Endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis: Beyond the diagnostic yield
ABSTRACT
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is the commonly used technique for pathological confirmation of clinically suspected sarcoidosis, mostly owing to its consistently high success rate in the detection of granulomas. However, other possible advantages, which are less appreciated and often poorly studied, may also contribute to the wider use of EBUS-TBNA in the future. These advantages include refinement of differential diagnoses through the study of lymph node characteristics during B-mode examination; reduction of complications associated with bronchoscopy, as well as improved triage of the specimen for ancillary studies with the use of rapid on-site evaluation; optimization of the quality of the sample through the selection of a target area for biopsy with minimal vascularity and absence of calcifications by using the colour Doppler and the B-mode; and prediction of the presence of extensive lymph node fibrosis by using the strain elastography module. Yet, limitations and possible clinical drawbacks should also be acknowledged. Indeed, due to the lack of specificity of the pathology findings in EBUS-derived samples, the diagnosis of sarcoidosis is one of the exclusion and should remain essentially clinical. The external validity of EBUS-TBNA results in sarcoidosis is questionable, as they mainly derive from studies in populations with a high disease prevalence. Finally, the risk exists that the low morbidity and high diagnostic yield of EBUS-TBNA may lead to its overuse in patients with clinical/radiological findings specific enough to secure a clinical diagnosis of sarcoidosis.
INTRODUCTION
Endoscopic sampling of the mediastinum using conventional transbronchial needle aspiration (c-TBNA), which was developed by the pioneering work of Ko-Pen Wang in the early 1980s, has revolutionized the diagnostic approach to many conditions, including sarcoidosis. Early studies of c-TBNA in sarcoidosis patients demonstrated that the detection of granulomas was feasible, even though the success rate was quite variable (46–90%).1-9 A systematic review and meta-analysis that included 21 studies for a total of 915 sarcoidosis patients and summarized the literature regarding c-TBNA in sarcoidosis up until 2012 attributed a cumulative diagnostic accuracy of 62% to the procedure.10
The introduction of endosonography (EUS: endoscopic ultrasound; EBUS: endobronchial ultrasound), and in particular the publication of the first studies that assessed the success rate and safety of EBUS,11, 12 started a process that, over a few years, has led EBUS-TBNA to become the first-choice technique for pathological confirmation of sarcoidosis in most centres worldwide.
WHY HAS EBUS BECOME INVALUABLE FOR THE DIAGNOSIS OF SARCOIDOSIS?
The frequency and pattern of involvement of intrathoracic lymph nodes in sarcoidosis favour their sampling with EBUS-TBNA
Lymphadenopathy is, by far, the most common manifestation of sarcoidosis across all ethnic groups.13-16 In addition, the computed tomography (CT) pattern of intrathoracic lymphadenopathy seen in most sarcoidosis patients strongly suggests that a diagnosis of sarcoidosis can be established with endoscopic techniques able to sample the mediastinum.14, 17 The extensive involvement of the intrathoracic lymph nodes, coupled with the reliability with which EBUS can safely and in real-time sample virtually any node in contact with a large airway, explains its consistently high success rate in diagnosing sarcoidosis. Most individual studies, as well as the only two available meta-analyses of EBUS-TBNA in sarcoidosis, in fact, revealed a detection rate for granulomas of more than 80%.11, 12, 18-23
In conclusion, the frequency and pattern of lymph node involvement seen in most patients with sarcoidosis, coupled with the accuracy and safety of EBUS-TBNA, makes this approach very useful for histological confirmation of clinically suspected sarcoidosis.
