variety of resistance patterns was described (Cilia et al., 2021; Zottola et al., 2013). In contrast, other studies described only a few AMR patterns, which might be attributed to differences in sampling methods and/or in environmental conditions of the wild boar population from which bacteria were isolated (Bonardi et al., 2019; Donazzolo, 2017; Methner et al., 2010).
Among AMR genotypes detected in this work, those found most frequently were sul1, gyrA and strA-strB-sul2-tetA, in agreement with phenotypic profiles detected, although 12 isolates did not express AMR determinants during in vitro growth. These cryptic determinants could be activated under certain conditions or stressors and might constitute a potential reservoir for AMR spread (Srikumar et al., 2015). In contrast, and despite searching for 16 AMR determinants, to our knowledge the deepest screening performed in Salmonella isolates from wild boar, genotypes were not identified for 46.2% of the isolates expressing AMR. This is the case for sulphonamide-resistant Salmonella, among which any sul1 to sul3 genes were detected in only 34.6% of isolates. Previous studies in wild boar vary greatly in the prevalence of sul genes, ranging from 72% of sul1 (Caleja et al., 2011a) to none detected among sulphonamide-resistant isolates (Pinto et al., 2010), although sul1 is the most frequently reported sulphonamide resistance determinant (Antunes et al., 2005; Argüello et al., 2018; Caleja et al., 2011a). Our data show a similar prevalence of sul1 and sul2 and also the frequent association between sul1 and sul3 genes, two uncommon characteristics (Argüello et al., 2018; Guerra et al., 2004).
Streptomycin is the aminoglycoside against which the highest prevalence of resistance has been found in this study, similarly to previous reports on Salmonella isolates from wild boar (Bonardi et al., 2019; Caleja et al., 2011a; Cilia et al., 2021; Donazzolo, 2017; Methner et al., 2010; Pinto et al., 2010; Zottola et al., 2013). The high proportion of isolates sharing streptomycin resistance is consistent with the frequency of genetic determinants found. Thus, strA, strB and aadA genes were found in 40.9%, 38.6% and 11.3% of resistant isolates, respectively. Similarly, high occurrences have been described in pigs (Leekitcharoenphon et al., 2019), while aadA was the major determinant found in Salmonella from wild boar, but at a much lower frequency (Caleja et al., 2011a; Pinto et al., 2010). Interestingly, intermediate sensitivity to streptomycin and to neomycin were frequently detected in this work. A recent study in Italian wild boar obtained similar results, with very similar percentages of intermediate sensitivity to streptomycin (Razzuoli et al., 2021). This is often a preliminary step preceding the rise of resistant strains (Koch et al., 2014) that could be selected and stably maintained in environments with low antibiotic concentration because this phenotype might not interfere with the biological fitness of bacteria (Howden et al., 2014).
After sulphonamides and aminoglycosides, resistance to tetracyclines was also frequently found. However, the detection of AMR determinants to tetracyclines was higher than in the case of aminoglycosides or sulphonamides, reaching levels close to 80%. The major gene found was tetA, reflecting results from other studies in wild boars (Caleja et al., 2011a), swine (Argüello et al., 2018; Okubo et al., 2019) and others animals and food (Gargano et al., 2021; Tawyabur et al., 2020). This resistance gene is highly persistent in the wild, even in the absence of selective pressure (Tamminen et al., 2011), and is capable of faster propagation than the other genes which code resistance against tetracyclines (Yu et al., 2015).
AMR against β-lactams and quinolones are very rarely found in isolates of Salmonella spp. from wild boar (Bonardi et al., 2019; Donazzolo, 2017; Leekitcharoenphon et al., 2019); however, we described percentages of resistance to β-lactams close to 20% and of around 6% to nalidixic acid. The first is mainly carried by the serovar Typhimurium, subspecies salamae, with 6/7 and 5/17 of isolates expressing resistance to ampicillin, respectively. These findings are in line with studies carried out in wild boar and that focused on the S. typhimurium serovar, reporting resistance values to ampicillin around 70–80% (Caleja et al., 2011a; Pinto et al., 2010). In contrast, the resistance to nalidixic acid showed in the present study is above that previously reported in Salmonella from wild boar in Europe. In Spain, only one strain with this characteristic has been described (Navarro-Gonzalez et al., 2012) and, in Italy, a 1.8–0.8% of resistance to nalidixic acid was reported (Zottola et al., 2013), besides a 3.4% rate in Salmonella from other wild animals (Botti et al., 2013). All nalidixic acid-resistant isolates from the present study displayed an intermediate sensitivity to enrofloxacin, as previously described (Astorga et al., 2007; Caleja et al., 2011a; Foti et al., 2018; Gomez-Laguna et al., 2011). Although MIC determination by microdilution is more appropriate for fluoroquinolone resistance in Salmonella (Parry et al., 2010) than the disk diffusion technique used in this study, the pattern observed must be related to detected gyrA genotypes, D87Y or D87N, closely linked to nalidixic acid resistance (Levy et al., 2004; Palomo Guijarro, 2011; Rahmani et al., 2013). Furthermore, gyrB mutations were not detected in isolates from this study, which is commonly described in Salmonella expressing fluoroquinolone resistance (Hopkins et al., 2005), whereas the single gyrA mutations found are enough for nalidixic acid resistance and additional polymorphisms in this gene would be required to cause fluoroquinolone resistance (Ruiz et al., 1997). The resistance against quinolones described in the present study is also common in pigs and other species (García-Feliz et al., 2007; Morshed & Peighambari, 2010; Rad et al., 2010) and could be related to treatment failures of invasive gastrointestinal infections with these agents (Aarestrup et al., 2003).
