Nitrogen Assimilation Plays a Role in Balancing the Chloroplastic Glutathione Redox Potential Under High Light Conditions
ABSTRACT
Nitrate reduction requires reducing equivalents produced by the photosynthetic electron transport chain. Therefore, it has been suggested that nitrate assimilation provides a sink for electrons under high light conditions. We tested this hypothesis by monitoring photosynthetic efficiency and the chloroplastic glutathione redox potential (chl-EGSH) of plant lines with mutated glutamine synthetase 2 (GS2) and ferredoxin-dependent glutamate synthase 1 (GOGAT1). Mutant lines incorporated significantly less isotopically-labelled nitrate into amino acids than wild-type plants, demonstrating impaired nitrogen assimilation. When nitrate assimilation was compromised, photosystem II (PSII) proved more vulnerable to photodamage. The effect of the nitrate assimilation pathway on the chl- EGSH was monitored using the chloroplast-targeted roGFP2 biosensor (chl-roGFP2). Remarkably, while oxidation followed by reduction of chl-roGFP2 was detected in WT plants in response to high light, oxidation values were stable in the mutant lines, suggesting that chl-EGSH relaxation after high light-induced oxidation is achieved by diverting excess electrons to the nitrogen assimilation pathway. Importantly, similar ΦPSII and chl-roGFP2 patterns were observed at elevated CO2, suggesting that mutant phenotypes are not associated with photorespiration activity. Together, these findings indicate that the nitrogen assimilation pathway serves as a sustainable energy dissipation route, ensuring efficient photosynthetic activity and fine-tuning redox metabolism under light-saturated conditions.
1 Introduction
Growing under highly variable light conditions, plants must constantly sense and adjust to daytime photon fluxes. Under high light conditions, NADPH and ATP production in the light reactions might exceed their consumption in the downstream Calvin-Benson cycle (CBC). Efficient coupling between energy generation and consumption is required to avoid damage to photosystems and over-reduction of the chloroplastic electron transport, maintaining photosynthesis-derived reactive oxygen species (ROS) under harmless low levels. Several energy dissipation mechanisms, including non-photochemical chlorophyll fluorescence quenching (NPQ) (Muller, Li, and Niyogi 2001), the water-water cycle (WWC) (Biehler and Fock 1996; Asada 1999; Miyake 2010; Mehler 1951) and plastid terminal oxidase (PTOX) activity (Nawrocki et al. 2015; Kambakam et al. 2016; Saroussi et al. 2019; Rog et al. 2022), have evolved in photosynthetic organisms to quench excessive light energy and achieve homoeostasis and optimal photosynthetic performance under HL. Compared with the linear electron transport in which electrons derived from water splitting in PSII reduce NADP+, the activity of energy dissipation mechanisms leads to a net loss of photonic energy or reducing equivalents, protecting from excess reductive activity and helping in adjusting ATP/NADPH ratio. An additional way to protect the photosynthetic apparatus from excess illumination while conserving energy is to channel energetic electrons into reduced carbon products such as starch and lipids (Treves et al. 2016; Saroussi et al. 2019). Besides carbon fixation, photosynthesis generates ATP and reducing power that is used to assimilate inorganic nutrients into organic substances, with nitrate assimilation being the most energy-consuming process. Compared with CO2 reduction, nitrate assimilation requires more electrons and less ATP, thus playing a role in balancing ATP/NADPH (Noctor 1998). However, the direct role of nitrogen assimilation in the alleviation of high light-induced photodamage and maintaining chloroplast redox homoeostasis is not fully resolved.
In most environments, nitrate (NO3–) is the primary nitrogen form available (Miller, Bowman, and Suding 2007). Its assimilation occurs in the roots and shoots of vascular plants, with the shoot being the predominant site in most cases (Scheurwater et al. 2002; Xu, Fan, and Miller 2012). The nitrate assimilation pathway consists of several key enzymes and is spatially separated between the cytoplasm and plastids/chloroplasts. Nitrate reductase (NR) catalyses nitrate reduction in the cytosol, forming nitrite, a highly reactive nitrogen compound. Nitrite is transported from the cytosol to the chloroplast, where it is reduced to ammonium via nitrite reductase (NiR) activity. The ATP-dependent incorporation of ammonium into amino acids is catalysed by the glutamine synthetase (GS)/glutamate synthase (GOGAT) cycle (Lea and Miflin 1974), in which GS catalyses the production of glutamine (Gln) from ammonium and glutamate (Glu). The reductive transfer of the amide group from Gln to α-ketoglutarate, which produces two Glu molecules, is catalysed by GOGAT. Glu and Gln serve as N-donors for the production of various N-containing compounds, such as the biosynthesis of amino acids and nucleic acids (Coruzzi 2003).
Two types of GS were found in plants (Lea and Miflin 2003). In Arabidopsis, GS1 type is encoded by five genes (GLN1; 1–5) and is active in the cytosol of roots and leaf cells. GS2 is encoded by a single gene (GLN2) and is located in plastids/chloroplasts. It has been suggested that GS2 plays a central role in the nitrate assimilation pathways as well as in the re-assimilation of NH4+ released during photorespiration (Bernard and Habash, 2009; Ferreira et al. 2019; Marino, Cañas, Betti 2022). Indeed, barley, legume Lotus japonicus and tomato mutants lacking GS2 failed to thrive and showed severe stress symptoms under normal air conditions while growing normally under non-photorespiration conditions (i.e., O2-depleted or CO2-enriched air) (Blackwell, Murray, and Lea 1987; Wallsgrove et al. 1987; Orea et al. 2002). In contrast, the recently characterised Arabidopsis GS2 mutants had no severe phenotypes, demonstrating that GS2 activity is not critical for plant survival and questioning its central role in primary N metabolism (Ferreira et al. 2019; Hachiya et al. 2021; Lee, Chung, and Hsieh 2022; Marino, Cañas, Betti 2022; Kobercová, Melo, and Fischer 2024).
