Different tools for different trades: contrasts in specialized metabolite chemodiversity and phylogenetic dispersion in fruit, leaves, and roots of the neotropical shrubs Psychotria and Palicourea (Rubiaceae)
Abstract
- Plants produce an astonishingly diverse array of specialized metabolites. A crucial step in understanding the origin of such chemodiversity is describing how chemodiversity manifests across the spatial and ontogenetic scales relevant to plant–biotic interactions.
- Focusing on 21 sympatric species of Psychotria and Palicourea sensu lato (Rubiaceae), we describe patterns of specialized metabolite diversity across spatial and ontogenetic scales using a combination of field collections, untargeted metabolomics, and ecoinformatics. We compare α, β, and γ diversity of specialized metabolites in expanding leaves, unripe pulp, immature seed, ripe pulp, mature seed, and fine roots.
- Within species, fruit tissues from across ontogenetic stages had ≥α diversity than leaves, and ≤β diversity than leaves. Pooled across species, fruit tissues and ontogenetic stages had the highest γ diversity of all organs, and fruit tissues and ontogenetic stages combined had a higher incidence of organ-specific mass spectral features than leaves. Roots had ≤α diversity than leaves and the lowest β and γ diversity of all organs. Phylogenetic correlations of chemical distance varied by plant organ and chemical class.
- Our results describe patterns of specialized metabolite diversity across organs and species and provide support for organ-specific contributions to plant chemodiversity. This study contributes to the growing understanding within plant evolutionary ecology of the biological scales of specialized metabolite diversification. Future studies combining our data on specialized metabolite diversity with biotic interaction data and experiments can test existing hypotheses on the roles of ecological interactions in the evolution of chemodiversity.
INTRODUCTION
Plants harbour a high diversity of specialized metabolites, many of which are undescribed, both in form and function. These specialized metabolites are hypothesized to influence ecological and evolutionary interactions across levels of biological organization, from fitness of individual plants to species diversity and niche differentiation of plants and the organisms with which they interact (e.g., Kursar et al. 2009; Iason et al. 2011; Richards et al. 2015; Endara et al. 2017; Salazar et al. 2018; Whitehead et al. 2022). Yet despite the insights from these and many other compelling studies, major components of the evolutionary and ecological dynamics underlying the diversity, structure, and function of specialized metabolites remain to be addressed. In particular, the patterns and processes of specialized metabolite diversity have yet to be thoroughly explored within and across organisms. To date, most research characterizing plant chemodiversity has focused on vegetative tissues, in particular leaves, across species (e.g., Richards et al. 2015; Sedio et al. 2017; Sedio, Parker, et al. 2018; Salazar et al. 2018; Endara et al. 2023; Forrister et al. 2023), or when comparing organs, has focused on certain structural classes of specialized metabolites (e.g., Kessler & Halitschke 2009; van Dam et al. 2009; Agrawal & Hastings 2023; Anaia et al. 2024; Ziaja & Müller 2025). However, all plant organs contain specialized metabolites of numerous structural classes and, as such, represent components of the plant metabolome – its full suite of specialized metabolites as transcribed from its genome. In response to organ-specific selective pressures, each organ may play a significant part in shaping the evolution of the plant metabolome. In turn, the evolution of organ-specific specialized metabolites may play a significant part in shaping the vast chemodiversity of the plant kingdom. Thus, quantifying the patterns and hierarchical structure of organ-specific chemodiversity within a group of related species represents a crucial step in understanding the evolutionary origins and ecological ramifications of plant chemodiversity.
In addition to their different functions in plant growth and reproduction, fruits, leaves, and roots occupy different physical environments and interact with different guilds and species of organisms. Fruits experience a variety of different environments and shifting selection pressures across stages of their lifetime, from fruit development, seed dispersal, and through to seed germination at a new location (Cipollini & Levey 1997; Dalling et al. 2020). Fruits interact with a community of partners, including a diverse array of antagonists and mutualists, whose composition and identity shift throughout the stages of plant recruitment. Specialized metabolites contained in seeds and pericarp are hypothesized to influence these plant–biotic interactions at each stage (Cipollini & Levey 1997; Dalling et al. 2020). For animal-dispersed species, which represent over half of seed plant species globally (Rogers et al. 2021), specialized metabolites in ripe fruit function not only in defence against antagonistic consumers but also in attraction or manipulation of mutualistic seed dispersers (Cipollini & Levey 1997).
Compared to fruits, leaves undergo relatively little change over their lifetimes with respect to the types of biotic interactions in which they are involved. Of these interactions, antagonistic interactions with folivores and pathogens and mutualistic interactions with endophytes represent the major types involving plant specialized metabolites (Wink, 2003; Rodriguez et al., 2009; Porras-Alfaro and Bayman, 2011; Endara et al. 2023). Although the types of biotic interactions in which leaves are involved change little over leaf lifetimes, there may be turnover in the assemblage of folivore species upon leaf maturation associated with increased physical defence and changes in the composition of the specialized metabolite profile (Stamp 2003; Moore et al. 2014).
Below ground, roots are generally involved in the same types of specialized metabolite-mediated interactions as leaves, including antagonistic interactions with herbivores and pathogens and mutualistic interactions with microbes. Root–microbial interactions are widespread, with over 50% of terrestrial plant species relying on root-mutualistic fungi and bacteria to some extent for growth, survival, and/or defence against pathogens (van der Heijden et al. 2008; Pozo et al. 2010; Jung et al. 2012). The soil is also home to root-eating arthropods and nematodes (Rasmann et al. 2011; Mundim et al. 2017), and roots often receive substantial allocations of constitutive or induced defence specialized metabolites, suggesting that root herbivory can significantly affect plant fitness (Rasmann & Agrawal 2008; van Dam 2009; Johnson et al. 2016).
Few studies have quantified metabolome-wide (i.e., untargeted) patterns of diversity for specialized metabolites across ecologically disparate plant organs, such as leaves, roots, and fruit (Schneider et al. 2021, 2023; Weinhold et al. 2022). Studies comparing reproductive organs to leaves include an assemblage of sympatric Piper species (Schneider et al. 2021) and two sympatric Psychotria species (Schneider et al. 2023). The genera Piper L. (Piperaceae) and Psychotria L. (Rubiaceae) are both known to possess distinctive and chemically diverse specialized metabolite traits (Piper: Kato & Furlan 2007; Richards et al. 2015. Psychotria: Sedio et al. 2017; Berger et al. 2021). In both studies, plant organ identity explained significant portions of the variation in specialized metabolite richness and molecular structural characteristics. One study comparing roots, root exudates, and leaves of four unrelated subtropical tree species also found significant organ-specific metabolomic variation, as well as differences in responses of organ-level chemical diversity to species richness of the tree community (Weinhold et al. 2022).
In this study, we compare patterns of metabolomic variation and distribution across organs and ontogenetic stages in 21 sympatric, closely related species of Psychotria and Palicourea shrubs with bird-dispersed fruit. For these species, we describe patterns of specialized metabolite diversity across leaves, fruit pericarp, seeds, and roots and through ontogeny for reproductive organs. We apply metrics of α, β, and γ diversity to quantify chemodiversity across fruit ontogeny, across organs within individual plants, and among closely related species within a clade. Further, in a macroevolutionary context, we compare the correlations of each organ's specialized metabolite composition with plant species phylogeny. By characterizing the spatial and ontogenetic variation of specialized metabolite composition within and among species, and chemo-phylogenetic correlations among species, we help fill crucial knowledge gaps in ecological patterns relevant to the evolution of plant chemodiversity and facilitate future testing of diversification hypotheses (Wetzel & Whitehead 2020; Whitehead et al. 2022; Thon et al. 2024).
