Idiopathic chronic productive cough and response to open-label macrolide therapy: An observational study
Abstract
Background and objective
Adult patients with chronic productive cough of unknown cause are commonly seen in respiratory clinics. We have previously described a subgroup of these patients who have a short-lived response to standard antibiotic treatment but a prolonged response to 3 months of low-dose azithromycin therapy.
Methods
This observational study describes the physiological, radiological and pathological features of this patient cohort along with their response to a 12-week open-label trial of 250 mg azithromycin thrice weekly.
Results
A total of 30 subjects with a mean age of 57 were recruited. The majority demonstrated airway dilatation on high-resolution computed tomography (HRCT) scan without evidence of established bronchiectasis (n = 21) and non-specific chronic inflammatory changes on bronchial biopsy (n = 15/17). Twenty-nine subjects completed 3 months of azithromycin with a significant improvement in median Leicester Cough Questionnaire (LCQ) score (−6.3 points, P < 0.00001), reduction in median 24-h sputum volume (−5.8 mL, P = 0.0003) and improvement in sputum colour (P = 0.003). Patients responsive to azithromycin (n = 22) demonstrated neutrophilic or paucigranulocytic airway inflammation, whereas five subjects with eosinophilic airways inflammation did not respond symptomatically to azithromycin.
Conclusion
We describe a cohort of patients with chronic productive cough not adequately described by existing disease labels whose symptoms responded well to low-dose azithromycin. Many of the features are similar to the paediatric condition protracted bacterial bronchitis.
INTRODUCTION
A cohort of adult patients with chronic productive cough which improves with antibiotic treatment but quickly relapses has been described previously.1 In these cases, investigations including spirometry, bronchoscopy, immunodeficiency screen and high-resolution computed tomography (HRCT) scans failed to provide a satisfactory explanation for their symptoms, although many have been diagnosed with ‘asthma’ and treated with inhaled corticosteroids.1
We have frequently observed a marked and sustained symptomatic improvement in these patients following a 3-month course of low-dose azithromycin. Azithromycin is a macrolide antibiotic with demonstrated efficacy in the treatment of respiratory conditions including diffuse panbronchiolitis,2 chronic obstructive pulmonary disease (COPD),3 bronchiectasis4 and asthma.5 In addition to antibiotic effects, azithromycin has demonstrated immunomodulatory and anti-inflammatory effects6 particularly in subjects with underlying neutrophilic airway inflammation.7-9
The aims of this study were to (i) describe the clinical, pathological and radiological features of this cohort and (ii) determine the response to a 12-week course of azithromycin and assess if any of the baseline characteristics measured could predict response to azithromycin.
METHODS
Design
This was a single-centre study with detailed description of baseline clinicopathological features followed by an open-label trial of 12 weeks of low-dose azithromycin (250 mg thrice weekly) in subjects with idiopathic chronic productive cough.
Subjects
Patients with a productive cough of ≥3 months duration who had not smoked for ≥10 years with a <20 pack-year smoking history were recruited from respiratory clinic if initial investigations found no cause for their symptoms. Subjects were excluded if they had evidence of immunodeficiency, established bronchiectasis on HRCT, history of inhaled irritant exposure, a contraindication to azithromycin treatment (prolonged QT interval on electrocardiogram (ECG)/significant cardiac pathology or liver function tests >2× the upper normal limit) or documented macrolide hypersensitivity. The study was approved by a local Research Ethics Committee (Ref 13/YH/0245). All participants meeting the inclusion criteria attended a baseline visit where written informed consent was obtained. Investigations were carried out over five study visits V1-V5 (Fig. 1).
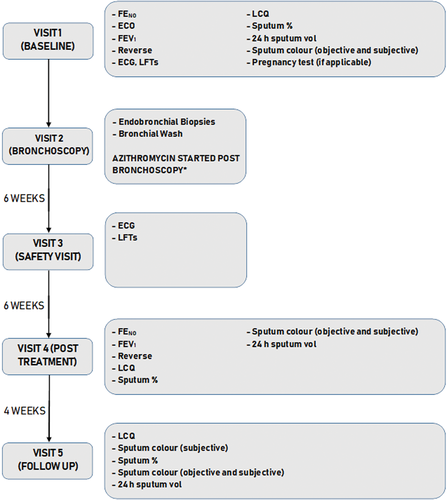
Outcomes
The main aims were to describe the baseline clinicopathological features of the patients and identify responders and non-responders to 12-week low-dose azithromycin, using change in Leicester Cough Questionnaire (LCQ) as the primary outcome.
