Natural variation-based genetic screen in rice identifies the isopentylamine biosynthetic gene that modulates brown planthopper behaviour
Abstract
- We previously identified isopentylamine as a novel defence metabolite accumulated in rice (Oryza sativa L.) infested by brown planthoppers (BPHs, Nilaparvata lugens) and caterpillars, however the key biosynthetic gene(s) remained unknown.
- An analytical screen of genetically diverse rice cultivars was used to identify natural variation in isopentylamine production in rice, revealing that it can be produced at high levels in cv. Nipponbare but not in cv. Kasalath. Genetic approaches with chromosome fragment substitution lines and backcross inbred lines of Nipponbare and Kasalath were then used to identify genes potentially involved in biosynthesis of isopentylamine. The main candidate biosynthetic gene was knocked out with CRISPR/Cas9 to obtain three independent Nipponbare mutant lines, and correlations between gene function and isopentylamine were examined analytically.
- The gene Os10g0400500 was identified as the main biosynthetic gene for production of isopentylamine in rice. Furthermore, behavioural responses to isopentylamine in two-choice host assays were examined by placing BPHs in containers with mutant and wild-type Nipponbare leaves. Although BPHs were not always attracted to either wild-type or mutants, more BPHs, at least at some points, were found on the mutant leaves.
- Our results suggest that BPHs may show a preference for isopentylamine-deprived plants relative to wild types, which further corroborates the potential role of isopentylamine as defence against insect herbivores in chemically diverse rice species.
INTRODUCTION
Plants defend themselves from herbivorous insects with various strategies. One of the main defence strategies involves biosynthesis and accumulation of specialized metabolites that are toxic to herbivores or function as antifeedants. As an example, benzoxazinoids (BXDs) in wheat (Triticum aestivum), rye (Secale cereale), and maize (Zea mays) have toxic and antifeeding effects on herbivorous insects (Niemeyer 1988, 2009; Sicker et al. 2000). Interestingly, proportions of BXDs vary between species and among tissues within plants. The main BXD in rye is 4-hydroxy-1,4-benzoxazin-3-one glucoside (Oikawa et al. 2001), while 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside accumulates in aerial parts of wheat and maize (Cambier et al. 2000; Oikawa et al. 2001), and 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one is the dominant BXD in maize roots (Robert et al. 2012). BXDs are also vary in chemical reactivity, depending on their structure. Such chemical variation leads to diversification in defence compound mode of action as toxins or antifeedants (Wouters et al. 2016). Furthermore, cardenolide toxin mixtures from milkweed plants enhance resistance against herbivores, compared to equal concentrations of single compounds (López-Goldar et al. 2024). This shows that phytochemical diversity greatly enhances plant defence against herbivores and therefore is central to development of pest-resistant crops.
The BXDs are a class of nitrogen-containing secondary metabolites mainly found in grasses (Poaceae), but other nitrogen-containing defence chemicals, hordenine and gramine in barley, also have deterrent properties against insects (Zuniga & Corcuera 1986; Bernays et al. 2000). Structurally simpler nitrogen-containing compounds, the amines, also function as chemical defence against herbivores. For example, high concentrations of tryptamine in Catharanthus roseus have anti-oviposition activity toward whiteflies (Bemisia tabaci; Thomas et al. 1995), and anti-feeding activity in tobacco and poplar toward Malacosoma disstria and Manduca sexta caterpillars (Gill et al. 2003). Dopamine functions as a defence substance in the green alga Ulvaria obscura and reduces feeding damage by sea urchins (Strongylocentrotus droebachiensis), snails (Littorina sitkana), and isopods (Idotea wosnesenskii) (Van Alstyne et al. 2006). Serotonin accumulates in rice (Oryza sativa) leaves in response to pathogen or herbivore infestation (Ishihara et al. 2008a, 2008b). Increased serotonin levels after insect feeding have also been reported in foxtail millet (Setaria viridis) and Japanese barnyard millet (Echinochloa esculenta) (Ishihara et al. 2015, 2017). S. viridis seedlings grown on media supplemented with serotonin have higher endogenous serotonin levels, which reduce Rhopalosiphum padi aphid survival and body weight (Dangol et al. 2022). In contrast, Lu et al. (2018) and Wang et al. (2019) showed that feeding artificial diets supplemented with serotonin increased survival of brown planthopper (BPH, Nilaparvata lugens) and body weight of striped stem borer (Chilo suppressalis). Overall, the effects of serotonin on insects vary, but given that serotonin is a signalling molecule that controls insect feeding behaviour (Howarth et al. 2002; Liscia et al. 2012), the concentration added may have been responsible for differences in results.
