Virus viability in spiked swine bone marrow tissue during above-ground burial method and under in vitro conditions
Rafael Ebling and Willian Pinto Paim equally contributed to this study.
Abstract
The emergence of high consequence animal diseases usually requires managing significant mortality. A desirable aspect of any carcass management method is the ability to contain and inactivate the target pathogen. The above-ground burial (AGB) technique was recently developed and proposed as an alternative carcass management method. Here, we investigate the tenacity of swinepox virus (SwPV), as a surrogate model for African swine fever virus (ASFV) in swine carcasses during the AGB process. For this, SwPV was inoculated intrafemorally in 90 adult swine carcasses, which were subsequently disposed under AGB conditions. Bone marrow samples were recovered periodically throughout 12 months and virus viability was assessed by virus isolation (VI), whereas the presence of SwPV DNA was evaluated by quantitative polymerase chain reaction (qPCR). Additionally, an in vitro study assessed the inactivation rate of SwPV, Senecavirus A (SVA), and bovine viral diarrhoea virus (BVDV). Viral suspensions were mixed with bone marrow material and maintained at 21–23°C for 30 days. Virus viability was assessed by VI and viral titration. In the field study, SwPV remained viable only in 11 (55%) bone marrow samples collected on day 7; only viral DNA (and not infectivity) was detected afterwards. SwPV inactivation was estimated to have occurred by day 11. The in vitro testing revealed a variable tenacity of the studied viruses. The viability period was estimated in 28, 80, and 118 days, respectively, for BVDV, SwPV, and SVA. Overall, these findings indicate that the AGB technique was effective in quickly inactivating SwPV. Additionally, the SwPV inactivation rate is comparable to ASFV under field studies and poses a potential model for preliminary ASFV inactivation studies with reduced biosecurity requirements. Moreover, this study contributes to understanding the inactivation kinetics of viruses under specific conditions, which is critical when designing and applying countermeasures in case of biosecurity breaches in sites managing animal mortality.
1 INTRODUCTION
The emergence and spread of the African swine fever virus (ASFV) in Asia, Europe, and recently in the Dominican Republic are a significant disruptor to the global pork industry. The introduction of ASFV or any high consequence animal disease affecting livestock into major producing areas requires the euthanasia and safe disposal of a large number of carcasses (Moennig, 2000). For instance, a 2019 report estimates that the ongoing ASFV outbreaks resulted in over six million pigs being slaughtered in several countries (FAO, 2019).
Carcass disposal methods available for large-scale depopulation during high consequence animal disease outbreaks are limited. This is especially challenging for carcasses of adult pigs, cattle, or other large animals. Despite being a common carcass disposal technique among the available options, deep burial requires the excavation of deep trenches and presents a constant contamination risk for groundwater (Eamens et al., 2011; Moennig, 2000). Rendering was successfully used to manage livestock carcasses during the foot-and-mouth disease virus (FMDV) outbreak in the United Kingdom in 2001 (Taylor, 2002). However, it involves transporting infectious animals or carcasses over long distances to the rendering plant location, increasing chances for virus dissemination. In addition, composting may be restricted due to space limitations, scarcity of carbon sources, or lack of functioning static compost piles during the winter months (Pepin et al., 2020; Schwarz & Bonhotal, 2015). Suboptimal composting conditions are especially critical in subtropical, temperate, and subpolar regions with significant pork production.
Two critical aspects of carcass disposal methods involve limiting the environmental impact and providing containment and inactivation of the target pathogen (FAO, 2019). The newly developed above-ground burial (AGB) technique, also knwon as large scale shallow burial with carbon, emerges as an attractive alternative for carcass management (Miller & Flory, 2018). The method involves placing the animal carcasses in shallow trenches, over a layer of organic compost, such as wood shavings, and covering the carcasses with a thin layer of soil (Flory et al., 2017). The superficial burial method allows for the effective aerobic decomposition of the tissues by the active microbiome found in the soil's upper layers (Flory et al., 2017). The technique also has the potential to solve some of the logistical, practical, and environmental problems associated with other carcass management options (Flory et al., 2017).