MATERIAL AND METHODS
Study site
All samples were collected at Barro Colorado Island, Panama (9° 9′N, 79° 51′W), hereafter referred to as BCI. BCI encompasses a lowland, moist tropical forest with average annual rainfall of 2.6 m (Leigh et al. 1996). The precipitation in central Panama is concentrated within a rainy season between May and December, with a pronounced dry season between January and April (Leigh et al. 1996). Seasonality is evident in both the vegetative and reproductive phenology of the woody plants of BCI, with a peak in leaf and seed production in the late dry season and early wet season (Leigh et al. 1996; Zimmerman et al. 2007) and a second, smaller peak of seed production in the mid-late wet season (Wright et al. 1999; Zimmerman et al. 2007).
Study species
The genus Psychotria L., tribe Psychotrieae (Rubiaceae), and the closely related genus Palicourea Aubl., tribe Palicoureeae, together represent over 2000 species of tropical and subtropical woody plants (Govaerts et al. 2021), often occurring as a “species swarm” (Gentry 1989) with high site-level congeneric species richness. The Psychotria of BCI have seeds that are dispersed mainly by birds of the families Pipridae and Turdidae, with six species among these families accounting for 97% of Psychotria fruits recovered in a mist-netting survey (Poulin et al. 1999) that included 19 of the 21 Psychotria-allied species in the present study. Further, 70% of birds carrying Psychotria seeds had consumed more than one Psychotria species in a feeding bout (Poulin et al. 1999), consistent with a generalist approach to a diet varying in toxin and/or nutrient content. Regarding antagonists, the Psychotria-allied species of BCI have been found to host five species of insect seed predator, none of which were found on more than one Psychotria species (Basset et al. 2021), and 127 species of insect folivores which were found to contain an average of 3.46 Psychotria species in their diet (Sedio 2013). Insect folivores included 69 species of Coleoptera, 22 species of Lepidoptera, and 21 species of Orthoptera (Sedio 2013).
Included in the present study were all species on BCI belonging to the Palicoureeae and Psychotrieae, 20 in total, with the exception of Ps. poeppigiana Müll. Arg., which we were not able to locate in sufficient numbers for sample collection. We have combined the members of Palicoureeae and Psychotrieae in our study because of the complex and continually revised phylogenetic relationships among the species of these groups (Razafimandimbison et al. 2014; Berger et al. 2021). Also included is Ronabea emetica (L. f.) A. Rich., which has been reclassified from Psychotrieae to Lasiantheae (Taylor 2004). We have retained R. emetica in our study due to its sharing of avian seed disperser species with sympatric Psychotrieae and Palicoureeae (Poulin et al. 1999) and its phytochemical significance as a traditional medicinal plant (Berger et al. 2011).
Among our 21 focal species, mature fruit production is ontogenetically aggregated between the mid-wet season and early dry season (August–January), with the exceptions of Ps. limonensis and Ps. marginata which are less synchronous in reproductive phenology and exhibit an additional peak in mature fruit production in the early wet season (May–July) (Croat 1978; Poulin et al. 1999). For each of the 21 species, we collected samples of expanding leaves, unripe fruit, and ripe fruit. In a subsequent sampling effort, we collected samples of fine roots for 10 of the 21 species. A full list of the 21 species is provided in Table S1.
Sampling methods
We collected expanding leaves, immature fruit, and ripe fruit in September 2019–January 2020. Fine roots (≤2 mm diameter) were collected during follow-up sampling in July–August 2022. For all sample types, only undamaged plant material was collected. Expanding leaves were collected from distal ends of stems or of uppermost branches. Roots were traced to stems to confirm species identity. We sampled plant material from at least three individual plants of each species and collected each organ type from each individual when feasible. For each organ type, we collected from at least three individuals, but for Ps. marginata we were only able to collect immature fruit from one individual and for Ps. horizontalis we collected roots from two individuals. Samples from the five individuals of Ps. tenuifolia were pooled for analysis due to the small size of the fruits of this species, the low number of fruits produced by each individual, and the scarcity of the species at the study site. Species-level sample coverage is provided in Table S1. For fruit samples, pericarp and seed were separated from one another and analysed separately. However, the seeds of unripe fruit could not be dissected satisfactorily due to the early stage of organ differentiation at which unripe fruit were collected, and may therefore contain some unripe pericarp. While we include immature seed in the analysis, we cautiously interpret results of immature seeds with this caveat in mind. For root samples, at least 2 g (fresh weight) of material was collected for each sample and samples were gently rinsed with distilled water to remove soil and dead organic matter. The quantity of plant material collected for leaf and fruit samples and the phenological and environmental criteria for all sampling were as described in Schneider et al. (2023). All samples were freeze-dried and stored at −20°C awaiting specialized metabolite extractions, using sample preparation protocols as described in Schneider et al. (2023).
Chemical extractions and untargeted metabolomics
Chemical extractions
Chemical extraction of plant specialized metabolites and preparation of samples for UPLC–MS/MS analysis were carried out at Utah State University using the methods described in Schneider et al. (2023). For each sample, 80 mg homogenized powder was weighed into a 1.8 mL polypropylene screw-top tube using a microbalance. The extraction solvent used was 99.9% ethanol with 0.1% formic acid. Each sample was extracted with a total of 7.0 mL of the extraction solvent, conducted over five iterations of the following procedure. A glass syringe was used to add 1.4 mL of extraction solvent to the 1.8 mL tube containing the sample. The sample was mixed with the extraction solvent for 5 min in a vortexer at 1500 rpm and then centrifuged for 5 min at 13,800×g, after which the supernatant was removed and added to a 20 mL glass scintillation vial. The supernatant from each of the five iterations was combined in the same 20 mL vial. The combined extract was dried at room temperature using a vacuum-centrifuge. The dried extract was then weighed and stored at −20°C until analysis.
UPLC–MS/MS data acquisition
We used untargeted mass spectrometry-based metabolomic analysis to characterize specialized metabolite diversity (Wang et al. 2016; Sedio et al. 2017; Aron et al. 2020). For our UPLC–MS/MS analysis, dried extracts were resuspended at 10 mg mL−1 in 75:25 water: acetonitrile +0.1% formic acid, with 2.0 μg mL−1 ginsenoside as internal standard. The extract was then sonicated for 10 min, after which a 20 μL aliquot was taken and diluted 10-fold with 75:25 water:acetonitrile +0.1% formic acid. The diluted aliquot was then vortexed and centrifuged (10 min, 13,000×g) and an aliquot (180 μL) transferred to an LC–MS vial for analysis. Blanks were prepared using the same batch of resuspension solvent, internal standard, and LC-MS vials, as was used for the samples. Chromatography and mass spectrometry data were collected at the Chemistry Mass Spectrometry Facility at the University of Utah (Salt Lake City, Utah, USA) using an Acquity I-class UPLC coupled to a Waters Xevo G2-S q-ToF mass spectrometer (Waters, Milford, MA, USA). MS/MS fragmentation data were collected with the instrument set to data-dependent acquisition (DDA) mode and positive ion mode. Blanks were run after every 10 sample data collection runs. For instrument parameters, including column specification, temperatures, flow rates, and solvent gradient, see Supplementary Methods.