The LCQ is a validated questionnaire designed to assess health-related quality of life in patients with chronic cough.10 It has a good level of internal consistency and reliability11 and a minimal clinically important difference (MCID) of 1.3 points12 (Appendix S1, Supplementary Information).
Specified secondary outcomes were change in forced expiratory volume in 1 s (FEV1), fractional exhaled nitric oxide (FENO), subjective and objective sputum colour, 24-h sputum volume, sputum inflammatory type and sputum supernatant cytokine levels. Baseline blood eosinophil counts and previous sputum microbiology were also assessed.
FENO concentration was measured using an offline electrochemical analyser (NOBreath, Bedfont Scientific, Harrietsham, UK) at a flow of 50 mL/s. Sputum colour was assessed by subjects and investigators using a commercially available sputum colour chart (BronkoTest, Heredilab Inc., Salt Lake City, UT, USA). To obtain a 24-h sputum collection volume, subjects were instructed to collect all spontaneously produced sputum in universal sample containers in the 24-h period prior to their next visit. Any obvious salivary portion of the sample was discarded before measurement of the volume in a measuring cylinder with 0.1 mL graduations. Sputum samples were induced using hypertonic saline and processed to obtain a sputum differential cell count as described previously.13 Aliquots of sputum supernatant were frozen at −80°C for later quantification of IL-8, IL-1β, IL-17A and TNF-α levels using a multiplex immunoassay system (Bio-Plex, Bio-Rad, Hemel Hempstea, UK).
Bronchial wash samples and biopsies were obtained as described (Appendix S2, Supplementary Information) and biopsies were processed and reviewed by a consultant histopathologist blinded to subjects’ response to azithromycin (Appendix S2, Supplementary Information). An aliquot of bronchial wash fluid was processed (Appendix S3, Supplementary Information) to obtain a differential cell count.
Subjects’ HRCT scans were reviewed by a consultant thoracic radiologist (K.P.) blinded to their azithromycin response using a checklist of important features developed following initial review of selected scans (Appendix S4, Supplementary Information).
Statistical analysis
Demographics and baseline clinical measures were determined using Stata v11.0 (StataCorp., TX, USA). For data that were not normally distributed and could not be transformed to normality, pre- and post-treatment median values were calculated and compared using the Wilcoxon signed-rank test. FEV1 was normally distributed and mean FEV1 values pre- and post-treatment were compared with the paired T-test. Frequency tables for pre- and post-treatment objective and subjective sputum colour scores were constructed and compared using the Wilcoxon signed-rank test. Pre- and post-treatment IL-1β levels were logarithmically transformed to normality and pre- and post-geometric means compared using a paired t-test.
Subjects were divided into ‘responders’ or ‘non-responders’ based on an LCQ score of greater or less than the MCID of 1.3 points respectively.12 Analyses for the primary and secondary endpoints were repeated in these two groups. Subjects were classified as ‘neutrophilic’ if the baseline differential neutrophil count was ≥61%, ‘eosinophilic’ if the differential eosinophil count was ≥3% or ‘paucigranulocytic’ if neither of these criteria were met.14
The sensitivity, specificity and positive and negative predictive values of HRCT scan features to predict azithromycin response were calculated. Receiver operating characteristic (ROC) analysis of blood eosinophil count to predict azithromycin response was performed.
RESULTS
Participants
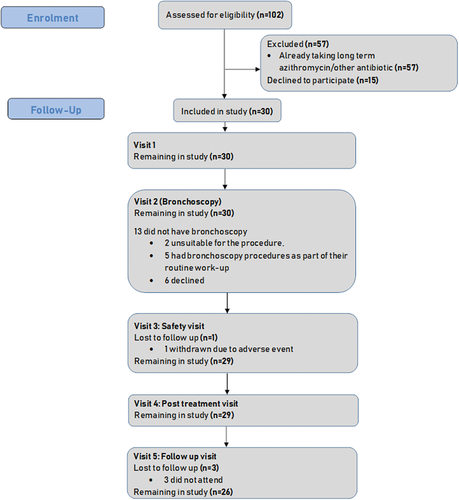
Between January 2014 and January 2016, 102 eligible subjects were identified. Fifty-seven of these were already taking azithromycin or another long-term antibiotic and 15 declined the study (Fig. 2). The baseline demographics and clinical features of the 30 participants are shown in Table 1. Diagnosis of asthma was based on a previous clinical diagnosis. Nine subjects had sputum samples positive for pathogenic bacteria in the 12 months prior to the study (Tables 1, S1 (Supplementary Information)).