Despite the benefits of the AGB method, the relative superficial location of the infected tissues increases the vulnerability to biosecurity breaches due to extreme environmental conditions, such as heavy rain and flooding. In addition, it facilitates the carcass access to scavengers. Oral transmission by infected tissues is a concern for high consequence animal disease viruses, including ASFV and classical swine fever virus (CSFV) (Blome et al., 2020; Cowan et al., 2015; Edwards, 2000; Zani et al., 2020). Therefore, it is critical to understand the level of infectious virus remaining in decomposing tissues over time towards the rational development and application of countermeasures during biosecurity breaches. However, there is a paucity of data related to the time-course evaluation of infectious viruses in decomposing tissue, especially in bone marrow, which contains cells that support the replication of relevant viruses, including ASFV, CSFV, and FMDV (Fischer et al., 2020; Gómez-Villamandos et al., 2003; Stenfeldt et al., 2020).
To further build knowledge on the inactivation rate of viruses in swine carcasses during decomposition, two studies were conducted. The first study involved the field inoculation of swinepox virus (SwPV), as a surrogate for ASFV, into the medullary cavity of 180 femurs from 90 cull sow carcasses to evaluate the viral infectivity and viral DNA presence for 12 months. ASFV is the sole virus in the Asfarviridae family. Although it remains a matter of debate, the tentative order of Megavirales would contain, among other families, the Asfarviridae and Poxviridae families (Andrés et al., 2020; Iyer et al., 2006). The selection of SwPV as an ASFV model was based on the structural similarities with ASFV, the high resistance of these viruses under environmental conditions (Smith, 2007), and due to the SwPV endemic status in the United States. The inoculation of SwPV intrafemorally was chosen due to local containment and because it is one of the latest structures to decompose and, therefore, would allow sample recovery over the 12-month period. The second study was conducted using an in vitro system, and the viability of SwPV, Senecavirus A (SVA), and bovine viral diarrhoea virus (BVDV) was assessed in spiked bone marrow tissues maintained at 21–23°C over a 30-day period.
2 MATERIALS AND METHODS
2.1 Viruses and cells
The SwPV (NVSL catalogue number 002-PDV) was amplified in PK-15 cells (porcine kidney), whereas the SVA strain HI/2012-NADC40 (kindly provided by Drs. Lager and Buckley – USDA ARS) was propagated in swine testis (ST) cells. The BVDV strain Singer (NVSL catalogue number 140-BDV) was amplified in Madin–Darby bovine kidney (MDBK) cells. Cell lines were cultured at 37°C with 5% CO2 in MEM medium (Corning) supplemented with 10% fetal bovine serum (Seradigm), 2 mM l-glutamine (Corning), 1% Antibiotic-Antimycotic 100X (Gibco), and gentamicin (50 μg/ml; Corning).
When the cytopathic effect was observed in more than 90% of the monolayers, the flasks were submitted to a freeze-thaw cycle, followed by centrifugation at 1200× g for 5 min. The clarified viral stock was titrated using limiting dilution assay, and the titre was calculated using the method of Reed and Muench (1938). Viral stocks were titrated in triplicates and stored at −80°C until use. The viral stocks’ titers were 106.8, 109.5, and 107.5 tissue culture infectious dose (TCID50/ml) for SwPV, SVA, and BVDV.
2.2 Virus isolation and titrations
For the virus isolation (VI) assay, spiked bone marrow samples were centrifuged for 5 min, at 1200× g and the supernatant was diluted (1:20) in phosphate buffered saline (PBS). The diluted samples were inoculated in 24-well plates about 70% confluent using the appropriate cell line for each virus as described above. Samples were submitted to five passages of 3–6 days each. The original material of the VI positive samples were subsequently submitted to virus titration assay (Reed & Muench, 1938).