UPLC-MS/MS data processing
Raw data were converted from Waters. RAW proprietary format to .mzML for processing and analysis. The program Waters2mzML v. 1.2.0 (Prisching 2022) was used to conduct the file conversion. Chromatogram alignment, feature curation, and deconvolution of parent and fragmentation mass spectra were conducted using MZmine3 v. 3.4.14 (Schmid et al. 2023). Feature detection parameters were as follows: signal intensity threshold = 1000 counts (MS1), 500 counts (MS2); subtraction of background signal below intensity threshold; minimum consecutive scans = 5; further MZmine analysis parameters are provided in (data and code repository to be finalized prior to publication). Features detected in blanks were subtracted from feature lists of samples after GNPS molecular networking workflow (see below). For annotation of MS/MS spectra, including in-silico structure prediction, the deconvoluted spectra were exported in .mgf format from MZmine for processing in SIRIUS v. 5.6.3 (Dührkop et al. 2019). Within SIRIUS, the core molecular formula prediction workflow was utilized together with the full suite of structure prediction and classification workflows: ZODIAC network-informed molecular formula prediction refinement, CSI:FingerID molecular fingerprint prediction and structure database search, and CANOPUS compound class prediction. The parameters used for each SIRIUS module are provided in Supplementary Methods. Finally, molecular networking and chemical structural similarity analyses, including cosine distance matrix calculation, were conducted using the GNPS Feature-Based Molecular Networking workflow v. 28.2 (Nothias et al. 2020). The parameters used for the GNPS workflow are provided in Supplementary Methods.
Spatial and ontogenetic chemical diversity comparisons
Quantifying diversity
We characterized and compared chemical diversity of expanding leaves, roots, unripe and ripe pericarp, and immature and mature seeds. The development of fruit and the differentiation of its tissues are likely to represent key spatial and ontogenetic axes of interaction diversity. To characterize spatial and ontogenetic axes in the diversity of specialized metabolite traits across scales, we apply α, β, and γ diversity metrics commonly used in community ecology and modified for chemical traits (Wetzel & Whitehead 2020). We calculated α, β, and γ diversity of specialized metabolites based on the mass spectral feature presence/absence data produced by the UPLC-MS/MS analysis described above. Mass spectral features are hereafter referred to as “features”. Since dose–response data are not available for Psychotria specialized metabolites in the diets of the organisms that consume Psychotria tissues, and extraction efficiencies of a given solvent likely differ among organs, we focus on quantifying chemical richness. We define chemical richness as the sum or total count of putatively distinct specialized metabolites which met the UPLC-MS detection criteria. To calculate richness, ion intensity data were converted to raw incidence (presence/absence) format. Singleton features, or those only detected in one sample of the focal sample group for each analysis (species with organs pooled, organ with species pooled, or organ within species), were excluded from diversity calculations for that analysis. For α diversity, raw incidence data was averaged across each organ within a species to obtain mean chemical richness per sampling unit (defined as an organ within an individual of each species). We calculated β diversity as the variance in chemical composition across sampling units: (1) across species for a given organ as well as (2) across organs for a given species. To quantify β diversity, we use pairwise dissimilarity in chemical structural diversity to calculate multivariate dispersion (Anderson et al. 2011; Schneider et al. 2021, 2023). We examined the larger scale of γ diversity across samples from two perspectives: that of the plant and that of potential consumers. From the plant perspective, we examine γ diversity as the total chemical richness for all organs within a given species, which we denote as species-level γ diversity. From the perspective of a potential organ-specific consumer, we examine γ diversity as the total chemical richness for each organ, pooled across species, which we denote as organ-level γ diversity.
Spatial chemical diversity comparisons
To characterize spatial variation of chemical diversity, we quantified α, β, and γ diversity of features that co-occur at a given point in time across spatial scales, such as across organs of an individual plant or within organs across species. We included all samples of all organs to analyse the spatial chemical landscape of our focal species assemblage that a mutualist or antagonist might encounter at a given point in time. Our observations of overlap in leaf flush and fruiting phenology and of within-individual asynchrony of fruit ripening indicate that this is a realistic representation of the spatial chemical landscape of our focal species assemblage.
Ontogenetic chemical diversity comparisons
To characterize ontogenetic variation of chemical diversity, we quantified α, β, and γ diversity of features that occur across fruit development. We focus on ontogenetic variation in the chemistry of reproductive organs over the course of fruit ripening, as ontogenetic variation in vegetative organs has been the subject of numerous studies (e.g., Brenes-Arguedas et al. 2006; Wiggins et al. 2016; Sedio et al. 2017) and was found to be minimal in a subset of our focal species (Sedio et al. 2017; Schneider et al. 2023). We used a cross-sectional rather than a time-series design, because of practical impediments to the latter. Specifically, a true time-series comparison using unripe and ripe organs of the same individual fruit would be impractical due to the necessity of destructive collection for chemical analysis and due to potential defensive induction of specialized metabolites in the remaining tissue. Further, Psychotria species within our focal assemblage have been found to exhibit minimal intraspecific chemical variation within a given organ (Schneider et al. 2023), from which we infer that the potential for within-individual variation to obscure ontogenetic variation is remote.
Analysing diversity
To analyse differences in α diversity, we used linear mixed models (‘lmer’ function in ‘lme4’ package in R; Bates et al. 2015). As predictors, we included species, organ, and their interaction as fixed effects and plant identity as the random effect in the full model. Samples across individuals of P. tenuifolia were pooled and hence were given a single plant identification number for the purposes of the random effect in the linear mixed models. We compared this full model to a reduced model without the interaction term using a conditional F test with the Kenward-Roger approach for approximating degrees of freedom (‘KRmodcomp’ function in package ‘pbkrtest’, Halekoh & Højsgaard 2014). We conducted post-hoc pairwise comparisons between organ types within species and adjusted P-values to control for multiple comparisons using Tukey's HSD with five estimates per species without the root comparison and six estimates per species with the root comparison using the ‘emmeans’ package (Lenth 2024). To determine statistical significance, we used a significance threshold of P = 0.05.
To quantify β diversity, we analysed variation in structural composition based on the chemical structural dissimilarity matrices (Aron et al. 2020) produced by the GNPS molecular networking analysis described above. This method of β diversity quantification is described in detail by Schneider et al. (2021). We used PERMANOVA to assess patterns of variation in chemical structural composition among organ spatial and ontogenetic components and species (‘adonis2’ function in R package ‘vegan’). We evaluated a PERMANOVA model including organ type, species, and their interaction, which included all organ components and species. We also evaluated separate PERMANOVA models for each species, comparing organ components within species. PERMANOVA is sensitive to differences in group dispersions and significant results could be due to either shifts in centroids or different group dispersions. We used the function ‘betadisper’ in the R package ‘vegan’ to compare the dispersion of samples around the centroid for each group and tested for differences in dispersion among organ components using permutation tests separately for each species (N = 999) followed by Tukey's HSD tests for pairwise group comparisons as described above. To visualize variances within and among species and organ components, we conducted Nonmetric Multidimensional Scaling (NMDS) analysis using the R package ‘vegan’ and its function ‘metaMDS’. The NMDS analysis was run with 1000 maximum iterations, 500 maximum random starts, and four dimensions. As with the β diversity analysis, NMDS was performed on the chemical structural dissimilarity matrices produced by the GNPS workflow.