EBUS-TBNA improves diagnostic accuracy of patients with isolated intrathoracic lymphadenopathy
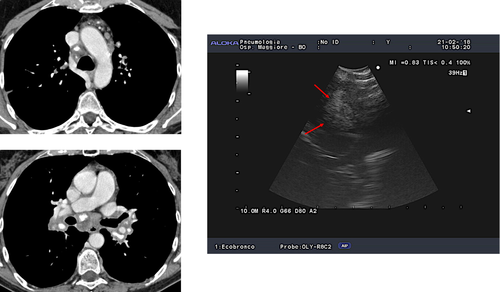
While transbronchial lung biopsy (TBB) has been associated with high diagnostic accuracy for the detection of pulmonary granulomas in sarcoidosis patients with parenchymal abnormalities on imaging, most studies have demonstrated that diagnostic accuracy in patients with Scadding stage I is suboptimal (12–66%), at least when four to five biopsies per patient are obtained, as per common clinical practice.24, 25 Higher success rates (78–100%) have been obtained with TBB in stage I sarcoidosis in three studies in which 8–10 biopsies were obtained,26-28 but a 13% complication rate was also observed in one trial28; moreover, such a high number of biopsies is seldom obtained in clinical practice. Conversely, EBUS shows an average detection rate for granulomas close to or higher than 90% in patients with radiological stage I disease.11, 12, 18-21 More importantly, endosonography is the only non-surgical procedure that can strengthen the clinical suspicion of sarcoidosis or suggest an alternative diagnosis in patients with normal lung parenchyma on high-resolution CT scan and in whom the pre-test clinical probability of a diagnosis of sarcoidosis is not as high as in typical cases. Examples of these conditions include, but are not limited to, isolated lymphadenopathy in patients with a history of (current/previous and treated/untreated) malignancy; isolated lymphadenopathy in patients with demographic, clinical or radiological findings that make tuberculosis (TB) or lymphoma a diagnostic possibility29, 30; and isolated lymphadenopathy in patients with suspected extrathoracic sarcoidosis (Fig. 1).
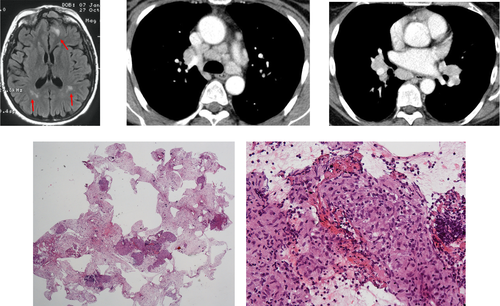
In conclusion, the high success rate and safety of EBUS-TBNA have made it the first-choice technique in the diagnostic approach to patients with suspected stage I sarcoidosis who require a pathological confirmation. The procedure is particularly useful in patients without a high pre-test clinical probability of a diagnosis of sarcoidosis, as in these cases the chance of EBUS changing the initial diagnosis is significantly high.
B-mode examination of lymph node density using EBUS provides important information for differential diagnosis
The differential diagnosis of a patient with isolated intrathoracic lymphadenopathy is challenging. While suspecting and confirming malignant lymph node involvement is usually straightforward when imaging and pathology findings are combined, differentiating sarcoidosis from infectious lymphadenopathy (especially isolated tuberculous (TB) lymphadenopathy) is far more difficult, especially in countries or ethnic groups with a high prevalence of TB.31 Symptoms such as fever, cough and fatigue can be present in both sarcoidosis and TB, yet they can be absent or very subtle in both conditions. As for imaging features, even though TB lymphadenopathy is characterized significantly more often than sarcoidosis by asymmetric hilar involvement and areas of reduced attenuation on CT, these findings are not always present.32, 33 Cytology of needle aspiration specimens may reveal non-necrotizing granulomas in both sarcoidosis patients as well as in a significant percentage of patients with TB lymphadenopathy.34-37 Finally, the interferon-gamma release assays are generally negative in patients with sarcoidosis, but they can also be negative in up to 20% of patients with active TB.38, 39 Interestingly, the tuberculin skin test (TST) is the most useful non-invasive test for differentiating the two conditions. One study in India has demonstrated that the TST is negative in the vast majority of sarcoidosis patients, owing to their typical anergy, regardless of the rate of TST positivity in the general population.40 Consequently, a positive TST in a patient thought to have sarcoidosis should prompt a thorough diagnostic work-up to rule out TB.40, 41
In the last decade, studies aiming at comparing the diagnostic yield of endosonography for the diagnosis of TB and sarcoidosis have shown that the ultrasound B-mode evaluation can improve the differential diagnosis between the two conditions.42-46 The most common sonographic features of sarcoidosis lymph nodes include homogeneous echotexture, tendency to cluster, oval shape, distinct margins and increased vascularity (Videos S1, S2, Supplementary Information).42-45 In the largest study comparing the sonographic characteristics of sarcoidosis and TB lymphadenopathy, 358 nodes from 165 patients (118 sarcoidosis and 47 TB) were studied and sampled.42 Both heterogeneous echotexture (53.4% vs 12.6%, P < 0.001) and coagulation necrosis (26.1% vs 3.3%, P < 0.001) were significantly more common in tuberculous lymph nodes than in sarcoidosis lymph nodes. The combination of a positive TST and either heterogeneous echotexture or coagulation necrosis had a diagnostic specificity of 98% and a positive predictive value (PPV) of 91% for TB. The presence of these sonographic findings, even in a sputum-negative patient with isolated intrathoracic lymphadenopathy, may warrant empiric anti-TB treatment pending results of culture of needle aspiration material (Fig. 2).