2.3 Field study design
To evaluate the viability of the SwPV in decomposing swine bone marrow tissues under a field study, two trenches were constructed (designated West and East trench). A total of 100 cull sows, 50 for each trench, with an average weight of 200 kg, were used. The project was approved by the Institutional Animal Care and Use Committee, protocol number VM19-19. The animals were separated into two groups of 50, 1 week apart. Euthanasia was conducted with a penetrating captive bolt. The animal death was confirmed by the absence of a heartbeat, absence of a corneal reflex, and cyanosis of mucous membranes. After the animal death was confirmed, the carcasses were transported to the burial site. Carcasses were placed in lateral decubitus position over a layer of about 30 cm of woodchips. The use of 30 cm of carbon layer has been adequate to absorb leachate and facilitate biological activity during recent trials employing the AGB technique (Flory, personnel observation). The 50 carcasses covered about 30 m in length of each trench. The trenches measured approximately 2 m wide and 0.6 m deep.
Inside the trench, the medial aspect of the femur was accessed. Femurs from five carcasses in each trench were collected on day 0 and served as negative controls during subsequent tests. For the remaining 45 carcasses in each trench, a drill was used to create a passage (22 mm in diameter) to the bone marrow. A portion of bone marrow was removed to facilitate the inoculation of 10 ml of SwPV with a 106.8 TCID50/ml titre. Subsequently, a stainless-steel plug was placed to seal the bone passage.
A longitudinal incision was made in the abdominal wall of the carcasses to prevent gas accumulation during the decomposition. Two Onset HOBO U12 temperature data loggers were used to measure temperatures in each trench. Each logger was composed of eight channels. The temperature probes were placed on the 12th, 24th, 36th, and 48th carcass on top of the chest cavity and inside the abdomen. The temperature for each probe was recorded hourly. The carcasses were then covered with the soil excavated during trench preparation. Ten inoculated femurs were harvested per trench on days 7, 14, 21, 28, and around months 2, 3, 6, and 12 post-inoculation to evaluate virus viability. A total of 10 inoculated femurs were collected on day 0 in each trench and used as positive controls during subsequent testing. The pelvic portion of the carcasses was exposed for sample collection, femurs were identified, the plug was removed, and the bone marrow content was aspirated using a syringe. The samples remained in a liquid-to-viscous state in most femurs up to approximately day 60 post-inoculation, and between 2 and 5 ml were collected per femur. After day 60, or in the absence of a liquid sample, 10 ml of PBS pH 7.2 (Corning) was used to rinse the internal femur surface and then recovered as the sample. Finally, the samples were aliquoted in microtubes and immediately stored in an isothermal box with ice for subsequent laboratory testing. The samples were submitted to viral isolation, titration, and qPCR assays.
The duration of SwPV viability in inoculated femurs was estimated using a linear calibration regression model. The model considered the logarithm concentration of the initial virus titre retrieved from two syringes containing the SwPV inoculum and two inoculated femur samples collected at day 0. The titres from these samples were about 106.5 TCID50/ml. The regression model also considered that the detection limit of the virus titration was 101.8 TCID50/ml.
2.4 SwPV inoculum biosafety
The SwPV virus stock used to inoculate the bone marrow in the field study was tested for adventitious viruses for biosafety purposes. For this, the sample was sequenced using an Illumina platform. The virus was amplified, and an aliquot of 50 ml was purified as previously described (Paim, Bauermann, et al., 2021). The DNA library was prepared with 1 ng of purified DNA using the Illumina Nextera XT DNA Library Preparation Kit and sequenced using the Illumina iSeq 100 System with Illumina Reagent Kit V1 (2 × 150 paired-end reads). The data was de novo assembled on BaseSpace Cloud (Illumina) with the metaSPAdes genome assembler (version 3.0). Assembled contigs were examined for similarities to viral nucleotide using Blastn in a database created from the RefSeq virus database (taxid: 10239). The analyses were conducted using Geneious Prime software (version 2020.2.1). To assemble the SwPV genome, the viral contigs were mapped against a reference SwPV genome using Geneious Prime app as previously described (Paim, Maggioli, et al., 2021).