Species-level γ diversity
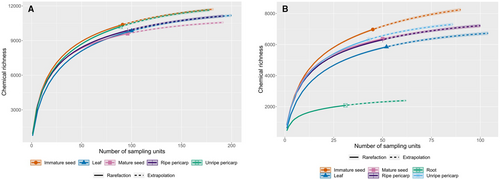
γ diversity from the plant perspective – was calculated as the total count of unique features which were each detected in at least two samples across any of the focal organ components within a species. Organ-level γ diversity – γ diversity from the consumer perspective – was calculated as the total count of unique features which were each detected in at least two samples across any of the focal species within an organ component. Further, to supplement our quantification of observed chemical diversity with estimates of population- or community-level diversity, we used rarefaction-extrapolation analysis in the R package ‘iNEXT’ (Chao et al. 2014; Hsieh et al. 2022) to estimate the total richness and sample completeness for each species and organ component.
Phylogenetic reconstruction and chemical similarity dendrograms
In evaluating the involvement of a trait or set of traits in niche differentiation among congeners, any such trait must be considered in a phylogenetic context. Previous studies of divergence in chemical traits within taxonomic groups have utilized and developed distance-based comparative methods which allow parallel comparisons of phylogenetic and chemical similarity (Kursar et al. 2009; Endara et al. 2017; Sedio, Boya, et al. 2018; Sedio, Parker, et al. 2018). Chemical traits that show intraspecific divergence as a result of selection by spatially or ontogenetically specific ecological interactions can be expected to exhibit divergent patterns when their spatial or ontogenetic components are separated and compared across phylogeny. We compared specialized metabolite-based traits across ontogenetic and spatial scales drawing from the aforementioned methods developed for phylogenetic-chemical distance comparisons.
Phylogenetic reconstruction
In order to maximize the accuracy of pairwise phylogenetic distances used for comparisons with chemical distances, we used all DNA or RNA marker loci in NIH GenBank that were available for at least 10 of the study species, and which resulted in each of our study species being represented by at least two of five loci. There was one exception in the case of Ps. gracilenta, for which sequence data were available from only one locus. The five loci used were 18 s rRNA, matK, psbA-trnH, rbcL, and rps16. Species coverage of loci is detailed in Table S2. Voucher data are provided in (data and code repository). Following Sedio et al. (2012), Coussarea curvigemmia Dwyer. (Rubiaceae) was used as outgroup.
Once sequence data were obtained, these data were subjected to a partition homogeneity test using PAUP* 4.0a build 169 (Swofford 2021), which confirmed (P = 0.22) that the five marker loci could be combined for subsequent phylogenetic tree construction (Farris et al. 1994). The sequence data were then subjected to an hLRT likelihood ratio test in MrModeltest2 v. 2.4 (Nylander 2018) which selected HKY + G as the best-fit model. Under the HKY + G model, the sequence data were concatenated and analysed using 3 × 107 MCMC generations in MrBayes v. 3.2.7a (Ronquist et al. 2012). MrBayes produced five Bayesian consensus trees of equal posterior probability. For visual representations of the phylogeny in our results, one of these trees was selected at random. For use in analyses involving phylogenetic distance, a mean phylogenetic distance matrix was calculated using pairwise distance matrices from each of the five consensus trees. Pairwise phylogenetic distance matrices were obtained by converting MrBayes output trees to Newick format using FigTree v. 1.4.4 (Rambaut 2018) then calculating pairwise distances using the function ‘cophenetic’ in R v. 4.2.3 (R Development Core Team 2023).
Chemical similarity dendrogram construction
We constructed dendrograms to display organ-level hierarchical clustering of the study species based on their chemical structural similarity. Hierarchical clustering analysis was conducted for each chemical structural similarity matrix with 1 × 104 bootstrap replicates using the R package ‘pvclust’ v. 2.2–0 (Suzuki & Shimodaira 2006).
Phylogeny–chemical similarity dendrogram comparisons
In order to visualize comparisons of phylogenetic and chemical similarity among the study species, we used the R package ‘dendextend’ v. 1.17.1 (Galili 2015). Utilizing the ‘tanglegram’ function in ‘dendextend’, we generated side-by-side comparisons of a Bayesian consensus tree and the hierarchical clustering-based chemical similarity dendrogram.
Distance matrix comparisons
We implemented Mantel tests to compare correlations of chemical dissimilarity matrices with phylogenetic distances. We conducted these tests with the full chemical dataset and subsequently conducted analogous tests with the chemical dataset subdivided by chemical pathways, which were classified by SIRIUS using NPClassifier taxonomy (Kim et al. 2021). Mantel and partial Mantel tests were performed using the R package ‘vegan’ v. 2.6-4 (Oksanen et al. 2022).
RESULTS
Alpha diversity varies across organs and ontogeny
The interaction among species and organ components was retained in the linear mixed model of α diversity of expanding leaf, unripe pericarp, immature seed, ripe pericarp, and mature seed of the 21 species (F80,295 = 5.93, P < 0.001). In general, α diversity in reproductive organ components was on par with or greater than the α diversity of expanding leaves within species (Fig. 1). Nine species had higher α diversity in at least one tissue or ontogenetic component of reproductive organ compared to that of expanding leaves. In Pa. guianensis, Ps. acuminata, Ps. limonensis, and Ps. cyanococca, ripe pericarp and mature seed had significantly higher diversity than expanding leaves. In Ps. limonensis and Ps. cyanococca, all tissues and ontogenetic stages of reproductive organs had higher diversity than expanding leaves. In Pa. guianensis and Ps. acuminata, ripe pericarp, mature seed, and immature seed had higher diversity than expanding leaves, while α diversity of unripe pericarp was similar to that of expanding leaves. In Ps. deflexa, immature and mature seeds had the highest α diversity across organs while α diversity in other organs did not differ. In Carapichea ipecacuanha, α diversity in mature seeds was higher than that in expanding leaves and unripe pericarp and similar to that in mature pericarp and immature seeds. In Ps. psychotriifolia, unripe pericarp had higher α diversity than expanding leaves and seeds. Compared to all other organ components within each species, α diversity was highest in unripe pericarp in Ps. micrantha and in immature seed in Ps. capitata (Fig. 1) (potentially due to our limitations in separating seed from pericarp, see METHODS).
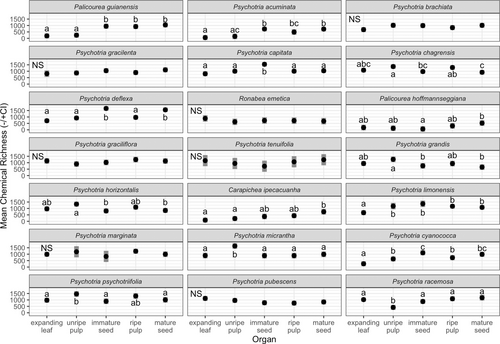
In the rest of the species, α diversity in reproductive organs was similar to or lower than α diversity observed in expanding leaves (Fig. 1). In seven species, α diversity did not vary among organ components (Ps. brachiata, Ps. gracilenta, Ps. graciliflora, Ps. tenuifolia, Ps. marginata, Ps. pubescens, Ronabea emetica). In four species (Ps. chagrensis, Ps. grandis, Ps. horizontalis, Pa. hoffmannseggiana), α diversity of reproductive organs did not differ statistically from expanding leaves, but α diversity differed among reproductive organ components. In Ps. chagrensis α diversity of pericarp was higher than in seeds across ontogeny, and, in Ps. grandis and horizontalis, unripe pericarp had higher α diversity than immature and mature seeds. Finally, in Ps. racemosa, unripe pericarp had lower α diversity than all other organs.