Previously, we identified isopentylamine as a novel amine compound that significantly increased in rice leaves (cv. Nipponbare) in response to feeding damage by herbivorous insects in both sucking (Nilaparvata lugens) and chewing (Mythimna loreyi) feeding guilds (Aboshi et al. 2021). When rice seedlings supplemented with isopentylamine were fed to BPHs, mortality increased, suggesting that isopentylamine has defence potential in rice. Therefore, functional studies of this novel defence chemical will strengthen our knowledge of rice chemical defence potential against herbivores. Isopentylamine can be produced in plants by decarboxylation of leucine (Trevor 2013), and experiments using stable isotopes have shown that isopentylamine in rice is also produced from leucine (Aboshi et al. 2021). In rice, although glutamate decarboxylase (Akama et al. 2020), tyrosine, and tryptophane decarboxylase genes (Kang et al. 2007) have been reported, no leucine decarboxylase genes have yet been identified. Therefore, in this study, we searched for genes involved in biosynthesis of isopentylamine, using chromosome segment substitution lines (CSSLs) and backcross inbred lines (BILs) of isopentylamine-producing and non-producing rice cultivars. CSSL and BIL populations are powerful QTL mapping tools that have been used to elucidate the molecular basis of many important traits in plants. Since isopentylamine accumulation was promoted by jasmonic acid (JA) treatment, as well as by feeding damage of herbivores (Aboshi et al. 2021), we decided to streamline the key genes in isopentylamine biosynthesis by examining the presence and absence of this compound in JA-treated CSSLs and BILs. This study provides novel insights into the role of isopentylamine by identification of its key biosynthetic gene in rice that can potentially be expanded to other isopentylamine-producing plant species.
MATERIAL AND METHODS
Plants and insects
The experiments were carried out with the wild-type rice (cv. Nipponbare, Kasalath, Kameji, Omachi, Tupa 729, Jena 035, Keiboba, Shoni, Tupa121-3, Surjamukhi, Ratul, Bandari Dhan, Kaluheenati, Milyang 23, Deejiaohualuo, Hong Cheuh Zai, IR64), CSSLs, BILs and three mutant lines. These 17 wild-type rice varieties were used to determine production of isopentylamine. The seeds were obtained from the Genebank at the National Agriculture and Food Research Organization (NARO), Japan. Chromosome segment substitution lines (http://www.rgrc.dna.affrc.go.jp/ineNKNCSSL48.html) and backcross inbred lines (https://www.rgrc.dna.affrc.go.jp/ineNKBIL98.html) were derived from a cross between Nipponbare and Kasalath, and seeds were provided by the Rice Genome Research Center, Japanese Ministry of Agriculture, Forestry, and Fisheries.
Seeds were soaked in 1.2% sodium hypochlorite solution with shaking for 1 min, rinsed with distilled water four times, and soaked in fresh water overnight. Seeds were planted 1 cm deep in cultivation soil Naeichiban (Ranpoku, Niigata, Japan) in plastic pots (length 5.0 cm, width 5.0 cm, height 5.6 cm), and pots were placed in a Biotron LH-300 cabinet (Nihon-ika, Osaka, Japan) under 18 h/6 h photoperiod, light of 150 μmol photons m−2 s−1 at 28°C and ambient humidity. Plants were watered daily. Unless otherwise noted, all plants were used for experiments when they were 3–4 weeks old. When treated with JA to induce isopentylamine, rice leaves were sprayed with 1 mM JA four times over 2 days. Control plants were treated with distilled water.
The BPHs were from colonies maintained at Okayama University, originally collected in a paddy field in Koshi (Kumamoto Prefecture, Japan). BPHs were kept on a constant supply of 3–4-week-old rice seedlings (cv. Nipponbare).