Additionally, the virus stock was tested for bovine respiratory syndrome virus (BRSV), porcine reproductive and respiratory syndrome virus (PRRSV), SVA, and swine influenza virus (SIV) using qPCR. The tests were performed following standard diagnostic protocols at the Oklahoma Animal Disease Diagnostic Laboratory (OADDL).
2.5 SwPV DNA extraction and qPCR
According to the manufacturer's instructions, viral DNA was extracted from bone marrow samples using the MagMAX Viral RNA / DNA kit (Life Technologies) in an automated nucleic acid extractor (King Fisher Purification System, Thermo Fisher Scientific).
The SwPV genome retrieved in this study was used to design the qPCR assay using the PrimerQuest Tool (Integrated DNA Technologies). The used primers were the PoxF 5′-TCAGTACATCCAATTGTCAAGGA-3′, and PoxR 5′-CTGGCTAAATAGAATGAGTGAAACG-3′, and the probe [6FAM]ACTTCCAGAAACGAGTAATCCTTACAAGAC[BHQ-2] (Integrated DNA Technologies). The conditions of the qPCR were as follows: 1 cycle at 95 °C for 60 s, followed by 40 cycles of 50°C for 30 s, 95°C for 10 s and 62.5°C for 30 s. The assay was performed on Applied Biosystems 7500 thermocycler.
2.6 In vitro study design
An in vitro system was used to evaluate the viability of SwPV, SVA, and BVDV in decomposing bone marrow kept at room temperature (21–23°C). The SVA and BVDV, respectively, belong to the same viral families of FMDV and CSFV. Both FMDV and CSFV have tropism to hematopoietic cells and are frequently identified in bone marrow tissues of infected animals (Gómez-Villamandos et al., 2003; Stenfeldt et al., 2020). Although the SwPV has no tropism for hematopoietic cells, it was included in the in vitro study to evaluate any possible effect of the bone marrow matrix to directly inactivating the SwPV and impacting the results of the field study. For the in vitro study, bone marrow tissue from the femurs of pigs older than 4 months was collected in a biosafety cabinet. About 500 mg of tissue was mixed with 500 μl of viral stock in microtubes. As controls, microtubes containing only virus suspensions were prepared. After inoculation, control and spiked samples were collected at days 0, 2, 7, 15, 20, and 30. Three microtubes containing spiked samples and three control microtubes were collected and independently titrated for each time point. The titre of the viral triplicates for each sampling point was used in a linear regression model to evaluate the virus viability decay. The analyses were conducted using GraphPad Prism (version 9.2.0).
3 RESULTS
3.1 AGB study—SwPV inoculum biosafety
Following metagenomics sequence and specific qPCR assays, no other swine viral pathogen was identified in the SwPV inoculum used in the AGB field study. Metagenomics of the SwPV inoculum allowed us to retrieve the near-complete genome sequence of the SwPV used in the current study. The average coverage for the genome was 194×. The genome has over 146,456 base pairs in length and is about 99.93% similar to the isolate 17077–99 (GenBank accession number AF410153.1). A total of 150 open reading frames were predicted. The sequence was deposited in GenBank under the accession number MZ682626.
3.2 AGB study—Carcasses temperature monitoring, SwPV viability, and viral DNA detection
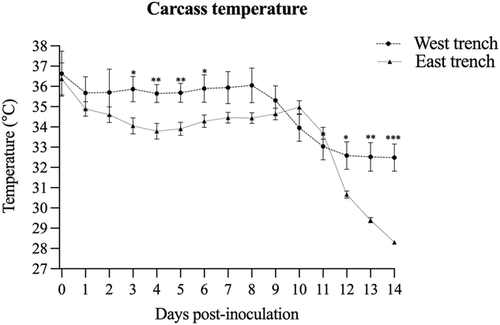
Temperature monitored by probes placed in the carcasses demonstrated that during the first 10 days of the study, the average daily temperatures were above 33°C in both trenches and gradually decreased up to day 14 (Figure 1). The temperature in the West trench trended higher, especially from day 3 to day 7, with an average temperature between 1.5 and 1.9°C higher than the East trench. The maximum and minimum air temperatures during the first 10 days of the study are presented in Table 1.