For most species, α diversity did not shift over the course of fruit development in pericarp and seed. Alpha diversity declined in the pericarp during ripening for Ps. micrantha and in the seed during maturation for Ps. capitata (Fig. 1). In contrast, α diversity increased in the pericarp during ripening for Pa. guianensis and Ps. racemosa and in the seed during maturation for Pa. hoffmannseggiana (Fig. 1).
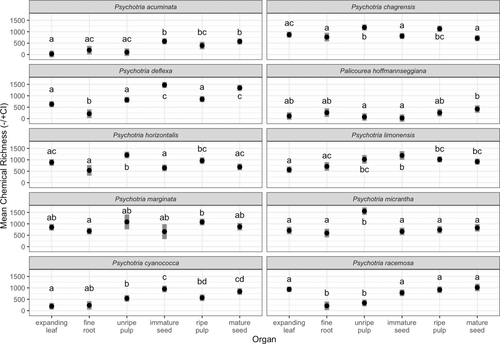
The interaction among species and organ was retained in the linear mixed model of α diversity of expanding leaf, fine roots, unripe pericarp, immature seed, ripe pericarp, and mature seed of the 10 species in which roots were also analysed (F45,178 = 11.44, P < 0.001). Across species, α diversity of roots was lower or similar to other plant organs (Supplementary Results, Fig. 2). Alpha diversity of roots was similar to that of expanding leaves in eight out of the ten species and lower than expanding leaves in Ps. deflexa and Ps. racemosa. Alpha diversity of roots was lower than that of all other organs in Ps. deflexa, and, at the other extreme, similar to all other organs in Pa. hoffmannseggiana. Other α diversity results were mostly qualitatively similar to those above (Fig. S2).
Beta diversity varies across organs and ontogeny
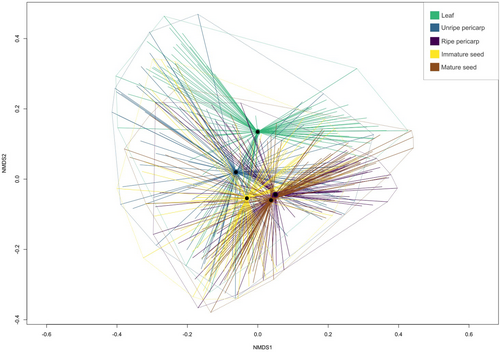
PERMANOVA indicated that organ (F4,471 = 30.6, P < 0.001), species (F20,471 = 19.1, P < 0.001), and their interaction (F60,471 = 3.97, P < 0.001) all were significant in explaining variance in chemical structural dissimilarity for the 21 species. Species explained the largest amount of variance (R2 = 0.32), followed by species–organ interaction (R2 = 0.27) and organ (R2 = 0.10). Within species, PERMANOVA results indicated that plant organ significantly explained variance in chemical structural dissimilarity for all species (Table S3). Because the interaction in the PERMANOVA was significant, we analysed multivariate dispersions of chemical structural dissimilarity among plant organs within species. There were no significant differences in dispersion around group centroids among plant organs (Table 1), except for in Ps. deflexa (n permutations = 999; F4,21 = 4.83, P < 0.01; Tukey's HSD Padj <0.06) and in Ps. racemosa (n permutations = 999, F4,25 = 5.86, P < 0.01; Tukey's HSD Padj <0.02). Pairwise post-hoc comparisons of Ps. deflexa indicated that expanding leaf and unripe pericarp had comparable multivariate dispersion, and the remaining fruit tissues and ontogenetic stages (immature seed, mature seed, and ripe pericarp) were comparable to one another in dispersion, but exhibited statistically lower dispersion than expanding leaf and unripe pericarp (Table 1). For Ps. racemosa, ripe pericarp had statistically lower dispersion than immature seed and unripe pericarp, while the remaining leaf and fruit tissues and ontogenetic stages were statistically similar (Table 1). Further comparison of centroids and group dispersion patterns in multivariate space via NMDS (k = 4, stress = 0.137) visually highlighted the variation in group centroid locations as well as in the perimeters of the dispersion clouds, with centroids of ripe pulp and mature seed close together, and the centroid of leaves further away from reproductive organs (Fig. 3). Results of PERMANOVA and analysis of multivariate dispersion among plant organs of the 10 species that also had roots analysed were qualitatively similar to the 21-species comparison (Tables S4 and S5). Roots had lower dispersion than other plant organs in Ps. deflexa, Ps. limonensis, Ps. marginata, and Ps. racemosa (Table S6).
| species | expanding leaf | unripe pericarp | ripe pericarp | immature seed | mature seed |
|---|---|---|---|---|---|
| Carapichea ipecacuanha | 0.302 | 0.354 | 0.288 | 0.399 | 0.315 |
| Palicourea guianensis | 0.332 | 0.373 | 0.311 | 0.272 | 0.369 |
| Palicourea hoffmannseggiana | 0.266 | 0.271 | 0.231 | 0.291 | 0.386 |
| Psychotria acuminata | 0.272 | 0.441 | 0.290 | 0.352 | 0.310 |
| Psychotria brachiata | 0.422 | 0.427 | 0.327 | 0.390 | 0.306 |
| Psychotria capitata | 0.398 | 0.388 | 0.371 | 0.294 | 0.438 |
| Psychotria chagrensis | 0.294 | 0.282 | 0.194 | 0.289 | 0.249 |
| Psychotria cyanococca | 0.268 | 0.389 | 0.310 | 0.365 | 0.291 |
| Psychotria deflexa | 0.375a | 0.361a | 0.227b | 0.229b | 0.223b |
| Psychotria gracilenta | 0.327 | 0.291 | 0.222 | 0.303 | 0.211 |
| Psychotria graciliflora | 0.255 | 0.227 | 0.224 | 0.261 | 0.265 |
| Psychotria grandis | 0.279 | 0.338 | 0.210 | 0.216 | 0.248 |
| Psychotria horizontalis | 0.262 | 0.285 | 0.241 | 0.261 | 0.314 |
| Psychotria limonensis | 0.330 | 0.254 | 0.272 | 0.226 | 0.305 |
| Psychotria marginata | 0.332 | NA | 0.233 | NA | 0.177 |
| Psychotria micrantha | 0.228 | 0.218 | 0.173 | 0.200 | 0.182 |
| Psychotria psychotriifolia | 0.257 | 0.272 | 0.194 | 0.270 | 0.253 |
| Psychotria pubescens | 0.334 | 0.347 | 0.321 | 0.329 | 0.276 |
| Psychotria racemosa | 0.375a,b | 0.436a | 0.235b | 0.428a | 0.323a,b |
| Ronabea emetica | 0.248 | 0.261 | 0.255 | 0.202 | 0.152 |
- Mean distances to centroid are calculated from PCoA eigenvalues. Comparisons among organs within species are not significantly different unless noted by letter superscripts. Non-shared letters in superscript indicate significant differences by Tukey's HSD post-hoc pairwise test (P < 0.06). n ≥ 3 for each organ within each species, except for Ps. marginata and Ps. tenuifolia. See Table S1 for number of replicates of each organ for each species.
