The CmTGA1–CmRbohD Cascade Confers Resistance Against Chrysanthemum White Rust by Promoting Reactive Oxygen Species Generation
Qi Chen and Ruibing Jin contributed equally to this work.
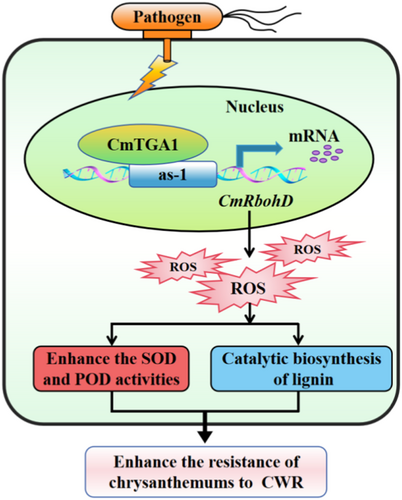
Core: CmTGA1 binds to CmRbohD, resulting in a ROS burst. The rapid accumulation of ROS enhances SOD and POD activities and catalysers lignin biosynthesis, thereby improving the resistance of chrysanthemum to CWR.
ABSTRACT
Chrysanthemum white rust (CWR), caused by Puccinia horiana Heen., is a serious disease of chrysanthemum worldwide. This disease reduces the quality and yield of Chrysanthemum morifolium, leading to significant losses for chrysanthemum growers and industries. It is often referred to as the ‘cancer’ of chrysanthemum. The most effective approach to managing CWR is to utilise host resistance. Reactive oxygen species (ROS) are conserved basic defence compounds in higher plants that are generated in response to biotic stresses. This study reported the TGACG-binding (TGA) transcription factor 1 (CmTGA1) in chrysanthemum. Subcellular localisation analysis revealed that CmTGA1 is localised in the nucleus and cytoplasm. Overexpression or knockout of CmTGA1 in chrysanthemum increased or reduced CWR resistance by regulating ROS generation, the activities of antioxidant enzymes, and CmRbohD (a gene mediating ROS generation) expression. Yeast one-hybrid, dual-luciferase, and electrophoretic mobility shift assays showed that CmTGA1 bound directly to the as-1 element in the promoter region of CmRbohD. Subcellular localisation analysis revealed that CmRbohD was localised in the cytomembrane and cytoplasm. CmRbohD was induced by P. horiana infection and enhanced CWR resistance by promoting ROS generation, activating the antioxidant enzyme system, and catalysing lignin biosynthesis. Our results showed that CmTGA1 activated CmRbohD to improve the CWR resistance via the ROS pathway in chrysanthemum. Our findings provided novel insights into the regulatory pathways involving the CmTGA1-CmRbohD cascade-mediated regulation of CWR resistance, demonstrating an effective strategy to improve tolerance to P. horiana in chrysanthemum.
1 Introduction
Chrysanthemum white rust (CWR), caused by Puccinia horiana Heen., is a crucial disease impacting chrysanthemum cultivation, leading to substantial yield losses (Torres et al. 2017). It not only affects the growth of chrysanthemum but also reduces the related economic benefits. Chrysanthemum affected by CWR develops a series of response mechanisms to resist damage from P. horiana. One of the response mechanisms is the induction of programmed cell death (PCD) (Veselin et al. 2015). Usually, PCD is induced by increasing ROS levels.
ROS are important signalling molecules in both pattern-triggered immunity (PTI) and effector-triggered immunity (ETI). When pathogens infect plants, they trigger a rapid transient ROS accumulation (Heath 2000). The rapid and localised accumulation of ROS at the site of pathogen infection can effectively inhibit pathogen growth, ultimately triggering a hypersensitive response (HR) in plants. In addition, ROS activate the antioxidant enzyme system and enhance cell wall structure by promoting lignin biosynthesis, thereby inhibiting pathogen infection (Shi et al. 2019). Therefore, ROS levels are closely associated with plant resistance. However, this process must be carefully regulated to prevent oxidative stress due to excessive ROS accumulation (Liu et al. 2021). TGACG-binding (TGA) transcription factors (TFs) belong to the bZIP TF family. The bZIP TF family is one of the most important and largest TF families in plants. TGAs can specifically bind to the TGACG (activation sequence-1; as-1) element to regulate the transcription of target genes and play an important role in the defence responses of plants against biotic and abiotic stresses (Wang et al. 2022). Previous studies have shown that TGAs play important roles in the ROS pathway. TaTGA1 in wheat (Triticum aestivum) and PpTGA1 in peach (Prunus persica) enhance resistance to biotic stresses by promoting ROS accumulation (Xu et al. 2018; Li et al. 2021). In Arabidopsis, AtTGA1 directly binds to the TGACG motif in the AtRbohD promoter, enhancing ROS accumulation and increasing the plant's resistance to bacterial wilt (Qi et al. 2022). However, the role of TGAs in the response to CWR has not yet been elucidated.
