Chemical, morphological, and genetic characterization of the floral scent and scent-releasing structures of Gynandropsis gynandra (Cleomaceae, Brassicales)
Abstract
- Flowering plants showcase a remarkable diversity in floral fragrances, colours, and structures, which function harmoniously as signals to attract and guide pollinators. Like visual signals, the scents emitted by flowers can be associated with the attraction of specific pollinator classes. As such, divergence in floral scent composition can be a key isolation mechanism for speciation.
- Between continents, the leafy vegetable Gynandropsis gynandra possesses differences in morphology, phenology, foliar chemodiversity, and pollinators. Importantly, G. gynandra is pollinated by hawkmoths in Africa, and bees and butterflies in Asia. Here, we combined chemical, morphological, and transcriptome analyses to assess differences in the floral scent and scent-releasing structures between African and Asian G. gynandra accessions, and within flowers of the same accession.
- The prevalence of nitriles and benzenoids in the floral fragrance of the African and Asian accessions, respectively, corresponds to features typically associated with their differing pollinator classes. Further, we uncovered differences in floral epidermal cell morphology, with papillae present on the petal claws and nectary of the African accession and absent (or reduced) for the Asian accession. Through transcriptomic analyses, we showed that the stalk-like floral structures are putatively involved in terpenoid biosynthesis and emission. However, the epidermal cell morphology and staining suggests that the petals, stamens, and stigma may be involved in scent production of other floral volatile classes (e.g., nitrogen-containing compounds).
- These additional phytochemical and morphological distinctions between African and Asian accessions suggest that the divergent forms of G. gynandra may merit taxonomic recognition at subspecies level.
INTRODUCTION
Most flowering plants exhibit an array of features that act synergistically as signals for pollinator attraction (Dobson 2006; Willmer 2011; Junker & Parachnowitsch 2015). Alongside visual displays, flowers present nearly limitless possibilities of fragrance blends, consisting of different volatile compounds and ratios, which can be learned and recognized by pollinators (Dobson 2006; Dötterl & Gershenzon 2023). The chemodiversity of floral scent is shaped by biotic and abiotic factors, such as pollinator interactions and environmental conditions (Raguso 2008; Farré-Armengol et al. 2020; Keefover-Ring 2022). Consequently, floral volatile profiles tend to vary within genera, as well as in populations and individuals of a single species (Delle-Vedove et al. 2017; Knudsen & Gershenzon 2020). Divergence of floral scent can be an important isolating mechanism for speciation, with each fragrance blend attracting different pollinators (Schiestl & Schlüter 2009). For example, Chess et al. (2008) reported differences in the floral volatile ratios of Linanthus dichotomus (Polemoniaceae) subspecies, which are morphologically similar but differ in geography, flowering time, and pollinators. Similarly, Waelti et al. (2008) showed that differences in the amount of a key floral volatile limit cross-pollination between two hybridizing Silene (Caryophyllaceae) species, with moths favouring one species and bumblebees, flies, and butterflies preferring the other.
Although floral fragrance is often released from the entire flower, its composition and emission may vary across structures (Knudsen & Gershenzon 2020; García et al. 2021; Kantsa et al. 2023). For instance, tissue involved in visual signalling (e.g., petals) tends to emit higher proportions of pollinator-attracting volatiles and can emit unique attractive compounds compared to non-visual signalling tissue (García et al. 2021). Alternatively, floral scent can be exclusively emitted from a particular structure or localized to specific regions of tissue (Effmert et al. 2006; Gonçalves-Souza et al. 2017; Wiemer et al. 2009). Regions of specialized scent-emitting tissue (i.e., osmophores) commonly display distinct cell types (Vogel & Renner 1990; Effmert et al. 2006). Regardless of the secretion location, floral volatiles are predominantly produced in epidermal cells and released into the atmosphere after synthesis (Vogel & Renner 1990; Maoz et al. 2020). Since the highest expression of genes involved in floral volatile biosynthesis occurs in the scent-emitting tissue of the flower, transcriptome analyses can provide insight into the structures involved in olfactory signalling (Pichersky 2023).
There are several chemical classes of floral volatiles that are widely distributed across flowering plants, including aliphatics (i.e., fatty acid derivatives), benzenoids, nitrogen-containing compounds, and terpenoids (Knudsen et al. 2006; Knudsen & Gershenzon 2020; Dötterl & Gershenzon 2023). Terpenoids, the most common class, are derived from the five-carbon building blocks isopentyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), which are products of the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways (Dudareva et al. 2013; Vranová et al. 2013). Terpene synthases catalyse the formation of terpenoids from IPP and DMAPP derivatives, with many capable of forming multiple terpenoids from a single precursor (Tholl 2006; Degenhardt et al. 2009). Although volatile biosynthesis and emission are ubiquitous processes among flowering plants and mediate plant–pollinator interactions, the floral scent of numerous species remain unexplored (Dötterl & Gershenzon 2023).
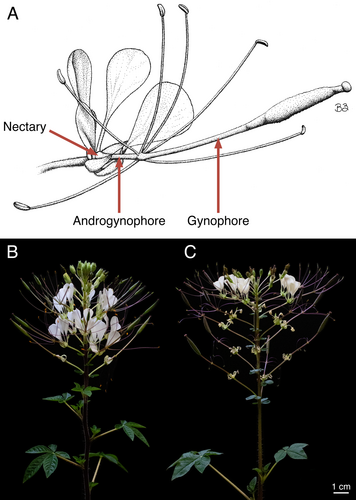
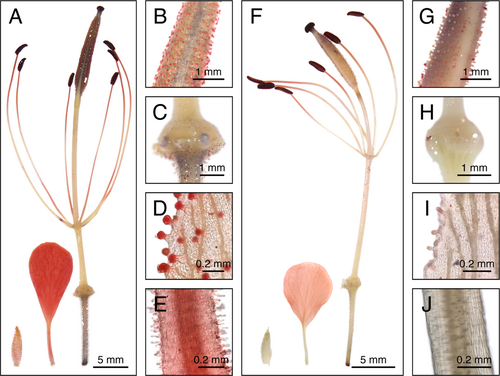
One such species that lacks floral fragrance characterization is Gynandropsis gynandra (L.) Briq. (Cleomaceae), a leafy vegetable native to Africa and Asia (Sogbohossou et al. 2018; Achigan-Dako et al. 2021; Mashamaite et al. 2022). The flowers of G. gynandra are arranged in bracteate racemes and typically have four sepals, four petals, an annular nectary, an elongated androgynophore and gynophore (i.e., stalk-like structures subtending the reproductive organs and pistil, respectively), six stamens, and a bicarpellate pistil (Fig. 1A) (Raju & Rani 2016; Zohoungbogbo et al. 2018; Das et al. 2022; Zenchyzen et al. 2023a, 2023b). Like most Cleomaceae taxa, G. gynandra flowers are monosymmetric (i.e., bilaterally symmetrical) due to an upward curvature of the floral structures (Patchell et al. 2011; Bayat et al. 2018). Monosymmetry is often considered an innovation connected to specialized pollination syndromes (Armbruster 2017; Bayat et al. 2018). Further, the androgynophore and gynophore may play a part in pollination by providing optimal reproductive organ positioning for pollinator contact (Rocha et al. 2015; Zenchyzen et al. 2023a).
