Eco-evolutionary factors contribute to chemodiversity in aboveground and belowground cucurbit herbivore-induced plant volatiles
Abstract
- When attacked by insect herbivores, plants emit blends of chemical compounds known as herbivore-induced plant volatiles (HIPVs). Although HIPVs are produced both aboveground and belowground, how HIPVs vary across plant tissues remains unresolved, as do the selective forces shaping interspecific HIPV emission patterns. Here, we compared foliar and root HIPVs within and among closely related plant species and evaluated if different eco-evolutionary forces, including plant domestication, coexistence histories with herbivores, or phylogenetic relatedness, explain HIPV blends.
- To examine aboveground and belowground patterns in HIPVs, we compared leaf and root volatile profiles for six species in the Cucurbitaceae that differed in domestication status and coexistence history with specialist insect herbivores. We predicted that within-species HIPVs from different tissues would be more similar than HIPV blends among different species, and that plant volatile chemodiversity was reduced by domestication and enhanced by coexistence histories with herbivores.
- We found that herbivory induced both quantitative and qualitative changes in volatile emissions across all plant species, which were more pronounced aboveground than belowground. Each species produced tissue-specific HIPVs, and foliar and root HIPVs differed among species. Contrary to our predictions, plant domestication enhanced foliar volatile diversity, while coexistence histories with herbivores reduced foliar and root volatile diversity. Additionally, phylogenetic relatedness did not correlate with aboveground or belowground volatiles.
- Overall, this work furthers our understanding of the eco-evolutionary forces driving patterns in aboveground and belowground HIPV emissions, elucidating an important and previously undescribed component of within-plant variation in chemodiversity.
INTRODUCTION
A major goal in the field of chemical ecology is to understand the ecological and evolutionary drivers of plant chemodiversity (Wetzel & Whitehead 2020; Thon et al. 2024). Under normal physiological conditions, plants constitutively produce an enormous diversity of specialized metabolites that influence surrounding ecological communities (Kessler 2015). Specialized metabolites are often differentially produced, translocated, and stored across plant tissues, leading to within-plant spatial variation in phytochemistry (Kaplan et al. 2008a; Tissier et al. 2014; Tsunoda et al. 2017). In addition to within-plant phytochemical variation, chemodiversity is also found among different plant species, and insect herbivory is often implicated as a strong selective force on the generation and maintenance of plant chemodiversity (Uesugi & Kessler 2013; Poelman & Kessler 2016; Kalske et al. 2019). Specialized metabolites produced constitutively reduce herbivore attack, but plants can also use an inducible defence strategy to increase specialized metabolites, both quantitatively and qualitatively, to enhance antiherbivore defence (Karban 2020). This includes emitting herbivore-induced plant volatiles (HIPVs), which provide direct protection from subsequent herbivores or indirectly defend plants by recruiting natural enemies (De Moraes et al. 2001; Baldwin 2010; Turlings & Erb 2018). HIPV emissions can vary depending on the attacking herbivore species (Timilsena et al. 2020) or feeding guild (Davidson-Lowe & Ali 2021) and across plant species (McCormick et al. 2012), genotypes (Keskitalo et al. 2001; Grof-Tisza et al. 2021; Russavage et al. 2024) or the attacked plant tissue (Sun et al. 2022). For instance, while leaves and roots both emit ecologically important HIPVs, their volatile profiles and temporal patterns of emission can be different (Danner et al. 2015). In contrast to our understanding of other specialized metabolites, interspecific emission patterns in HIPVs among closely related species are not well understood (Pearse et al. 2013; Courtois et al. 2016), and whether these patterns differ between leaves and roots remains an open question. Here, we examine how foliar and root HIPVs differ within and among closely related plant species, and test the relevance of different evolutionary drivers, including plant domestication and coexistence histories with herbivores, in shaping patterns of HIPV emissions.
Eco-evolutionary factors influencing plant chemodiversity can be human-mediated, including plant domestication and the introduction of plants to novel geographic regions. Agricultural crop domestication involves selection for traits of agroeconomic value, such as increased plant growth or yield (Fuller et al. 2023), which can inadvertently alter other plant traits relative to wild ancestors, including HIPV emissions (Rowen & Kaplan 2016; Whitehead et al. 2017). For instance, roots of European maize lines and their wild relatives produce a key HIPV – (E)-β-caryophyllene – that is attractive to root-feeding herbivores and their associated natural enemies (Rasmann et al. 2005; Robert et al. 2012), but North American varieties lost the ability to produce (E)-β-caryophyllene during plant domestication (Köllner et al. 2008). Emerging evidence has revealed how domestication altered HIPVs of tomato (Paudel et al. 2019; Lee Díaz et al. 2022), blueberry (Urbaneja-Bernat et al. 2021), and cranberry (Rodriguez-Saona et al. 2011b) relative to wild ancestors, but a multitude of other crops remain underexplored. Moreover, a history of coexistence with an herbivore is predicted to optimize plant defence over time (Desurmont et al. 2011). When plants are introduced to novel geographic regions, coexistence histories are often disrupted as plants encounter new herbivores (Thompson et al. 2022b). Little is known about the importance of such coexistence histories in HIPVs, although recent findings point towards emission of unique HIPVs when challenged by unfamiliar herbivores (Danner et al. 2018), as well as plants losing the ability to produce HIPVs over evolutionary time in novel geographic regions (Lin et al. 2021a). Therefore, a deeper understanding of how plant domestication and coexistence histories with herbivores alter HIPV emissions and patterns among species is needed.