| Frequency (%) | ||
|---|---|---|
| Total number included for analysis | 30 | |
| Mean age (range) | 57.3 (25–77) | |
| Sex: male | 13 (43) | |
| Ethnic group | ||
| Black or Black British | 1 (3) | |
| White or White British | 29 (97) | |
| Median BMI (IQR) | 29.9 (8.4) | |
| Smoking history | ||
| Ex-smokers | 12 (40) | |
| Non-smokers | 18 (60) | |
| Diagnosis of asthma | 17 (57) | |
| On inhaled steroid treatment | 17 (57) | |
| History/symptoms of GORD | 6 (20) | |
| History/symptoms of PNDS | 6 (20) | |
| Positive sputum culture in 12 months prior to study entry | 9 (30) | |
| Mean (SD) | Range | |
| ICS dose (BDP equivalent μg) | 800 (1000) | 0–4000 |
| FEV1% predicted | 96.4 (22) | 49–131 |
| FEV1/FVC ratio (%) | 76 (8.5) | 60–90 |
| Median (IQR) | Range | |
| Baseline (V1) sputum % neutrophils | 65.6 (41.3) | 4.5–99.25 |
| Baseline (V1) sputum % eosinophils | 0.68 (1.5) | 0–58 |
| LCQ score | 11.5 (3) | 7.8–18.2 |
| FENO (ppb) | 19 (20.5) | 0.5–52.5 |
| Sputum volume (mL) | 8.1 (5.5) | 3–31.1 |
- BMI, body mass index; FENO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GORD, gastro-oesophageal reflux disease; ICS, inhaled corticosteroid; IQR, interquartile range; LCQ, Leicester Cough Questionnaire; PNDS, post-nasal drip syndrome.
Histopathological features
Histopathological examination of bronchial biopsies revealed chronic airway inflammatory changes in 15 of 17 subjects who underwent bronchoscopy (Fig. 2). Inflammatory infiltrates were lymphocytic or plasmacytic in nature and the severity of inflammation ranged from mild to severe. Basement membrane thickening was noted in 9 of 17 subjects.
Radiological features
Twenty-nine HRCT scans were available for review. The two most frequently identified abnormalities were mild airway dilatation (n = 21) and bronchial wall thickening (n = 13) with 10 patients having both.
Effect of 12 weeks azithromycin on LCQ
Azithromycin treatment resulted in a significant improvement in the primary outcome measure of median LCQ score (+6.3 points, P < 0.001) (Table 2). LCQ score increased by ≥MCID (1.3 points) in 22 of 29 subjects (76%). This improvement was largely sustained 4 weeks post-treatment, with a follow-up median LCQ score of 15.9 (vs 11.5 at baseline, P < 0.001) and 17 of 26 (65%) subjects still reported LCQ scores above their baseline value by ≥MCID. Four subjects (15%) reported worsening symptoms post-azithromycin with visit 5 LCQ scores within the MCID from baseline or lower than their baseline scores.
| V1 | V4 | V1 − V4 difference | Significance (P) |
V5 | V1 − V5 difference | Significance (P) |
|
|---|---|---|---|---|---|---|---|
| LCQ score | 11.5 (3) | 17.8 (5.9) | 6.3 | <0.00001 | 15.9 (8.3) | 4.4 | 0.0006 |
| 24-h Sputum volume (mL) | 7.9 (5.5) | 2.1 (7.2) | −5.8 | 0.0001 | |||
| FENO level (ppb) | 19 (19.5) | 12.5 (12) | −6.5 | 0.14 | |||
| FEV1 (L)† | 2.77 (0.99) | 2.75 (1.0) | −0.02 | 0.78 |
- Values shown are median (IQR).
- † Values are mean (SD).
- FENO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; IQR, interquartile range; LCQ, Leicester Cough Questionnaire.
Secondary outcomes
Azithromycin treatment resulted in significant improvements in median 24-h sputum volume (−5.8 mL, P < 0.001) and objective sputum colour (P < 0.001, Table 2). There were no statistically significant differences in FEV1, FENO or subjective sputum colour (Table 2).