With its extensive geographic distribution, G. gynandra exhibits intra- and inter-continental variation in morphology, phenology, and foliar phytochemistry (Blalogoe et al. 2020; Houdegbe et al. 2022; Sogbohossou et al. 2020, 2019; Wu et al. 2018). African accessions are taller with greater biomass and larger seeds, have a lower germination rate, and take longer to reach seed maturation than Asian accessions (Wu et al. 2018; Sogbohossou et al. 2019; Blalogoe et al. 2020; Houdegbe et al. 2022). For some traits, west African accessions are more similar to Asian accessions than southeast African accessions (Sogbohossou et al. 2020, 2019). For instance, the leaves of west African and Asian accessions are lower in carotenoids and chlorophylls, higher in tocopherols, and overall have higher similarity in semi-polar metabolite profiles than southeast African accessions (Sogbohossou et al. 2020, 2019). Geographic differences in the flowers include floral structure length, with west African and Asian accessions having shorter stamen filaments and gynophore than southeast African accessions (Sogbohossou et al. 2019).
In addition to the variation in vegetative and floral traits, the insect floral visitors of G. gynandra differ between continents (Table S1). In Asia (India), visitors include bees (Amegilla cingulata, Apis cerana, Apis florea, Tetragonula iridipennis, and Xylocopa latipes), butterflies (Catopsilia pomona, Danaus chrysippus, Pachliopta aristolochiae, and Papilio polytes), ants (Crematogaster sp.), and flies (unknown species) (Burkill 1916; Chandra et al. 2013; Raju & Rani 2016). Raju & Rani (2016) noted that only bees and butterflies are effective pollinators, while ant and fly species act as nectar robbers. As in Asia, G. gynandra visitors in southeast Africa (Kenya and Tanzania) include bees (Amegilla spp. and Lasioglossum sp.), ants (unknown species), and flies (Syrphidae sp.) (Werth 1942; Oronje 2011). However, Oronje (2011) and Werth (1942) stated that these species are pollen and nectar thieves and do not contribute to pollination. Rather, hawkmoths (Sphingidae) are responsible for G. gynandra pollination in southeast Africa with short-tongued hawkmoth visitors (Basiothia medea, Daphnis nerii, Hippotion celerio, H. eson, H. osiris, Hyles sp., Nephele aequivalens, N. comma, and Temnora sp.) more effective in pollination than long-tongued hawkmoth visitors (Agrius convolvuli, Coelonia sp., and Xanthopan morganii) (Monteiro 1875; Werth 1942; Oronje 2011; Martins & Johnson 2013).
Just as flower morphologies converge to exploit the sensory capabilities and preferences of particular pollinators, there are convergent floral scents associated with the attraction of specific pollinator groups (Dobson 2006; Willmer 2011). Despite these trends and the abovementioned differences in G. gynandra traits and pollinators – which suggest the possibility of distinct, geographically separated subspecies – it is unknown whether G. gynandra populations have unique floral scent profiles. Given that G. gynandra is an important vegetable in some African countries, and food security is a global concern (Onyango et al. 2013; Sogbohossou et al. 2018; Achigan-Dako et al. 2021), understanding its floral features related to pollinator interactions is a vital component for ensuring its reproductive success. Therefore, we characterized and compared the floral scent blends of African (Malawi, southeast Africa) and Asian (Malaysia, Asia) G. gynandra accessions (Fig. 1B,C). To identify possible scent-releasing structures and integrate emitted floral volatiles with biosynthetic pathway expression profiles, we described the floral cell morphology, stained for osmophore tissue, and analysed gene expression patterns across floral organs.
MATERIAL AND METHODS
Plant material
Gynandropsis gynandra accessions TOT8917 from Malawi and TOT7200 from Malaysia (Sogbohossou et al. 2020), collected from multiple inbred plants, were grown from seed in professional growing mix (Sun Gro Horticulture, Agawam, MA, USA). Plants of each accession were grown in a CMP3244 growth chamber (Environmental Growth Chambers, Chagrin Falls, OH, USA) set to a photoperiod of 12 h light/12 h dark, 28°C/22°C. The time from sowing to flowering was ca. 2 months. Voucher specimens were deposited at the University of Alberta Vascular Plant Herbarium (ALTA), with the following accession numbers: 143368 and 143369 (TOT8917), 144831 (TOT7200). Plants were photographed using an EOS Rebel T7i DSLR camera with an EF-S 18–55 mm STM lens (Canon, Tokyo, Japan).
Floral volatile collection
In vivo active sampling (i.e., dynamic headspace technique; Raguso & Pellmyr 1998; Tholl et al. 2006) was used to collect the floral volatiles of G. gynandra African and Asian accessions following Schmidt et al. (2024). For each accession, floral volatiles were collected from the first inflorescence of five plants. The plants were transported from the growth chamber to the sampling apparatus located in a fume hood (Fig. S1). The inflorescence was enclosed within a PYREX 3 L glass beaker (Corning, Corning, NY, USA; modified by Glass Shop, Department of Chemistry, University of Alberta) with a Teflon base containing two gas ports and a retractable piece (Machine Shop, Department of Chemistry, University of Alberta). Once the inflorescence was in position, glass wool was placed around the stem and the retractable piece was inserted into the base to seal the chamber. Prior to entering the chamber, air was purified through a capillary-grade hydrocarbon trap (Restek, Bellefonte, PA, USA) and an activated charcoal trap (Glass Shop). Based on preliminary experiments, floral volatiles were collected for 24 h – to capture the entire suite of compounds released in a day – by pulling air from the chamber through a universal stainless steel thermal desorption tube containing Tenax TA/Carbograph 1TD/Carbosieve SIII (product no. C3-AAXX-5139; Markes International, Bridgend, UK) using a GilAir-3 pump (Sensidyne, St. Petersberg, FL, USA) at a flow rate of 50 mL min−1. The traps, chamber, tube, and pump were connected using Teflon tubing. During sampling, the fume hood lights were turned on between 07:40–08:40 h and off between 17:00–18:40 h for a total 9.25–10.25 h of light. A method blank was collected from the empty chamber under the same conditions. After sampling, thermal desorption tubes were immediately capped with brass caps, wrapped in aluminium foil, and stored at 4°C until analysis. A TC-20 (Markes International) was used to condition and dry-purge thermal desorption tubes between samples.