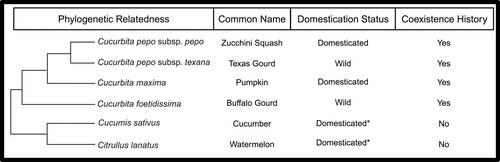
Plants in the gourd family (Cucurbitaceae) encompass wild and domesticated species that interact with diverse insect herbivores both aboveground and belowground. Plants in the genus Cucurbita are native to North America, which includes wild species like buffalo gourds (Cucurbita foetidissima) and Texas gourds (Cucurbita pepo subsp. texana), as well as domesticated species such as pumpkins (Cucurbita maxima) and zucchini squash (Cucurbita pepo subsp. pepo) (Chomicki et al. 2020). In contrast, cucumbers (Cucumis sativus) and watermelons (Citrullus lanatus) were domesticated outside of North America (Chomicki et al. 2020), although both are grown as agricultural crops throughout the world. Cucurbit plants grown in North America, including native and non-native species, are attacked by specialist insect herbivores, such as striped cucumber beetles (Acalymma vittatum) and squash bugs (Anasa tristis). These herbivores have coevolved with cucurbits and are adapted to the cucurbit defensive metabolites, cucurbitacins, which are toxic and bitter non-volatile triterpenoids (Metcalf 1986). For most animals, cucurbitacins are highly distasteful, repellent, or toxic (Shapiro & Mauck 2018), but for specialist herbivores, cucurbitacins are phagostimulants and increase herbivore feeding (Deheer & Tallamy 1991). Intriguingly, cucurbit plant domestication selected for fruits with lower cucurbitacin content (Chomicki et al. 2020), which appears to have inadvertently reduced or eliminated cucurbitacins in the leaves, roots, and flowers of most domesticated species or cultivars (Theis et al. 2014; Brzozowski et al. 2019; Ye et al. 2024). In essence, cucurbitacins are not present in many domesticated species and, even if present, cucurbitacins do not deter specialist herbivores, indicating plants must rely on other defensive strategies to combat these herbivores.
To defend against specialist herbivores, cucurbits appear to rely on HIPVs. Mounting evidence shows HIPVs mediate a diversity of ecological interactions in cucurbit systems, including herbivore host plant selection (Brzozowski et al. 2020; Thompson et al. 2022a), plant communication (Marmolejo et al. 2021; Thompson et al. 2024), and natural enemy recruitment (Agrawal et al. 2002; Kappers et al. 2011; Grunseich et al. 2020). Evidence from prior research also shows that specialist herbivores and their associated natural enemies exhibit strong preferences for different cucurbit species or cultivars (Brzozowski et al. 2016; Cornelius 2018; Cornelius et al. 2022), indicating HIPVs likely play a role in the foraging behaviours of herbivores and natural enemies. Although cucurbit foliar HIPVs have received the most attention, cucurbit roots also produce HIPVs for direct and indirect defence, repelling cucumber beetle larvae and attracting natural enemies (Grunseich et al. 2020). However, our understanding of HIPVs produced by different cucurbit species is currently lacking, and whether HIPVs are tissue-specific remains unknown for cucurbits, as well as the eco-evolutionary factors driving patterns among species.
The overall goal of this study was to characterize tissue-specific HIPVs within and among cucurbit plant species and to examine the possible role of eco-evolutionary factors in shaping chemodiversity aboveground and belowground. We predicted foliar and root HIPVs of cucurbit plants would contain similar compounds within species – as maize plants emit some of the same terpenes, including (E)-β-caryophyllene, from herbivore-damaged leaves and roots (Köllner et al. 2004; Rasmann & Turlings 2008; Bernal et al. 2023) and Brassica plants emit sulfur-containing volatiles aboveground and belowground (van Dam et al. 2012; Danner et al. 2015). We expected to find larger differences in HIPVs among species, correlated with phylogenetic relatedness. We also expected to find signatures of both domestication and coexistence history in HIPV blends, hypothesizing domestication would reduce volatile chemodiversity while coexistence history would enhance HIPVs due to extensive interactions between plants and herbivores over evolutionary time. To test these predictions, we selected six cucurbit species that differed in their domestication status and coexistence histories with specialist insect herbivores (Fig. 1). For each of the cucurbit species, we collected and characterized foliar volatiles from non-damaged controls and plants damaged by squash bugs (A. tristis). In separate experiments for each plant species, we collected and characterized root volatiles from non-damaged controls and plants damaged by striped cucumber beetle (A. vittatum) larvae. We assessed herbivory-induced quantitative and qualitative changes in volatile emissions both aboveground and belowground, and we compared foliar and root HIPVs within and across cucurbit species. Overall, this work advances our understanding of the diversity of HIPVs produced by specific plant tissues aboveground and belowground and also sheds light on the eco-evolutionary forces shaping volatile chemodiversity among plant species.
MATERIAL AND METHODS
Plants
For each cucurbit species, separate cohorts of plants were grown for foliar or root volatile collection experiments (detailed below). For all experiments, plants were grown from seed and seeds were obtained from the following sources: zucchini squash (Cucurbita pepo subsp. pepo cv. Raven) and cucumber (Cucumis sativus cv. Diva) – Johnny's Selected Seeds, Fairfield, ME, USA; watermelon (Citrullus lanatus cv. Sugar Baby) – Burpee, Warminster, PA, USA; pumpkin (Cucurbita maxima cv. Big Max) – Ferry-Morse, Norton, MA, USA; buffalo gourd (Cucurbita foetidissima) – fruits collected in Hillsboro, TX, USA; Texas gourd (Cucurbita pepo subsp. texana) – collaborators at Pennsylvania State University in State College, PA, USA. Before use in each volatile collection experiment, plants were kept under the same environmental conditions in an insect-free room with accessory lighting (16 h light:8 h dark; 29 °C:22 °C; 56% RH). Plants were grown in 10-cm diameter pots with top-soil mix and 3 g Osmocote fertilizer (15-9-12, N-P-K). To standardize experimental plants by phenological stage, plants were used for volatile collection experiments at 4–5 true leaves, which ranged from 3 to 5 weeks of growth across plant species.