| West trench | East trench | ||||||
|---|---|---|---|---|---|---|---|
| Day | Date | Max temp | Min temp | Day | Date | Max temp | Min temp |
| Day 0 | 9/4/19 | 33.3 | 18.9 | Day 0 | 9/11/19 | 32.8 | 20.6 |
| Day 1 | 9/5/19 | 34.4 | 20.0 | Day 1 | 9/12/19 | 28.9 | 20.0 |
| Day 2 | 9/6/19 | 35.6 | 18.3 | Day 2 | 9/13/19 | 27.8 | 18.9 |
| Day 3 | 9/7/19 | 35.6 | 20.0 | Day 3 | 9/14/19 | 32.8 | 18.3 |
| Day 4 | 9/8/19 | 34.4 | 19.4 | Day 4 | 9/15/19 | 34.4 | 19.4 |
| Day 5 | 9/9/19 | 33.9 | 21.1 | Day 5 | 9/16/19 | 33.3 | 20.0 |
| Day 6 | 9/10/19 | 33.9 | 22.2 | Day 6 | 9/17/19 | 33.3 | 20.6 |
| Day 7 | 9/11/19 | 32.8 | 20.6 | Day 7 | 9/18/19 | 32.8 | 20.6 |
| Day 8 | 9/12/19 | 28.9 | 20.0 | Day 8 | 9/19/19 | 32.2 | 20.0 |
| Day 9 | 9/13/19 | 27.8 | 18.9 | Day 9 | 9/20/19 | 28.9 | 21.1 |
| Day 10 | 9/14/19 | 32.8 | 18.3 | Day 10 | 9/21/19 | 30.6 | 21.1 |
| Day 11 | 9/15/19 | 34.4 | 19.4 | Day 11 | 9/22/19 | 28.9 | 18.9 |
| Day 12 | 9/16/19 | 33.3 | 20.0 | Day 12 | 9/23/19 | 30.6 | 16.1 |
| Day 13 | 9/17/19 | 33.3 | 20.6 | Day 13 | 9/24/19 | 29.4 | 21.1 |
| Day 14 | 9/18/19 | 32.8 | 20.6 | Day 14 | 9/25/19 | 31.1 | 19.4 |
Infectious SwPV was isolated in cell culture in 11 samples (three from the West trench and eight from the East trench) on day 7. The viral cytopathic effect was observed only on the fourth passage, indicating a low titre remaining in the samples. This was subsequently confirmed by virus titration, in which all virus isolation positive samples had titres below the assay threshold (101.8 TCID50/ml). No infectious virus was identified in samples collected after day 7. Linear calibration regression estimated that 1 TCID50 was achieved in about 11 days. Due to sample limitations, a confidence interval (CI) could not be provided because no estimate of variability was available.
The quantitative polymerase chain reaction (qPCR) testing detected SwPV DNA in 93% of 160 bone marrow samples collected (Figure 2). Eight of the negative samples were in the West trench, whereas three were in the East trench. The cycle quantification (Cq) values of positive samples during the study demonstrated the stability of SwPV DNA under the AGB conditions. Additionally, the consistently positive results validated the sample recovery procedure from femurs throughout the study.
