Double combination inhalers in COPD: How to get your head around this data
Abstract
The development of inhalers containing both a long-acting muscarinic antagonist (LAMA) and a long-acting beta agonist (LABA) has increased the treatment options in chronic obstructive pulmonary disease (COPD). Early studies proved the advantage of LAMA/LABA combinations versus long-acting bronchodilator monotherapies.1, 2 Interest quickly moved to compare LAMA/LABA versus inhaled corticosteroid (ICS)/LABA combinations; studies in low exacerbation risk patients showed a lung function advantage for LABA/LAMA and a benefit on symptoms.1, 3, 4 Moreover, the FLAME study conducted in higher exacerbation risk patients (≥1 exacerbation in the past year) showed superiority for LAMA/LABA over ICS/LABA for exacerbation prevention over 1 year.5 Did this weight of evidence close the case, LAMA/LABA beats ICS/LABA?
Certainly not, the IMPACT (The Informing the Pathway of COPD Treatment) study created controversy.6 The IMPACT population was at high exacerbation risk; 54% had ≥2 moderate or severe exacerbations and 26% had ≥1 severe exacerbation (hospitalization) in the previous year. There was a 10% treatment difference on exacerbations over 1 year in favour of ICS/LABA (fluticasone furoate/vilanterol) over LAMA/LABA (umeclidinium/vilanterol). Why is this result opposite to FLAME? A simplistic and mostly unhelpful explanation is that different molecules were studied. This cannot account for a complete reversal of the result. The real answer lies in the populations studied and the nature of the run-in periods before randomization. The majority of FLAME participants had one exacerbation in the previous year (approximately 80%), while the IMPACT participants were at higher exacerbation risk. The FLAME study used a 6-week run-in period on tiotropium. This is much longer than most COPD randomized controlled trials and likely skewed the population towards patients with a lower disease burden who could manage with LAMA monotherapy for an extended run-in period. In contrast, the IMPACT study had a 2-week run-in period using the patient's own medication, thereby not introducing bias due to a treatment change during run-in period. After run-in period, there was a direct switch to randomized treatment in IMPACT which could include ICS withdrawal; clearly, this aspect may have favoured ICS-containing treatments. These run-in period issues reflect clinical trial design complexity, but the different exacerbation histories of the study populations are directly relevant to clinical practice. In the higher exacerbation risk IMPACT population, ICS/LABA was a better choice than LAMA/LABA, although triple therapy (ICS/LABA/LAMA) trumped both double combinations.
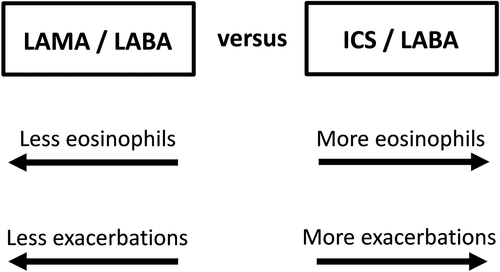
The current debate around double or triple combinations centres on the benefits versus risk of ICS.1, 7 Precision medicine in clinical practice involves using clinical and biological information to make individual treatment decisions that optimize the benefit versus risk ratio for pharmacological treatments.1 For ICS, the individual risk of exacerbations and side effects should be considered (clinical information) plus the blood eosinophil count to predict efficacy1, 7 (biomarker/biological information). In both IMPACT and FLAME studies, the comparison of ICS/LABA versus LAMA/LABA varied with the blood eosinophil count, as ICS effects increased at higher counts.6, 8 Figure 1 shows the key factors that should be used to decide which double combination to use in clinical practice, with more exacerbations and more eosinophils favouring ICS/LABA use.
In this issue of the journal, Frith et al. report the FLASH (Assessment of switching salmeterol/Fluticasone to indacateroL/glycopyrronium in A Symptomatic COPD patient coHort) study, which compared glycopyronium/indacaterol (LAMA/LABA) with fluticasone propionate/serevent (ICS/LABA) for 12 weeks in patients at low exacerbation risk (39.8% and 60.2% with one or zero exacerbations, respectively, in the previous year).9 There are other similar studies,3, 4 so was another one really needed? The clinical importance of this study relates to the direct switch (‘flash’) from ICS/LABA to LAMA/LABA at randomization, plus the sensible decision to recruit only patients who were taking ICS/LABA in real life before study entry. This study design resembles clinical practice in low exacerbation risk patients where there are concerns about long-term ICS side effects. LAMA/LABA treatment was better than ICS/LABA for lung function, while there was a benefit on dyspnoea that just failed to reach statistical significance. The relatively short study duration means that exacerbations were not fully evaluated, but nevertheless there was no increase in exacerbation signal due to ICS withdrawal in the short term. Overall, the results support a flash switch from ICS/LABA to LAMA/LABA in this specific COPD subgroup.
The GOLD (Global initiative for chronic Obstructive Lung Disease) ABCD assessment was designed to be applied after initial diagnosis, in treatment-naïve individuals.10 The associated pharmacological treatment algorithms provide advice on initial treatment and the subsequent follow-up choices. GOLD ABCD was not intended to be applied to patients already on maintenance treatment during long-term follow-up.7 The FLASH study recruited patients who were already on maintenance treatment; only the complete historic knowledge of each individual's clinical history would allow accurate allocation to ABCD. The FLASH study authors discuss the GOLD categorization of the study population; I disagree with this approach which is not supported by the GOLD report. Understanding the characteristics of the FLASH study population is more important than the letters ABCD; the homogeneous nature of the population regarding current treatment and exacerbation risk makes it an easily recognizable group in clinical practice.
The population enrolled. The nature of the run-in period. These are key factors that determine the outcome of pharmacological COPD clinical trials. The results of LAMA/LABA versus ICS/LABA studies will swing according to the previous exacerbation history and the type of run-in period. By the way, don't forget the blood eosinophil count.
Disclosure statement
D.S. has received sponsorship to attend and speak at international meetings, honoraria for lecturing or attending advisory boards from the following pharmaceutical companies: Apellis, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GlaxoSmithKline, Glenmark, Johnson and Johnson, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Skypharma, Teva, Therevance and Verona.