Isopentylamine analysis
Analysis of isopentylamine was as described previously (Aboshi et al. 2021). Briefly, about 100 mg undamaged fresh rice leaves were cut with scissors and ground with steel balls in 200 μl of 50% methanol using a μT-12 bead crusher shaker (Taitec, Saitama, Japan). The extracts were centrifuged at 10,000 × g for 5 min and the supernatants derivatized using the 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) reagent. Aliquots of plant extracts of 10 μl were mixed with 60 μl of 200 mM borate buffer (pH 8.8) and 5 μl of 1 μmol mL−1 β-alanine (internal standard, IS). The reaction was initiated by addition of 20 μl of 3 mg mL−1 AQC reagent in acetonitrile, followed by incubation for 10 min at 55°C. From each sample, 5 μl were injected into a Mightysil RP-18 GP II column (50 × 2.0 mm; Kanto Chemical, Tokyo, Japan) and separated using a Waters Acquity UHPLC system (Milford, MA, USA). Solvent A was water containing 0.1% formic acid and solvent B was acetonitrile containing 0.1% formic acid. The gradient was 0–3 min, 1% B, 3–12 min, linear gradient to 40% B, 12–13 min, linear gradient to 99% B, 13–17 min, 99% B, 17–18 min, linear gradient to 1% B, 18–22 min, 1% B. Flow rate was 0.2 mL min−1 and column temperature was 40°C. Mass spectra were recorded using a Synapt G2 HDMS (Waters). Mass spectra acquisition was performed under the following conditions: Ionization mode, electrospray ionization positive mode; capillary voltage, 2.0 kV; cone voltage, 30 V; desolvation temperature, 550°C; desolvation gas, 90 L h−1; source temperature 120°C. Detected ion range was set between m/z 150 and 800. For each plant line and treatment, three to five replicates were analysed. The amount of isopentylamine was calculated using calibration curves with an isopentylamine standard (Wako Pure Chemical Industries, Osaka, Japan).
Generation of Os10g0400500 knockout lines by CRISPR/Cas9
We used CRISPR/Cas9 to generate Os10g0400500 knockout lines in the Nipponbare genetic background following Mikami et al. (2015). Twenty bases upstream of the protospacer adjacent motif (PAM) were selected as candidate target sequences (Fig. S1). The primers for two target sequences in the open-reading frame region of Os10g0400500 are listed in Table S1. These DNA fragments were cloned into pU6gRNA then subcloned into the pZDgRNA_Cas9 vector. Calli generated from Nipponbare were transformed using Agrobacterium tumefaciens EHA105 according to Hiei et al. (1994). Sequence information of the resultant mutants is provided in Fig. S1.
To genotype the resultant mutants, we extracted genomic DNA from leaves of transgenic plants with the conventional CTAB method (Doyle & Doyle 1987), followed by PCR amplification using primer pairs listed in Table S1. The PCR products were purified using the LaboPass Gel Extraction kit (Hokkaido System Science, Sapporo, Japan) and sequenced directly using the commercial sequencing facility of Azenta, Japan. The target metabolite for verification of gene functions was detected in mature plants by LC/MS, as described above.
The BPH choice assay
In the BPH choice experiment, mutants with a 27 bp deletion and a 43 bp deletion as knockout mutants of the OsIPAS1 were used. Leaves of the Nipponbare (wild type) and mutant rice plants grown for 6 weeks were inserted into a plastic cylinder (88 mm diameter × 263 mm high) and covered with a plastic Petri dish and mesh (Fig. S2). About 20 BPHs were placed in each cylinder, and the number of BPHs on leaves was counted at regular intervals. The percentage of BPHs residing on the leaves of both wild-type and mutants was calculated. Experiments were repeated three times, in January 2023, August 2023, and January 2024.
Bioinformatics analysis
Nucleotide sequence of Os10g0400500 from 17 rice varieties used in the study were retrieved from the genome browser TASUKE+ v. 20231214 (https://agrigenome.dna.affrc.go.jp/tasuke/ricegenomes/), including coding region of each gene flanked with 5′UTR (2000 nt) and 3′UTR (1500 nt) sequences (Fig. S3A). As genes are oriented in the minus orientation in the rice genome, retrieved sequences were reverse-complemented using the Sequence Manipulation SuiteVer. 2, http://www.bioinformatics.org/sms2/). Missing and incorrect nucleotide information in Tupa121_3 sequence was amended with information from the PCR product obtained from Tupa121_3 genomic DNA using 5′ → 3′ forward (ATGGGTAGCTTGCCACTCG) and reverse (CGAGATCTGCCAGTCCTTGT) primers (Fig. S3B), following a general amplification protocol provided with KOD One® PCR Master Mix (Toyobo, Japan) at Tm = 55°C. Gel-purified PCR products (FastGene gel/PCR extraction kit; Nippon Genetics, Japan) were sequenced in a commercial facility (Eurofins Genomics, Japan). Multiple nucleotide sequence alignment was performed by CLUSTALW with default parameters using Kyoto University Bioinformatics Center website (https://www.genome.jp/tools-bin/clustalw). The maximum likelihood phylogenic tree was constructed from the CLUSTALW aligned sequences by PhyML with default bootstrap value using software provided on the above website.