Gamma diversity varies from both plant and consumer perspectives
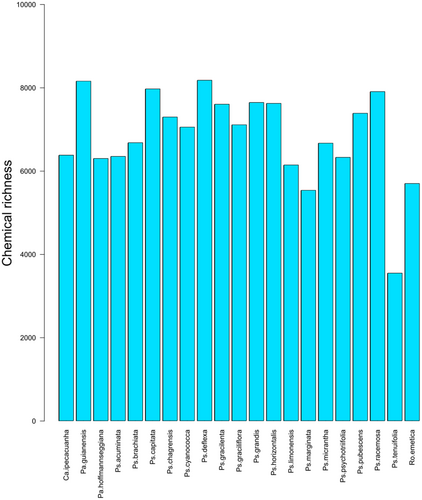
Total chemical richness exhibited substantial variation from the plant perspective (species-level, all organs pooled) and from the consumer perspective (organ-level, all species pooled). The total number of non-singleton features detected was 14,982. Of these, 5,225 features were annotated as specialized metabolites (alkaloids, amino acids and peptides, polyketides, shikimic acids and phenylpropanoids, or terpenoids). A further 669 features were annotated as primary metabolites (carbohydrates or fatty acids) and were excluded from subsequent analyses. Rarefaction-extrapolation analysis indicated that sample coverage at the species level exceeded 75% for all species except Ps. tenuifolia (68%), and that interspecific differences in richness were generally robust to variation in sample coverage (Fig. S1). Reduced sample coverage in P. tenuifolia was attributable to the reduced effective sample size resulting from the pooling of sample materials for this species (see Methods). Species-level γ diversity ranged from 5,533 for Ps. marginata to 8,174 for Ps. deflexa (Fig. 4) excluding root samples and the samples of P. tenuifolia that were pooled due to low sampling coverage. When root samples were included and quantified in a separate analysis (excluding the 11 species without root sampling), species-level γ diversity ranged from 1,745 in Ps. hoffmannseggiana to 5,398 in Ps. racemosa (Fig. S2). Among the 10 species included in the root analysis, rarefaction-extrapolation analysis indicated that sample coverage exceeded 90% for all but Ps. hoffmannseggiana (84%) and that interspecific differences in richness were robust to variation in sample coverage (Fig. S1).
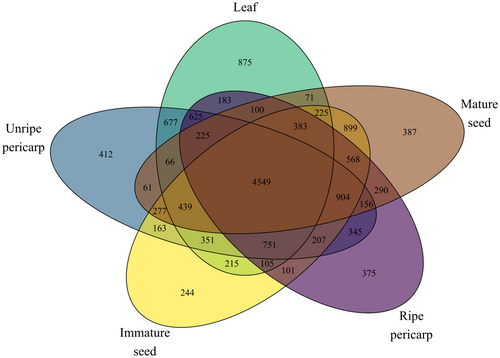
At the organ level, γ diversity of reproductive organs was similar or higher than that of leaves and lowest for roots. Organ-level γ diversity among the full 21 species pool, with roots and pooled samples of P. tenuifolia omitted, varied less than species-level diversity, ranging from 9599 for mature seed to 10,381 for immature seed, with intermediate and convergent values for leaves (9840), ripe pericarp (9866), and unripe pericarp (10,208) (Fig. 5). The 95% confidence intervals for these richness values as calculated by rarefaction-extrapolation analysis are also shown in Fig. 5. Rarefaction-extrapolation analysis further indicated that sample coverage was in excess of 95% for all organs (Fig. S3A). Across the 21 species, approximately one-third of non-singleton features were shared among organs (4549 out of 14,313; Fig. 6), but slightly more than one-third were unique to fruit (summed over ontogenetic stages and tissues of fruit, including those features shared among specific ontogenetic stages and tissues: 5389 out of 14,313). The number of total unique features found in fruit was six-times higher than in leaves, with the caveat that fruit were sampled four times more than leaves (Fig. 6). Comparing across organs and/or ontogenetic stages across the 21 species, the numbers of organ−/stage-specific features were highest in leaves (875); intermediate in unripe pericarp (412), mature seeds (387), and ripe pericarp (375); and lowest in immature seeds (244) (Fig. 6). The organ-specific number of alkaloids, amino acids and peptides, polyketides, shikimates and phenylpropanoids, and terpenoids tended to be higher for expanding leaves than for fruit organs and ontogenetic stages (Table 2).
| alkaloids | amino acids & peptides | polyketides | shikimates & phenylpropanoids | terpenoids | |
|---|---|---|---|---|---|
| Expanding leaf | 76 (1065) | 47 (599) | 10 (192) | 30 (854) | 15 (597) |
| Unripe pericarp | 45 (1228) | 36 (612) | 7 (196) | 7 (903) | 19 (666) |
| Ripe pericarp | 34 (1229) | 28 (595) | 5 (186) | 5 (877) | 14 (620) |
| Immature seed | 30 (1372) | 13 (609) | 1 (168) | 3 (1010) | 4 (549) |
| Mature seed | 58 (1353) | 12 (482) | 2 (161) | 21 (1020) | 6 (503) |
- Total numbers of features are presented in parentheses following numbers of organ-specific features. In each case, the total number includes the number of features specific to an organ plus those found in multiple organs. n ≥ 3 for each organ within each species, except Ps. marginata and Ps. tenuifolia. See Table S1 for number of replicates of each organ for each species.
Organ-level γ diversity among the 10 species including root samples ranged from 2082 for roots to 6970 for immature seed, with intermediate values of 5849 for leaves, 6310 for ripe pericarp, 6326 for unripe pericarp, and 6372 for mature seed (Fig. 5B). Among these 10 species, the 95% confidence intervals for these richness values as calculated by rarefaction-extrapolation analysis are also shown in Fig. 2B. Sample coverage exceeded 95% for all sample types (Fig. S3B). Across the 10 species that include roots samples, summing over fruit ontogenetic stages and tissues, including those features shared among specific ontogenetic stages and tissues, there were 4727 features unique to fruit (Fig. S4). The numbers of organ−/stage-specific features were highest in leaves (698), intermediate in immature seed (542) and ripe pericarp (483), and lowest in mature seed (328), unripe pericarp (318), and roots (310; Fig. S4). The organ-specific number of alkaloids, amino acids and peptides, polyketides, shikimates and phenylpropanoids, and terpenoids tended to be higher for expanding leaves than fruit organs and ontogenetic stages and roots (Table S7).
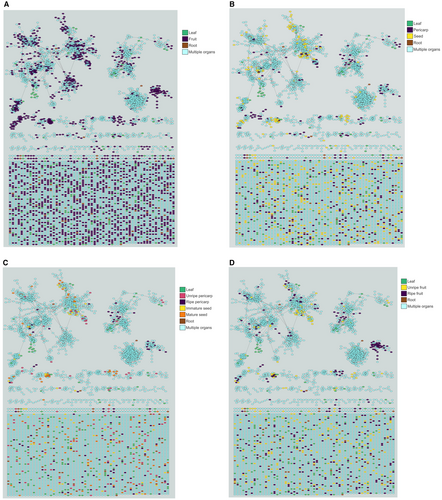
Colour-coding of features within the molecular network diagram provided a visual representation of the chemical structural diversity represented among organ-specific features (Fig. 7–D). While both vegetative and reproductive organs contained unique features from numerous molecular neighbourhoods, reproductive organs exhibited a higher degree of clustering within network neighbourhoods, indicating groups of features with variations on a broadly similar structure (Wang et al. 2016; Aron et al. 2020; Nothias et al. 2020).