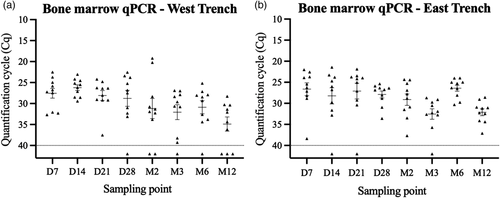
3.3 In vitro study—Virus viability in bone marrow samples
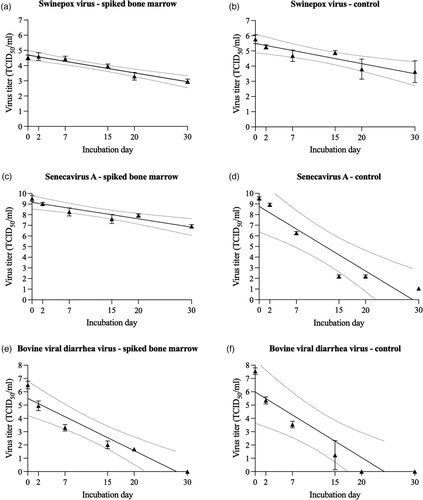
Using an in vitro system, the viability of SwPV, SVA, and BVDV was periodically assessed in virus spiked bone marrow tissue kept at 21–23°C for 30 days. Variable levels of virus inactivation were observed among the studied viruses in the spiked bone marrow matrix and the viral control solution (Figure 3a–f). The SwPV viability in spiked bone marrow decreased from about 104.5 TCID50/ml on day 0 to 103.0 TCID50/ml on day 30. The analyses of SwPV titre decay using the regression model demonstrated a coefficient of determination (R2) of 0.95, and the X-intercept point (virus inactivation) was estimated to be 80 days (63–114 days with 95% CI). Similar to SwPV, SVA remained viable over the study period. In bone marrow-spiked samples, the titre decreased from 109.5 to 106.5 TCID50/ml over the 30 days. SVA regression model indicated an R2 of 0.89 with an estimated virus inactivation of 118 days, ranging from 83 to 223 days with a 95% CI. Conversely, BVDV tenacity in bone marrow was reduced compared to SwPV and SVA. BVDV titre in bone marrow samples ranged from 106.0 TCID50/ml to undetectable viable virus on day 30. However, on day 20, the samples had a titre of 101.8 TCID50/ml. The inactivation was estimated to be achieved in 28 days. The regression model determined an R2 of 0.92, and the inactivation of BVDV was estimated to range from 22 to 40 days considering a 95% CI. Comparing the viral titres from bone marrow-spiked samples to the virus solution used as control, the bone marrow extends the virus viability for the SVA. The estimated viability for the control samples was estimated in 83, 29, and 24 days respectively, for SwPV, SVA, and BVDV. The R2 for the control groups was, respectively, 0.85, 0.89, and 0.85. When considering a 95% CI, the viability was estimated in 57–158 days, 22–46 days, and 17-44 days for SwPV, SVA, and BVDV, respectively.
4 DISCUSSION
The emergence and spread of high consequence animal diseases create the need for prompt actions towards pathogen containment and eradication, which in most cases will require depopulation of affected herds and safe carcass management. The current alternatives for carcasses management during large depopulation events are limited. Among the options, the recently developed AGB method was previously tested during a sheep mortality event caused by peste des petits ruminants virus (PPRV) (Miller & Flory, 2018). However, studies did not evaluate the viability length of these viruses in the carcasses. In our field study, the viability of SwPV as a surrogate for ASFV was evaluated. While the SwPV DNA was consistently retrieved in most samples throughout the 1-year sampling period, viable virus was only detected in samples collected at day 7 after inoculation.
Statistical analyses suggested that virus inactivation was achieved around day 11 post-inoculation in the specific conditions of this study. These results align with the findings from an ASFV field project conducted in Lithuania (Zani et al., 2020). In that study, ASFV naturally infected wild boar carcasses were buried using a shallow burial method, and carcasses were excavated in various locations. By the time of the bone marrow sampling, carcasses were buried for 18–440 days, and none of the samples yielded positive infectious virus (Zani et al., 2020). Another study using bone marrow tissues of pigs naturally infected with ASFV only detected infectious virus at samples kept at room temperature on day 0, and no viable virus was detected after the first week of the study, although, ASFV DNA was detected for up to 2 years on tested samples (Fischer et al., 2020). Conversely, ASFV was viable in the bone marrow of Parma ham for 94 days (McKercher et al., 1987). Differences in the initial viral titre in the samples may affect the viability length. For instance, in the study conducted by Fischer et al. (2020), there were significant higher titres of ASFV in bones rich with red marrow compared to bones rich in fat marrow (yellow marrow). This is likely related to the concentration of ASFV permissive cells in the different types of marrow.