ROS are produced by multiple enzymatic systems, including the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. Plant NADPH oxidases, called Rboh, transfer electrons from NADPH to O2− during plant-pathogen interactions. When plant cells are stimulated by pathogens, they produce Ca2+ ions, which bind to the EF structure of Rboh and lead to excessive ROS accumulation (Flury et al. 2013; Liu and He 2016). Rboh-mediated ROS generation is crucial in responses against various biotic stresses. In barley (Hordeum vulgare) and potato (Solanum tuberosum), HvRbohA and StRbohB enhance resistance to barley powdery mildew and potato blight, respectively, by promoting ROS formation (Kobayashi et al. 2007; Nagano et al. 2017). In wheat, TaWRKY19-mediated inhibition of TaNOX10 transcription impairs ROS formation and enhances the susceptibility of wheat to stripe rust (Wang et al. 2022). In cotton (Gossypium barbadense and G. hirsutum), GbRboh5/18 and GhRbohD enhance the plant's resistance against V. dahliae by elevating ROS accumulation (Chang et al. 2020; Huang et al. 2021). In citrus (Citrus sinensis), CsRbohD also enhances the resistance to P. digitatum via the ROS pathway (Wang et al. 2022). Thus, Rbohs are crucial for disease resistance in plants (Kadota, Shirasu, and Zipfel 2015).
C. morifolium is one of the most valuable ornamental species susceptible to P. horiana. Our previous study elucidated the mechanism underlying the response to CWR via the salicylic acid (SA) signalling pathway (Bi et al. 2021; Gao et al. 2022). However, the molecular regulatory network underlying ROS generation in chrysanthemum in response to CWR remains unclear. In the present study, transcriptomic analyses, molecular interaction assays, and biochemical assays were used to explore how CmTGA1 impacts the responses of chrysanthemum against CWR via the ROS signalling pathway. Transcriptome analysis showed that CmTGA1 was differentially expressed in response to P. horiana. Our results showed that CmTGA1 contributes to ROS generation and CWR resistance. Yeast one-hybrid (Y1H) assay, dual-luciferase (LUC) assay, and electrophoretic mobility shift assays (EMSA) showed that CmTGA1 directly bound to the as-1 element within the CmRbohD promoter. CmRbohD, a gene mediating ROS generation, enhances ROS production, activates the antioxidant enzyme system, and catalysers lignin biosynthesis, enhancing resistance to CWR. Thus, the present study provided a novel insight into the mechanical resistance response of chrysanthemum to CWR.
2 Results
2.1 Isolation and Sequence Analysis of CmTGA1
In our previous study, CmTGA1 was found to be upregulated after P. horiana inoculation in chrysanthemum (log2 fold change = 2.0145, https://www.ncbi.nlm.nih.gov/, login number: PRJNA797165), suggesting that CmTGA1 might be associated with the resistance of chrysanthemum to P. horiana (Gao et al. 2022). In the present study, we assessed CmTGA1 expression in P. horiana-inoculated chrysanthemum using quantitative real-time polymerase chain reaction (qRT-PCR). As shown in Figure S1A, CmTGA1 was significantly upregulated at 24 h postinfection (hpi; p < 0.05). This result was consistent with the transcriptional profiles generated.
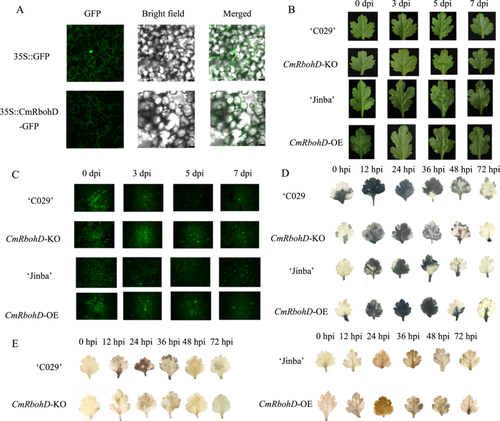
To analyze the molecular properties of CmTGA1, we isolated the 657-bp full-length cDNA of this gene, encoding a predicted 219 amino acids long protein, from ‘C029’ (Figure S1B). The theoretical isoelectric point and predicted molecular weight of this protein were 4.99 and 25,294.95, respectively. Sequence-based homology showed that CmTGA1 was highly identical to TGA1 of Artemisia annua. ExPASy analysis revealed that CmTGA1 primarily comprised leucine, valine, glutamic acid, alanine, and threonine residues, accounting for 10.6%, 9.6%, 10.6%, 6.9%, and 6.4% of all amino acid residues, respectively (Figure S1C). The molecular formula of the protein was C1131H1778N298O339S10. Based on the predicted protein structure, we used self-optimised prediction method with alignment (SOPMA) to predict its secondary structure (Figure S1D). To investigate the subcellular localisation of CmTGA1, we transiently expressed CmTGA1 in tobacco by infiltrating the tobacco leaves with Agrobacterium tumefaciens suspension carrying the 35S::CmTGA1-GFP expression construct or the control vector. Our results showed that CmTGA1 localised in the nucleus and cytoplasm (Figure 1A).
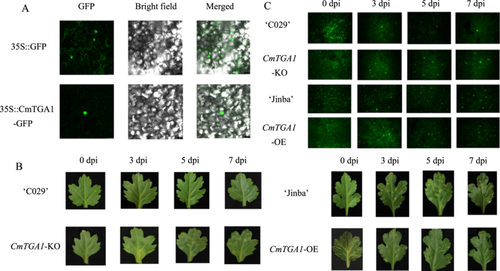
2.2 CmTGA1 Positively Regulates the resistance to P. horiana in chrysanthemum
To further investigate the function of CmTGA1, we created one CmTGA1 overexpression line (CmTGA1-OE) and one CRISPR-Cas9-mediated mutant line (CmTGA1-KO) (Figure S2A). qRT-PCR showed that the transcription level of CmTGA1 in the CmTGA1-OE line was significantly higher (3.65-fold) than that in ‘Jinba’ (Figure S2B). Hence, CmTGA1-OE was selected for further analysis. Then, we analyzed the CmTGA1 sequence in the mutant line and found two amino acid substitutions and two amino acid deletions in the corresponding protein sequence (Figure S2C). Therefore, this line, denoted as CmTGA1-KO, was selected for further analysis.