Similar percentages of resistance to those described in the previous paragraph appear against chloramphenicol, which has been banned for use in livestock in several countries, including the EU, but is still used in human health in the treatment of infections by Salmonella with low susceptibility to other compounds (EFSA & ECDC, 2020). Since AMR against phenicols is still lower in Salmonella in humans (6.5%) than from pig samples (14.6%), results in the present study suggest that Salmonella from wild boar are nearest to human isolates, probably indicating that this animal is relatively free of exposure to these antimicrobials in comparison with the management required for swine production. Similarly, this work indicates that gentamicin and colistin are more effective against Salmonella from Spanish wild boars than from swine (EFSA & ECDC, 2020), from other wild animals in Spain (Darwich et al., 2019) or from wild boar in Italy (Zottola et al., 2013).
Genetic determinants of antimicrobial resistance are commonly associated with mobile elements in bacterial genomes, mainly plasmids and/or integrons (Frye & Jackson, 2013). Class 1 integrons, among the most important elements contributing to the spread of antimicrobial resistance in Salmonella (S. Chen et al., 2004), were detected in 7 isolates from the present study and 4 of them harboured different gene cassettes conferring resistance to β-lactam (blaPSE-1), streptomycin (aadA) and trimethoprim (dfrA), a linkage previously described in Salmonella and E. coli from wild boar (Caleja et al., 2011a; Literak et al., 2010). These mobile multidrug resistance determinants have the potential for horizontal transfer to other Salmonella and could potentially acquire and integrate new GC into their genome structure (Levings et al., 2005; Toleman et al., 2006). Apart from integrons, plasmids are the other most influential genetic elements in bacterial antimicrobial resistance (McMillan et al., 2020). Studies of antimicrobial-resistant Salmonella have determined that specific plasmid replicon types are associated with resistance, geographic origin, and source host (Carattoli, 2009; Frye & Jackson, 2013; Frye et al., 2011; Lindsey et al., 2009). Results from the present study showed an increment in AMR associated with the carriage of IncFIIA replicons, which has been recognized as the major virulence-associated replicon in Salmonella (Carattoli et al., 2005). This plasmid is also the most frequently reported in cases of clinical salmonellosis in livestock (Abraham et al., 2014; Leekitcharoenphon et al., 2019). On the other hand, the presence of plasmids in the isolates did not ensure AMR in these isolates (Leekitcharoenphon et al., 2019). Several strains from our samples carried replicons and did not manifest any AMR. Moreover, resistance genes have been identified in isolates with no replicons detected, which could be due to the location of such gene/s on an undetected plasmid or elsewhere in the genome (Frye et al., 2011). Apart from the association with AMR, it is evident that strains from the collection do carry mobile genetic elements, such as integrons and plasmids, that may facilitate the acquisition of additional resistance determinants if selection is further sustained or intensified.
In conclusion, the AMR traits detected in Salmonella isolates from wild boar highlight the transcendence of the interface between wildlife and anthropogenic environments, where microbiological surveillance is a valuable element of the One Health approach to contain the potential for the AMR spread.
ACKNOWLEDGEMENTS
This research has been sponsored by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO, currently MICINN, Grant PID2020-118405RB-I00), and the Regional Government of Extremadura (Consejería de Economía e Infraestructuras) through the research projects IB16073, IB18047, GR15075 and IB20181, as well as by the European Regional Development Funds (FEDER). AG thanks his current contract (Government of Extremadura and European Social Fund). The authors would like to thank Gemma Gaitskell-Phillips (BVetMed) for her assistance in the preparation of the manuscript.
AUTHOR CONTRIBUTIONS
MGM, JR, PFLL and AQ conceived and planned the study; MGM, PG and DR collected the samples; DR, FEMC and MGM analysed the data; AG, FEMC and MGM drafted the manuscript; AQ revised the manuscript; JR and AQ obtained the funding.
CONFLICT OF INTEREST
All the authors have read the manuscript and have approved this submission. The authors report no conflicts of interest.
ETHICAL STATEMENT
Ethical statement is not applicable because samples have been gathered from death animals.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