Statistical analysis
Statistical analyses were carried out using Microsoft Excel (Student's t-test).
RESULTS
Isopentylamine biosynthesis gene candidate
To determine which rice varieties produce isopentylamine, we analysed 17 varieties after JA treatment. There were six other rice cultivars (Kameji, Omachi, Tupa 729, Jena 035, Keiboba, Hong Cheuh Zai) besides Nipponbare in which isopentylamine was detected in leaves treated with JA, but isopentylamine was not detected in the 10 remaining rice cultivars (Kasalath, Shoni, Tupa121-3, Surjamukhi, Ratul, Bandari Dhan, Kaluheenati, Milyang 23, Deejiaohualuo, IR64) after JA treatment (Fig. 1A).
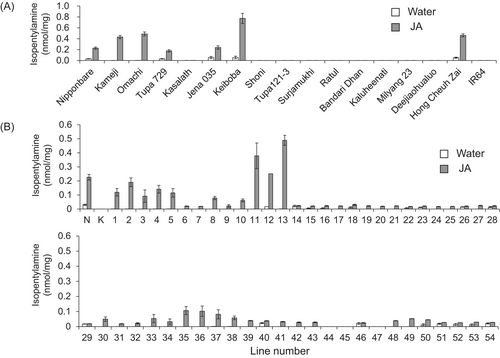
To identify the genetic basis of varieties in which isopentylamine is detected and those in which it is not, CSSLs derived from a cross between Nipponbare and Kasalath were used to map isopentylamine accumulation as a quantitative trait. Among 54 tested CSSLs, 51 had a phenotype similar to that of Nipponbare, showing accumulation of isopentylamine in leaves with JA treatment (Fig. 1B). Three CSSLs did not show any detectable foliar isopentylamine. These three CSSLs have the Kasalath genotype at markers R2174 and R1629 on the chromosome inserted in the Nipponbare genetic background (Fig. 2). Next, we selected lines with recombination in the R1933-R2447 region of chromosome 10 from the Nipponbare × Kasalath BILs, designed PCR markers targeting the Indel polymorphism between Nipponbare and Kasalath to create a more detailed genetic map, and attempted to narrow the genetic region involved in the isopentylamine biosynthesis. Among 10 tested BILs, isopentylamine was detected in six lines but was absent in four lines (Fig. 3). The six backcross inbred lines with isopentylamine have the Nipponbare genotype at markers C10-33 and C10-54, while the four BILs without isopentylamine have the Kasalath genotype in this region (Fig. 4).
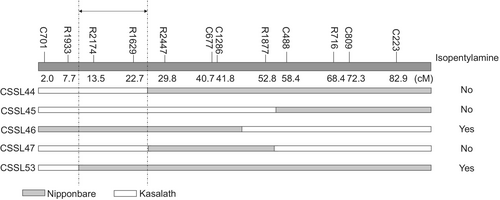
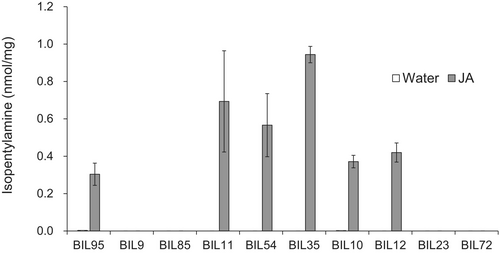
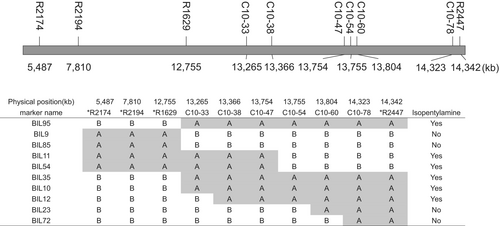
Analysis of the Nipponbare genome sequence (https://rapdb.dna.affrc.go.jp/) (Kawahara et al. 2013) of chromosome 10 between markers C10-33 and C10-54 revealed genes listed in Table S2. Among these genes, we considered Os10g0400500 (Michigan State University Rice Annotation Project LOC_Os10g26110) as the most promising candidate for isopentylamine synthase gene 1 (IPAS1) because it was annotated as “similar to pyridoxal-dependent decarboxylase conserved domain containing protein, expressed”. This is consistent with the fact that isopentylamine is most likely produced by decarboxylation of leucine in rice, and most amino acid decarboxylases involved in amine biosynthesis are pyridoxal-5′-phosphate (PLP)-dependent enzymes (Sandmeiere et al. 1994).