Next, we performed a pathogen infection test. P. horiana infection assays revealed that CmTGA1-OE exhibited higher resistance to P. horiana while CmTGA1-KO was more susceptible than wild type (WT) (‘Jinba’ and ‘C029’) (Figure 1B). Then, we performed fluorescence staining to quantify the spores in the leaves and determine whether the plants were susceptible to P. horiana infection (Figure 1C). Immediately after inoculation, the findings from WT and transgenic lines were similar. However, the CmTGA1-KO leaves harboured more pathogenic spores than ‘C029’ leaves at 3, 5, and 7 days postinfection (dpi). In contrast, the CmTGA1-OE leaves harboured fewer pathogenic spores than ‘Jinba’ leaves at 3, 5, and 7 dpi. Taken together, these findings suggested that CmTGA1 overexpression improved resistance to P. horiana in chrysanthemum, while the knockout of CmTGA1 made the plant more susceptible.
2.3 CmTGA1-Mediated ROS Production Contributes to CWR Alleviation
To further investigate the mechanisms underlying CmTGA1-mediated resistance to CWR, ROS levels were analyzed via 3, 3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining at 0, 12, 24, 36, 48, and 72 hpi. We observed a higher and lower ROS accumulation in CmTGA1-OE and CmTGA1-KO, respectively, compared to WT after P. horiana inoculation (Figure 2A,B). However, CmTGA1-KO exhibited a higher H2O2 accumulation at 24 hpi than that in ‘C029’. We hypothesised that CmTGA1 primarily affects O2− accumulation, and the mild H2O2 accumulation cannot compensate for the large O2− deficiency. Overall, CmTGA1 knockout weakened the ROS burst during the initial stages of P. horiana infection and reduced the resistance of chrysanthemum to CWR.
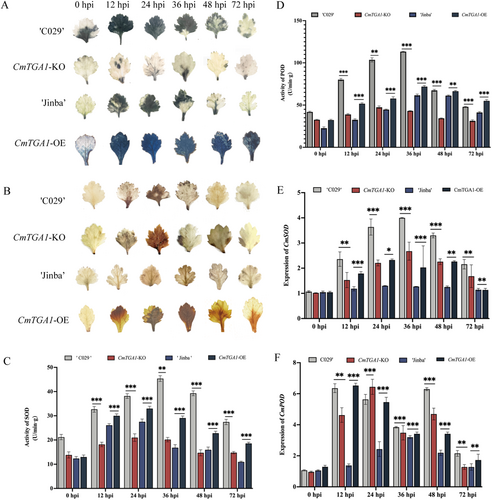
ROS homoeostasis in plants is regulated by antioxidant enzymes, such as SOD and POD. Therefore, we investigated the SOD and POD activities and the expression of corresponding genes in WT and transgenic lines. We found a rise in the SOD and POD activities in CmTGA1-OE but a significant reduction in CmTGA1-KO. SOD and POD activities peaked earlier in CmTGA1-KO than in ‘C029’; however, the mutant line exhibited only 0.6- and 0.46-fold SOD and POD activities, respectively, compared to ‘C029’ (Figure 2C,D). The variations in CmSOD and CmPOD expression levels were similar to those in SOD and POD activities, respectively (Figure 2E,F). Notably, CmPOD expression was higher in CmTGA1-KO than in ‘C029’ at 24 hpi. We hypothesised that after P. horiana inoculation, CmTGA1-KO expressed higher CmPOD levels but for a short duration, decreasing rapidly at 48 hpi. This change could not compensate for the lack of CmSOD. These findings suggested that CmTGA1 might enhance resistance to P. horiana via the ROS pathway.
2.4 CmRbohD Plays an Important Role in the Response of Chrysanthemum to CWR
In plants, the Rboh gene family mainly mediates the production of ROS. To further investigate the role of Rboh gene family in response of chrysanthemum to CWR, we first performed BLAST analysis with Rboh genes reported in Arabidopsis to identify all CmRbohs, revealing a total of 12 Rboh genes (Table 1). Then, we assessed Rbohs transcription levels at 48 hpi in P. horiana-inoculated ‘C029’ using qRT-PCR (Figure S3A). Among them, evm. TU. scaffold_1224.151 was significantly induced by P. horiana. The closest ortholog of evm.TU.scaffold_1224.151 in Arabidopsis is AtRbohD (Figure S3B). We thus designated evm.TU.scaffold_1224.151 as CmRbohD.
| > evm. TU. scaffold_1225.87 | > evm. TU. scaffold_790.157 | > evm. TU. scaffold_1263.69 |
| > evm. TU. scaffold_1263.69 | > evm. TU. scaffold_790.157 | > evm. TU. scaffold_1201.175 |
| > evm. TU. scaffold_321.87 | > evm. TU. scaffold_508.31 | > evm. TU. scaffold_213.89 |
| > evm. TU. scaffold_1224.151 | > evm. TU. scaffold_944.114 | > evm. TU. scaffold_1658.163 |
To gain insights into the mechanism underlying CmTGA1-mediated ROS production, we quantified CmRbohD expression in CmTGA1-KO and ‘C029’ after P. horiana inoculation. In ‘C029’, the transcription level of CmRbohD at 48 hpi was 6.15-fold higher than that at 0 hpi. Furthermore, CmTGA1 knockdown led to a significant CmRbohD downregulation (Figure S3C). These data suggested that CmRbohD might respond to CWR incidence and that there might be a transcriptional regulatory association between CmTGA1 and CmRbohD.