Ten subjects produced paired pre- and post-treatment sputum samples with 4 producing non-viable baseline samples and 15 unable to produce post-treatment samples. There was a significant decrease in sputum differential neutrophil count (pre-treatment median 86.1% vs post-treatment 69.4%, P = 0.049) but no change in sputum differential eosinophil count (Table 3).
| Mean % pre-treatment (SD) | Mean % post-treatment (SD) | V1 − V4 difference | Significance (P) |
|
|---|---|---|---|---|
| Sputum differential neutrophil count (%) | 86.1 (33.5) | 69.4 (18.6) | −16.7 | 0.049 |
| Sputum differential eosinophil count (%) | 0.75 (1.5) | 0.5 (7) | −0.25 | 0.64 |
Responders versus non-responders
Baseline characteristics of responders and non-responders are shown in Table 4. Of the 22 responders, 14 (63.6%) had neutrophilic inflammation on sputum/bronchial wash, 5 (22.7%) demonstrated paucigranulocytic inflammation and 3 (13.6%) did not produce viable sputum samples or have baseline bronchoscopies (Table 4). Significant improvements were seen in median 24-h sputum volume (−4.9 mL, P < 0.0001), subjective sputum colour (P = 0.01) and objective sputum colour (P = 0.001). Median FENO decreased significantly (−6 ppb, P = 0.009) in responders.
| Responders (post-treatment LCQ > MCID) | Non-responders (post-treatment LCQ < MCID) | ||
|---|---|---|---|
Frequency (%) |
Frequency (%) |
Significance (P) | |
| Total number included for analysis | 22 | 7 | |
| Mean age (range) | 55.5 (25–77) | 63.9 (55–70) | 0.20 |
| Sex: male | 7 (31.8) | 5 (71.4) | 0.09 |
| Ethnic group | |||
| Black or Black British | 1 (4.6) | 0 (0) | |
| White or White British | 21 (95.4) | 7 (100) | 1.0 |
| Smoking history | |||
| Ex-smokers | 7 (31.8) | 4 (57.1) | |
| Non-smokers | 15 (68.2) | 3 (42.9) | 0.38 |
| Diagnosis of asthma | 11 (50) | 6 (85.7) | 0.19 |
| On inhaled steroid treatment | 11 (50) | 6 (85.7) | 0.19 |
| History/symptoms of GORD | 4 (18.2) | 1 (14.3) | 1.0 |
| History/symptoms of PNDS | 4 (18.2) | 2 (28.6) | 0.61 |
| Sputum/bronchial wash inflammatory type (n = 29) | |||
| Neutrophilic (>61%) | 14 (63.6) | 1 (14.3) | <0.001 |
| Eosinophilic (>3%) | 0 (0) | 5 (71.4) | |
| Paucigranulocytic | 5 (22.7) | 1 (14.3) | |
| Missing sample | 3 (13.6) | 0 (0) | |
| Mean (SD) | Mean (SD) | Significance (P) | |
| ICS dose (BDP equivalent μg)† | 800 (800) | 900 (800) | 0.12 |
| Blood eosinophil count (×109/L)† | 0.2 (0.1) | 0.4 (0.45) | 0.0004 |
| FENO (ppb)† | 18 (17) | 19 (37.5) | 0.68 |
| FEV1 (% predicted) | 103.6 (18.8) | 73.6 (17.3) | 0.0009 |
| FEV1/FVC ratio (%) | 78.4 (7) | 67.6 (8) | 0.0019 |
| Baseline (V1) sputum % neutrophils (n = 26) | 73.2 (21.9) | 46.8 (34.2) | 0.06 |
| Baseline (V1) sputum % eosinophils (n = 26) | 0.5 (0.75) | 13.7 (24.8) | 0.03 |
- † Values shown are median and IQR.
- FENO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GORD, gastro-oesophageal reflux disease; ICS, inhaled corticosteroid; IQR, interquartile range; LCQ, Leicester Cough Questionnaire; MCID, minimal clinically important difference; PNDS, post-nasal drip syndrome.