To confirm that Os10g0400500 encodes IPA synthase (IPAS) activity, we made knockout mutations in the Nipponbare genetic background using CRISPR/Cas9. We obtained three independent T0 transgenic plants in Nipponbare after Agrobacterium-mediated transformation. Genomic DNA was extracted from the leaves to investigate the CRISPR/Cas9-induced mutations at designated target sites. Sequencing analysis revealed that these three mutants had 1 bp, 27 bp, and 43 bp deletions in the OsIPAS1 gene, two of which resulted in frame-shift mutations in OsIPAS1 (Fig. S1). By sequence analysis, the 1 and 43 bp deletion mutants harboured out-of-frame mutations resulted in a premature termination codon in the predicted protein sequence (Fig. 5). The 27 bp deletion mutant harboured an in-frame mutation that resulted in a deletion of nine amino acid residues. From T2 populations, mutant homozygotes were selected by sequencing the OsIPAS1 gene and used in the following experiments. When the seedlings of three OsIPAS1 knockouts were treated with JA, isopentylamine was not detected in any mutant, indicating that OsIPAS1 was functionally disabled (Fig. 6).

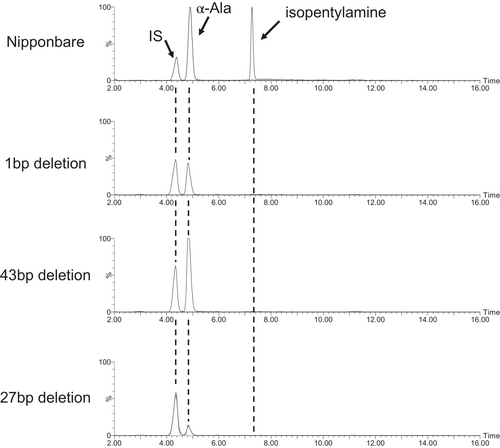
Sequence analysis of isopentylamine synthase genes
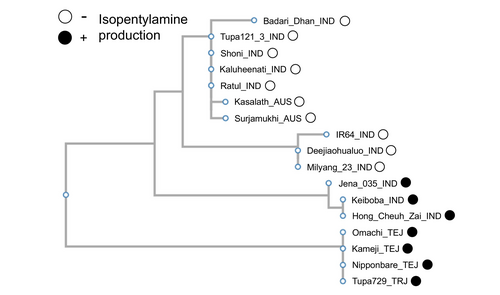
Nucleotide sequences of rice varieties examined in Fig. 1A (Fig. S3A) were retrieved from the TASUKE+ public database that contains genomes of 685 rice varieties generated by genome re-sequencing approach (Kumagai et al. 2019). After partial re-sequencing of Tupa 121_3 coding region to improve the low quality of the original sequence deposited in the TASUKE database (Fig. S3B), alignment of the coding regions (1509 nt) flanked with 5′UTR (2000 nt) and 3′UTR (1000 nt) sequences revealed the presence of 40 single nucleotide polymorphisms (SNPs) in 5′UTR promoter regions, seven SNPs in protein coding sequences, and 19 SNPs in 3′UTRs, when using the RAP-DB gene annotation model. Of these, only one SNP in 5′UTR at nucleotide position −1505 was consistent with the isopentenylamine production in examined rice cultivars, suggesting possible attenuation of gene transcription in isopentylamine-deficient rice. Interestingly, one SNP at position +454 in the protein coding region was also consistent in all non-producing vs. producing cultivars. As this nucleotide change results in Gly to Cys substitution in the Gly-rich region of the isopentylamine synthase protein (Figs. S4 and S5), it is possible that this amino acid plays an important role in the enzyme activity. In addition, a phylogenetic tree of isopentylamine synthases from 17 cultivars showed that this metabolic loss has likely occurred after separation of Indica and Japonica rice (Fig. 7), as some Indica varieties still produce isopentylamine, while others have lost it, and all Japonica varieties are isopentylamine producers.