2.5 CmTGA1 Directly Activates CmRbohD by Binding to Its Promoter
Next, we investigated whether CmTGA1 regulates CmRbohD. We cloned the CmRbohD promoter and analyzed its cis-acting elements. The promoter harboured several motifs, including W-box, MYB element, MYC element, TATA-box, and as-1 element. The as-1 element acts as a binding site for TGA TFs. Hence, for further analysis, the CmRbohD promoter was divided into two parts: P1 (with as-1 element) and P2 (without as-1 element) (Figure S3D). Next, we performed a Y1H assay, wherein the yeast cells containing P1 transformed with pGADT7-CmTGA1 were grown on SD/−Leu medium containing 400 ng·mL−1 aureobasidin A (AbA). The baits with the negative control pGADT7 were unable to grow on SD/−Leu medium (Figure 3A). The Y1H assays showed that CmTGA1 interacted with P1 but not P2. EMSA was used to investigate whether CmTGA1 directly binds to the CmRbohD promoter in vitro. Our results showed that GST-CmTGA1, but not GST alone, strongly bound to the probes containing P1. The migration bands gradually became shallower with the addition of cold probes (Figure 3B). Overall, these results indicated that CmTGA1 directly binds to CmRbohD in vitro.
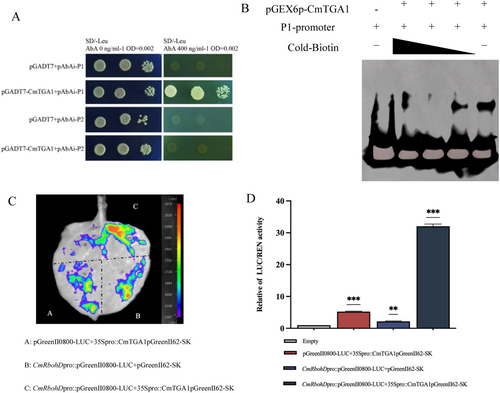
Then, we performed a dual-luciferase reporter system to evaluate the regulatory effects of CmTGA1 on CmRbohD expression in vivo. The plasmids containing luciferase (LUC) driven by the CmRbohD promoter and Renilla (REN) driven by the CmTGA1 were co-transformed into tobacco leaves (Figure 3C). Our findings revealed that the co-expression of CmTGA1 with CmRbohD significantly increased the LUC/REN ratio, indicating that CmTGA1 activates CmRbohD expression (Figure 3D). Overall, these results indicated that CmTGA1 bound to P1 and activated it both in vitro and in vivo.
2.6 CmRbohD Positively Regulates CWR Tolerance in Chrysanthemum by Promoting ROS Production
Next, we isolated the 2750-bp full-length cDNA of CmRbohD, encoding a predicted 916 amino acids long protein, from ‘C029’ (Figure S4A). The theoretical isoelectric point and predicted molecular weight of this protein were 8.82 and 102,789.69, respectively. This protein contains typical NADPH domains with a NAD-binding motif distributed between amino acids 721 and 898, and an EF-hand motif distributed between amino acids 241 and 304 (Figure S4B). Sequence-based homology showed that CmRbohD was highly identical to RbohD of Lactuca sativa (Figure S4C). ExPASy analysis revealed that CmRbohD primarily comprised alpha-helical structures, Tt helical structures, Ss spiral structures, and random coil structures, accounting for 43.34%, 13.54%, 4.80%, and 38.32% of all amino acid residues, respectively (Figure S4D). Based on the predicted protein structure, we used SOPMA to predict its secondary structure (Figure S4E). To investigate the subcellular localisation of CmRbohD, we transiently expressed CmRbohD in tobacco by infiltrating the tobacco leaves with Agrobacterium tumefaciens suspension carrying the 35S::CmRbohD-GFP expression construct or the control vector. Our results showed that CmRbohD localised in the cytomembrane and cytoplasm (Figure 4A).
To further investigate the role of CmRbohD in responses against P. horiana infection, we created three CmRbohD overexpression lines (CmRbohD-OE1, CmRbohD-OE2, and CmRbohD-OE4) and one CRISPR-Cas9-mediated mutant line (CmRbohD-KO) (Figure S5A). qRT-PCR showed that the transcription level of CmRbohD in the CmRbohD-OE4 line was significantly higher (8-fold) than that in ‘Jinba’ (Figure S5B). Hence, CmRbohD-OE4 was selected for further analysis. We analyzed the CmRbohD sequence in the knockout line and found two amino acid substitutions in the corresponding protein sequence. Therefore, this line was selected for further analysis (Figure S5C).