NADH-dependent and ferredoxin (Fd)-dependent GOGAT enzymes have been identified in plants, with the latter being unique to photosynthetic organisms and responsible for most GOGAT activity in leaves (Somerville and Ogren 1980; Suzuki and Rothstein 1997). In the Arabidopsis genome, two genes encoding Fd-GOGAT were found: GLU1 and GLU2, with GLU1 playing an active role in re-assimilating photorespiratory NH4+ as well as in nitrogen assimilation in shoots, whereas GLU2 is involved in root nitrogen assimilation (Coschigano et al. 1998). Nitrate reduction and subsequent ammonium assimilation require a considerable amount of reducing equivalents in the form of Fd or NADPH, which, in leaves, are directly supplied by the photosynthetic electron transport chain (Stitt et al. 2002; Foyer, Noctor, and Hodges 2011). Depending on the environmental conditions and nitrate availability, nitrate reduction can consume 5%–25% of reducing equivalents generated in chloroplasts, rendering it the second largest sink for low potential electrons generated by the photosynthetic electron transport chain (Bloom et al. 1989; Edwards and Baker 1993; Stitt et al. 2002). Accordingly, nitrate assimilation is considered the primary factor contributing to the gap between electron requirements for oxygen evolution and CO2 assimilation (de la Torre, Delgado, and C Lara 1991; Edwards and Baker 1993; Noctor 1998). As a major electron-consuming pathway, nitrate assimilation activity is tightly linked to the photosynthetic light and carbon reactions. The tight regulation of electron partitioning between nitrate assimilation and other electron-consuming pathways is achieved, at least in part, by the redox regulatory systems, as indicated by the redox sensitivity and Trx-based regulation of various N assimilation enzymes (Lemaire et al. 2007; Muthuramalingam et al. 2013; Rosenwasser et al. 2014; González et al. 2019).
ROS detoxification via the ascorbate–glutathione cycle involves the drawing of electrons from the glutathione (GSH) pool (Foyer and Noctor 2011; Awad et al. 2015). The resultant GSH disulfide (GSSG) is reduced by the electron carrier NADPH in a reaction catalysed via GSH reductase (GR). Thus, changes in the balance between ROS and reducing power production can be reflected in the GSH redox potential (EGSH), which is dependent on GSH level and the [GSH]/[GSSG] ratio (Foyer and Noctor 2011; Rahantaniaina et al. 2013). Recently, the role of chloroplastic EGSH in maintaining efficient photosynthesis has been demonstrated using GR knockout lines in the model moss Physcomitrella patens (Müller-Schüssele et al. 2020). High spatiotemporal resolution monitoring of EGSH can be achieved using redox-sensitive green fluorescent proteins (roGFPs) (Meyer et al. 2007; Meyer 2008; Meyer and Dick 2010), which carry two engineered cysteine residues that form an intramolecular disulfide bridge which impacts its fluorescence characteristics (Dooley et al. 2004; Hanson et al. 2004). Recently, daily patterns in chloroplast-specific EGSH under normal and high light conditions and the effect of NPQ and cyclic electron flow pathways in shaping these patterns were resolved using chloroplast-targeted roGFP2 (Haber et al. 2021). However, the possible role of nitrate assimilation in preventing over-reduction and ROS generation under HL conditions, thereby balancing the chl-EGSH, has not been investigated.
The present work aimed to examine the possible role of the nitrogen assimilation pathway as an alternative reducing power sink under high light, by examining the flux from nitrate into glutamine and glutamate, PSII efficiencies and chl-EGSH dynamics in plant mutated in GS2 and GOGAT1. Here, we demonstrated the induction of nitrate assimilation pathways under HL and the impairment of de-novo nitrogen assimilation in the examined mutant lines. We also showed that the decrease in nitrate assimilation rates observed in the mutant lines is associated with a lower PSII efficiency and abnormal daily chl-EGSH dynamics.