Floral volatile analyses
Floral volatile samples were analysed via GC/GC-TOF/MS with a 7890A gas chromatograph (GC; Agilent Technologies, Santa Clara, CA, USA) and BenchTOF-Select mass spectrometer (MS; Markes International). Volatile compounds were introduced to the GC by thermal desorption (TD100-xr thermal desorption system; Markes International) at 250°C for 25 min under nitrogen gas with a split flow rate of 10 mL min−1 and inlet in splitless mode. The GC was equipped with a Rxi-5 column (30 m length, 0.25 mm inner diameter, 0.25 μm film thickness; Restek) and a Rtx-17 column (5 m length, 0.25 mm inner diameter, 0.25 μm film thickness; Restek) for the first- and second-dimension separations, respectively. The oven temperature was held at 40°C for 3 min, increased by 8°C min−1 to 280°C, and held at 280°C for 4 min. Helium was used as carrier gas with flow rates of 0.73 mL min−1 for the first dimension and 15.0 mL min−1 for the second dimension, and the modulation period set to 2.2 s. The setup included a purged microfluid splitter (Markes International) dividing flow to the flame ionization detector (FID) and the MS. The ion source and transfer line temperatures were 250°C. Volatiles were ionized by electron impact at 70 eV. Mass spectra were acquired in the range of m/z 40–600 at 100 Hz.
The GC/GC-TOF/MS data were processed using GC Image GC × GC (v. 2022r1; GC Image, Lincoln, NE, USA) with the following parameters: smoothing, 0.1 and 1.0 for the first and second dimensions, respectively; sensitivity level, 3; minimum differentiation interval, 2; minimum area, 50; minimum volume, 40; minimum peak height, 10. An aligned peak table was produced, following comprehensive template matching fingerprinting from Cordero et al. (2010) using GC Image MDC Investigator (v. 2022rl). Compounds present in the blank were removed from the aligned peak table unless upregulated in a sample by a factor of at least 5, in which case the peak area of the blank was subtracted from that of the sample. Raw aligned peak areas were normalized to the total peak area for each sample. Compounds were putatively identified by comparing mass spectra and linear temperature-programmed retention indices (LTPRIs) to those of the NIST/EPA/NIH Mass Spectral Library (NIST 17; v. 2.4). LTPRIs were calculated based on a series of n-alkanes (C7–C28) and traces of hexane found in some of the samples. For terpenoid compounds, mass spectra and retention indices were compared to those of 19 terpenoid standards. The n-alkane standard mix and cannabis terpenes standard #1 (Restek) were diluted in hexane and methanol, respectively, spiked onto a thermal desorption tube, and analysed as described above.
To assess dissimilarity in floral scent profiles between the African and Asian accessions, non-metric multidimensional scaling (NMDS) and permutational multivariate analysis of variance (PERMANOVA) were performed using the ‘metaMDS’ and ‘adonis2’ functions from the ‘vegan’ R package (Dixon 2003) based on Bray-Curtis dissimilarities calculated from a square-root transformed, normalized dataset. To compare floral scent compositions between accessions, we categorized compounds by biosynthetic class, following Knudsen et al. (2006) (except C5 branched-chain compounds, which were grouped with aliphatics) using scripts that were applied to every peak of each chromatogram (Nam et al. 2021). Due to the large number of compounds, biosynthetic classes were only manually determined for peaks of interest. Pie charts were generated from the normalized dataset by averaging the proportion of each compound across replicates then summing the average proportion of compounds from each biosynthetic class. To identify compounds with the largest statistical difference in percentage composition between accessions, we used Metaboanalyst (v. 5.0) (Pang et al. 2021) to conduct hierarchical cluster analysis on the normalized dataset and generate a heatmap.
Identification of potential osmophores
As volatile biosynthesis and emission can spatially vary within a flower, and scent emitting regions tend to display distinct cell types, we used scanning electron microscopy (SEM) to characterize the epidermal cell morphology across the flower of the two accessions. For each accession, anthetic (i.e., open) flowers from three G. gynandra plants were fixed in FAA (formalin-aceto-alcohol; 10% formalin:5% acetic acid:50% ethanol), vacuum infiltrated for 30 min while on ice, and stored at 4°C. These specimens were processed either for SEM or osmophore staining. Specimens processed for SEM were dehydrated in an ethanol series and critical point dried with carbon dioxide using a CPD 030 critical point dryer (Bal-Tec, Liechtenstein). Sepals, petals, nectary, androgynophore, stamens, gynophore, and pistil were dissected from the dried specimens. The floral structures were mounted on SEM stubs with conductive carbon tabs and sputter-coated with gold using a Hummer 6.2 Sputter Coater (Anatech, Sparks, NV, USA). Mounted structures were imaged using a ZEISS Sigma 300 VP or ZEISS EVO 10 SEM (Carl Zeiss, Oberkochen, Germany). Contrast and brightness of the micrographs were adjusted in Photoshop (Adobe, Mountain View, CA, USA).
Traditionally, histological stains have been used to establish presumptive evidence for osmophore tissue in flowers (Vogel 1963; Stern et al. 1986). We fixed and stained the flowers of the two accessions using a combination of Lugol (stains starch dark blue or purple), oil red O (stains lipophilic substances orange-red), and neutral red (stains vacuoles of glandular tissue, such as osmophores and nectaries, red) (Proescher 1927; Johansen 1940; Vogel & Renner 1990; Hernández & Katinas 2019), following a modified version of the protocol described in Hernández & Katinas (2019). After fixation, specimens were rinsed with distilled water and partially rehydrated in 15% ethanol for a minimum of 24 h. As FAA sufficiently removed pigmentation from the specimens, and 5–10% sodium hypochlorite greatly reduced structural integrity of the tissue, the discolouration step was excluded from the original protocol. Partially rehydrated specimens were rinsed with distilled water, stained with Lugol (i.e., iodine/potassium iodide solution; product no. 62650; Sigma-Aldrich, St. Louis, MO, USA) for 10 min, 5 mg mL−1 oil red O (Sigma-Aldrich) in 80% ethanol for 10 min, 5 mg mL−1 neutral red (Thermo Fisher Scientific, Waltham, MA, USA) in distilled water for 10 min, and rinsed with distilled water. Stained specimens were examined under a SMZ1500 stereo microscope (Nikon, Tokyo, Japan) or mounted on cavity slides (Eisco, Victor, NY, USA) with glycerol and examined with an Eclipse 80i light microscope (Nikon). Photographs were taken with a Pixel 5 smartphone (Google, Menlo Park, CA, USA) attached to the stereo- or light microscope with a NexYZ 3-axis universal smartphone adaptor (Celestron, Torrance, CA, USA). Backgrounds were removed from the photographs using the ‘Magic Eraser Tool’ in Photoshop. Staining is not considered an absolute indicator of osmophore tissue; for instance, neutral red can be retained in non-glandular anthers, pollen, stigmas, and necrotic cells, as well as oil-secreting trichomes (Vogel & Hadacek 2004; Hernández & Katinas 2019). Nevertheless, in conjunction with additional analyses, staining can provide valuable insight into floral scent emission.