Insects
Cucurbit-specialized insect herbivores were selected to investigate foliar or root herbivore-induced plant volatiles: aboveground squash bug (Anasa tristis) nymphs and belowground striped cucumber beetle (Acalymma vittatum) larvae. Squash bugs (A. tristis) feed exclusively on aboveground cucurbit foliage as both nymphs and adults (Doughty et al. 2016). As lacerate-and-flush piercing-sucking herbivores, squash bugs inflict substantial damage to host plants (Bonjour et al. 1991) and can also transmit a lethal plant pathogen (Serratia marcescens) (Bruton et al. 2003). Based on their destructive feeding habits and status as disease vectors, squash bugs are a major aboveground threat to cucurbits. Relatedly, striped cucumber beetle (A. vittatum) larvae are important belowground root-feeding herbivores that can stunt plant growth, particularly when larvae attack young cucurbit plants (Latin & Reed 1985). Striped cucumber beetle larvae are chewing herbivores that complete their lifecycle on cucurbits, feeding belowground before pupating and emerging from the soil as adult beetles (Haber et al. 2021). Adult beetles feed on aboveground cucurbit foliage and, like squash bugs, adult beetles can transmit a lethal plant pathogen (Erwinia tracheiphila) (Rojas et al. 2015). Squash bugs and striped cucumber beetles are native to North America and share a long coexistence history with plant species in the Cucurbita genus (Shapiro & Mauck 2018), likely exerting selective pressures on both wild and domesticated Cucurbita plant populations. Most research to date on cucurbit herbivore chemical ecology has focused on their behavioural responses to pheromones or pathogen-induced plant volatiles (Mauck et al. 2010; Shapiro et al. 2012; Weber 2018; Brzozowski et al. 2020, 2022), but very little is known about plant defence responses to these herbivores, prompting us to focus on plant volatiles induced by aboveground squash bug or belowground striped cucumber beetle larval herbivory.
To challenge plants with herbivory during foliar or root volatile collection experiments, we used laboratory reared insects. A laboratory colony of squash bugs (A. tristis) was reared on zucchini squash (C. pepo subsp. pepo cv. Raven), while a colony of striped cucumber beetles (A. vittatum) was maintained on both zucchini squash (C. pepo subsp. pepo cv. Raven) and cucumber (C. sativus cv. Max Pack) prior to experiments. Squash bugs were originally collected from College Station, TX, USA, while beetles derived from State College, PA, USA, and both colonies were periodically supplemented with wild-caught individuals collected near College Station, TX, USA. Using previously described methods (Thompson et al. 2022a), both colonies were kept at 25 °C on a 16 h light:8 h dark schedule in College Station, TX, USA.
Foliar volatile collection experiments
To determine the emission of foliar constitutive volatiles and HIPVs across cucurbit plant species, we characterized volatiles from non-damaged controls and plants damaged by squash bug nymphs. We collected foliar volatiles using dynamic headspace sampling following the methods of Marmolejo et al. (2021). Due to a limited number of volatile collection chambers, separate foliar volatile collection experiments were conducted for each of the six cucurbit species using identical methodologies described here. Prior to collections, we caged 14 fourth-instar squash bug (A. tristis) nymphs on bug-damaged plants, while control plants remained non-damaged (for replicate numbers, see Table S1). Squash bugs (A. tristis) fed for 48 h prior to collections, at which time plants with actively feeding bugs or non-damaged controls were placed inside individual 4-l glass chambers (Rogers Custom Glass, Warriors Mark, USA). Foliar volatiles were collected for 8 h during the photophase (14:00–22:00), as filtered air was pushed into chambers at a rate of 2.6 l·min−1 while air was pulled out of chambers at 1.0 l·min−1 through an adsorbent filter containing 45 mg HayeSep Q (Hayes Separations, Bandera, USA). At the conclusion of foliar volatile collections, squash bug (A. tristis) nymphs were removed, and aboveground plant tissues were collected, dried at 35 °C, and weighed to quantify the emission of volatiles per gram of plant tissue.
Root volatile collection experiments
To examine root constitutive volatiles and HIPVs emitted by different cucurbit plant species, we characterized volatiles of non-damaged controls and roots damaged by belowground striped cucumber beetle (A. vittatum) larvae. Root volatiles were collected using dynamic headspace sampling following the methods of Grunseich et al. (2020). For each of the six cucurbit plant species, identical and separate root volatile collection experiments were executed as detailed here. Prior to collections, plants were placed into individual glass pots (5-cm diameter; Rogers Custom Glass, Warriors Mark, USA) containing clean sand (10% water W/V) and allowed to acclimate for 24 h. Following acclimation, 5 second-instar striped cucumber beetle (A. vittatum) larvae were introduced to the roots of plants and allowed to feed for 24 h prior to volatile collections, while non-damaged controls received no larvae (for replicate numbers, see Table S1). During the photophase, volatiles were collected for 8 h (14:00–22:00) by gently pulling air over roots at 0.2 l·min−1 through an adsorbent filter trap containing 45 mg of HaySep Q (Hayes Separations). Following root volatile collections, larvae were recovered and confirmed to be feeding. Roots of all plants were harvested, washed and dried. Root tissues were dried at 35°C and weighed to quantify the emission of volatiles per gram of plant tissue.
Foliar and root volatile analysis
Adsorbent volatile filters were eluted with 150 μl dichloromethane and 5 μl of an internal standard containing nonyl acetate (80 ng·μl−1) were added to each sample. To analyse volatiles, we used an Agilent 7890B gas chromatograph and 5977B mass spectrometer with a splitless injector held at 250 °C and helium as the carrier gas. We injected 1 μl of each sample and the column (HP-5MS 30 m × 0.250 mm-ID, 0.25 μm film thickness; Agilent Technologies, Santa Clara, USA) was held at 40 °C for 5 min before the temperature increased at 20 °C·min−1 to 250 °C. Electron impact ionization ionized compounds at 70 eV and mass spectra were acquired by scanning from 40 to 300 m/z at 5.30 scans·s−1. Target compounds were tentatively identified by comparison with mass spectral libraries (NIST17 and Adams2 [Allured Publishing]). When possible, we confirmed structure assignments by comparison of mass spectra and retention times with authentic standards. These analyses identified gaseous low-molecular and largely lipophilic plant volatiles belonging to diverse chemical classes, including terpenes and non-terpenes, such as alcohols, aldehydes, alkanes, aromatics, esters, jasmonates, and ketones (Tholl et al. 2006). Compounds were quantified relative to standard concentrations and calculated as ng g−1 dried plant tissue (leaf or root mass).
Statistical analyses
Volatile induction values equal to 1 indicate herbivore-induced emissions were equal to the comparative non-damaged control plants. Volatile induction values >1 signify that herbivory increased total volatile emission relative to the baseline control, whereas volatile induction values <1 show reduced emissions relative to baseline. After obtaining total volatile induction values for each herbivore-damaged plant, we used a two-way anova to assess quantitative changes in volatile emissions with insect herbivory across different cucurbit species and plant tissue types.