2 Results
2.1 Growth Phenotype of GS2 and GOGAT1 Mutant Lines
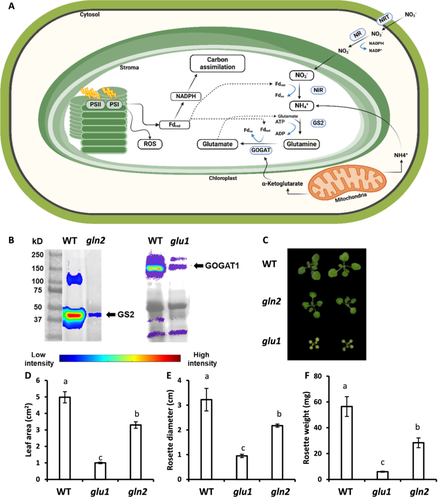
To assess the essentiality of nitrate assimilation as an energy dissipation route, gln2 (Ferreira et al. 2019) and glu1 (Somerville and Ogren 1980) mutant lines, defective in GS2 and GOGAT1, respectively (Figure 1A) were studied. Immunoblot analysis confirmed a significant reduction in GS2 and GOGAT1 levels in gln2 and glu1 lines, respectively, compared to WT (Figure 1B). The appearance of weak bands in protein extractions derived from the mutant lines may reflect residual expression levels. No severe phenotype was observed in gln2 mutant lines despite being slightly smaller than WT. For example, gln2 leaf area was ~70% of that of WT plants (Figure 1C-F). These results are in agreement with recent reports which showed that Arabidopsis gln2 knockout mutants are viable under photorespiratory conditions (Lee, Chung, and Hsieh 2022; Kobercová, Melo, and Fischer 2024), but lie in contrast to the severe phenotype of GS2 mutants in barley and L. japonicus (Blackwell, Murray, and Lea 1987; Orea et al. 2002). The glu1 plants showed a chlorotic phenotype and were small, with a five-fold reduction in leaf area compared to WT plants (Figure 1C–F). These results are in agreement with previous observations of the typical photorespiratory phenotype of glu1 plants (Somerville and Ogren 1980; Coschigano et al. 1998).
2.2 The Primary Nitrate Assimilation Pathway Involves GS2 and GOGAT1 Activity and Is Enhanced Under HL
To directly explore the in vivo activity of the nitrogen assimilation pathway, the rate of nitrogen assimilation was evaluated by following the transfer of isotope-labelled 15N from nitrate into Gln (glutamine) and Glu (glutamate) in hydroponically grown plants (Figure 2A). Gln contains both amide and amine nitrogen groups, while Glu contains one amine. Accordingly, labelled Glu was detected by a 1 Da mass shift while labelled Gln was identified as either a 1 Da or 2 Da mass shift, indicated as 15N1-Gln and 15N2-Gln, respectively. The analysis was carried out using high-resolution mass spectrometry, allowing the resolving of naturally occurring 13C isotopes from experimentally introduced 15N isotopes (Supporting Information S1: Figures S1–S3). Isotopically-labelled 15N, derived from 15NO3- was efficiently incorporated into Gln and Glu in WT plants maintained for 24 h under normal growth conditions (NL, 120 μmol m−2 s−1, 16 h light/8 h dark), with ∼23.5% of the total Glu marked with 15N and ~42.3% and ~6.1% of the total Gln marked with 15N1 and 15N2, respectively (Figure 2B). The structure of the nitrogen assimilation pathway can explain this hierarchy in the rate of accumulation of the labelled amino acids. As shown in Figure 1A, Gln is the first amino acid assimilated with labelled nitrogen via GS activity, explaining its fastest labelling rate. Then, in a GOGAT-catalysed reaction, the amide group of Gln is transferred to a 2-oxoglutarate molecule, yielding two glutamates. The lower abundance of 15N-Glu compared to 15N1-Gln is likely the result of the diversion of some of 15N1-Gln to amino acid or protein biosynthesis instead of to 15N-Glu. As predicted, the relative abundance of 15N2-Gln was lower than 15N1-Gln, as it was generated through GS2 activity with 15N-Glu serving as a substrate. Minor 15N incorporation into Glu and Gln was observed in plants maintained for 24 h in the dark, with the percentage of labelled amino acids being 0.73%, 1.75% and 0.04% for 15N-Glu, 15N1-Gln and 15N2-Gln, respectively. In comparison, values less than 0.006% were recorded in leaf samples not exposed to 15N, representing naturally occurring isotopes. These results clearly demonstrate the light dependency of the nitrogen assimilation pathway (Figure 2B).
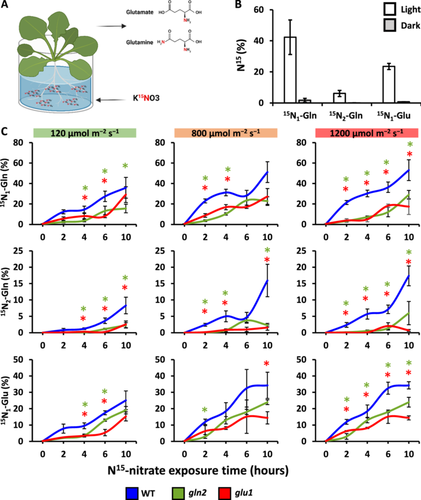
To determine whether GS2 and GOGAT1 activities are required for the de-novo nitrate assimilation pathway and how light intensities affect this process, WT, gln2 and glu1 plants, grown under NL, were treated with 15NO3− and immediately exposed to 800 (ML) or 1200 μmol m−2 s−1 (HL) for 10 h or left under NL as control (Figure 2C). An increase in the relative abundance of labelled Gln and Glu was detected during the experiments in all tested lines, demonstrating their ability to acquire and assimilate the labelled nitrate. Higher incorporation rates were measured under HL compared to NL in WT plants, with, for example, 27.4% and 36.3% of 15N1-Gln after 6 h under NL and HL, respectively. The most pronounced effect of HL treatment was evident at 4 h and 6 h, (p = 0.01, 0.0064, 0.04, for 15N1-Gln, 15N2-Gln and 15N-Glu, respectively). An increase in the average relative abundance of labelled amino acids was also detected in plants after 4 h and 6 h under ML compared to NL, although the effect of ML was less significant. These results show that high light intensities stimulate the nitrogen assimilation pathway.