RNA sequencing of floral structures
Unlike the long-distance transport of many plant hormones, floral volatiles are primarily synthesized in epidermal cells and directly emitted into the atmosphere (Jetter 2006; Lynch et al. 2020). Therefore, to explore the spatial variation in floral volatile biosynthesis and emission, we performed RNA sequencing across floral structures of the African accession. Adaxial and abaxial petals, androgynophore, filaments, and gynophore were excised from anthetic flowers between 14:00 h and 16:00 h, flash frozen in liquid nitrogen, and stored at −80°C. As both floral and vegetative glandular trichomes are suspected to exclusively produce defence compounds (Effmert et al. 2005, 2006), we excluded the floral structures with glandular trichomes (sepals and pistil) from our RNA-seq analyses. Floral structures were pooled from four (androgynophore, filaments, gynophore) or five (adaxial and abaxial petals) flowers on the same plant from a total of four plants (i.e., four biological replicates per floral structure). Pooled structures were manually ground in liquid nitrogen, and RNA was extracted from the ground structures using a RNeasy Micro Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol, with the subsequent modifications: (1) following the initial vortexing, ground structures were incubated in buffer RLT for 5 min to enhance lysis; (2) after adding RNase-free water, the RNeasy MinElute spin column was incubated for 5 min before centrifugation and the resulting eluate was dispensed into the same spin column, and centrifuged again to maximize RNA yield. A NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) and 2100 Bioanalyzer (Agilent Technologies) were used for RNA quantification and qualification. A cDNA library was generated for each sample using a TruSeq Stranded mRNA Library Prep Kit (cat. no. RS-122-2101; Illumina, San Diego, CA, USA) following the manufacturer's low sample protocol with NucleoMag NGS Clean-up and Size Select magnetic beads (Macherey-Nagel, Düren, Germany). Adaxial and abaxial petals and androgynophore libraries were normalized, pooled, and sequenced with a HiSeq 2500 System (Illumina) by The Centre for Applied Genomics (TCAG) at the Hospital for Sick Children (Toronto, Canada). Filaments and gynophore libraries were normalized, pooled, and sequenced with a HiSeq X System (Illumina) by Canada's Michael Smith Genome Sciences Centre at BC Cancer (British Colombia, Canada). Variation related to the change in sequencing platform have been considered and previously investigated (Zenchyzen et al. 2023a); however, error rate is typically larger between samples than between sequencing platforms, and samples that are prepared by the same lab group tend to have consistent error rates (Stoler & Nekrutenko 2021).
Transcriptome assembly and analyses
Raw reads were trimmed with Trim Galore! (v. 0.6.6) and quality checked with FastQC (v. 0.11.9), then assembled de novo using Trinity (v. 2.12.0) (Grabherr et al. 2011). The raw reads are available at the Sequence Read Archive (SRA; BioProject ID PRJNA680567 and PRJNA1030768), and the assembly has been deposited at EMBL Nucleotide Sequence Database, GenBank, and the DNA Data Bank of Japan (accession SUB14081551). To confirm no batch effects were introduced by the sequencing platforms, a Principal Component Analysis (PCA) was performed on transcript counts using the Trinity toolkit ‘PtR’ script. The transcriptome assembly completeness was evaluated using Benchmarking Universal Single Copy Orthologs (BUSCO; v. 5.1.2) with the ‘brassicales_odb10’ dataset (Manni et al. 2021). The transcriptome was annotated using BLASTn (v. 2.13.0) (Altschul et al. 1990) with default parameters and the Araport 11 database (Cheng et al. 2017). Transcripts with the highest bit-score were selected as representatives for the genes of interest. In cases where two transcripts had similar bit-scores and lengths, the transcript with higher TPM (transcripts per million) expression was selected for biological significance. Further, transcripts with expression <5 TPM in at least three replicates for all structures, were excluded. The close evolutionary relationship between Arabidopsis thaliana (Brassicaceae; sister family to Cleomaceae) and G. gynandra allows for relevant comparisons of their genetic networks (Bayat et al. 2018). Since terpenoid biosynthesis is the most thoroughly studied of the floral volatile biosynthetic pathways and is well-characterized in A. thaliana (Vranová et al. 2013; Muhlemann et al. 2014; Lynch et al. 2020), we examined the expression of genes involved in this pathway in detail. A heatmap was generated using the ‘gplots’ R package with representative transcripts for the A. thaliana genes from the Kyoto Encyclopedia of Genes and Genomes (KEGG) terpenoid backbone biosynthesis pathway (ath00900) (Kanehisa et al. 2023) and A. thaliana terpenoid synthase genes summarized in Aubourg et al. (2002) and Tholl & Lee (2011).
RESULTS
Floral scent profiles differ between African and Asian accessions
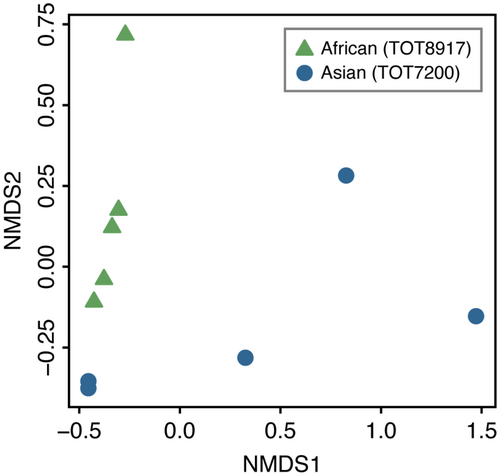
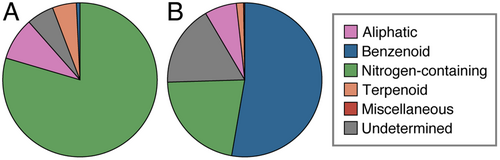
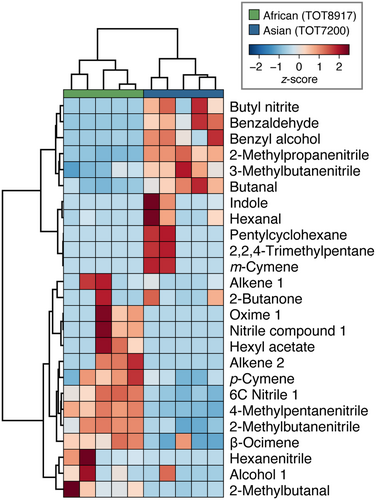
A total of 485 (257 ± 60) and 636 (333 ± 277) compounds were detected in the floral headspace of the African and Asian accessions, respectively, with 480 compounds shared between the two accessions (Table S2). Many of these compounds were present in trace amounts (<0.005% composition). The Asian accession had larger variation in the number of compounds between biological replicates than the African accession, with two replicates containing at least three-times as many compounds as the other three replicates (Fig. 2). The dissimilarity in floral scent profiles between accessions shown in the NMDS ordination plot (stress = 0.02; Fig. 2), was supported by PERMANOVA results, which indicated a significant difference between the African and Asian accessions (F1,8 = 2.63, p < 0.05). The floral fragrance of both accessions consisted of aliphatics, benzenoids, nitrogen-containing compounds, and terpenoids. The average proportions of these chemical classes varied considerably between accessions, with the majority of the floral scent profile comprised of nitrogen-containing compounds for the African accession (79.57%; Fig. 3A) and benzenoids for the Asian accession (52.72%; Fig. 3B). Further, the floral scent of each accession was dominated by two or three compounds (Table 1), the nitrogen-containing compounds 4-methylpentanenitrile (49.40 ± 6.69%), 2-methylbutanenitrile (11.95 ± 3.14%), and a 6-carbon nitrile (11.25 ± 4.01%) for the African accession, and the benzenoids benzaldehyde (28.21 ± 14.63%) and benzyl alcohol (22.91 ± 14.59%) for the Asian accession. These dominant compounds significantly differed in proportion between accessions (Fig. 4).