To evaluate qualitative changes in plant volatile emissions after herbivory, we quantified chemodiversity metrics for volatile blends using the package vegan v2.6-4 (Oksanen et al. 2013). Chemodiversity metrics included Shannon volatile diversity (e.g., alpha diversity), volatile richness, and volatile evenness (Neuhaus-Harr et al. 2024). Volatile richness shows the number of different volatiles in each blend, while volatile evenness accounts for the similarity in volatile abundances within a blend, as a value of 0 for evenness indicates completely varied volatile abundances and a value of 1 indicates equal abundances across all volatiles within a blend. Shannon volatile diversity indexes account for both volatile richness and evenness within a blend (Oksanen et al. 2013). We used three-way anovas to compare the effects of herbivore damage, plant tissue type, and plant species on volatile diversity, richness, and evenness.
To simultaneously assess quantitative and qualitative differences in volatile blends (e.g., beta diversity), we compared blends with Bray-Curtis dissimilarity matrices analysed by permutational analysis of variance (PERMANOVA; number of permutations = 1000) using the function adonis2 from the package vegan v2.6-4 (Oksanen et al. 2013). Volatile blends were visualized using nonmetric multidimensional scaling ordinations. First, differences in volatile blends within-species were determined by comparing non-damaged controls and herbivore-damaged plants, as well as foliar and root tissues. Then, differences among plant species were analysed for foliar HIPVs (e.g., comparing bug-damaged leaves across species) and root HIPVs (e.g., comparing larvae-damaged roots across species). To determine induction of specific volatiles in response to herbivory, we averaged the amount of each volatile compound emitted by non-damaged controls, as well as their herbivore-damaged counterparts. We repeated this process for each plant species and tissue type. After average emission values were obtained for each volatile compound, we calculated the differences in emission of each volatile between controls and herbivore-damaged plants by tissue and species. These differences were then visualized to show volatile compounds emitted under herbivory relative to controls as heatmaps using the package pheatmap v1.0.12 (Kolde & Kolde 2015). Chemicals were retained for each tissue after curation of at least 50% species retention across samples, which focused our analyses on the most common volatiles for both leaves and roots. Relative differences in emission of each volatile chemical are displayed on the heatmap, allowing for comparison among species. Similarities in volatile emission are clustered by species (columns) and compound (rows) using Euclidean distances and represented as dendrograms.
To examine if different eco-evolutionary forces, including plant domestication and coexistence histories with herbivores, contribute to patterns in HIPV emissions across species, we measured the effects of these characteristics on volatile emissions, as well as testing each volatile for phylogenetic signal (Fig. 1). For plant domestication, to control for relatedness, we focused our analysis of volatile diversity, richness and evenness in the herbivore-damaged plants, by tissue, on the four Cucurbita species (Cucurbita pepo subsp. pepo, Cucurbita pepo subsp. texana, Cucurbita maxima, Cucurbita foetidissima; Fig. 1) using two-way anovas. Differences in volatile blends across wild and domesticated species for foliar and root HIPVs were also assessed using PERMANOVA. For coexistence history with specialist foliar and root herbivores, we regrouped herbivore-damaged plants and tissue types by ‘coexistence history’ and ‘no coexistence history’ and evaluated volatile diversity, richness, and evenness using two-way anovas, as well as differences in volatile blends with PERMANOVA. To account for the influence of species relatedness, we determined phylogenetic signals for each volatile using quantities from foliar and root tissues. To do so, we curated 26 existing alignments of nuclear genes from Kates et al. (2017) using Christoph Hahn's docker container (chrishah/alicut-aliscore-docker:2.31) via Alicut v2.3 and Aliscore v2.0 (Misof & Misof 2009; Kück et al. 2010). A maximum likelihood tree was generated using a single supermatrix of each gene alignment, with independent DNA mutational models determined from iqtree v1.6.12 based on 2000 bootstraps and a 50% majority rule (Nguyen et al. 2015). After rooting the tree to the watermelon (Citrullus lanatus) outgroup, we tested two measures of phylogenetic signal: Blomberg's K and Pagel's Lambda using the phylosig program in the package phytools v2.1-1 (Revell 2024). Values above 1 for Blomberg's K and at 1 for Pagel's Lambda indicate a high phylogenetic signal, suggesting that traits are phylogenetically conserved among closely related species. Conversely, values below 1 for both metrics indicate a low phylogenetic signal, suggesting that traits are more derived and vary more than what is predicted by their evolutionary relationships.
RESULTS
Quantitative changes in plant volatile emissions were stronger aboveground than belowground following herbivory
To investigate quantitative changes in total volatile emissions following herbivory, we calculated total volatile induction values for each herbivore-damaged plant. We found that insect herbivory increased total volatile emissions relative to non-damaged control plants for both aboveground foliar tissues and belowground root tissues, as indicated by total volatile induction values >1 (Fig. 2). When comparing foliar and root volatile induction, we found stronger foliar volatile induction than root volatile induction (compare y-axes Fig. 2; anova F = 23.70, P < 0.0001). However, plant species did not differ in quantitative volatile induction (Fig. 2; anova F = 1.33, P = 0.26) and there was no significant interaction between plant species and tissue type (Fig. 2; anova F = 1.81, P = 0.12).

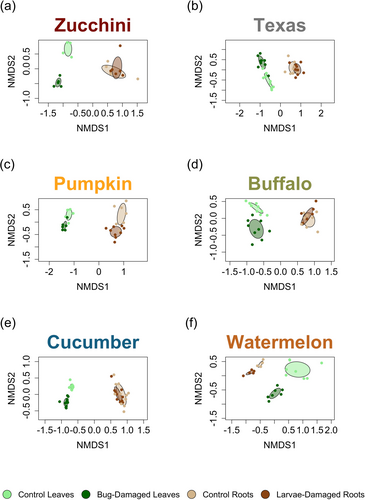
Plant volatile blends are tissue-specific and modified by herbivory
To determine differences in volatile blends across tissues within plant species, we compared volatiles emitted by non-damaged and bug-damaged leaves, as well as non-damaged and larvae-damaged roots, for each species. All plant species showed the same result: herbivore damage altered volatile blends and volatiles differed across plant tissue types (Fig. 3a–f; Table S2; PERMANOVA Herbivore Damage P < 0.05, Plant Tissue P < 0.05). Squash bug herbivory induced strong changes in foliar volatile blends relative to non-damaged controls for all plant species (Fig. 3). In general, changes in larvae-damaged root volatiles relative to non-damaged control roots were more subtle, except in pumpkin and watermelon roots where belowground larval herbivory strongly modified volatile blends (Fig. 3). Regardless of herbivore damage, foliar and root tissues produced strikingly different volatile blends across all plant species, indicating tissue specificity in volatile emissions (Fig. 3).