Under all three light regimes and throughout the experiment, significantly lower rates of labelled nitrate incorporation were measured in gln2 and glu1 compared to WT, demonstrating the impaired nitrogen assimilation pathway in these mutants (Figure 2C). For example, the relative abundance of 15N-Glu after 6 h in NL was 17.5%, 12.9% and 6.2% for WT, gln2 and glu1, respectively. Unlike the light-stimulated nitrate assimilation observed in WT, no significant effect of light intensities on the assimilation rate was detected in glu1, while moderate stimulation in gln2 was observed under ML but not HL. Taken together, the absence of GS2 and GOGAT1 does not entirely abolish nitrogen assimilation but significantly reduces its rate.
2.3 Nitrate Assimilation Alleviates High Light-Induced PSII Photoinhibition
It was hypothesised that nitrate assimilation under high light prevents damage to PSII by consuming excessive photosynthetic electrons. In such a case, the poor nitrate assimilation in gln2 and glu1 was expected to result in a lower electron flux through PSII. To test this hypothesis, ΦPSII was assessed based on chlorophyll fluorescence measurements in WT, gln2 and glu1 plants under high light conditions. ΦPSII recorded in darkness, which represents the maximum quantum efficiency of PSII, was lower for glu1 (ΦPSII = 0.73) compared to gln2 and WT (ΦPSII = 0.78), with no significant differences between gln2 and WT lines (Figure 3). Severely impaired photosynthetic efficiency was observed in gln2 and glu1 plants exposed to high light intensities, reaching ΦPSII values of 0.3–0.4 in plants illuminated at 800 and 1200 µmol m−2 s−1 (Figure 3A,B). In comparison, ΦPSII values of approximately 0.6 and 0.5 were recorded for WT plants exposed to the same conditions.
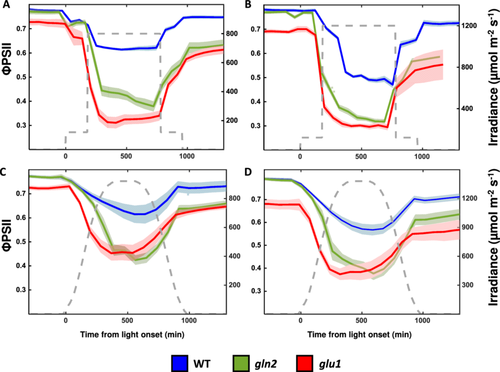
To mimic physiologically relevant light conditions, plants were exposed to light intensities gradually increasing to a maximum value of either 800 or 1200 µmol m−2 s−1, followed by a gradual decrease in light intensities towards the dark period (Figure 3C,D). In general, a decrease in ΦPSII in response to increasing light intensity followed by increasing ΦPSII when the light was dimmed toward the end of the day was found in all three lines, under both light treatments. As expected, in all examined lines, the lowest ΦPSII values were recorded towards the middle of the day, as the light reached its maximum intensity, with the lower values observed in plants exposed to 1200 µmol m−2 s−1 compared to 800 µmol m−2 s−1. Importantly, lower ΦPSII values were measured in gln2 and glu1 lines compared to WT. For example, mid-day ΦPSII values of 0.44, 0.43 and 0.61 were recorded for gln2, glu1 and WT, respectively, in response to 800 µmol m−2 s−1 and 0.36, 0.37 and 0.53 were recorded in response to 1200 µmol m−2 s−1. In conclusion, these data demonstrate that impairment of nitrogen assimilation enzymes resulted in lower photosynthetic efficiency, suggesting that the induction of nitrogen assimilation under high light conditions serves as a photoprotective mechanism.
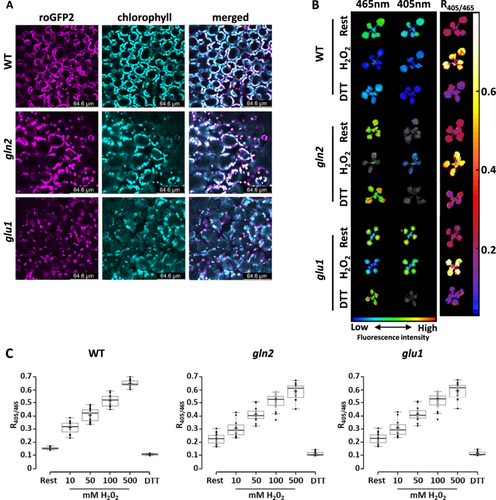
2.4 Gradual Relaxation of chl-EGsh Under High Light Is Dependent on the Nitrogen Assimilation Pathway
Considering the role of nitrogen assimilation as an electron outlet, which preserves PSII activity, we hypothesised that it also plays a role in preventing ROS overproduction, thereby shaping chl-EGSH dynamics under high light. To test this hypothesis, we produced plants expressing the chl-roGFP2 in the background of WT, gln2 and glu1 lines. Antibiotic-resistant plants with intense chl-roGFP2 fluorescence levels were selected to monitor the degree of chl-roGFP2 oxidation (Figure 4A,B). No phenotypic differences were observed between the original lines and their correspondence lines expressing chl-roGFP2 (Supporting Information S1: Figure S4). Chloroplast localisation of chl-roGFP2 was validated using confocal microscopy, as shown by colocalization of roGFP2 and chlorophyll autofluorescence (Figure 4A). The probe's response to redox changes was monitored in the resting state and following the application of hydrogen peroxide (H2O2) or dithiothreitol (DTT) to induce full probe oxidation and reduction, respectively (Figure 4C). An increase from resting state 400/488 nm fluorescence ratio was observed in H2O2-treated plants of WT, gln2 and glu1 chl-roGFP2 expressing lines. DTT treatment resulted in a moderate decline in the 400/488 ratio compared to the resting state. A similar dynamic range (400/488 nm under a fully oxidised state divided by 400/488 under fully reduced state) of approximately 5.5 was recorded for all chl-roGFP2 lines. These results demonstrate the sensitivity of the probe to changing redox conditions and are consistent with probe characteristics previously observed in plant cells (Schwarzländer et al. 2008; Haber et al. 2021; Hipsch et al. 2021). Notably, dose-dependent responses of chl-roGFP2 to elevated H2O2 concentrations suggest no difference, among the different lines, in the antioxidant capacity to detoxify H2O2 (Figure 4C).