Subjects with neutrophilic airway inflammation (n = 15) demonstrated significant improvements in median LCQ score (pre-treatment 11.4 vs post-treatment 18.5, P < 0.001), median 24-h sputum volume (−5.3 mL, P = 0.002), objective sputum colour (P = 0.004) and subjective sputum colour (P = 0.009). Subjects with paucigranulocytic inflammation (n = 6) also demonstrated a significant improvement in median LCQ score from 11.9 to 15.9 (P = 0.03) but no significant change in other measures. None of the responders exhibited eosinophilic airway inflammation.
Five of the 7 (71%) non-responders demonstrated eosinophilic inflammation in sputum or bronchial wash (Table 4). Blood eosinophil count was significantly higher in non-responders (0.4 vs 0.2 × 109/L, P = 0.0004) with a sensitivity of 95% to predict treatment response using a cut-off point of ≤0.3 × 109/L (Fig. S1, Supplementary Information).
The severity of inflammation on bronchial biopsies tended to be more marked in responders (n = 10) with mild (n = 5), moderate (n = 3) or severe (n = 2) inflammation seen. The six non-responders had no inflammation (n = 2) or mild inflammation (n = 4) only.
Of the baseline radiological features, airway dilatation was sensitive (0.86) at predicting azithromycin response, but not specific (0.56) whilst bronchial wall thickening had low sensitivity and specificity (Table S2, Supplementary Information).
Cytokine profiling of sputum samples
Adequate sputum samples for cytokine analysis were obtained from 28 and 15 subjects at visits 1 and 4, respectively. In these 15 subjects, there was no significant difference in the sputum IL-17, TNF-α or IL-8 post-treatment, but sputum IL-1β decreased significantly (pre-treatment geometric mean 943.8 pg/mL vs post-treatment 372.4 pg/mL, P = 0.02) (Table S3, Supplementary Information).
DISCUSSION
This study supports previous observations of a cohort of patients with idiopathic chronic productive cough whose symptoms respond well to prolonged low-dose azithromycin.1 There was a marked improvement in the primary outcome of LCQ score, and the secondary outcomes of 24-h sputum volume and objective sputum colour with azithromycin treatment.
None of the subjects with eosinophilic airway inflammation (n = 5) responded symptomatically to azithromycin. In contrast, the AMAZES trial5 reported a reduction in exacerbations and symptomatic improvement with long-term azithromycin in eosinophilic and non-eosinophilic asthma subtypes. These apparently conflicting findings may be due to: (i) differences in patient selection, as all subjects in the AMAZES study had a diagnosis of asthma or (ii) a difference in outcome measures as exacerbation rate and asthma quality of life questionnaire were primary outcome measures in AMAZES. The response of subjects with neutrophilic airway inflammation to azithromycin is consistent with the clinical improvement seen in other cohorts of patients with sputum neutrophilia7, 15 or neutrophilic predominant airway disease such as bronchiectasis and COPD.3, 4, 16-18 The improvement seen in the paucigranulocytic group was less marked and of uncertain benefit. Our finding that a raised blood eosinophil count was highly predictive of a poor response to azithromycin needs confirmation but could represent a simple predictor to guide therapy in patients with chronic productive cough. Our findings contrast with previous studies that concluded macrolide treatment was not beneficial in chronic cough.19, 20 We hypothesize that this is due to our study only including patients with a productive cough, which is probably more associated with airway inflammation and/or infection than a dry cough.
Analysis of paired sputum samples from 10 of 29 subjects revealed a significant decrease in sputum differential neutrophil count consistent with previous studies of azithromycin treatment.15, 21, 22 High levels of IL-8, IL-1β, IL-17A and TNF-α have previously been noted in neutrophilic airway disease.23, 24 Sputum concentrations of these cytokines were higher in our patients than those with asthma or COPD25, 26 and comparable to those detected in subjects with bronchiectasis.27 Azithromycin treatment resulted in no change in sputum concentration of IL-8, IL-17A or TNF-α, whereas IL-1β levels decreased significantly. IL-1β is a potent pro-inflammatory cytokine which induces pulmonary neutrophil airway inflammation and airway damage in mice,24 and the reduction in IL-1β with azithromycin is consistent with findings from murine studies28, 29 and suggests a mechanism for decreased neutrophilic inflammation.
Several key features of this airways disease phenotype have been identified. First, although the number of subjects (n = 30) recruited to the study in a 24-month period suggests a low incidence of this phenotypic characteristic, many more met the inclusion criteria on screening. Most eligible subjects could not be included because they were already being treated with low-dose azithromycin, suggesting recognition of azithromycin response is widespread.