Plant volatile chemodiversity changes to a greater extent aboveground than belowground with respective herbivore challenge
To assess changes in volatile blends following herbivory, we quantified chemodiversity metrics for volatile blends including volatile diversity, richness, and evenness. Volatile diversity differed among plant species (Fig. 4, Table S3; F = 9.05, P < 0.0001), although herbivory (Fig. 4, Table S3; F = 2.94, P = 0.09) and plant tissue type (Fig. 4, Table S3; F = 0.11, P = 0.74) did not influence diversity variance. We also detected a number of interactions in the volatile diversity model (Table S3), most notably an interaction between plant tissue type and species (Fig. 4, Table S3; F = 15.83, P < 0.0001), e.g., Texas gourd plants exhibited higher volatile diversity in roots relative to leaves regardless of herbivore damage (Fig. 4). In contrast, pumpkins showed greater volatile diversity in leaves compared to roots and herbivory increased volatile diversity across both tissue types (Fig. 4), while watermelon volatile diversity increased following foliar bug damage but decreased after belowground larvae damage (Fig. 4).
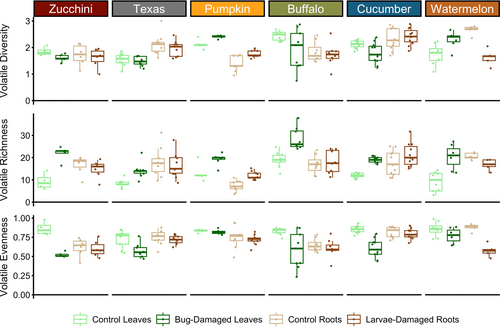
Herbivore damage altered volatile richness (Fig. 4, Table S3; F = 60.61, P < 0.0001) and these effects differed among plant species (Fig. 4; Table S3; F = 17.26, P < 0.0001) but not plant tissue types (Fig. 4, Table S3; F = 0.004, P = 0.95). The volatile richness model detected many interactions (Table S3), intriguingly with herbivore damage and plant tissue type (Fig. 4, Table S3; F = 40.20, P < 0.0001). For all six cucurbit species, we found foliar volatile richness increased substantially after squash bug herbivory (Fig. 4). In contrast, root volatile richness changed less consistently across species following larval feeding damage (Fig. 4). After herbivory, root volatile richness did not change for most species, while pumpkin volatile richness increased and watermelon volatile richness decreased with larval damage (Fig. 4). These findings are explained by two volatile classes that were emitted more frequently by bug-damaged leaves than larvae-damaged roots: green leaf volatiles and sesquiterpenes. Although green leaf volatile production from foliage is fairly intuitive, we were surprised that so few sesquiterpenes were produced belowground as previous work on root HIPVs in maize and cotton not only found many sesquiterpenes in root HIPV blends, but also determined sesquiterpenes regulate important root defences against herbivory (Rasmann & Turlings 2008).
Similar to our findings for richness, volatile evenness was affected by herbivore damage (Fig. 4, Table S3; F = 69.59, P < 0.0001) and plant species (Fig. 4, Table S3; F = 10.41, P < 0.0001), but plant tissue type had no effect on volatile evenness (Fig. 4, Table S3; F = 1.01, P = 0.32). Different interactions were detected in the volatile evenness model (Table S3), including an effect of herbivore damage and plant tissue type on volatile evenness (Fig. 4, Table S3; F = 19.33, P < 0.0001). Across all plant species, squash bug herbivory reduced volatile evenness, most prominently in zucchini and cucumber (Fig. 4). Taken together with the volatile richness results, these patterns indicate squash bug herbivory induced the emission of different types of volatiles and some of these volatiles were emitted in very high abundances relative to other volatiles in bug-damaged blends (Fig. 4). These highly abundant volatiles included compounds such as methyl salicylate or DMNT (Table S4), which are well-known natural enemy attractants for indirect plant defence (Turlings et al. 1990; Mallinger et al. 2011; Rodriguez-Saona et al. 2011a; Li et al. 2018). In contrast, root volatile evenness did not change with larval herbivory (Fig. 4), except in watermelons, which showed a strong reduction in root volatile evenness after larval damage (Fig. 4).
Variation in foliar and root HIPVs among species is driven by volatile from diverse chemical classes
To evaluate differences in HIPV blends among species, we compared volatile emissions from bug-damaged leaves (i.e., foliar HIPVs) and larvae-damaged roots (i.e., root HIPVs) across the six cucurbit species. Overall, species-level variation was observed among foliar HIPVs (Fig. 5a; PERMANOVA F = 7.57, P = 0.001) and root HIPVs (Fig. 5b; PERMANOVA F = 10.28, P = 0.001). A total of 99 different volatile compounds from diverse chemical classes – including alcohols, aldehydes, alkanes, aromatics, esters, jasmonates, ketones, and terpenes – were detected in foliar and root HIPVs across all plant species (Table S4). Many compounds were tissue-specific, found only in leaves or in roots for one or two plant species (Table S4).