WT, gln2 and glu1 lines expressing chl-roGFP2 were exposed to 3 h of normal growth conditions (120 µmol m−2 s−1) at the beginning of the day, followed by a phase of ML or HL (i.e., 800 or 1200 µmol m−2 s−1) for a 10 h period. After the ML and HL phase, the light was dimmed to the normal growth conditions for an additional 3 h period. chl-roGFP2 oxidation degree (OxD) was measured using an automated system that continuously measures fluorescence signals from living plants (Haber et al. 2021; Lampl et al. 2022). The chl-roGFP2 OxD values were normalised with the OxD values collected under NL, measured the day before the experiment began. Upon transition from NL to HL conditions, an increase in chl-roGFP2 OxD was observed in all lines (Figure 5A,B). Interestingly, when plants were returned to NL after the HL period, a decrease in chl-roGFP2 OxD to steady-state levels was clearly seen for WT but not for gln2 and glu1 lines. A return to steady-state levels was only detected in the mutant lines when plants were placed in the dark (Figure 5A,B).
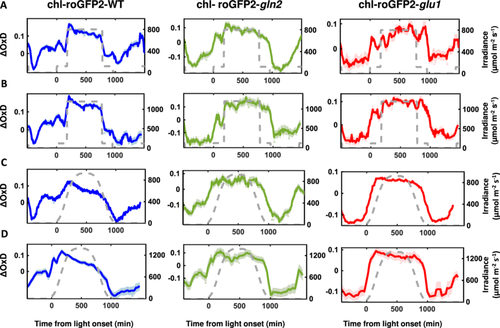
When chl-roGFP2-expressing WT plant lines were exposed to gradual increases in light intensities, peaking at 800 m−2 s−1, followed by a gradual decrease, identical to the conditions introduced in the ΦPSII experiments (Figure 3C), OxD gradually increased, peaking (ΔOxD = 0.127) when the light intensity was ∼600 µmol m−2 s−1. From that point onward, a gradual decrease in chl-roGFP2 oxidation was observed, reaching its lowest oxidation degree (ΔOxD = −0.113) at the beginning of the dark period (Figure 5C). A similar pattern of initial oxidation followed by reduction during the high light period was also observed in plants exposed to gradual increases in light intensities, peaking at 1200 m−2 s−1 (Figure 5D). These results agree with our previous reports showing oxidation followed by relaxation of chl-roGFP2 in repose to increasing light intensities (Lampl et al. 2022). Remarkably, chl-roGFP2 oxidation patterns in roGFP2-expressing gln2 and glu1 lines were different than those of WT. When exposed to increasing light intensities, mutant lines showed increased chl-roGFP2 oxidation, similar to WT, suggesting that the nitrate assimilation pathway does not affect the initial light-driven changes in chl-EGSH. However, no relaxation was observed during the high light phase in the mutant lines. (Figure 5C,D). Inspection of the chl-roGFP2 oxidation patterns in these lines revealed that the reduction in chl-roGFP2 OxD values occurred when light intensities were decreased towards darkness. These results demonstrate the effect of nitrogen assimilation in alleviating oxidative stress under light stress and suggest that the reduction in chl-roGFP2 OxD during the high light period in WT plants is achieved by diverting excess electrons to the nitrogen assimilation pathway.
2.5 Decreasing Photorespiration Rates by Threefold Did Not Affect ΦPSII and EGSH Patterns in gln2 and glu1
De-novo nitrogen assimilation as well as the re-assimilation of ammonia released in the photorespiration pathway require the activity of the GS/GOGAT cycle, indicating that both pathways are interconnected. Accordingly, the observed phenotypes in gln2 and glu1 mutants may be associated with the impairment recycling of photorespiratory metabolites rather than pointing to a direct link between nitrogen assimilation, PSII photoinhibition, and EGSH homoeostasis. To assess the contribution of hampered photorespiration to the observed phenotypes, ΦPSII and EGSH were measured in gln2, glu1, and WT plants under elevated CO2 (Figure 6A). To adapt plants to higher CO2 levels, plants were grown at 700 ppm CO2 for 3 weeks (Supporting Information S1: Figure S5). Growing plants at higher CO2 concentrations resulted in adverse effects in all genotypes. Under elevated CO2, WT and gln2 leaves were indispensable (Figure 6B), consistent with a recent report made by Kobercová, Melo and Fischer (2024). Consisting with the role of Fd-GOAGT in photorespiration, the growth of glu1 also improved under elevated CO2 compared to ambient conditions. Nevertheless, glu1 remained smaller than WT even under elevated CO2 (Figure 6B).