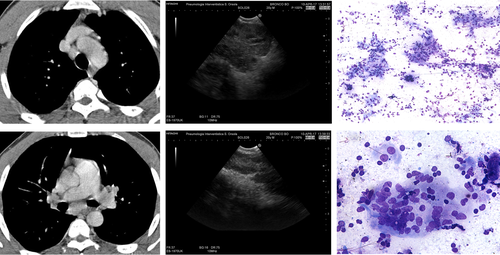
In conclusion, in the appropriate clinical setting, the B-mode examination of intrathoracic lymph nodes during EBUS can help differentiate sarcoidosis from infectious lymphadenopathy.
EBUS-TBNA material can undergo rapid on-site cytological evaluation
Rapid on-site evaluation (ROSE) of cytological material from lymph nodes sampled with needle aspiration procedures allows clinicians to check sample adequacy, establish a preliminary diagnosis and appropriately triage the specimen for ancillary studies.47, 48 Although three randomized trials failed to demonstrate an advantage in terms of diagnostic yield or specimen adequacy associated with its use, they clearly showed that ROSE helps clinicians avoid biopsies from additional targets without a loss in diagnostic yield, thus reducing the risk of bronchoscopy-related complication.49-51 Most studies investigating the diagnostic value of bronchoscopy in patients with suspected sarcoidosis were performed without the availability of ROSE. Such studies showed that: (i) the success rate is higher if sampling from both the mediastinum (TBNA or EBUS-TBNA/EUS with fine needle aspiration (EUS-FNA)) and from the lung parenchyma (TBB) is carried out5, 7, 9, 19, 20, 29, 52; (ii) the vast majority of the complications (pneumothorax and bleeding) are caused by TBB20, 26, 27; and (iii) the number of EBUS needle passes recommended to optimize the diagnostic yield is four to five.53, 54 Based upon these data, there are numerous advantages possibly associated with the use of ROSE in sarcoidosis. Because most complications of bronchoscopy come from TBB,20, 26, 27 the identification of non-necrotizing granulomas using ROSE on EBUS-TBNA material might also prevent the operator from sampling the lung parenchyma, thus avoiding additional costs and potential complications (Fig. S1, Supplementary Information). Furthermore, a positive ROSE could theoretically help reduce the number of needle passes, which, in turn, would reduce the overall time needed for bronchoscopy and the potential complications associated with endosonography itself.55-57 Finally, ROSE might help effectively triage the EBUS-TBNA material for ancillary studies, especially in cases where sarcoidosis is in the differential diagnosis, but is not the final diagnosis (Fig. 3).
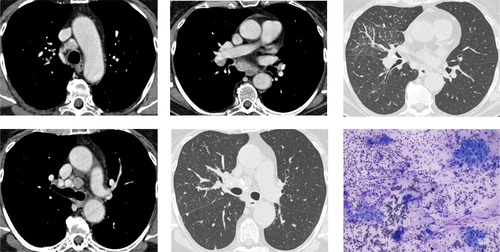
Several studies have assessed the value of ROSE in patients with suspected sarcoidosis undergoing EBUS-TBNA.28, 52, 58 Plit et al. performed EBUS-TBNA with ROSE followed by TBB and bronchial biopsies in a prospective cohort of 60 patients with suspected sarcoidosis. EBUS-TBNA detected granulomas in 45 of 49 patients with a final diagnosis of sarcoidosis.28 ROSE produced 43 true-positive results and 1 false-positive result for the diagnosis of sarcoidosis. Given the high accuracy of EBUS-TBNA with ROSE, in addition to the high concordance rate between the ROSE result and the final pathological diagnosis, the authors concluded that EBUS combined with ROSE should be considered the first-line approach in patients with suspected sarcoidosis.28 Interestingly, the only complications observed (pneumothorax in 8% of patients and moderate bleeding in 5% of patients) were caused by TBB.28 While EBUS alone may identify granulomas, bronchoscopic specimens such as bronchial and needle wash should still be sent out to rule out mycobacterial or fungal infections. Furthermore, the pre-test clinical suspicion of sarcoidosis must be considered carefully before assuming that the presence of granulomas on a ROSE-EBUS specimen equates with sarcoidosis, as malignancy, infection and other inflammatory diseases can lead to mediastinal granulomatous reactions.