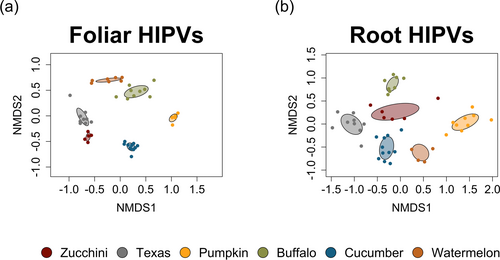
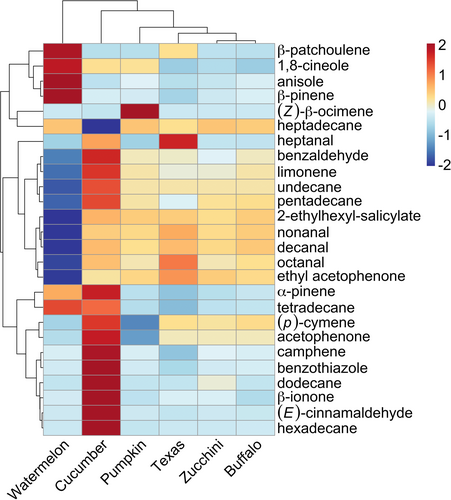
To analyse changes in individual volatiles among plant species, we focused our analyses on the most common volatiles found across all species for both leaves and roots, as these are great candidate compounds for further studying ecological mechanisms in this system. For leaves, when comparing compounds emitted from herbivore-damaged plants relative to controls, we found herbivory induced relative changes among plant species in volatile emissions for a range of different chemicals (Fig. 6). Clustering by plant species indicated cucumbers, Texas gourds, and zucchinis were more similar to one another in foliar HIPV emissions than pumpkins, buffalo gourds, and watermelons (Figs 5a and 6). Relative induction of specific volatiles among species highlighted a few compounds strongly induced by cucumbers, Texas gourds, and zucchinis, including (Z)-β-ocimene, methyl salicylate, (E)-β-ocimene, and linalool (Fig. 6). Strong relative induction of specific volatiles appears to distinguish pumpkins (Fig. 6; α-gurjunene, δ-cadinene, β-cubebene, germacrene D), buffalo gourds (Fig. 6; β-myrcene, DMNT, (E)-nerolidol, (E)-α-farnesene, benzothiazole, β-caryophyllene, ethyl acetophenone, nonanal, decanal), and watermelons (Fig. 6; benzaldehyde, (p)-cymene, octanal, (Z)-3-hexenyl acetate, β-pinene, α-pinene, tetradecane).

For roots, investigating changes in herbivore-damaged plants relative to controls showed herbivory induced the emission of many different volatiles (Fig. 7), some of which were shared with foliar tissues and others unique (Figs 6 and 7). Clustering by plant species revealed root HIPV emissions are more similar among buffalo gourds, zucchinis, and Texas gourds relative to pumpkins, cucumbers, and watermelons (Figs 5b and 7). Generally, species in the Cucurbita genus (buffalo gourds, zucchinis, Texas gourds, pumpkins) emitted relatively low levels of volatiles overall, with the exception of heptanal for Texas gourds and (Z)-β-ocimene for pumpkins (Fig. 7). In contrast to Cucurbita species, herbivory strongly induced an array of compounds in cucumbers relative to the other species (Fig. 7; camphene, benzothiazole, dodecane, β-ionone, (E)-cinnamaldehyde, hexadecane). For watermelons, herbivory also strongly induced a few compounds (Fig. 7; anisole, β-pinene) while showing intense relative reductions in emission for many other compounds relative to other species (Fig. 7; benzaldehyde, limonene, undecane, pentadecane, 2-ethylhexyl-salicylate, nonanal, decanal, octanal, ethyl acetophenone).
Differences among species in foliar and root HIPVs are associated with plant domestication and coexistence history with herbivores, but not phylogenetic relatedness
To explore how different eco-evolutionary factors, such as plant domestication, coexistence history with herbivores, or phylogenetic relatedness drive patterns in HIPV emissions among species, we first compared ‘wild’ and ‘domesticated’ HIPVs for both foliar and root tissues by focusing on two domesticated and two wild Cucurbita species, which controlled for plant species relatedness. Evaluating chemodiversity metrics revealed domestication alone did not affect volatile diversity (Table S5, Figure S1; F = 0.43, P = 0.51), richness (Table S5, Figure S1; F = 2.93; P = 0.09), or evenness (Table S5, Figure S1; F = 2.20, P = 0.14). However, the volatile diversity model detected an interaction between domestication and plant tissue type (Table S5, Figure S1; F = 4.85, P = 0.03), as volatile diversity was much higher in bug-damaged leaves of domesticated plants than their wild counterparts (Figure S1). We found HIPV blends for domesticated and wild species were distinct (Table S6; PERMANOVA F = 3.64, P = 0.001) and that the difference depended on tissue type (Table S6; PERMANOVA F = 4.87, P = 0.001). Next, we compared foliar and root HIPVs of plant species ‘with’ and ‘without’ a coexistence history with herbivores. We found coexistence history altered volatile diversity (Table S5, Figure S2; F = 7.86, P = 0.006), but not volatile richness (Table S5, Figure S2; F = 1.86, P = 0.18) or evenness (Table S5, Figure S2; F = 3.74, P = 0.06), as HIPV diversity was higher in leaves and roots of plants without a coexistence history with specialist herbivores (Figure S2). Moreover, HIPV blends for plants with and without coexistence histories were different (Table S6; PERMANOVA F = 7.68, P = 0.001) and this distinction depended on plant tissue type (Table S6; PERMANOVA F = 6.49, P = 0.001).
Finally, we selected common (50% retained) volatiles emitted across species from foliar and root tissues and tested for the role of phylogenetic relatedness in the emission of each compound. Overall, we found a low phylogenetic signal (values <1) for the vast majority of volatile compounds (Figure S3a–d). For non-damaged control leaves, the only volatile compound with a phylogenetic signal was tetradecane (Figure S3a), while bug-damaged leaves produced seven volatile compounds with phylogenetic signals: (p)-cymene, octanal, benzaldehyde, β-pinene, α-pinene, tetradecane, and (Z)-3-hexenyl acetate (Figure S3b). For non-damaged control roots, no compounds showed any phylogenetic signal (Figure S3c), whereas larvae-damaged roots produced one volatile with a phylogenetic signal, nonanal (Figure S3d).