The temperature monitoring in the carcasses demonstrated slightly lower temperatures (1.5–1.9°C) in the East trench compared to the West trench between days 3 and 6 post-inoculation. It is important to note that carcasses in the two trenches were buried 7 days apart. The temperature difference in the trenches is likely associated with weather conditions; however, intrinsic conditions to each trench may also be involved. Although comparison of the inactivation rate between the two trenches was out of the scope of the present study, 73% of the virus positive samples were from the East trench. Whereas it is impossible to strictly correlate these results with the carcass temperature, previous studies have demonstrated that minor temperature variations significantly impact viral survival (Edwards, 2000).
It is well described that temperature, pH, and the matrix containing the virus will directly affect virus inactivation (Cowan et al., 2015; Depner et al., 1992; Edwards, 2000; Farez & Morley, 1997; Fischer et al., 2020). Additionally, it is conceivable that factors associated with specific environmental conditions in each mortality management site, including weather, soil biochemical characteristics, and local insect activity, will create unique factor combinations influencing virus inactivation (Benninger et al., 2008; Fischer et al., 2020; Heaton et al., 2014; Zani et al., 2020). Moreover, different organs may promote variable levels of virus tenacity, and the matrix effect may prolong viability for months (Edwards, 2000; Fischer et al., 2020). In addition to the microenvironment of each carcass management site, the inactivation rate may vary based on the characteristics of each virus (Dee et al., 2018).
Whereas it is impossible to fully replicate under laboratory settings the environmental factors affecting carcass decomposition under natural conditions, the evaluation of SwPV viability using the in vitro system suggests that the SwPV viability could be prolonged under lower temperatures. Over the 30-day period, viable SwPV was isolated from spiked bone marrow samples. Importantly, the in vitro method demonstrated no significant direct effect of bone marrow tissue in inactivating the SwPV or in promoting significant interference in viable virus recovery. Similarly, high levels of infectious SVA have been observed in the bone marrow-spiked samples. SVA has demonstrated high tenacity in previous studies under various combinations of temperature, humidity, and matrices (Caserta et al., 2021; Dee et al., 2018). Like SVA, FMDV is also a member of the Picornaviridae family. A study with FMDV demonstrated viability in bone marrow infected samples maintained at 4°C for up to 7 months (Cottral, 1969), and viral titres over 103 TCID50/ml were observed in the bone marrow of carcasses stored at 4°C for 77 days (Stenfeldt et al., 2020).
The BVDV has been used as a CSFV model in inactivation studies, and under specific conditions, demonstrated similar tenacity to CSFV (Depner et al., 1992). The decreased tenacity of BVDV (estimated in 28 days) compared to the other studied viruses has been demonstrated (Cowan et al., 2015; Depner et al., 1992; Edwards, 2000). Studies have shown a reduction of 2.7 logs in the CSFV titre after 3 days at room temperature (Kubin, 1967). On the other hand, CSFV demonstrated prolonged stability at 4°C, with a reduction of 1.6 logs after 4 days (Kubin, 1967). Another study showed that at 21°C in neutral pH the half-life of CSFV virus was 50 h, compared to 7 h at 37°C (Depner et al., 1992). In organs of pigs infected with CSFV, the viability at 25°C in lymph node, fat, and muscle was estimated in 32 h, 36 h and 148 h, respectively (Cowan et al., 2015).
These results further validated the potential use of the AGB method in the United States. The technique is now outlined as an approved method by the USDA-APHIS to be used during animal mortality emergencies (available at https://www.aphis.usda.gov/animal_health/emergency_management/downloads/agb-emergency-policy.pdf). Whereas SwPV may be a suitable surrogate for preliminary testing of virus viability as a model for ASFV, time course studies using AGB and ASFV infected pigs are needed to support these findings. Additionally, this study extended the knowledge of virus viability in the decomposing bone marrow and may provide critical information towards rational design of countermeasures in cases of biosecurity breaches in sites managing animal mortality.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal have been adhered to and the appropriate ethical review committee approval has been received.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