Next, we performed a pathogen infection test on ‘Jinba’, ‘C029’, CmRbohD-OE4, and CmRbohD-KO. P. horiana infection assays revealed that CmRbohD-OE4 and CmRbohD-KO exhibited higher and lower resistance to P. horiana, respectively, than WT (‘C029’ and ‘Jinba’) (Figure 4B). We performed fluorescence staining to quantify the fungal spores in the leaves of all lines and determine the susceptibility of plants to P. horiana infection. The findings from all samples were similar at 0 dpi. However, at 3, 5, and 7 dpi, the CmRbohD-OE4 and ‘C029’ leaves exhibited fewer pathogenic spores than the ‘Jinba’ and CmRbohD-KO leaves, respectively (Figure 4C). Furthermore, we used DAB and NBT staining in the leaves of all lines to assess ROS accumulation. Our results showed that both CmRbohD-OE4 and ‘C029’ exhibited a prominent and more intense ROS burst at 36 hpi than ‘Jinba’ and CmRbohD-KO, respectively. After 36 hpi, the ROS burst started to decrease in all plants (Figure 4D,E). These results suggested that ROS production is associated with CmRbohD in the initial stages of response to pathogen infection. CmRbohD expression promoted ROS accumulation, improving CWR resistance in chrysanthemum.
2.7 CmRbohD Activates the Antioxidant Enzyme System and Promotes Lignin Biosynthesis
Next, we measured the activities of antioxidant enzymes in WT and transgenic lines. The SOD activities in both ‘Jinba’ and CmRbohD-OE4 peaked at 24 hpi; however, CmRbohD-OE4 exhibited 1.17-fold higher SOD activity than ‘Jinba’. In contrast, the POD activities in ‘Jinba’ and CmRbohD-OE4 peaked at 48 and 24 hpi, respectively. Furthermore, the SOD and POD activities in ‘C029’ and CmRbohD-KO peaked at 36 hpi, with CmRbohD-KO exhibiting substantially lower enzyme activities than ‘C029’ (Figure 5A,B). Next, we assessed the CmSOD and CmPOD expression levels in WT and transgenic lines. We found markedly higher CmSOD and CmPOD expression levels in CmRbohD-OE4 than in ‘Jinba’. Among all the lines, the lowest CmSOD and CmPOD expression levels were detected in CmRbohD-KO (p < 0.05) (Figure 5C,D). These results indicated that the CmRbohD overexpression improved SOD and POD activities and, thus, enhanced CWR tolerance by activating the antioxidant defence.
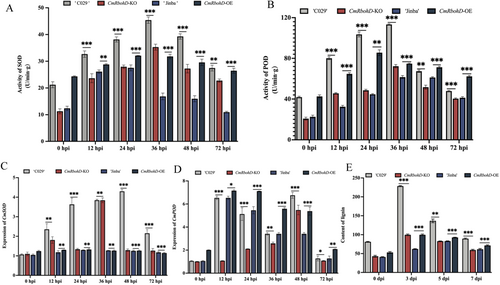
ROS generated by AtRbohD activity might regulate lignin biosynthesis, thereby enhancing resistance to pathogens. Hence, we evaluated lignin accumulation in WT and transgenic lines after P. horiana inoculation. CmRbohD-OE4 and CmRbohD-KO exhibited higher and lower lignin accumulation, respectively, than their WT counterparts. These findings suggested that CmRbohD plays an important role in conferring P. horiana resistance in chrysanthemum by enhancing lignin levels (Figure 5E).
3 Discussion
3.1 Chrysanthemum CmRbohD Promotes ROS Generation During the Initial Response Stages to CWR
The innate immune system of plants can recognise and respond to external stresses by recognising pathogen-associated molecular patterns (PAMPs) (Fiorin et al. 2018; Liu et al. 2021). When plants recognise PAMPs, they can trigger PTI, which is associated with ROS production. Rapid ROS accumulation within a short period can help plants resist more pathogens. In Brassica rapa, BrMYB108 binds to the BrRbohF promoter, promoting Verticillium wilt resistance by triggering localised ROS production (Su et al. 2023). In citrus plants, the CsAP2-09-CsWRKY25-CsRboh2 cascade enhances citrus bacterial canker (CBC) resistance by promoting ROS accumulation (Li et al. 2024). In wheat, TaRBP1 knockdown confers strong resistance to Pst by promoting ROS accumulation in the host plant (Li et al. 2023). Thus, ROS are important components of plant innate immunity and play important roles in response to pathogen infections (Chen et al. 2023).
In the present study, we found that CmRbohD overexpression enhanced the resistance to CWR, while its knockdown substantially increased the plant's sensitivity to the pathogen. At 12–36 hpi, short ROS bursts were observed in the WT and transgenic lines. Notably, these ROS levels decreased at 48 hpi, which was consistent with the results of previous studies (Chen et al. 2021; He et al. 2018; Liu et al. 2021). Furthermore, in our study, ‘C029’ and CmRbohD-OE showed higher antioxidant activities and lignin biosynthesis. These results indicated that during early infection stages, plants respond by enhancing ROS levels. The rapid ROS accumulation enhanced SOD and POD activities and lignin biosynthesis, thereby improving the resistance of chrysanthemum to CWR. After their accumulation, SOD and POD scavenged excess ROS to a certain extent to maintain ROS homoeostasis (Figure 6).