DISCUSSION
Plants biosynthesize a huge array of specialized metabolites aboveground and belowground, but discerning the ecological functions or evolutionary drivers of such chemodiversity remains challenging. Plant volatiles are a promising class of specialized metabolite for such investigations, as volatiles are emitted constitutively and induced by herbivory across plant tissue types. Plant leaves and roots rely on HIPVs for defence, but relatively little is known about the eco-evolutionary determinants of plant volatile chemodiversity. Our study compared foliar and root HIPVs within and among closely related cucurbit species, and evaluated how plant domestication and coexistence histories with herbivores, as well as phylogenetic relatedness among species, may relate to patterns of HIPV emissions. We found both aboveground and belowground herbivory quantitatively increased total volatile emissions, but induction was much stronger for leaves than roots (Fig. 2). We also observed qualitative differences, as each species produced distinctive foliar and root volatiles that changed with herbivory (Fig. 3a–f). Furthermore, aboveground herbivory induced greater volatile richness but lower evenness relative to belowground herbivory (Fig. 4). Although each species produced unique HIPVs, we observed clustering of different species in foliar or root HIPV blends (Figs 5-7). Our data also suggest that plant domestication and coexistence histories with specialist herbivores may shape HIPVs (Table S5, Figures S1 and S2), but phylogenetic relatedness among plant species did not correlate with most foliar or root volatiles (Figure S3a–d). Taken together, our findings demonstrate aboveground and belowground HIPVs differ both within and among plant species and that eco-evolutionary factors may contribute to such emission patterns, providing novel insights into the origins and maintenance of tissue-specific chemodiversity in plants.
Plant volatile emissions typically increase after insect herbivory, but whether aboveground or belowground feeding differentially induces volatiles is currently unclear. Our findings show that aboveground herbivory induced greater changes relative to belowground herbivory, which indicates leaves are more herbivore-inducible than roots. Stronger inducibility in leaves could suggest greater reliance on HIPVs for different ecological processes. For instance, plants may utilize indirect defence more frequently aboveground than belowground as foliar HIPVs recruit diverse natural enemies ranging from arthropods to birds (Hiltpold & Shriver 2018; Pearse et al. 2020), while belowground HIPVs are known to attract only entomopathogenic nematodes in a few systems (Turlings & Erb 2018). HIPV-mediated plant communication is also common aboveground (Karban et al. 2014), but seemingly rare belowground (van Doan et al. 2021; Pan et al. 2022), further implying herbivore-inducibility may be greater and more ecologically relevant aboveground. Another possible explanation is the difference in aboveground and belowground abiotic environments. Aboveground, unique abiotic factors such as wind, UV light, ozone, and humidity can drastically alter volatiles and their ecological functions (Gouinguené & Turlings 2002; Karban et al. 2006; Blande et al. 2014; Heil 2014), which could suggest plants need to produce more volatiles to send reliable signals aboveground. Belowground, volatile diffusion is restricted in the soil (Hiltpold & Turlings 2008) and root volatiles appear to regulate localized ecological processes only (Arce et al. 2021). An alternative and intriguing explanation is that roots constitutively produce higher relative amounts of volatiles than leaves and thereby have a higher inducibility threshold (Kessler 2015). Considering roots interact with abundant microbial communities in the soil, roots may need to constitutively produce large quantities of volatiles to regulate microbial interactions, such as deterring microbial pathogens or attracting beneficial microbes (Dudareva et al. 2006; Schulz-Bohm et al. 2018), prior to any attack from insect herbivores.
Within-plant phytochemical variation is well characterized across aboveground and belowground systems for many specialized metabolites (Zangerl & Rutledge 1996; Kaplan et al. 2008b; Rasmann et al. 2009), but our understanding of within-plant variation in volatiles lags behind. To address this, we compared foliar and root volatile emissions within each cucurbit species and profiled blends with and without herbivory. Our results revealed that each species emitted distinctive aboveground and belowground volatile blends, which were further modified by herbivory. Finding strong within-plant variation for all six cucurbit species did not match our predictions and was rather surprising. However, root HIPVs have been characterized for only a handful of plant species and even fewer with complementary profiling of foliar HIPVs, making it challenging to know if tissue-specific volatile emissions are the norm or the exception. It is also important to note that herbivore feeding guilds or varied feeding rates on different host plants may have played a role in the observed differences in our study, but we argue that this represents a realistic herbivory scenario. In other words, potential variation in specialist herbivory is ecologically relevant and reflects how plants would receive and respond to these herbivores in natural field settings. Thereby, HIPVs profiled in our study reveal important chemical information transmitted by cucurbit plants to their surrounding aboveground and belowground environments, which could influence behaviours of herbivores and natural enemies, although these behaviours remain to be studied. Our findings complement previous work on different herbivore species feeding aboveground or belowground (Erb et al. 2009, 2011; Karssemeijer et al. 2020) and studies that utilized general herbivore elicitors, such as methyl jasmonate (Frost 2023). Ultimately, we found clear tissue-specificity in constitutive volatile emissions and HIPVs, shedding light on an important component of within-plant chemodiversity.
Insect herbivory often induces the emission of additional volatile compounds not produced constitutively, qualitatively changing volatile blends. We found foliar volatile richness (i.e., the number of different volatiles) generally increased after aboveground herbivory, while minimal changes in richness followed belowground herbivory. Our findings for volatile richness may correspond to the different types of herbivores that plants encounter, as insect herbivore richness is generally much higher aboveground than belowground (Johnson et al. 2016). Herbivore diversity can positively correlate with phytochemical diversity (Richards et al. 2015), which may indicate foliar volatile richness corresponds to a larger number of different types of herbivores aboveground than belowground. When considering cucurbit herbivore communities, there are many herbivores that feed exclusively aboveground, including squash bugs and other Hemipterans, such as aphids and whiteflies, and thrips (Doughty et al. 2016; Messelink et al. 2020), while generalist cucumber beetles feed aboveground on cucurbits but prefer to oviposit belowground on grasses (Eben & Espinosa de Los Monteros 2013). It is possible that cucurbits encounter fewer herbivores belowground, except for specialist striped cucumber beetle larvae and perhaps a few other generalists, and that these differences in the number of aboveground or belowground herbivores may explain the general patterns we detected in volatile richness. Additional studies are needed to explicitly test whether herbivore diversity predicts volatile diversity or richness across aboveground and belowground plant systems, as well as to determine any costs to plants for emitting additional compounds and the scale at which these interactions operate. It is also important to point out that although herbivore richness may be lower belowground, microbial richness and abundance are much higher, and future work should aim to disentangle how quantitative and qualitative changes in root volatiles may correspond to the diversity of belowground herbivores or microbes.