| biosynthetic class | putative compound | experimental RI | library RI | mean (± SD) % composition | |
|---|---|---|---|---|---|
| African (TOT8917) | Asian (TOT7200) | ||||
| Aliphatic | Butanal | 588 | 593 | 0.17 ± 0.09 | 0.84 ± 0.35 |
| 2-Butanone | 609 | 601 | 0.10 ± 0.19 | 0.12 ± 0.16 | |
| 2-Methylbutanal | 657 | 662 | 4.99 ± 4.16 | 0.56 ± 0.34 | |
| 2,2,4-Trimethylpentane | 676 | 693 | n.d. | trace | |
| Alkene 1 (55, 84, 41, 54, 53, 56, 43, 83) | 746 | N/A | 0.09 ± 0.12 | 0.01 ± 0.02 | |
| Hexanal | 801 | 801 | 0.04 ± 0.04 | 0.38 ± 0.44 | |
| Hexyl acetate | 985 | 1011 | 0.05 ± 0.06 | n.d. | |
| Alkene 2 (67, 41, 82, 43, 55, 54, 42, 57) | 1010 | N/A | 0.15 ± 0.14 | n.d. | |
| Pentylcyclohexane | 1142 | 1135 | n.d. | 0.02 ± 0.03 | |
| Alcohol 1 (95, 81, 67, 119, 41, 55, 82, 138) | 1185 | N/A | 0.05 ± 0.05 | 0.02 ± 0.04 | |
| Aliphatic 1 (55, 41, 43, 59, 58, 57, 56, 42) | 2390 | N/A | 1.13 ± 2.54 | 0.06 ± 0.13 | |
| Benzenoid | Benzaldehyde | 978 | 966 | 0.18 ± 0.14 | 28.21 ± 14.63 |
| Benzyl alcohol | 1063 | 1040 | 0.28 ± 0.59 | 22.91 ± 14.59 | |
| Nitrogen-containing | Butyl nitrite | 566 | 576 | n.d. | 0.16 ± 0.11 |
| 2-Methylpropanenitrile | 628 | 626 | 0.24 ± 0.15 | 3.00 ± 0.46 | |
| Nitrile compound 1 (54, 41, 81, 52, 53, 66, 80, 51) | 701 | N/A | 0.03 ± 0.03 | n.d. | |
| 2-Methylbutanenitrile | 727 | 723 | 11.95 ± 3.14 | 2.48 ± 1.73 | |
| 3-Methylbutanenitrile | 732 | 731 | 2.86 ± 0.91 | 6.01 ± 1.61 | |
| 4-Methylpentanenitrile | 865 | 847 | 49.40 ± 6.69 | 6.37 ± 3.09 | |
| Hexanenitrile | 878 | 874 | 0.48 ± 0.63 | 0.02 ± 0.02 | |
| 6C Nitrile 1 (55, 54, 41, 56, 43, 52, 42, 59) | 881 | N/A | 11.25 ± 4.01 | 1.21 ± 1.20 | |
| 6C Nitrogen-containing compound 1 (43, 41, 55, 54, 59, 42, 56, 68) | 896 | N/A | 1.14 ± 1.65 | 0.01 ± 0.03 | |
| Oxime 1 (57, 41, 59, 55, 42, 54, 43, 56) | 969 | N/A | 0.09 ± 0.09 | n.d. | |
| Indole | 1316 | 1295 | n.d. | 0.56 ± 0.77 | |
| Terpenoid | m-Cymene | 1029 | 1022 | n.d. | 0.01 ± 0.02 |
| p-Cymene | 1033 | 1026 | 0.14 ± 0.09 | 0.04 ± 0.04 | |
| β-Ocimene | 1053 | 1050 | 1.80 ± 0.48 | 0.53 ± 0.91 | |
| Geraniol | 1266 | 1290 | 2.35 ± 5.00 | 0.02 ± 0.03 | |
- Note: For compounds assigned to a putative class, the eight most abundant mass fragments (m/z) are listed in descending order.
- Abbreviations: n.d., not detected; RI, retention index.
Epidermal cell morphology is diverse across floral structures and differs between accessions
Epidermal cell morphology varied drastically among floral structures and within the sepals, petals, and pistil, with notable differences between the two accessions (Figs. S2 and S3). The following descriptions apply to both accessions, except when stated otherwise. The epidermal cells of the sepals primarily consisted of elongate jigsaw-shaped cells (Fig. 5A,C). Scattered among these cells on the abaxial surface and near the margin of the sepal were stomata and multicellular capitate-stalked glandular trichomes (hereafter referred to as glandular trichomes) (Fig. 5A). The Asian accession had shorter, less abundant glandular trichomes than the African accession (Fig. 5C). The epidermal cells of the petal blades were jigsaw-shaped with disordered surface striations (Fig. 5B). On the adaxial surface near the middle of the petal blade, gaps between the jigsaw-shaped cells formed circular cavities, which were less abundant and prominent on the abaxial surface and for the Asian accession (Fig. 5D,F). Near the apex of the petal claw, the epidermal cells were elongate with linear surface striations (Fig. 5E). At the midsection of the petal claw, the elongate epidermal cells had smooth surfaces, and for the African accession, some had spherical papillae (Fig. 5G). For the African accession, the epidermal cells near the base of the petal claw and throughout the receptacular nectary had extended papillae (Fig. 5H,J,K) (Zenchyzen et al. 2023b). The Asian accession had shorter and less abundant papillae on the petal claw and entirely lacked papillae on the nectary (Fig. 5I,L). Stomata modified for nectar secretion (i.e., nectarostomata) were mainly scattered throughout the apical half of the nectary (Fig. 5K,L) (Zenchyzen et al. 2023b).
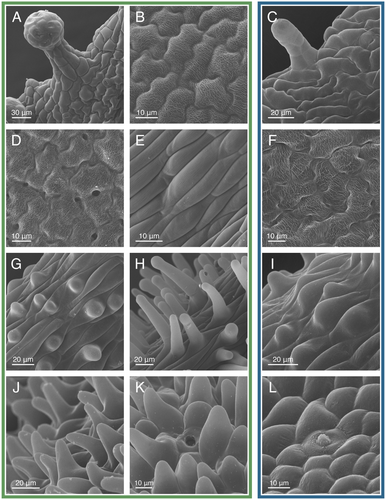
Gynandropsis gynandra flowers have three stalk-like structures (Fig. 1A): an androgynophore (Fig. 6A–C), stamen filaments (Fig. 6D–F), and a gynophore (Fig. 6H). All three structures had elongated epidermal cells. For the African accession, the elongated cells near the base of the androgynophore and filaments were inter-woven (i.e., overlapping with adjacent cells; Fig. 6A,D) (Zenchyzen et al. 2023a). Inter-woven cells were absent from the Asian accession (Fig. 6C,F). Stomata were scattered throughout the elongated cells of the gynophore, but were rare on the androgynophore and filaments (Fig. 6H). The filaments were the only stalk-like structures with linear surface striations (Fig. 6E). These striations were absent from the proximal section of the filaments (Fig. 6D,F). At the junction where the stalk-like structures diverge, epidermal cells were circular to oblong, and for the African accession, some were bulbous or had papillae similar to the nectary (Fig. 6G,I). Stomata were located throughout this junction.