In conclusion, unlike any other currently available bronchoscopy-based sampling method [Bronchoalveolar lavage (BAL), bronchial and transbronchial biopsy], c-TBNA and endosonography-derived samples from patients with suspected sarcoidosis can be examined by ROSE. Although ROSE proved to be sensitive and highly concordant with the definitive slides, key advantages associated with its use, such as the reduction of the costs and complications of bronchoscopy, as well as a more effective triaging of the aspirated material (especially when it shows features suggestive of alternative diagnoses) have not been systematically studied in patients with suspected sarcoidosis.
EBUS allows the selection of the best possible areas for sampling, thus avoiding regions with increased vascularity and extensive calcification
Clinical experience and data from the literature suggest that prominent vascularization is frequently seen during colour Doppler evaluation in patients with sarcoidosis undergoing endosonography (Fig. 4).45, 59 While the risk of bleeding associated with the puncture of vessels is thought to be very low, the retrieved sample may be of limited quality due to the excess of blood. Furthermore, the application of suction during the aspiration manoeuvres, especially if hypertrophic bronchial arteries are punctured, may lead to the formation of small intra-nodal haematomas, which may negatively affect the quality of the material obtained from repeated aspirations. Likewise, calcification of hilar/mediastinal lymph nodes caused by sarcoidosis or infection is common.14, 60 Although the distribution and pattern of calcification in lymph nodes of patients with infection and sarcoidosis may differ due to varying routes of lymphatic drainage of pulmonary TB and granuloma necrosis,60 cytological and microbiological confirmation may be warranted especially in patients without parenchymal involvement. In such cases, endosonography allows clinicians to elegantly identify and avoid lymph node areas with extensive calcification while selecting areas with ‘vital’ tissue for biopsy (Fig. 5).
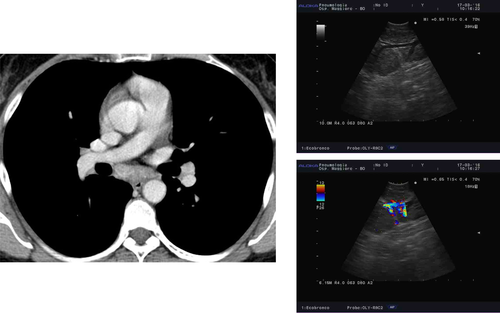
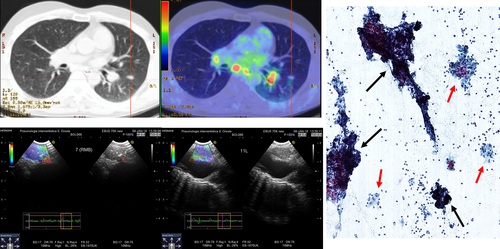
In conclusion, the evaluation of the presence of prominent vascularity or extensive calcification in the lymph nodes using the colour Doppler or the B-mode, respectively, may allow clinicians to optimize the quality of samples by selecting the best possible target areas for needle aspiration/biopsy.
Evaluation of lymph node elasticity
Although the diagnostic success of EBUS-TBNA in detecting granulomas in sarcoidosis lymph nodes is very high, the procedure is not foolproof. Clinical experience suggests that at least some of the false-negative EBUS-TBNA results in sarcoidosis patients may be caused by the presence of lymph nodes with extensive fibrosis. In such cases, the operator feels a very high level of resistance upon needle advancement during aspiration attempts, and the node tends to move as a whole while the needle is advanced back and forth into it (Video S3, Supplementary Information). To date, no study has formally assessed the impact of lymph node fibrosis (a common finding in sarcoidosis lymph nodes61) on the diagnostic yield of EBUS-TBNA. This might partly be due to the fact that imaging tools commonly used in patients with suspected sarcoidosis (e.g. chest CT) cannot reliably identify fibrotic lymph nodes, whereas they can identify fibrotic changes in the lung parenchyma quite reproducibly.