Correlations between chemical and phylogenetic similarity vary by organ
All organs exhibited a positive, significant (P < 0.05) Mantel correlation between chemical structural similarity and phylogenetic similarity (Fig. 7). In the 21-species group, the Mantel correlation coefficient r ranged from 0.24 for unripe pericarp to 0.34 for leaves, and the rest of the reproductive organs/ontogenetic stages were higher (0.46 for ripe pericarp, 0.48 for immature seed, and 0.68 for mature seed; Fig. 8). For expanding leaves, unripe fruit, mature seed, and ripe pericarp of this species group, the hierarchical clustering analysis of the chemical structural similarity data yielded dendrograms in which 42%–63% of nodes were statistically supported (approximately unbiased probability; P ≥ 0.95; Fig. 8). The well-defined structure of these chemical similarity dendrograms indicates robust separation of organs and species in chemical trait space, despite the varying degrees of congruence between chemical and phylogenetic clustering patterns.
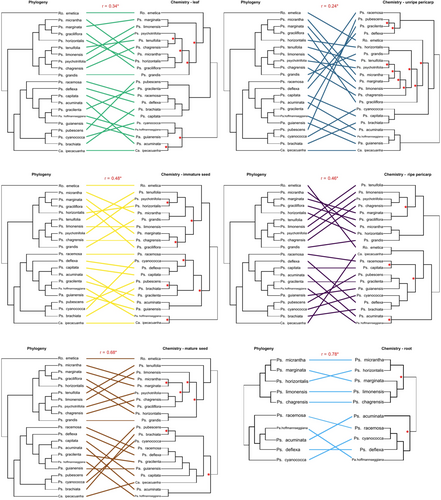
In the 10-species group for which roots were sampled, the Mantel correlation coefficient was 0.78, higher than any of the coefficients exhibited by leaf or fruit organs (Fig. 8). The hierarchical clustering analysis of chemical structural similarity yielded a dendrogram in which 22% of nodes were statistically supported (Fig. 8). Only two basal nodes were supported at P ≥ 0.95 (approximately unbiased probability). The indistinct structure of the chemical similarity dendrogram indicates weak separation of species in chemical trait space and limited chemical divergence for root specialized metabolites at the phylogenetic scale in question.
Correlations between chemical and phylogenetic similarity vary by chemical class
The annotation of features performed during the SIRIUS workflow classified each feature by metabolic pathway, e.g., alkaloids, terpenoids, polyketides, etc., based on NPClassifier taxonomy (Kim et al. 2021). Five chemical classes accounted for over 95% of specialized metabolites in our samples: alkaloids, amino acids and peptides, polyketides, shikimates and phenylpropanoids, and terpenoids. Based on these classifications, we calculated molecular networks and chemical structural similarity matrices for each chemical class within each organ. The class-level chemical structural similarity matrices were then analysed using hierarchical clustering analysis and phylogenetic comparison, as has been described for the full, combined-class chemical dataset. The Mantel correlation coefficients for each chemical class-by-organ type comparison with phylogeny are provided in Table 3. With the exception of shikimates and phenylpropanoids in unripe pericarp, all chemical class-level correlation coefficients were either indistinguishable from or lower than the coefficients for the full chemical dataset (Table 3). Notably for alkaloids, polyketides, and terpenoids in certain organ types, the Mantel correlation of chemical similarity with phylogenetic similarity was not significantly different from zero, while the coefficients for the full chemical dataset were significantly positive for all organs (Table 3).
| chemical class | leaf | fine root | unripe pericarp | ripe pericarp | immature seed | mature seed |
|---|---|---|---|---|---|---|
| All | 0.34 | 0.78* | 0.24* | 0.46* | 0.48* | 0.68* |
| Alkaloids | 0.21 | 0.66* | 0.18 | 0.31* | 0.46* | 0.72* |
| Amino acids & Peptides | 0.39 | 0.36* | 0.28* | 0.40* | 0.23* | 0.52* |
| Polyketides | 0.01 | 0.36* | 0.29* | 0.19 | 0.43* | 0.46* |
| Shikimates & Phenyl-propanoids | 0.32 | 0.71* | 0.37* | 0.36* | 0.43* | 0.62* |
| Terpenoids | 0.19 | 0.30* | 0.20 | 0.41* | 0.08 | 0.18 |
- Colour-coding of class-level coefficients indicates an increase (red) or decrease (blue) of ≥|0.05| in correlation compared to the all-chemical coefficient. Asterisks denote correlation coefficients that are significantly different from zero (P ≤ 0.05).
DISCUSSION
Among the 21 focal species studied, fruits, leaves, and roots exhibited marked differences in patterns of specialized metabolite diversity and in metabolomic–phylogenetic correlations. These results are consistent with organ-specific evolutionary responses in the plant metabolome to selective pressures imposed by the contrasting ecological interactions of each organ. Fruits, leaves, and roots all exhibited organ-specific specialized metabolites, each contributing to species-level chemodiversity. In terms of their α, β, and γ diversity, the aboveground organs, fruits, and leaves made similarly large contributions to metabolomic diversity at the level of individual species (Figs. 1 and 2) and the 21-species assemblage (Fig. 6). While roots contributed less to γ diversity than did fruits and leaves, the proportion of specialized metabolites which were unique to roots was similar to the proportions of organ-specific metabolites in other organs (Fig. S4).
In this group of bird-dispersed species, ripe pericarp is under the intensely divergent selective pressures of defending against antagonists while remaining palatable to mutualists, which can result in diversification of specialized metabolites that have stronger physiological effects on antagonist taxa than on mutualist taxa (Tewksbury & Nabhan 2001; Gunasekaran et al. 2021; Trabelcy et al. 2023). Potentially reflecting such selection, specialized metabolites of ripe pericarp in our study exhibited α, β, and γ diversity that was either higher than or comparable to that of leaves for most of our species. Further, while phylogenetic distance was more positively correlated with ripe pericarp chemical dissimilarity than with leaf chemical dissimilarity (Fig. 7), the significant correlation of both organs with phylogeny was characterized by the separation of the two major clades represented by the Palicoureeae and Psychotrieae tribes. Within tribes, there was substantial metabolomic–phylogenetic non-congruence for both ripe pericarp and leaves (Fig. 7). Here, we speculate that diversifying selection on specialized chemistry in pericarp may be weaker than that in leaves due to the divergent selective pressures imposed by antagonists being opposed by the stabilizing selection from shared mutualists. Meanwhile, the diversity patterns in leaves – intermediate to high α, β, and γ diversity and low metabolomic-phylogenetic correlation – suggest an evolutionary history of chemical diversification that maximizes divergence among sympatric congeners. Such a pattern is consistent with the scenario of evolutionary escape from natural enemies that has long been a leading hypothesis in plant–herbivore evolutionary ecology (Ehrlich & Raven 1964; Stamp 2003). Indeed, over 100 species of leaf-feeding insects have been found consuming one or more of our 21 focal plant species (Sedio 2013).
The specialized metabolites of mature seeds of our focal species are presumed to be under selection mainly by pathogens and seed predators. While mature seeds exhibited α, β, and γ diversity metrics comparable to those of ripe pericarp or leaves (Tables 1 and 2; Figs. 1, 2, 5, and 6), the metabolomic–phylogenetic correlation of the mature seed metabolome was the highest of all tissues examined in the 21-species analysis (Table 3; Fig. 8). Although there are insufficient ecological data to thoroughly address these patterns, the low rates of late-stage pre-dispersal seed predation among our study species (Basset et al. 2021) suggest that mature seeds acquired chemical defences early in the evolutionary history of the Psychotrieae and Palicoureeae that remain effective and have faced relatively little diversifying selection from seed predators.