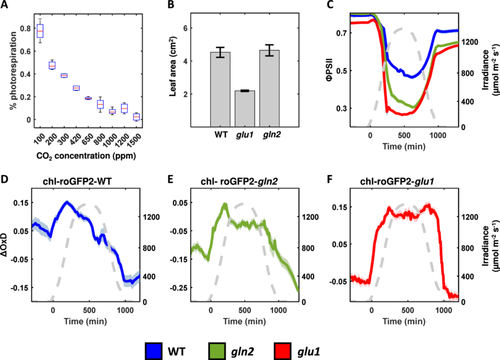
ΦPSII and EGSH were recorded in plants adapted to CO2 exposed to high light (gradual increases in light intensities, peaking at 1200 m−2 s−1, followed by a gradual decrease) and elevated CO2 concentrations of ~900 ppm (Figure 6C–F). Based on A/Ci curves conducted under conditions of 21% and 1% in air, such CO2 levels are expected to decrease photorespiration to a low level (9%), but not to completely suppress the Rubisco oxygenation (Figure 6A). Importantly, similar to data recorded in ambient conditions (Figure 3), lower ΦPSII was measured in gln2 and glu1 lines compared to WT with mid-day values of 0.30, 0.26, and 0.46 for gln2, glu1, and WT, respectively (Figure 6C). Examination of chl-roGFP2 oxidation pattern throughout the day also yielded results similar to those observed under ambient conditions, displaying oxidation followed by relaxation in WT plants and no relaxation under high light conditions in both mutants (Figure 6D–F). These results suggest that the increased PSII photoinhibition and deviation from the WT chl-EGSH pattern observed in gln2 and glu1 are not related to the activity of the photorespiration pathway.
3 Discussion
Tight coordination between the production of highly energised electrons in the photosynthetic light reaction and their consumption by downstream carbon reactions is crucial for preventing the overproduction of photosynthesis-generated ROS. Several photoprotective mechanisms have evolved in plants to protect the photosynthetic machinery from over-reduction of the photosynthetic electron chain. In some of them, energy is dissipated (e.g., quenched through heat production), while in others, over-reduction is avoided by electron flow to alternative electron-consuming pathways (Saroussi et al. 2019). As the nitrogen assimilation pathway consumes photosynthesis-derived reduced Fd and NADPH either in the chloroplast for nitrite reduction or in the cytosol through NR and the malate shunt activity, it can serve as an alternative electron-consuming pathway (Noctor 1998). Accordingly, it has been suggested that nitrogen assimilation plays a photoprotective role, avoiding energy imbalance induced by excessive reducing power and ATP accumulation (Noctor 1998; Busch, Sage, and Farquhar 2018). Using plants mutated in the nitrogen assimilation pathway, this study aimed to experimentally test the effectiveness of nitrogen assimilation in alleviating light stress.
By tracing the incorporation of 15N-nitrate into amino acids, we demonstrated decreased nitrogen assimilation activity in gln2 and glu1 mutant lines under normal and high light conditions, confirming the role of GS2 and GOGAT1 in the de-novo nitrate assimilation pathway. Yet, while lower than WT, the noticeable rate of nitrogen assimilation observed in these two mutant lines suggests that other molecular components can partially compensate for the lack of GS2 and GOGAT1. In the case of GS2, it was suggested that cytosolic GS activity could compensate for the lack of GS2 (Lam et al. 1996). Similarly, it is possible that GOGAT2 activity partially compensates for the lack of GOGAT1 activity in glu1, although GOGAT2 expression is mainly concentrated in roots (Coschigano et al. 1998). Alternatively, the activity of NADH-GOGAT may compensate for the lack of Fd-GOGAT1 activity (Lancien et al. 2002; Selinski and Scheibe 2014; Selinski and Scheibe 2019).
The compromised nitrate assimilation observed in the two mutant lines may affect multiple cellular processes involving nitrogenous compounds. This may make it challenging to discern between the effect of the nitrate assimilation per se and the potential effects of inadequate nitrogen availability. For example, alterations in chloroplastic EGSH may be a result of the decreased GSH content, as the synthesis of amino acids might be limited as nitrate assimilation is less efficient. Yet, several lines of evidence suggest a direct link between nitrate assimilation, photosynthetic efficiency and GSH redox potential. The similar ΦPSII levels detected in WT and gln2 under normal growth conditions indicate that the vulnerability of gln2 to HL is directly caused by impairments in nitrogen assimilation pathways rather than a global change in leaf nitrogen status. In contrast, the low ΦPSII levels in glu1 were likely related to the overall defects of this mutant when grown in ambient CO2 levels. Indeed, extensive changes in gene expression profiles in glu1 plants have been demonstrated in transcriptomic analyses, including the downregulation of photosynthesis-related pathways and the induction of stress-related genes (Kissen et al. 2010). Notably, despite phenotyping differences, chl-roGFP2 OxD values were similar in WT, gln2 and glu1, under steady-state and in response to H2O2, implying no differences in overall GSH pool size among the plant lines under normal growth conditions (Figure 4C). It should be noted, however, that since chl-EGSH is dependent on both glutathione concentration and degree of oxidation (GSH/GSSG, Meyer et al. 2007), it is not possible to extract data regarding the total quantity of glutathione in the chloroplasts of the examined lines. Importantly, the similar deviation from WT patterns observed for chl-roGFP2 OxD in gln2 and glu1, despite being different in other physiological aspects such as growth rate and PSII activity, strengthens the notion that de-novo nitrate assimilation serves as an electron sink, which contributes to the maintenance of redox homoeostasis under light stress.