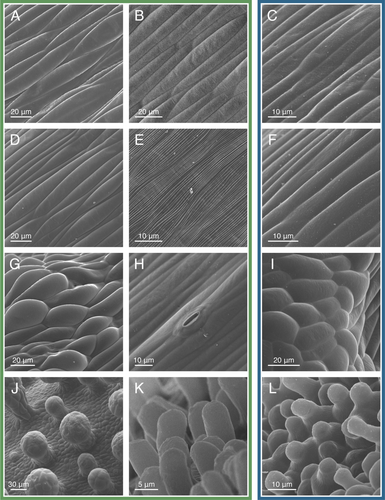
The filaments and gynophore subtended the anthers and pistil, respectively. The anthers had an assortment of cell shapes, all having disordered surface striations (Figs. S2, S3). Stomata were located between the two thecae. The ovary had square to rectangular epidermal cells with stomata scattered throughout (Fig. 6J). Glandular trichomes were located on the valves of the ovary but not the replum. Epidermal cells of the stigma were papillate, with shorter papillae for the Asian accession (Fig. 6K,L). In summary, cell morphology varied from circular or rectangular to more complex jigsaw shapes (sepals and petals), unicellular papillae (petals, nectary, and stigma for the African accession; petals and stigma for the Asian accession), glandular trichomes (sepals and ovary), and stomata (nectary, sepals, gynophore, and ovary) (Figs. S2 and S3; Table S4). Cell surfaces ranged from smooth to adorned with intricate striations (petals, filaments, and anthers). The major difference between the two accessions was shorter and fewer papillae for flowers of the Asian accession.
Petals, stamens, and stigma are potential scent-releasing structures
Several floral structures stained red, indicating possible osmophore tissue. For flowers of the African accession, the petals, glandular trichomes on the sepals and ovary, filaments, anthers, and stigma stained red (Fig. 7A–E; Table S4). Among features with papillae (petals, nectary, and stigma), the petals (both the blade and claw) and stigma stained red (Fig. 7A,C,E). Of the stalk-like floral structures, only the filaments stained red (Fig. 7A). The staining was not uniform along the length of the filament, rather accumulated at the apical section (below the anthers) and at the distal section (at or near the junction of the stalk-like structures), giving a ‘striped’ appearance. The anthers and stigma stained dark red. Dark staining of starches was apparent in the pistil and pedicel; starches are used as a carbon and energy source for volatile biosynthesis (Effmert et al. 2006; Hernández & Katinas 2019). In comparison to the African accession, flowers of the Asian accession retained less stain overall (Fig. 7F–J; Table S4). The staining pattern of the Asian accession differed from that of the African accession: petal claws were not stained (Fig. 7F,J); glandular trichomes were not uniformly stained (Fig. 7G,I), filaments were only stained at the apical section (Fig. 7F), and the gynophore and apical half of the androgynophore were faintly stained (Fig. 7F).
Terpenoid biosynthesis pathway gene expression is highest in the stalk-like floral structures
The transcriptome of the floral structures was of high quality. The read depth, averaged across replicates, ranged from 18.6 million (filaments) to 35.4 million (abaxial petals). A minimum of 10 million reads is sufficient for differential expression analyses (Liu et al. 2014), and optimal statistical power is typically achieved at a read depth of 20 million with a minimum of four biological replicates (Ching et al. 2014; Lamarre et al. 2018). Across all structures, median Phred quality scores were not below 32 for each base position; a Phred quality score of 30 represents a base call accuracy of 99.9% (Shi et al. 2016). The assembled transcriptome had a total of 221,985 transcripts at 93.6% completeness (Tables S5 and S6). This percentage represents the number of conserved single-copy and duplicated Brassicales genes present in our assembly. In the PCA of transcript reads, PC1 and PC2 explained 46.28% and 12.69% of the variance, respectively (Fig. S4). Replicates clustered by floral structure with little variance between adaxial and abaxial petals; although the petals differ in position within the flower, they are morphologically identical (i.e., uniform colour, size, and shape).
The MVA and MEP pathways, which are conserved across flowering plants, produce the essential building blocks for terpenoids (Tholl & Lee 2011; Vranová et al. 2013; Lynch et al. 2020). The MVA pathway results in IPP alone, whereas the MEP pathway yields a 6:1 ratio of IPP and DMAPP; both of which are required for terpenoid synthesis (Tholl & Lee 2011; Lynch et al. 2020). In the petals and stalk-like floral structures of G. gynandra, most genes involved in the MVA and MEP pathways were expressed, apart from GgAACT2 and GgPMK in petals (MVA pathway) and GgMCT and GgCMK in petals and filaments (MEP pathway), which had <5 TPM (averaged between replicates; Fig. 8; Tables S4 and S8). Of note, A. thaliana has two functionally redundant MDD and DXS genes (Vranová et al. 2013; de Luna-Valz et al. 2021), while only one putative copy of each was expressed in G. gynandra petals and stalk-like structures.
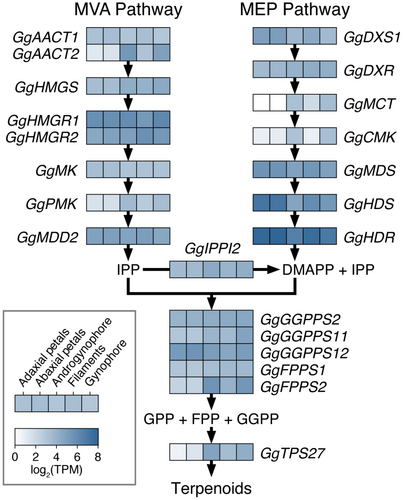
The IPP isomerases balance the equilibrium of IPP and DMAPP by converting IPP to DMAPP (Tholl & Lee 2011; Vranová et al. 2013). In A. thaliana, there are two IPP isomerases, IPPI1 and IPPI2; however, these are only partially redundant as IPPI2 is also involved in perianth development (Phillips et al. 2008; Tholl & Lee 2011). In the petals and stalk-like structures of G. gynandra, only GgIPPI2 was expressed (Fig. 8). Geranyl diphosphate (GPP), farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP) synthases catalyse the formation of GPP, FPP, and GGPP using DMAPP and IPP (Tholl & Lee 2011; Vranová et al. 2013). GPP is a precursor for monoterpenes, while FPP and GGPP are used for the synthesis of larger molecules, including terpenoids (diterpenes, triterpenes, and sesquiterpenes), plant hormones (abscisic acid, brassinosteroids, and gibberellins), and pigments (carotenoids and chlorophyll) (Tholl & Lee 2011; Vranová et al. 2013). Seven monoterpenes and one sesquiterpene were detected in the floral headspace of G. gynandra, although pigments and hormones are presumably also present in the sequenced floral structures. In A. thaliana, a heterodimer of GGPP11 and GGPP12 is the only enzyme presumed to synthesize GPP (Wang & Dixon 2009; Vranová et al. 2013). Three GGPP synthase genes, including GgGGPP11 and GgGGPP12, and two FPP synthase genes were expressed in G. gynandra, however, GgFPPS2 expression was <5 TPM in the petals (Fig. 8).