Strain elastography is a new imaging method used for the measurement of relative tissue elasticity through qualitative (colour pattern) or semi-quantitative (strain ratio or strain histogram) methods. Because malignant lesions are usually stiffer, several studies have demonstrated the ability of elastography to differentiate between malignant and benign lymph nodes, but none of them has assessed this approach in patients with suspected sarcoidosis.62, 63 However, one ex vivo study in abdominal lymph nodes has shown a correlation between strain elastography and the extent of fibrosis in patients with Crohn's disease.64 Very recently, the first report was published showing clear pathological evidence of a significant amount of fibrosis in EBUS-TBNA specimens from a sarcoidosis patient in whom elastography suggested the presence of lymph node fibrosis.65 This report will likely encourage clinical research investigating the influence of lymph node fibrosis on the density of granulomas, starting with a method (i.e. strain elastography) that seems most promising for identifying fibrotic lymph nodes before sampling (Fig. 6).
In conclusion, the evaluation of lymph node elasticity using strain elastography may help identify fibrotic nodes, which might have a low density of granulomas. While waiting for robust scientific data on the possible role of EBUS strain elastography, the identification of potentially fibrotic lymph nodes in a patient with suspected sarcoidosis might suggest that the operator should pursue additional sampling (i.e. TBB) to minimize the risk of missing the diagnosis, especially if ROSE is unavailable or inconclusive.
NOT ALL THAT GLITTERS IS GOLD: THE DOUBTS AND CLINICAL DRAWBACKS OF USING EBUS-TBNA TO DIAGNOSE SARCOIDOSIS
The pathology findings obtained using EBUS are not specific for sarcoidosis
The main limitation, and possibly danger, of endosonography for the diagnosis of sarcoidosis is the lack of specificity, which makes it crucial that the pathology findings are interpreted in the individual clinical context, and that the final diagnosis remains essentially a clinical one. Non-necrotizing granulomas, which are the pathological hallmark of sarcoidosis, can be found in a variety of conditions ranging from infectious diseases to malignancy. This is discussed below in more detail using some common examples.
Tuberculous lymphadenitis
Tuberculous lymphadenitis (TBLA) is the most common extrapulmonary manifestation of TB across all ethnic groups in both USA and UK.34, 66 Isolated intrathoracic TBLA, which accounted for 9% of all cases reported in the UK in 2009,34 is particularly challenging from a diagnostic standpoint because sputum culture and traditional bronchoscopic techniques are rarely helpful in such cases.34 On the other hand, the increasing prevalence of isoniazid-resistant and multidrug-resistant disease makes it extremely important to culture the causative organism for drug susceptibility testing.67, 68
c-TBNA and endosonography represent the only non-surgical methods to retrieve lymph node material for cytological and microbiological testing, and they are increasingly used in patients with suspected intrathoracic TBLA.33-36, 68, 69 Although individual studies and meta-analyses suggest that the success rate of these methods for the diagnosis of TBLA is quite high (79–95%), the criteria used to diagnose TB deserve some comments to help clinicians putting these results into clinical context.33-36, 68, 69
First, a microbiological confirmation (i.e. a positive PCR or culture results) is only achieved in 40–60% of patients.33-36, 68 Even the Xpert MTB/RIF (Cepheid Inc., Sunnyvale, CA, USA) assay, a World Health Organization (WHO)-recommended automated PCR-based test that can detect both TB and rifampicin resistance, has very good specificity (97.9%) and PPV (92.9%) for the diagnosis of TBLA on EBUS-derived samples, but lacks sensitivity (49.5%).70 As a consequence, cytology findings are not only used to corroborate the clinical suspect of TB and to guide empirical anti-mycobacterial treatment pending culture results, but also quite often represent the only ‘diagnostic test’ for TB patients who have negative PCR and culture results on EBUS-derived material. Furthermore, in areas where TB is endemic, PCR lacks specificity to rule out latent versus active infection. Newer techniques may be necessary to avoid false-positive PCR results.