Besides being an electron consumer, nitrate assimilation also affects photosynthesis by its interconnection with the photorespiration pathway. This interaction arises from the dual role of the chloroplastic GS/GOGAT cycle in nitrate assimilation and photorespiration and the need for photorespiratory carbon skeletons for amino acid biosynthesis (Noctor 1998; Broncano et al. 2023). Indeed, inhibition of nitrate assimilation has been demonstrated under non-photorespiratory conditions, while improvement in carbon assimilation rates has been achieved upon dual activation of the nitrate assimilation and photorespiratory pathways (Rachmilevitch, Cousins, Bloom 2004; Busch, Sage, and Farquhar 2018). While the viable phenotype of gln2 mutant line in ambient conditions raised questions about its involvement in photorespiration (Hachiya et al. 2021; Lee, Chung, and Hsieh 2022), our data, demonstrating improved gln2 growth under elevated CO2 agrees with a recent report that pointed for its contribution to the photorespiratory pathway (Kobercová, Melo, and Fischer 2024). Notably, the comparable maximum quantum efficiency of PSII in WT and gln2 (Figure 3) and slight growth phenotype of gln2 under ambient conditions are in contrast to the enhanced levels of photoinhibition reported in photorespiration-defective mutant lines upon exposure to air (Takahashi, Bauwe, and Badger 2007). Thus, while the slower incorporation rates of 15N in gln2 compared to WT clearly show the role GS2 plays in primary nitrate assimilation (Figure 2), its involvement in photorespiration still requires more research.
The typical photorespiratory phenotype of glu1, which developed into small, chlorotic plants with a low maximum quantum efficiency of PSII when grown under air, was in line with previous observations (Somerville and Ogren 1980; Coschigano et al. 1998). The low incorporation of 15N into Gln and Glu in this line (Figure 2C) confirmed the role of Fd-GOGAT in the de-novo nitrate assimilation pathway, locating Fd-GOGAT in a central position in plant nitrogen metabolism. Notably, the similar all-day ΦPSII and chl-EGSH patterns observed for WT, gln2 and glu1 under ambient and high CO2 concentrations, which slows down significantly photorespiration rates (Figures 3, 5 and 6) suggest that the de-novo nitrogen assimilation, rather than photorespiration shapes PSII efficiency and chl-EGSH under dynamic light conditions.
The pronounced decrease in ΦPSII in both mutants under high light (Figure 3) highlights the importance of nitrate assimilation in electron consumption to alleviate PSII photodamage. In line with these observations, increased activities of NR and GS were recently reported in cotton plants exposed to HL conditions (Guilherme et al. 2019). In addition, plants with high nitrate supply showed favourable photochemical characteristics such as higher electron flux through PSII and lower limitation in the acceptor side of PSI than those provided with minimal nitrate levels (Guilherme et al. 2019). Considering the high sensitivity of PSI to conditions in which electron flow from PSII exceeds the capacity of their consumption by PSI electron acceptors (Sonoike 2011), the higher PSII photoinhibition levels observed in the mutant lines can be viewed as an alternative photoprotection mechanism (Tikkanen, Mekala, and Aro 2014). This mechanism is enhanced when the diverting of reducing power to nitrate assimilation is not adequate to prevent overreduction of the photosynthetic electron transfer chain. This hierarchy may indicate the higher tendency to route incoming energy to sustainable sinks rather than quenching through heat production.
The importance of nitrate assimilation in managing excitation energy downstream to PSI was evident by the observed chl-EGSH patterns. chl-roGFP2 oxidation state reflects the balance between H2O2 production and NADPH availability for GR activity (Meyer et al. 2007; Meyer 2008). Here, chl-roGFP2 relaxation, observed upon transfer of WT plants from HL to NL (Figure 5A,B) or exposing plants to gradually increasing light intensities (Figure 5C,D), was not observed in nitrogen assimilation mutant lines. These results imply a shortage of NADPH supply required to relax chl-EGSH and chl-roGFP2 when nitrogen assimilation is impaired, possibly due to low ΦPSII and activation of alternative energy dissipation pathways. Interestingly, a recent report demonstrated the capability of NADPH-thioredoxin reductase C (NTRC) to mediate disulfide reduction of the roGFP2 moiety when both proteins were forced into close proximity in a fusion construct (Molinari et al. 2023). Whether unfused NTRC and roGFP2 interact similarly in chloroplasts and whether this reaction competes kinetically with glutaredoxin-mediated roGFP2 reduction remain unresolved. Nevertheless, considering both possible mechanisms for chl-roGFP2, the presented results indicate that the diversion of electrons for nitrate assimilation shapes the availability of high-energy electrons for antioxidant and redox signalling under dynamic light conditions.