Second, the symptoms experienced by this cohort do not appear to be related to recognized causes of chronic productive cough. Only six subjects (20%) had a diagnosis of gastro-oesophageal reflux disease (GORD) and six (20%) of post-nasal drip syndrome, none of whom responded symptomatically to conventional treatments for these conditions. These subjects’ symptoms are unlikely to be due to smoking-related chronic bronchitis due to our smoking-related exclusion criteria and uniformly low baseline exhaled carbon monoxide readings.
The relationship between asthma and this cohort of patients is harder to discern as no objective tests were conducted to confirm a clinical diagnosis of asthma. The majority (n = 17, 56.7%) had an asthma diagnosis, but their predominant symptom was productive cough rather than wheeze or dyspnoea. Ten (33%) patients had airflow obstruction, four of whom had evidence of eosinophilic inflammation and did not respond to azithromycin. These four patients probably had uncontrolled eosinophilic asthma with chronic mucus hypersecretion.30
The changes of airway dilatation with histological changes of chronic inflammation and high levels of Th1 and Th17 cytokines are all compatible with a diagnosis of early bronchiectasis. This cohort may represent subjects with ‘pre’-bronchiectasis, who have sustained an initial airway insult and have persisting neutrophilic inflammation and excessive airway secretions, but do not yet have macroscopic airway destruction identified as bronchiectasis on HRCT. Nine subjects had pathogenic bacteria isolated in their sputum in the 12 months preceding the study. Further elucidation of the role of the airway microbiota composition in the pathophysiology of the cohort was not possible as the marked treatment response to azithromycin meant we were unable to obtain a post-treatment sputum sample from most patients.
The study was subject to several limitations including its small size, the lack of a placebo group and post hoc tests on the response and non-response groups. Although the final number completing the trial was small (n = 29), the improvement in LCQ was highly significant due to a large reported improvement in symptoms by most participants. The true magnitude of this effect is difficult to discern, although placebo response alone is unlikely with the concurrent improvement in 24-h sputum volume and sputum colour and decreases in the sputum differential neutrophil count and IL-1β concentrations. Due to the relatively short study duration, we did not formally assess for unwanted effects of azithromycin including bacterial macrolide resistance and hearing loss. In clinical practice, the risk of such effects, which have been reported in previous trials of azithromycin of longer duration,3 must be weighed against the potential for symptomatic improvement.
Recognition of the heterogeneity and complexity of airways disease has led to proposals for a different system of classifying disease, based not on archetypal disease labels but on recognition of phenotypic or biological markers of disease (‘treatable traits’) that enable targeted treatment.31 The results of this study, irrespective of the exact underlying airway pathology, indicate that the symptom of chronic productive cough, especially with evidence of neutrophilic airway inflammation, may represent a trait responsive to prolonged low-dose macrolides. We have previously suggested a diagnostic label of adult-onset protracted bacterial bronchitis and that this represents a distinct ‘treatable trait’ within the spectrum of patients with airways disease. Further work needed to establish the validity of this theory includes a randomized controlled trial of azithromycin in patients with idiopathic chronic productive cough.
Data availability statement
Individual participant data will be available after de-identification along with the study protocol from publication until 5 years following publication. Data will be shared with any researchers who provide a methodologically sound proposal to achieve the aims in the approved proposal. Proposals should be directed to [email protected]
Acknowledgement
This study was funded by The Nottingham Respiratory Research Unit.
Disclosure statement
Professor D.E.S. reports Advisory Board fees from GlaxoSmithKline, Novartis and Astra Zeneca and travel fees from Novartis and Astra Zeneca. Professor T.W.H. reports Advisory Board/Speakers fees from GlaxoSmithKline, Vectura and Astra Zeneca. The rest of the authors declare no conflicts of interest.
Abbreviations
-
- FENO
-
- fractional exhaled nitric oxide
-
- FEV1
-
- forced expiratory volume in 1 s
-
- FVC
-
- forced vital capacity
-
- GORD
-
- gastro-oesophageal reflux disease
-
- ICS
-
- inhaled corticosteroid
-
- IL
-
- interleukin
-
- IQR
-
- interquartile range
-
- LCQ
-
- Leicester Cough Questionnaire
-
- MCID
-
- minimal clinically important difference
-
- PNDS
-
- post-nasal drip syndrome
-
- TNF-α
-
- tumour necrosis factor-alpha