3.2 Chrysanthemum CmTGA1 Enhances Plant Defence Against P. Horiana via the ROS Signalling Pathway
TGA TFs play an important role in responses to biotic stresses and respond to pathogens via multiple pathways, such as the SA, cytokinin, jasmonic acid (JA), ethylene (ETH), and ROS pathways (Huang et al. 2019). In Musa paradisiaca, MpTGA8 interacts with nonexpresser of PR genes 1 (MaNPR1) to regulate resistance to wilt disease via the SA pathway (Lin et al. 2021). In Arabidopsis, the expression of plant deffensin1.2 (AtPDF1.2) was impaired in the tga256 mutant after B. cinerea infection, suggesting that TGA TFs play an important role in the JA/ETH pathway (Zander et al. 2010). In Oryza sativa, disruption of OsbZIP53 increases H2O2 accumulation by promoting ROS generation, thereby regulating the rice immune responses via the ROS pathway (Lijuan et al. 2024).
In the current study, CmTGA1-KO exhibited a high susceptibility to pathogen infection. This mutant line exhibited substantially lower ROS accumulation than ‘C029’ after P. horiana inoculation. However, CmTGA1-OE exhibited a higher ROS accumulation and pathogen resistance. These findings indicated that the high sensitivity of CmTGA1-KO to pathogen infections might be attributed to the low ROS accumulation. These results indicated that CmTGA1 might be involved in the CWR response via the ROS pathway.
3.3 CmTGA1 Directly Activates CmRbohD at the Transcriptional Level
TGA is one of the most prominent families of transcription factors, playing a significant role in response to abiotic and biotic stress. Additionally, the correlation between the different TGA factors, including protein-protein and protein-DNA interactions, can effectively regulate abiotic and biotic stresses in plants. For example, the interaction between MdTGA2.1 and MdSCL14 enhances the salt tolerance in apple (Malus domestica) (Tian et al. 2023). Additionally, in potato, StTGA39 regulates S. tuberosum BRI1-associated receptor kinase 1 (StBAK1) expression, enhancing the tolerance to bacterial wilt (Tian et al. 2022).
the interaction between MdTGA2.1 and MdSCL14 enhances the salt tolerance in apple (Malus domestica) (Tian et al. 2023). Additionally, in potato, StTGA39 regulates S. tuberosum BRI1-associated receptor kinase 1 (StBAK1) expression, enhancing the tolerance to bacterial wilt (Tian et al. 2022).
TGA TFs possess a specific binding sequence, TGACG, which enables them to bind to the as-1 region of the target gene promoter. Both TGAI and TGAII TFs can activate the ROS-related genes by binding to the as-1 element, thereby regulating the plant's ROS-related pathways and ROS levels (Sun et al. 2018). For instance, AtTGA1 has been shown to bind to the as-1 element of the AtRbohD promoter, regulating the sensitivity of Arabidopsis to bacterial wilt (Qi et al. 2022). The current study aimed to investigate the mechanisms underlying CmTGA1-mediated regulation of ROS generation in chrysanthemum. To this end, we assessed the expression levels of CmRbohD. Our findings revealed a significant CmRbohD downregulation in CmTGA1-KO compared to WT at 24-48 hpi. Further, the Y1H assay, LUC assay, and EMSA showed that CmRbohD was directly regulated by CmTGA1.
The current study showed that CmTGA1 binds to the CmRbohD promoter and activates CmRbohD expression (Figure 6), providing an example of protein-DNA interaction between the genes of the TGA family. Furthermore, the elucidation of the CmTGA1-CmRbohD transcriptional cascade paves the road for investigating other TGA TFs at the transcriptional level.
4 Materials and Methods
4.1 Plant Materials
The resistant chrysanthemum cultivar ‘C029’ and the susceptible cultivar ‘Jinba’ were obtained from the Forestry College of Shenyang Agricultural University (ShenYang, China). The plants were grown in a growth chamber under 16-h light/8-h dark conditions at 25°C/18°C, respectively.
4.1.1 P. horiana inoculation and sampling
P. horiana teliospores were obtained from the abaxial sides of CWR-infected chrysanthemum leaves and placed in sterile water containing 0.05% (w/v) Tween 20. Noninfected plants from ‘C029’, ‘Jinba’, CmTGA1-OE, CmTGA1-KO, CmRbohD-OE4, and CmRbohD-KO lines were used for fungal infection. Ten leaves from each plant were selected. The abaxial sides of these leaves were sprayed evenly with the teliospore-containing solution. Each inoculated plant was then covered with a plastic film and incubated in the dark under 90% relative humidity. Leaves were harvested from WT and transgenic lines at 0, 24, 48, and 72 h postinoculation. The extracted leaves were immediately wrapped in an aluminium foil, frozen in liquid nitrogen, and stored at –80°C for sequencing experiments and assessment of physiological indices.
4.1.2 Gene Isolation and Phylogenetic Analysis
Total RNA was isolated from the extracted leaves using a Quick RNA isolation kit (CWBIO, China), and cDNA was synthesised using the PrimeScript RT reagent kit (TaKaRa, Tokyo, Japan). The full-length coding sequences of CmTGA1 and CmRbohD were cloned from ‘C029’ using Super Master Mix (CWBIO, China) with gene-specific primers CmTGA1-F/R and CmRbohD-F/R, respectively (Table 2). Then, they were cloned into the pEASYR-T1 vector using a Simple Cloning Kit (TransGen Biotech, Beijing). The plasmid was then transformed into Escherichia coli DH5α (AngYuBio, G6016), and the clones harbouring the inserts were identified for subsequent experiments.