Second, although a granuloma with necrosis is the pathological hallmark of TB, in a prospective study of 117 sarcoidosis patients and 181 sputum-negative TB patients, necrosis was identified in histological specimens in 10% of sarcoidosis patients versus 87% of TB patients.30 Several cytology patterns were found in the lymph nodes of patients with suspected TBLA who underwent endoscopic sampling. These patterns include necrotizing granulomas, non-necrotizing granulomas, necrosis with no granulomas and reactive findings with no granulomas.33-36, 68 Interestingly, non-necrotizing granulomas—very much like those seen in patients with sarcoidosis—represented the observed cytological pattern in 33–37% of TB patients in the two largest studies performed thus far, which assessed the role of endosonography in the diagnosis of TBLA.33, 34 While retrieving non-necrotizing granulomas in the EBUS-TBNA-derived material from a patient with a high clinical and radiological pre-test probability for TB can reasonably suggest starting an empiric anti-TB treatment pending culture results (Fig. S2, Supplementary Information), the same cytological pattern may lead to a wrong diagnosis of sarcoidosis (and possibly inappropriate and potentially harmful treatment) in a TB patient with a low clinical and radiological pre-test probability of the disease (Fig. S3, Supplementary Information).
Sarcoid-like reactions
The term ‘sarcoid-like reaction’ refers to granulomatous lesions that look like sarcoidosis but do not fulfil the criteria for a clinical diagnosis of sarcoidosis.24 Often, sarcoidosis remains in the differential diagnosis, but not enough so to provide some level of certainty. An example of sarcoidosis-like reaction includes non-caseating granulomas that are in the lymph nodes draining from cancer. The nodes may (Fig. S4, Supplementary Information) or may not (Fig. S5, Supplementary Information) harbour cancer cells besides the granuloma, but this is clearly associated with malignancy.71-73 Granulomas may also be found in lymph nodes of patients with lymphoma, especially Hodgkin's disease.74 Other examples include granulomatous lesions resulting from unknown reactions, such as those induced by several classes of drugs,75 those associated with immunodeficiency syndromes76 or those associated with inflammatory bowel disease.77
It transpires that clinical acumen and thoroughness of the clinical evaluation, rather than the retrieval of non-necrotizing granulomas with EBUS (in cases where a biopsy is taken from the intrathoracic lymph nodes), are key for preventing these cases from being wrongly diagnosed as sarcoidosis.
The external validity (generalizability) of EBUS-TBNA results in diagnosing sarcoidosis is questionable
Most individual studies11, 12, 18-21 and meta-analyses22, 23 of EBUS-TBNA in sarcoidosis indicate that the technique has a high sensitivity (usually >80%) for detecting granulomas. However, reliable calculation of sensitivity (and specificity) requires that an independent test is used for determining the final diagnosis. The diagnosis of sarcoidosis is established in the presence of a compatible clinical setting coupled with the demonstration of non-caseating granulomas and after exclusion of other causes of granulomatous inflammation.25 Recently, a sarcoidosis diagnostic score has been developed to standardize the clinical features in support of a diagnosis of sarcoidosis.78 As a ‘gold standard’ test is lacking in sarcoidosis, reporting the diagnostic yield for the detection of granulomas rather than its sensitivity for the diagnosis of sarcoidosis would be more accurate. Furthermore, even if a surrogate test is used as diagnostic gold standard (i.e. multidisciplinary meeting for adjudicating the diagnosis79), sensitivity (as well as specificity) is of no practical use when it comes to helping clinicians determine the probability of disease in individual patients.80 In contrast, PPV and negative predictive value (NPV) can describe a patient's probability of having the disease (sarcoidosis in this case) once the results of a given test (i.e. EBUS-TBNA) are known.80 The PPV and NPV of a test can be calculated by knowing its sensitivity and specificity, yet it is influenced by the prevalence of the disease in the study population.80 For example, a test with 80% sensitivity and 94% specificity will have a 40% PPV and 99% NPV when the prevalence of the disease is 5%, but a 96% PPV and 76% NPV when the prevalence of the disease is 60%.80 Because most trials of EBUS-TBNA in sarcoidosis have an inherent selection bias represented by the very high prevalence of the disease (often >90%) in the study population, the excellent results reported cannot be generalized to populations in whom the prevalence of sarcoidosis is lower.80 Ideally, EBUS-TBNA should be tested in consecutive patients for whom sarcoidosis is one of the diagnostic possibilities based on clinical and imaging entry criteria agreed upon beforehand and applied prospectively. This should allow for the selection of a study population more similar to that the average clinician deals within their clinical practice, and most certainly characterized by a lower prevalence of sarcoidosis. Such a study could also shed light on the role of EBUS in patients with moderate pre-test clinical probability of sarcoidosis, which is exactly the setting where a diagnostic test can make a difference by helping the clinician rule in or out a diagnosis that is truly uncertain.81