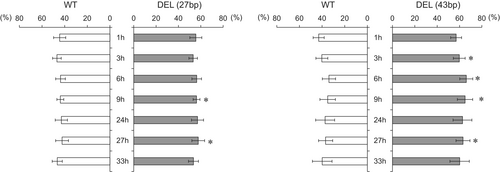
BPHs tend to choose OsIPAS1 knockout plants
As isopentylamine accumulation is not induced in OsIPAS1 knockout mutant rice, even when the plants are fed on by herbivores, we decided to examine the potential role of isopentylamine in host selection. Leaves of Nipponbare and mutant were inserted into a cylindrical container, and 20 BPHs added to each container to examine the relative proportion of BPHs residing on each genotype after 0–33 h of infestation. If isopentylamine has a defensive role against BPHs, it is likely that more BPHs will settle on OsIPAS1 knockout plants. The experiments were performed three times, in January 2023, August 2023, and January 2024. The proportions (%) of BPHs that distributed on the wild-type, mutant DEL (27 bp), and mutant DEL (43 bp) in January 2023 are shown in Fig. 8. In the choice between mutant DEL (27 bp) and wild type, the percentages of BPHs residing on the mutant DEL (27 bp) after 9 and 27 h were significantly higher than those on wild types. In choice between mutant DEL (43 bp) and wild type, the proportion of BPHs on mutants was again significantly higher relative to the wild type at 3, 6, 9, and 27 h. In the other two experiments, although we observed fewer statistically significant differences between BPHs on wild types and mutants, the percentage of BPHs on mutants at several time points was still higher than on isopentylamine-containing wild types (Figs. S6, S7), corroborating the overall trend for BPHs to choose the isopentylamine-deficient plants as preferred host plant. In all experiments, honeydew was secreted by BPHs, confirming that the BPHs used in the experiments were feeding on the rice plants.
DISCUSSION
In this study, we identified Os10g0400500 as a candidate for the isopentylamine biosynthetic gene in rice, using recombinant CSSLs and BILs derived from Nipponbare and Kasalath cultivars, which contain or lack isopentylamine, respectively. When the gene was knocked down by CRISPR/Cas9, isopentylamine was no longer produced in the mutant rice, even when seedlings were treated with JA, indicating that Os10g04005000 is the intrinsic isopentylamine synthase. This is supported by previous experiments using stable isotopes, which suggested that isopentylamine is produced in rice through the decarboxylation of leucine (Aboshi et al. 2021). Since Os10g0400500 is annotated as a gene similar to PLP-dependent decarboxylase, and taking into account the mutant plant phenotype, it is safe to conclude that it does catalyse the decarboxylation of leucine. However, as our current attempts at heterologous expression of this rice gene in Escherichia coli are not yet successful (data not shown), properties of Os10g0400500 as an enzyme have not yet been directly investigated.
The OsIPAS1 shares a high amino acid similarity with six other proteins in The Rice Annotation Project Database (RAP-DB, http://rapdb.dna.affrc.go.jp/), all annotated as tryptophan decarboxylase (TDC) or TDC-like proteins, or tyrosine/DOPA decarboxylases. A representative sequence Os08g0140300 has been functionally characterized as TDC, and the crystal structure was determined by Zhou et al. (2020). Generally, amino acid decarboxylases utilize PLP as a cofactor to catalyse decarboxylation of amino acids. The PLP cofactor is functionally conserved in all PLP-dependent decarboxylases and it binds to a conserved lysine to form one Schiff base (Sandmeiere et al. 1994). In OsTDC, a Schiff base bond is formed between K330 and PLP, and decarboxylation is completely inhibited in the K330A mutation, suggesting that K330 plays a fundamental role in decarboxylation catalysis. H214 is also involved in binding to PLP, and correct interaction of this residue with the substrate binding site Y359 is essential for enzyme activity (Zhou et al. 2020). Both K330 and H214 amino acid residues are also conserved in OsIPAS1, suggesting that OsIPAS1 is functional PLP-dependent decarboxylase (Fig. S8).