| Primer names | Primer sequences (5′–3′) |
|---|---|
| Primer F-CmRbohD | 5′-ATGCAAATGCAAAAAATGGGTA-3′ |
| Primer R-CmRbohD | 5′-AAAGTTCTCTTTATGGAAGTCGAA-3′ |
| Primer F-qRTCmRbohD | 5′-AGGCAGCAGGAGTGGAAGTAC-3′ |
| Primer R-qRTCmRbohD | 5′-GCAACTGAATCATCACCAACATCC-3′ |
| Primer F-CmRbohD-OE | 5′-ACGGGGGACTCTTGACCATGGATGCAAATGCAAAAAATGGG-3′ |
| Primer R-CmRbohD-OE | 5′-TAGAAATTTACCCTCAGATCTAAAGTTCTCTTTATGGAAGTCGA-3′ |
| PCmRbohDBsF | 5′-CTGATCTTGATCCATGGA-3′ |
| PCmRbohDBsR | 5′-CGATGTTAGCGATGAAGG-3′ |
| Primer F-CmTGA1-BD | 5′-GCCATGGAGGCCAGTGAATTCATGAGCATCCTTGAAACAACATTC-3′ |
| Primer R-CmTGA1-BD | 5′-CAGCTCGAGCTCGATGGATCCTCATAGTGTTTGAGTTGCCATCATC-3′ |
| Primer F-CmRbohD-AD | 5′-GGTACCCGGGGATCTGTCGACTAAAAAAACAAGATCGACCCACT-3′ |
| Primer R-CmRbohD-AD | 5′-ATACAGAGCACATGCCTCGAGCTTGATGTTCACAATCCAAATAGT-3′ |
| Primer F-CmRbohD-0800 | 5′-CTATAGGGCGAATTGGGTACCTAAAAAAACAAGATCGACCCACTA-3′ |
| Primer R-CmRbohD-0800 | 5′-CGCTCTAGAACTAGTGGATCCCTTGATGTTCACAATCCAAATAGT-3′ |
| Primer F-CmTGA1-62SK | 5′-CGCTCTAGAACTAGTGGATCCATGAGCATCCTTGAAACAACATTC-3′ |
| Primer R-CmTGA1-62SK | 5′-TCAGCGTACCGAATTGGTACCTCATAGTGTTTGAGTTGCCATCATC-3′ |
| Primer F-CmTGA1-6P | 5′-TTCCAGGGGCCCCTGGGATCCATGAGCATCCTTGAAACAACATTC-3′ |
| Primer R-CmTGA1-6P | 5′-GTCACGATGCGGCCGCTCGAGTCATAGTGTTTGAGTTGCCATCAT-3′ |
| Primer F-CmTGA1 | 5′-ATGAGCATCCTTGAAACAACATTC-3′ |
| Primer R-CmTGA1 | 5′-TCATAGTGTTTGAGTTGCCATCAT-3′ |
| PCmTGA1BsF | 5′-ATATATGGTCTCGATTGAAGCTAACCCTAATAGAAGGTT-3′ |
| PCmTGA1BsR | 5′-ATTATTGGTCTCGAAACGAATAAAGCTTGTTCGAATCAA-3′ |
| Primer F-qRTActin | 5′-TCCGTTGCCCTGAGGTTCT-3′ |
| Primer R-qRTActin | 5′-GATTTCCTTGCTCATCCTGTCA-3′ |
The online tool ExPASy2 was used to predict the physicochemical properties of CmTGA1 and CmRbohD. The SOPMA3 website and Phyre2 database were used to predict the protein structures. The MEGA5.0 software was used for the phylogenetic analysis of CmTGA1 and CmRbohD.
4.1.3 Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The relative expressions of the genes were detected using qRT-PCR. It was performed using SYBR Premix ExTaq II (Takara, Tokyo, Japan). The data was analyzed using the 2−ΔΔCT method. Chrysanthemum actin gene CmACTIN was used as a reference. Each qRT-PCR experiment comprised three biological replicates and three technical replicates. The primers used are listed in Table 2.
4.2 Chrysanthemum Transformation
To construct the overexpression vector, the open reading frame (ORF) of CmTGA1/CmRbohD was cloned into the SacI and BamHI sites of the pCAMBIA1301 vector. The online tool CRISPR v2.0 was used to determine the appropriate sequences of the CRISPR/Cas9 target sites based on the CmTGA1/CmRbohD gene sequence. A schematic representation of the target sites is shown in Table 3. The primers were designed according to basic design principles for target sites and single guide RNA (sgRNA) construction (Table 2). Using pCBC-DT1T2 as a template, the sgRNA expression cassette was constructed by overlapping PCR and connected to pHSE401 via Golden Gate assembly. The fusion vectors were introduced into Agrobacterium tumefaciens strain EHA105 (AngYuBio, G6040) and transformed into chrysanthemum via Agrobacterium-mediated transformation.
| Score | Sequence | Strand | Pos | %GC | |
|---|---|---|---|---|---|
| CmRbohD | 0.9311 | GTATGACCGGACTAAGTCCGGGG | + | 445 | 55 |
| 0.8673 | TACTAGGTCAAGAAACACCGAGG | + | 190 | 45 | |
| CmTGA1 | 0.8917 | GCTAACCCTAATAGAAGAGGATG | + | 118 | 45 |
| 0.9026 | TTGGATGAGGTCAAAGGGTTAAG | + | 913 | 50 |
- Abbreviation: %GC, GC content.