Functional investigations have been conducted on approximately 12 out of the 30 terpene synthase genes (TPS1-30) in A. thaliana (Aubourg et al. 2002; Tholl & Lee 2011). While these studies identified AtTPS03 as an (E)-β-ocimene synthase (Fäldt et al. 2003), none of the examined genes have been shown to primarily synthesize geraniol (Tholl & Lee 2011). In G. gynandra, only one terpene synthase orthologue (GgTPS27) was expressed in the stalk-like structures and none were expressed in the petals (Fig. 8); the (E)-β-ocimene synthase TPS03 was not expressed in any of the examined floral structures. In A. thaliana, the protein encoded by AtTPS27 converts GPP into ten monoterpenes, with 1,8-cineole being the major product (52%) (Chen et al. 2004). Although 1,8-cineole was not identified in the floral headspace of G. gynandra, several minor products of AtTPS27 were present (i.e., limonene, myrcene, (E)-β-ocimene, α-pinene, and β-pinene; Table S7). As AtTPS27 is not known to synthesize all seven terpenoids present in the floral scent profile of G. gynandra (Chen et al. 2004), these results imply that TPS27 synthesizes different products in G. gynandra compared to A. thaliana. Alternatively, G. gynandra may have terpene synthases that are either absent or not characterized in A. thaliana.
DISCUSSION
Our analysis of the floral fragrance of G. gynandra provides evidence for its putative role in the ecology and evolution of distinct pollination systems across continents, as the divergent floral scent profiles of the African and Asian accessions likely influence attraction of different pollinator classes. Hawkmoths were described as effective pollinators of G. gynandra in Africa (Table S1) (Monteiro 1875; Werth 1942; Oronje 2011; Martins & Johnson 2013). Hawkmoth-pollinated flowers typically emit a strong, sweet odour consisting of benzenoid esters, nitrogen-containing compounds, and oxygenated terpenoids (Dobson 2006; Willmer 2011; Stöckl & Kelber 2019). However, non-oxygenated terpenoids, such as (E)-β-ocimene, myrcene, and limonene, are also common (Knudsen & Tollsten 1993). The floral scent profile of the G. gynandra African accession is dominated by nitrogen-containing compounds and includes four nitriles, the oxygenated terpenoid geraniol, and the non-oxygenated terpenoid β-ocimene as major components (>1% composition; Fig. 3A; Table 1). As such, the floral fragrance of the African accession is comparable to that of other hawkmoth-pollinated taxa. Although nitrogen-containing oximes are emitted by many hawkmoth-pollinated flowers (Nielsen & Møller 2015; Cisternas-Fuentes et al. 2022; Eisen et al. 2022; Kantsa et al. 2023), we only tentatively identified one oxime in the floral fragrance of the African accession (Table 1).
In Asia, generalist pollination of G. gynandra was reported, with bees and butterflies as effective pollinators (Table S1) (Burkill 1916; Chandra et al. 2013; Raju & Rani 2016). Generalist flowering plants do not have unifying patterns of floral fragrance, except that they typically consist of the three major floral scent chemical classes (i.e., aliphatics, benzenoids, and terpenoids) and usually have one chemical class that dominates the other two (Dobson 2006; Willmer 2011). The floral scent profiles of bee-pollinated species are often dominated by benzenoids or terpenoids, and benzenoids, including benzaldehyde and benzyl alcohol, are common and abundant in the floral fragrance of butterfly-pollinated taxa (Andersson et al. 2002; Dobson 2006). Further, nitrogen-containing compounds may be secreted in low quantities by butterfly-pollinated flowers (Andersson et al. 2002; Dobson 2006). With benzenoids dominating and benzaldehyde and benzyl alcohol as major components (Fig. 3B; Table 1), the floral scent profile of the Asian G. gynandra accession shares features with bee- and butterfly-pollinated flowering plants. However, the proportion of nitrogen-containing compounds is greater than that of typical bee- and butterfly-pollinated flowers (21.8%; Fig. 3B; Andersson et al. 2002). Nitrogen-containing compounds, such as oxime, nitrile, and nitro compounds, are degradation products and/or biosynthetic precursors of glucosinolates, i.e., defence compounds that are ubiquitous across the Brassicales (including Cleomaceae) (Halkier & Gershenzon 2006; Edger et al. 2015; Bayat et al. 2018). This distinction from typical bee- and butterfly-pollinated taxa may reflect an ongoing evolutionary shift from hawkmoth pollination to bee and butterfly pollination for the Asian accession. However, the ancestral pollination system of G. gynandra has yet to be elucidated.
Of note, some of the volatile compounds from the floral headspace samples may have been emitted by the small leaf-like bracts along the axis of the inflorescence or from the fruit. For instance, several compounds are present in both the foliar and floral scent profiles of the African and Asian accessions (i.e., benzaldehyde, acetophenone, 3-methylbutanal, hexanal, methyl isothiocyanate, methyl thiocyanate) (see Sogbohossou et al. (2020) for G. gynandra foliar volatiles; Table S3). However, even if scent-releasing, these structures would likely contribute to the overall fragrance detected by pollinators due to their proximity to the flowers. Additionally, the role of floral scent is more complex than simply attracting pollinators, as flowers experience selection pressure from both pollinators and florivores (i.e., flower herbivores) (Sasidharan et al. 2023). The floral fragrance of G. gynandra presumably influences florivore behaviour, although studies involving florivore responses to floral volatiles are limited and warrant further investigation (Sasidharan et al. 2023).
Our morphological and transcriptome analyses indicate that the stalk-like floral structures of G. gynandra are involved in terpenoid biosynthesis, while the petals, stamens, and stigma may contribute to biosynthesis and emission of other volatile compounds. Glandular trichomes, like those on the abaxial surface of sepals and valves of the ovary of G. gynandra flowers, contain cells that synthesize and store specialized metabolites (Pichersky 2020; Schuurink & Tissier 2020). These specialized metabolites, which can include volatile compounds, presumably act as chemical weapons against herbivory or pathogens, rather than pollinator attractants (Muhlemann et al. 2014; Pichersky 2020; Schuurink & Tissier 2020). With the sepals and valves of the pistil safeguarding the developing flower and seeds, respectively (Willmer 2011), the presence of glandular trichomes on these structures provides an extra layer of protection. Further, the red staining of the glandular trichomes on the sepals and ovary is consistent with these structures housing specialized metabolites (Hernández & Katinas 2019). Unlike pollinator-attracting floral volatiles that are primarily emitted into the atmosphere after synthesis, glandular trichomes often hold and only release the specialized metabolites upon damage (e.g., florivory) (Effmert et al. 2006; Pichersky 2020; Schuurink & Tissier 2020). In contrast, epidermal tissue involved in pollinator-attracting floral volatile production and emission typically possess unique cell shapes (e.g., conical, papillate) (Effmert et al. 2006). Papillae are commonly abundant on the surface of osmophoric regions (Endress 1984; Vogel & Renner 1990; Vogel & Hadacek 2004; Gonçalves-Souza et al. 2017; Kettler et al. 2019; Gotelli et al. 2020), and Gonçalves-Souza et al. (2017) suggested that papillae might serve as an indicator of osmophores. In the African accession of G. gynandra flowers, papillae are located on the surface of the petal claws, nectary, stigma, and occasionally, the junction of the androgynophore, filaments and gynophore. Of these features, only the petals and stigma stained positively for osmophore tissue.