The risk of EBUS-TBNA overuse in patients with suspected stage I sarcoidosis
Most experts agree that the diagnosis of sarcoidosis can be reliably made on clinical grounds in asymptomatic patients with bilateral hilar lymphadenopathy (BHL), as well as in patients with typical clinical manifestations (i.e. Löfgren's syndrome and Heerfordt's syndrome).25, 82, 83 In 1973, Winterbauer et al. demonstrated that among 100 patients with BHL of different aetiologies, sarcoidosis was the cause in all 30 patients who were asymptomatic and had a negative physical examination.84 In a very thought-provoking study, Reich et al. surveyed the English-language medical literature to estimate the proportion of patients with TB, Hodgkin's disease, non-Hodgkin's lymphoma and sarcoidosis who presented with asymptomatic BHL.85 These researchers calculated that if 33 000 patients with asymptomatic BHL underwent mediastinoscopy, 32 982 (99.95%) would be found to have stage I sarcoidosis or, very rarely, an alternative disorder not requiring intervention.85 However, 407 patients would require hospitalization for complications at a cost in excess of $1 million, and 204 patients would experience major morbidity. Given these figures, a working clinical diagnosis of sarcoidosis associated with a watchful follow-up aimed at time-testing the initial hypothesis and at ruling out alternative diseases in a dynamic fashion seems to be the best option in this setting.82, 83
Keeping into account the above-mentioned considerations, the high accuracy of EBUS-TBNA in sampling the intrathoracic lymphadenopathy should not lead to its overuse, in spite of its reassuring safety profile,55, 56, 86 especially in patients in whom the benefit of a biopsy confirmation would be very low. While only a minority of the studies that assessed the role of EBUS-TBNA in diagnosing sarcoidosis mentioned the clinical features of their patients, the very high prevalence of the disease (especially stage I patients) found in most studies suggests that a sizeable proportion of patients with BHL were asymptomatic.
Importantly, the above-mentioned considerations may not apply to countries with high TB burden. In these areas, in fact, patients with TBLA are not infrequently asymptomatic or may show certain clinical features (i.e. uveitis and joint pain) that might even strengthen the clinical suspicion of sarcoidosis in countries with low TB burden. Indeed, TST and pathological/microbiological analysis (including the Xpert MTB/RIF assay) of samples retrieved from multiple endoscopic sampling procedures such as EBUS-TBNA, bronchial and transbronchial biopsy are commonly carried out to confirm or rule out TB reliably in areas with high TB prevalence.29, 42, 70, 87
INTRODUCTION ROUTE AND TECHNICAL ASPECTS
Endobronchial versus oesophageal ultrasound
Mediastinal lymph nodes can be sampled both from the airways (EBUS-TBNA) and from the oesophagus (EUS), in the latter case either with a regular echogastroscope (EUS-FNA) or with the EBUS scope (EUS-B-FNA). Very recently, the results of the first randomized trial aimed at comparing the diagnostic rate of EBUS-TBNA versus EUS-B-FNA in patients undergoing endosonography for suspected sarcoidosis have been presented in the form of an abstract at the 2018 European Respiratory Society in Paris .88 This study, which enrolled 332 patients from 13 hospitals in 9 countries and 4 continents, demonstrated no differences between EBUS-TBNA and EUS-B-FNA in terms of granuloma detection rate (75% vs 70.3%, respectively), sensitivity (84% vs 87%, respectively) and complications.88
These results will likely confirm the endobronchial route as the preferred approach for the endoscopic diagnosis of sarcoidosis in most centres, as it offers some key advantages. First, hilar (station #10) and interlobar (station #11) lymph nodes, which are the most commonly involved in sarcoidosis, can be sampled from the airways (EBUS) but not from the oesophagus (EUS/EUS-B). Second, the endobronchial route allows to perform, in a single session, both EBUS-TBNA and additional sampling procedures such as bronchoalveolar lavage, endobronchial and transbronchial biopsies. This strategy has been shown to optimize the diagnostic yield in sarcoidosis, especially in stage II.5, 7, 9, 19, 20, 29, 52 On the other hand, lymph nodes more difficult to sample (station #4L) or inaccessible (i.e. station #8L) from the airways can be sampled from the oesophagus using the EBUS scope, in the same diagnostic session, if the endobronchial approach fails or is unsatisfactory.89
Technical aspects
Besides ROSE,28, 52, 58 whose role has been discussed in detail in section EBUS-TBNA material can undergo rapid on-site cytological evaluation, a few technical aspects specific to EBUS-TBNA in sarcoidosis have been studied and deserve mentioning.