‘C029’ and ‘Jinba’ plants were used to obtain the CRISPR/Cas9 transgenic lines and overexpression transgenic lines, respectively. Putative transgenic ‘C029’ and ‘Jinba’ plantlets were rooted in solid media supplemented with kanamycin. The positive plants were screened via PCR and qRT-PCR using the primers CmTGA1/CmRbohD-F/R and qRT-CmTGA1/CmRbohD -F/R (Table 2).
4.2.1 Determination of Lignin Content, ROS Staining, and Enzymatic Activity
In WT and transgenic lines, H2O2 and O2– contents were measured at 0, 12, 24, 48, and 72 h post-fungal inoculation. For H2O2 determination, the leaves were immersed in the 3, 3′-DAB staining solution (containing 1 mg·ml−1 3, 3′-DAB and 10 mM Na2HPO4; pH 3.8) and incubated in the dark for 12–16 h and decolorised in 95% (w/v) ethanol. For O2– determination, the leaves were immersed in the nitro-blue tetrazolium (NBT) staining solution (containing 100 mg NBT and 50 mM Na2HPO4; pH 7.5) and incubated in the dark for 12–16 h and decolorised in 95% (w/v) ethanol.
Furthermore, frozen leaf samples from WT and transgenic lines were used to measure POD and SOD activities of defence enzymes according to a method previously described by Sun (2013). All experiments were performed with three biological replicates.
4.2.2 Fluorescent Staining
The leaves of the WT and transgenic lines were collected at 0, 3, 5, and 7 d postfungal inoculation and cut into small segments. Each segment was then treated with two drops of a 0.1% solution containing 50 mg Fluorescent Brightene 28 and 1 mL dimethyl sulfoxide (DMSO). After staining for 15 min, the segments were observed under a fluorescence microscope (Leica, DMi8).
4.2.3 Promoter Isolation and Analysis
CmRbohD promoter region was amplified using the Genome Walk Kit (Takara, Tokyo, Japan). The frequency of the TGACG element was identified using PlantCARE.
4.2.4 Yeast One-Hybrid (Y1H) Assays
CmRbohD promoter region was divided into two segments, P1 and P2, based on the analysis of cis-acting elements. P1 contained the critical regulatory TGACG element. P1 (0 to −630 bp) and P2 ( − 631 to −1447 bp) were cloned and separately constructed into the pAbAi vector to obtain the prey vectors pAbAi-P1 and pAbAi-P2. To determine identify whether CmTGA1 binds to the CmRbohD promoter, CmTGA1 was cloned into pGADT7. The bait vectors and prey plasmid pGADT7-CmTGA1 were co-transformed into the Y1H Gold yeast strain (AngYuBio, G6046). The empty vector pGADT7 was used as the negative control. Yeast cells were cultured on synthetic defined SD/−Leu plates, supplemented with AbA, for 3 days at 30°C. All experiments were performed with three biological replicates. The primer sequences are listed in Table 2.
4.2.5 Dual-Luciferase Assay
A dual-luciferase assay was used to analyze whether CmTGA1 can activate the CmRbohD promoter in chrysanthemum. P1 was inserted into pGreenII0800-LUC (CmRbohDP1-0800-LUC) as a reporter. CmTGA1 was cloned into pGreen-62SK (CmTGA1-62SK), driven by the 35S promoter, as an effector. CmTGA1-62SK and CmRbohDP1-0800-LUC recombinant plasmids were transferred into the GV3101 (pSoup) strain (AngYuBio, G6039-2). The Agrobacterium culture was prepared by mixing the cells with effector and reporter vectors at a ratio of 1:4. The OD600 of the Agrobacterium culture was adjusted to 0.5 using ½MS liquid medium containing 150 μmol·mL−1 acetylsyringone (AS), and then co-infiltrated into tabacc leaves. The leaves were analyzed for relative activity at 48 h after infiltration. The Tanon5200 multi-imaging apparatus (Tanon, Shanghai, China) was used to observe the luciferase activity. Luciferase activity was detected using a dual luciferase reporter assay system (Trans Gene).
4.2.6 Electrophoretic Mobility Shift Assay (EMSA)
CmTGA1 was inserted into pGEX6P, and the plasmid transformed into E. coli Rosseta (DE3). The cells containing the recombinant plasmid and the empty vector were incubated with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at 16°C for 12 h and purified using glutathione (GST) magnetic beads (Biyuntian, Beyotime, China). An EMSA probe biotin labelling kit (Biyuntian, Beyotime, China) was used to label the primer pairs (Table 2). Furthermore, a LightShift chemiluminescent EMSA kit (Biyuntian, Beyotime, China) was used to verify the interaction between P1 and the GST-CmTGA1 protein.
4.3 Data Analysis
Data were analyzed using analysis of variance (ANOVA) and one sample t and Wilcoxon test to determine significant differences. GraphPad Prism was used for statistical analysis.
5 Accession Numbers
The sequences of chrysanthemum genes involved in this work can be found in the Chrysanthemum Genome Database (http://210.22.121.250:8880/asteraceae/homePage), with the accession number evm. TU. scaffold_1224.151-Cmo (for CmRbohD) and National Center for Biotechnology Information (NCBI) BioProject database (https://www.ncbi.nlm.nih.gov/, login number: PRJNA797165), with the accession number Cluster-45258.111810 (for CmTGA1).
Acknowledgements
We thank the National Natural Science Foundation of China [31972447] and the Foundation of Liaoning Province Education Administration [LJKMZ20221029] for supporting our study.
Consent
All authors approve the manuscript and consent to the publication of the work.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.