Among unripe fruit organs, the high α, β, and γ diversity and low phylogenetic correlations observed were unexpected, but may be attributable to unforeseen diversity in the biotic interactions associated with unripe fruit: the overlap of folivores and fruit antagonists in early fruit development. Phenological overlap was observed during our sample collection in the leaf flush and early fruit development of our focal species, which may leave unripe fruit vulnerable to herbivores attracted by leaf production (White 2011; Martins et al. 2015). As a result of their relatively high protein content, unripe fruit are known to attract herbivorous vertebrates as well as invertebrates in a range of plant communities (White 2011). Alternative to or in conjunction with potentially more diverse ecological interactions than with leaves, the high fitness value and resource investment associated with unripe fruit tissue for the parent plant could also be expected to result in selection for higher defence metabolite diversity in unripe fruit than in leaves. Indeed, concentrations and/or diversity of specialized metabolites in unripe fruit have been found to exceed those in leaves of several plant taxa (Whitehead and Bowers, 2013; Whitehead et al., 2013) and in at least one notable plant–insect interaction involving an insect species which consumes both unripe fruit and leaves (Martins et al. 2015).
In exploring root chemodiversity, we found that roots had lower or similar α diversity to other plant organs, lower or similar β diversity, lowest γ diversity, and the lowest number of unique features. The influences of biotic interaction pressures on root chemodiversity can only be speculated upon as, to date, there has only been one belowground-inclusive comparison of herbivore species richness and host specificity by guild in a tropical forest community (Novotny et al. 2010). In this comparison, the estimated species richness of root-chewing insects per host plant species was an order of magnitude lower than that of leaf-chewing insects, but an order of magnitude higher than that of pericarp-chewing insects (seed-chewers were not evaluated) (Novotny et al. 2010). Further, arbuscular mycorrhizal fungi (AMF) have been found to mediate root chemical defence in numerous contexts (Pozo et al. 2010; Jung et al. 2012), and the prevalence of AMF-associated plant species in tropical forest communities (Öpik et al. 2006; Zhong et al. 2021) suggests that tripartite root–AMF–herbivore and root–AMF–pathogen interactions must be considered in identifying selection pressures on root chemical defence in these communities. The number and type of interactions belowground may result in lower diversity but a unique composition of features more effective in the disparate physical and abiotic environment of roots compared to aboveground plant organs. Overall, this further points to the importance of varying selective pressures across plant organs on the diversity of specialized metabolite traits found in plants. Further research should investigate how differing life history strategies, movement capabilities, and types of interactions of plant consumers across organs influence the diversity of chemical traits and the mechanisms under which that diversity arises (e.g., mobilization across organs, differential expression).
Further illustrating the potential dynamics of specialized metabolite diversification driven in parallel by organ-specific interactions and selection, metabolomic–phylogenetic correlation with respect to specific chemical classes varied by organ within each chemical class (Fig. 8; Table 3). With low phylogenetic correlation reflecting relatively rapid evolution of a trait, the differences across the five major specialized metabolite classes in which organ(s) exhibited the lowest phylogenetic correlation (Table 3) illustrated that organ-specific interactions could be semi-independently contributing to specialized metabolite diversification by acting in parallel through different specialized metabolite pathways. Specifically, leaves appear to be leading polyketide diversification with a correlation coefficient of 0.01, while immature seeds appear to be leading terpenoid diversification with a correlation coefficient of 0.08, both much lower than each organ's metabolome-wide correlation coefficient (0.34 and 0.55, respectively; Table 3; Fig. 8). While only 30% of features were found in all organs and ontogenetic stages (Fig. 3), it must also be noted that physiological and genetic constraints are almost certainly limiting the organ specificity at which specialized metabolites can be selected for or expressed (Adler et al. 2006, Adler & Irwin 2012; Kessler & Halitschke 2009; Keith & Mitchell-Olds 2019; but see Agrawal & Hastings 2023).
With ongoing research implicating plant chemodiversity as a defining factor in ever more types of plant–biotic interactions, it is also becoming clearer that the diversity of plant–biotic interactions for a given plant lineage is a defining factor in the evolution of its specialized metabolites. By examining chemodiversity in Psychotria and Palicourea, at the spatial and ontogenetic scales at which plant–biotic interactions take place, distinguishing between ecologically disparate spatial and ontogenetic components of the individual plant, and with the aid of accumulated genetic and ecological data, we have added to the evidence that can be used to test hypotheses regarding the evolutionary dynamics of such fine-scale interactions in the generation and maintenance of chemodiversity. Fleshy pericarp and roots tend to be under-studied organs in the generation of specialized metabolite diversity in plants and deserve greater attention in the future. As multi-omics tools and resources develop, future studies will be able to place the intra-organismal metabolomic and ecological patterns described here in the context of the underlying biochemical dynamics of the plants and interacting organisms. Indeed, organ-specific adaptations, such as modifications to metabolic pathways or the allocations of their end products, could facilitate subsequent adaptations in other organs, through shifts in expression for specialized metabolites that are already encoded in the genome. For example, a hypothetical plant species could acquire a novel chemical defence of its roots as a result of a mutation in gene expression for a specialized metabolite that was previously restricted to seeds. Finally, in order to apply these developments in the quantification of chemodiversity towards hypotheses surrounding the organ–organismal dynamics of chemodiversity across ecological and evolutionary scales (Whitehead et al. 2022), it will be crucial to develop and refine methods for quantifying the types and strengths of organ-specific biotic interactions, so that they can be compared across organs. Although ecological interaction data will need to be combined with observed metabolomic patterns in order to test evolutionary hypothesis for organ-level dynamics of metabolomic diversification, studies quantifying organ-specific metabolomic patterns across species and communities are still so few that ongoing exploratory work will be vital in identifying data collection priorities for organ-specific ecological interactions (Whitehead et al. 2022). The selective pressures imposed by biotic interactions are likely a product of both the taxonomic diversity of the interacting organisms and the diversity of their interaction types (e.g., chewing herbivore, seed-borer, necrotrophic pathogen), and there is currently no quantitative framework for integrating these metrics. Thus, by tracing the biochemical and ecological paths of folivory, frugivory, and other such spatially and ontogenetically specific interactions within individual plants, we will gain an integrated perspective of chemodiversity and its dynamics across ecological and evolutionary scales.
AUTHOR CONTRIBUTIONS
GFS designed the study with feedback from NGB. GFS supervised sample collection and conducted phylogenetic and metabolomic analyses. GFS and NGB conducted statistical analyses, wrote and revised the manuscript, and prepared figures. NGB acquired funding.
ACKNOWLEDGEMENTS
We are grateful to the following individuals for their contributions to this project: S.J. Wright for guidance on the taxonomy and ecology of Psychotria in central Panama; A. Billings and M. Ballard for collecting root samples; C. Carlson for collecting and processing samples from Ps. limonensis and Ps. marginata; J. Bryan, M. Hayden, and M. Gaitan for assistance with sample processing; S.R. Whitehead for insightful discussions; T.J. Massad, L.A. McCulloch, N.M. van Dam, C. Müller, and three anonymous reviewers for helpful comments on earlier versions of the manuscript; and the support personnel of the Smithsonian Tropical Research Institute for logistical assistance at Barro Colorado Island. This research was supported by the National Science Foundation grant no. IOS-1953934, including an REU for A. Billings and M. Ballard, National Science Foundation grant no. DEB-2231761, and start-up funds from Utah State University to N.G.B.
Open Research
DATA AVAILABILITY STATEMENT
The mass spectral data presented here are publicly available through GNPS MassIVE (MSV000097096). All other data presented in this manuscript and the R code used for analyses are publicly available through KNB (doi: 10.5063/F16W98J5).