Floral volatiles are likely released directly from the osmophore cells via a combination of active transport and passive diffusion through the cell membrane, cell wall, and cuticle (Effmert et al. 2006; Widhalm et al. 2015; Dötterl & Gershenzon 2023). However, modified stomata may also be involved in floral scent emission (Maiti & Mitra 2017). For example, in Agave amica (Asparagaceae), the petaloid tepals serve as the primary source of floral fragrance; stomata are predominately present on the adaxial surface of the petaloid tepals and respond positively to histological tests associated with scent emission (Maiti & Mitra 2017). In G. gynandra, stomata are located on the abaxial surface of the sepals, apical half of the nectary, gynophore, anthers, and ovary. Further, there is no evidence that surface striations, such as those located on the epidermal cells of the petals, filaments, and anthers of G. gynandra flowers, are involved in scent emission (Jetter 2006). Rather, these nanostructures seemingly contribute to the suite of visual cues for pollinators via structural colouration (Moyroud et al. 2017). Cells with disordered ridges scatter blue and ultraviolet radiation, producing a blue halo that is visible to insects (Moyroud et al. 2017).
Although the petals of the G. gynandra African accession contain papillae, which are commonly associated with osmophores, and stained positively for possible scent-releasing tissue (Table S4) (Gonçalves-Souza et al. 2017), low expression (<5 TPM) of GgPMK in the MVA pathway and GgMCT and GgCMK in the MEP pathway, and the absence of terpene synthase gene expression suggest that the petals are not predominately involved in terpenoid production and emission. However, volatile compounds and compositions can differ among floral structures (Effmert et al. 2006; Willmer 2011), i.e., floral structures that are not involved in terpenoid emission may release other volatiles (e.g., nitrogen-containing compounds). Gynandropsis gynandra filaments have <5 TPM expression of GgCMK from the MEP pathway, yet with the expression of genes throughout the MVA pathway and GgIPPI2, some of the IPP produced could be converted to DMAPP, providing the required precursors for terpenoid synthesis. Although the androgynophore and gynophore did not stain positively for osmophore tissue, gene expression throughout the MVA and MEP pathways indicate that these structures may also be involved in terpenoid synthesis and emission. The stalk-like structures may not act alone in terpenoid emission, since floral rewards, such as nectar and pollen, can also emit odours (Dobson & Bergström 2000; Raguso 2004). Future chemical studies on the intrafloral distribution of fragrance emission could assist in confirming the major sources of floral scent, and whether the emitted compounds differ between floral parts. For instance, Kantsa et al. (2023) collected and analysed the headspace of detached floral structures and nectar of Capparis spinosa (Capparaceae; sister family to Cleomaceae and Brassicaceae), and found that the petals and anther-bearing stamens have the greatest floral scent emission, and that petals emit the highest number of unique compounds.
Hawkmoth-pollinated flowers commonly have white or pale tubular or spurred petals with abundant nectar at their base, and a strong fragrance (Willmer 2011; Stöckl & Kelber 2019). Martins & Johnson (2013) noted that G. gynandra differs from other hawkmoth-pollinated flowers in Africa in that it has an open flower (i.e., petals are not tubular or spurred) and a weak fragrance. Although the floral phenotype of G. gynandra alone is atypical for hawkmoth-pollinated flowers, our study highlights the importance of examining the array of floral features, including those not visible to the human eye. As Kantsa et al. (2023) stated, important aspects of plant–pollinator interactions can be overlooked with strict adherence to floral phenotypes (i.e., syndromes) for predicting pollination system. Here, we reveal drastically different floral scent profiles between geographically distinct G. gynandra accessions, with the floral fragrance of the African and Asian accessions sharing aspects with floral scent profiles of other hawkmoth- and bee-/butterfly-pollinated flowers, respectively. Although the fragrance is faint to the human nose, these data suggest floral scent plays an important role in pollinator attraction for G. gynandra. In addition, we reveal discrepancies in epidermal cell morphology between African and Asian accessions (e.g., presence/absence of petal claw and nectary papillae; Table S4). The unique floral scent profiles, combined with differences in morphology, phenology, foliar phytochemistry, and pollinators (Blalogoe et al. 2020; Houdegbe et al. 2022; Sogbohossou et al. 2020, 2019; Wu et al. 2018), suggest that the divergent forms of G. gynandra may deserve taxonomic recognition as distinct subspecies – a potential taxonomic revision that requires broader geographic sampling (i.e., comparison of more individuals across a wider range of accessions).
Author Contributions
BZ and JCH conceived the idea and designed the study, with JCH overseeing the study. BZ and KM grew the plants. SAS optimized and collected floral scent data under the guidance of APM and JJH. SAS and BZ analysed the floral scent data. BZ and KM prepared specimens for SEM and captured the micrographs. BZ prepared and stained specimens for putative identification of osmophore tissue. BZ and KM analysed the morphological data. SC and BZ processed samples for sequencing and performed transcriptome assembly and analyses. BZ captured the photographs and prepared the figs. BZ wrote the manuscript with input from all co-authors.
Acknowledgements
The authors express their gratitude to the following individuals and facilities for their invaluable contributions to this research: M. Eric Schranz for generously supplying G. gynandra seeds; Bryce Thomas (Glass Shop, Department of Chemistry, University of Alberta) and Dirk Kelm (Machine Shop, Department of Chemistry, University of Alberta) for their role in constructing the floral volatile sampling apparatus; Nathan Gerein (Scanning Electron Microscopy Laboratory, Department of Earth and Atmospheric Sciences, University of Alberta) and Kacie Norton (Advanced Microscopy Facility, Department of Biological Sciences, University of Alberta) for their support with SEM; Troy Locke (Molecular Biology Service Unit, Department of Biological Sciences, University of Alberta) and Matthew Gerun for assistance with genetic sample quality control; Ewenet Mesfin, Gwen Nguyen, and Soein Wang for help setting up and cleaning the floral volatile sampling apparatus; Michael Barteski and Ryan Lewis for assistance with chemical purchasing and handling; and Maya L. Evenden for sharing her insight on volatile sampling and analysis. Insects in the graphical abstract are adapted from Johnson et al. (2017) and photographs by Vengolis and Gunjan Vasant Bonde. This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) [funding reference no. 5014131] and Canada Foundation for Innovation (CFI) through The Metabolomics Innovation Centre (TMIC).