In our previous study, we proposed that isopentylamine acts as a defence substance against BPHs, because a diet based on rice seedlings supplemented with isopentylamine showed increased mortality of BPHs. Therefore, we expected that knocking out OsIPAS1 may also affect BPH behaviour, for example, host plant selection. To test this possibility, we simultaneously presented mutant and wild-type rice leaves to BPH in preferential choice assays. Three experiments were conducted with paired plants of the same size, using either of the two independent knockout mutants of OsIPAS1, and the wild type. In all three experiments, statistically significant biases in choice of BPHs toward the isopentylamine-free plants were observed. The fact that knockout of the OsIPAS1 gene attracts more BPHs to plants shows, for the first time, that isopentylamine, in addition to mild toxicity (Aboshi et al. 2021), also may have a deterrent effect on BPHs, albeit a relatively weak one. This is not unexpected as isopentylamine is just one of the multi-layered defence tools in rice. For instance, rice possesses strong mechanical defences (Andama et al. 2020), and uses other chemicals, including volatile organic compounds, to affect insect behaviour (Mujiono et al. 2021). Other chemicals, such as phenolamides and momilactones, are also factors that respond to and affect insect behaviour in rice (Alamgir et al. 2016). However, in this experiment, we have not yet fully investigated whether the knockout of the OsIPAS1 gene affected phenotypic traits other than isopentylamine content. Although the knockout mutants we used did not seem to differ from Nipponbare in terms of leaf toughness, trichome condition or appearance, an objective evaluation and comparative metabolomics analyses are still pending tasks. Therefore, at this time, we cannot completely rule out the possibility that factors other than isopentylamine content may be involved in the preference of BPH for knockout plants. For instance, putative higher accumulation of leucine resulting from isopentylamine biosynthesis block could attract more BPHs that are likely to choose the nutritionally more valuable plants. Also, since planthoppers suck the phloem sap of plants, it will be necessary to investigate both composition and actual concentration of metabolites, including isopentylamine, in the phloem sap. Future comprehensive metabolite analyses of the mutants during feeding damage are also needed to determine the synergistic interactions of isopentylamine with other rice defences, which is now enabled by the existence of genetically modified rice resources, such as the knock-out mutants in the OsIPAS1 gene created in this study.
From a co-evolutionary perspective, the arms race between plants and herbivores is thought to drive diversity of plant secondary metabolites (Ehrlich & Raven 1964). Molecular mechanisms that promote plant secondary metabolite diversity include: gene and genome duplications and consequent exaptation (or ‘neofunctionalization’); accumulation of point mutations; and multi-locus control leading to variation in metabolic products (Kroymann 2011; Weng et al. 2012; Moore et al. 2014; Speed et al. 2015). In this study, sequence comparisons of isopentylamine synthase genes indicate that one SNP in the coding region at position 454 might be involved in the isopentylamine loss of function. In terpene synthase genes, point mutations are reported to produce completely different products from the same substrate (Kampranis et al. 2007). In the case of the isopentylamine synthase gene, however, we assume that point mutation resulted in the loss of a product, not a different product. Therefore, our study highlights that point mutations to biosynthetic genes result in variations in secondary metabolites among varieties. Nevertheless, as mentioned above, further metabolomics studies are required to confirm that the OsIPAS1 gene was not diverted to production of other amines, for example, by using an alternative amino acid for the decarboxylation reaction, which was putatively triggered by a single base change at nucleotide position 454 of the protein coding region.
AUTHOR CONTRIBUTIONS
TA, MT, and GI designed the research. TA, GI, and ST collected the data. MT and TY created the mutant rice plants. TA wrote the paper with the aid of MT and GI.
ACKNOWLEDGEMENTS
We thank Dr. Masaki Endo for providing pU6gRNA and pZDgRNA_Cas9 for generation of CRISPR/Cas9 lines. This study was supported by the Joint Research Program of the Institute of Plant Science and Resources at Okayama University, and a Grant-in-aid for (00721606, TY; no. 80514207, TS) from the Japan Society for the Promotion of Sciences.
CONFLICT OF INTEREST
The authors declare no conflicts of interest associated with this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data can be found within the manuscript and its supporting materials.