In contrast to volatile richness, foliar volatile evenness (i.e., similarity in the relative abundances of volatiles within a blend) decreased after aboveground herbivory while few changes in evenness were found after belowground herbivory. Evenness is currently an understudied metric of chemodiversity (Wetzel & Whitehead 2020), but emerging evidence points towards phytochemical evenness playing a role in plant abiotic stress responses (Diethelm et al. 2024), invasion ecology (Salgado et al. 2023), and herbivore host plant selection (Neuhaus-Harr et al. 2024). In the last study, evenness in constitutive volatile emissions negatively correlated with herbivore attraction to host plants, indicating herbivores preferred less-even volatile blends and likely homed in on a few key bioactive volatiles to find preferred hosts (Neuhaus-Harr et al. 2024). This may indicate that cucurbit foliar HIPV blends include high abundances of a few bioactive volatiles, which could aid in natural enemy recruitment for indirect defence or possibly make some plant species more susceptible to foraging herbivores. Whether volatile induction alone or changes in specific chemicals regulate herbivore or natural enemy behaviour for cucurbits remains relatively unknown; however, previous work documented volatile (E)-β-ocimene emitted from herbivore-damaged zucchini repels foraging squash bugs (Thompson et al. 2022a) and striped cucumber beetle larvae avoid initial emissions of cucumber root HIPVs (Grunseich et al. 2020). In root volatile evenness, we found few changes with belowground herbivory, except for watermelon roots which exhibited reductions in evenness with herbivory. Changes in watermelon root volatile evenness correspond to suppressed volatile emissions in HIPV blends relative to constitutive root volatiles, which requires further study to determine what is driving root volatile suppression in watermelon as this could possibly be regulated by a herbivore elicitor (Lin et al. 2021b).
We found foliar and root HIPVs differed among plant species, suggesting unique antiherbivore defence responses for each species. Notably, we also tested for correlations between volatiles and phylogenetic relatedness but detected little to no phylogenetic signal in constitutive volatiles or HIPVs both aboveground and belowground. Minimal phylogenetic signal indicates volatiles in our system are more derived and not highly conserved across closely related species, which is an emerging pattern in phylogenetic studies of plant chemodiversity (Forrister et al. 2023; Frost 2023; Wang et al. 2023). Phytochemical diversity appears to be an evolutionarily labile trait, which may allow plants to rapidly adapt to changing environmental conditions over time. This could indicate that plants evolve to produce distinct volatile blends and that selection acts against conserved HIPVs as a mechanism of defence. For cucurbits defending against specialist herbivores, plant species that evolve to emit unique volatiles are presumably at an ecological advantage, as these uncommon volatiles could allow plants to evade specialist herbivores that are likely targeting specific volatiles for host recognition.
Our findings also suggest eco-evolutionary factors can influence chemodiversity of plant volatile emissions. Plant domestication was associated with increased volatile diversity in bug-damaged leaves but reduced diversity of larvae-damaged roots, while coexistence history with specialist herbivores was associated with decreased volatile diversity of herbivore-damaged leaves and roots. Plant domestication is predicted to reduce phytochemical diversity through selection for traits of agroeconomic value (Whitehead et al. 2017), but other evidence points towards greater HIPV emissions in domesticated plants compared to wild relatives (Rowen & Kaplan 2016), indicating domesticated plants may rely more heavily on HIPV-mediated defences. Given their reduced cucurbitacin levels, it is possible that domesticated cucurbits evolved to shift resources towards HIPV-mediated defence strategies, while wild species rely on other defences. However, why this pattern differs for root HIPVs remains unknown and more work is needed to disentangle the influence of plant domestication on HIPVs. Importantly, we acknowledge these findings are based on two domesticated and two wild plant species and sampling additional species would bolster these conclusions to deepen our understanding of domestication and plant chemodivesity evolution. Moreover, plants with longer coexistence histories with herbivores are predicted to become better at detecting and defending against attack compared to ‘naïve’ plants with shorter coexistence histories. Contrary to our predictions, we found plants with no coexistence history exhibited higher volatile diversity relative to plants with coexistence histories. This may suggest these plants are “less familiar” with the herbivores and are activating a more generalized defence response with a broader range of chemicals. Future research should characterize cucurbit foliar or root HIPVs following attack from many different types of herbivore, which would identify broadly emitted HIPVs as well as more specific compounds.
CONCLUSION
Our study examined aboveground and belowground plant volatile emissions among closely related species and evaluated how eco-evolutionary factors may have shaped HIPV patterns. Despite detecting various species- and tissue-specific volatile compounds, overarching patterns emerged across species: quantitative and qualitative changes in HIPVs were stronger aboveground than belowground. In testing possible eco-evolutionary drivers of these patterns, we determined that phylogenetic relatedness did not explain volatile emissions, but domestication and coexistence history appear to have altered volatile diversity. This is the first study to profile a range of related plant species for both foliar and root HIPVs and explicitly investigate what factors could shape differences in plant volatile chemodiversity, providing an excellent springboard for future work on other plant families and herbivore groups. These results reveal novel evidence on tissue-specific plant volatiles modified by herbivory, shedding light on the evolutionary ecology of plant chemodiversity and defence against herbivory.
ACKNOWLEDGEMENTS
We thank members of the Helms lab for assistance with maintaining plants and colonies for experiments, and the Stephenson lab for Texas gourd seeds. Illustrations were created with BioRender.com. Funding from Texas A&M University College of Agriculture & Life Sciences Merit Fellowship and John A. Jackman Endowed Scholarship awarded to MNT, as well as Texas Ecological Laboratory Program, Texas A&M University, and HATCH project TEX0-2-9066 awarded to AMH, supported this work.
AUTHOR CONTRIBUTIONS
MNT and AMH conceived the ideas and designed experiments. MNT conducted the experiments and collected plant volatiles. MNT and DM analysed chromatograms. MNT and ZPC analysed the data. MNT wrote the manuscript. All authors contributed to the final version and approved the final submission.
Open Research
DATA AVAILABILITY STATEMENT
Data available upon request to the corresponding author.