In a study aimed at assessing the factors possibly influencing the diagnostic yield, Sun et al. found that performing more than one needle pass per lymph node was the only technical aspect of EBUS independently associated with a positive pathology at multivariate analysis.54 A more recent multicentre trial tried to assess specifically the number of needle passes needed to diagnose stage I/II sarcoidosis.53 The authors performed six needle passes for the main target lymph node lesion and calculated the cumulative yields for detecting non-caseating granulomas.53 The success rate did not increase significantly after four needle passes; the authors therefore recommended that such number of passes be performed in patients undergoing EBUS for suspected sarcoidosis in the absence of ROSE.53
As for the needle size, the only two randomized trials compared 22 versus 25 pro-core needles88 and 21- versus 22-gauge needles90 in patients undergoing endosonography for suspected sarcoidosis. Interestingly, no significant differences in the diagnostic yield for the detection of granulomas by needle size were observed.88, 90
Finally, the number of revolutions of the EBUS needle (20 vs 10) inside the target lymph node was not found to influence diagnostic yield, specimen adequacy or complication rate.91
SUMMARY
EBUS-TBNA represents a versatile, effective and safe tool to confirm pathologically the clinical suspicion of sarcoidosis. Application of the procedure to patients with clinical and radiological findings that are not specific enough to warrant a reliable diagnosis without a biopsy confirmation, and pathological interpretation of EBUS-obtained specimens in the clinical context of the individual patient maximize its clinical utility and limit its costs and possible complications. Prospective studies of patients for whom sarcoidosis is only one of the diagnostic options based on pre-defined inclusion criteria are urgently needed to verify the validity of EBUS-TBNA in populations without the bias represented by a very high prevalence of the disease.
Acknowledgement
The authors are grateful to Alessandra Cancellieri, MD, for providing the pathological images.
Disclosure statement
R.P.B. has received grants from Gilead, Bayer, National Institutes of Health, Foundation for Sarcoidosis Research and Genentech; and grants and personal fees from Mallinckrodt. D.A.C. has received consulting fees from Novartis, Actelion, Mallinckrodt and aTyr.
Abbreviations
-
- AFB
-
- Acid-fast bacilli
-
- BHL
-
- bilateral hilar lymphadenopathy
-
- c-TBNA
-
- conventional TBNA
-
- CT
-
- computed tomography
-
- EBUS
-
- endobronchial ultrasound
-
- EBUS-TBNA
-
- EBUS-guided TBNA
-
- EUS
-
- endoscopic ultrasound
-
- FNA
-
- fine needle aspiration
-
- MRI
-
- magnetic resonance imaging
-
- NPV
-
- negative predictive value
-
- PCR
-
- polymerase chain reaction
-
- PET
-
- positron emission tomography
-
- PPV
-
- positive predictive value
-
- ROSE
-
- rapid on-site evaluation
-
- TB
-
- tuberculosis
-
- TBB
-
- transbronchial lung biopsy
-
- TBLA
-
- tuberculous lymphadenitis
-
- TBNA
-
- transbronchial needle aspiration
-
- TST
-
- tuberculin skin test