Nitrate and ammonium are the primary nitrogen forms absorbed by plant roots, and their relative availability varies in different ecosystems and environmental conditions (Reed and Hageman 1980; Andrews 1986). Under light-limiting conditions, ammonia assimilation can be an adaptive strategy to fulfil the heightened demand for photosynthetically derived reducing power when nitrate is assimilated (Debiasi et al. 2021). Yet, ammonium might be toxic to plant cells when it accumulates to high concentrations, limiting its utilisation even under low light conditions. On the other hand, in environments where light is not a limiting factor and especially in stressful fluctuating environments where the risk for photodamage is high, the higher energetic requirements of nitrate compared to ammonium assimilation, renders nitrate assimilation a stronger sink for electrons and, therefore a better strategy to cope with light-induced stress. Indeed, faster net oxygen evolution was found in plants exposed to high light when receiving nitrate rather than ammonium as a nitrogen source (Walker et al. 2014; Bloom 2015). Therefore, sophisticated nitrogen fertiliser management, according to the spatiotemporal variability in light conditions in agricultural fields, could decrease PSII photodamage, slow NPQ activation and likely improve photosynthesis and crop yields. Additional experiments will be needed to determine if nitrogen fertilisation protocols can be fine-tuned to provide plants with adequate nitrogen and allow plants to better acclimate to high light-induced stress conditions.
Nitrate assimilation occurs both in roots and shoots, with high variation across plant species in their reliance on such activities in the root versus shoot, as explored by the differences in NR activity in the two plant parts and the relative abundance of nitrate and reduced nitrogen compounds in the xylem sap of various plant species (Crafts-Brandner and Harper 1982; Sprent and Thomas 1984; Smirnoff and Stewart 1985; Andrews 1986; Debiasi et al. 2021). Root assimilation, which relies on respiration-derived reductants, is more energetically costly than utilising reducing equivalents directly from the photosynthetic light reaction, although the reliance on respiratory fixed sugar was suggested to be an advantage in certain conditions, such as low temperatures (Andrews 1986). While the mechanisms that determine the partitioning of nitrate assimilation between root and shoot have not been resolved, the presented role of nitrate assimilation in protecting PSII photodamage and regulating the chloroplastic GSH redox potential might be a key factor in adopting root versus shoot assimilation strategies.
4 Methods
4.1 Plant Material and Growth Conditions
Arabidopsis thaliana WT (ecotype Columbia-0), gln2 (SALK_051953, At5g35630) and glu1 (CS8611, At5g04140) lines were used throughout this research. Seeds were sown on unfertilised Klasmann-Deilmann (K) Select soil (fertilised manually at sowing with 1 g/L of 20-20-20+Micro [E5020372KJSS12, Ecogan]), transferred to the dark at 4°C for 2 days and then grown under a 16/8 light-dark cycle with a photosynthetic photon flux density (PPFD) of 120 μmol m−2 s−1 (21°C, 60%–70% RH, ambient CO2), for 2–3 weeks. For 15N incorporation experiments, plants were grown in hydroponic nutrient solution for 3 weeks.
4.2 Hydroponics Solution
The hydroponics nutrient solution, based on Hoagland solution, comprised of 2 M KNO3 (or K15NO3), 1 M Ca(NO3)2, 1 M MgSO4, 1 M KH2PO4 and 0.1 M EDFS as macroelements and 0.5 mM CuSO4, 0.5 mM H2MoO4, 2 mM MnSO4, 50 mM KCl, 25 mM H3BO3 and 2 mM ZnSO4 as microelements. The solution pH was set to 5.7–5.8 using NaOH and HCl for adjustments (measured by Jenway 351000 3510 Bench pH/mV Metre).
4.3 LC-HRMS Analysis of Glutamine and Glutamic Acid
For the quantification of labelled 15N2-Gln, the above equation was used with M + 2 in the numerator.
4.4 Western Blot Analysis
Protein extracts were prepared, using a hand homogeniser, in 2 mL tubes with 200 µL extraction buffer containing 5 µL/mL NP40 and 1 µL/mL protease inhibitor (PI). Proteins were precipitated for 2 min on ice and centrifuged at 14,000 rpm for 17 min, at 4°C. The supernatant (100 µL) of each sample was transferred to fresh 2 mL tubes. DTT (10 mM, final concentration) and sample buffer (×3) (150 mM Tris-HCl, pH 6.8, 6% (w/v) SDS, 30% glycerol and 0.3% pyronin Y) were added to each sample. The samples were then boiled for 10 min and immediately transferred to ice for 2 min. Gel fractionation was carried out on handcast 10% polyacrylamide gel. Fractionated proteins were transferred to a polyvinylidene fluoride membrane (Bio-Rad), using the Trans-Blot Turbo Transfer System (Bio-Rad) with Trans-Blot Turbo Midi Transfer Packs. The membrane was incubated with anti-GS2 antibody (1:5000; agrisera) or anti-GOGAT1 antibody (1:1000; agrisera), both followed by goat anti-rabbit horseradish peroxidase (HRP)-conjugated IgG (1:20,000; agrisera). Chemiluminescence was detected using the Advanced Molecular Imager HT (Spectral Ami-HT, Spectral Instruments Imaging LLC. USA).
4.5 Chl-roGFP2 Transformation
The entire gene sequence of chl- roGFP2 was chemically synthesised for the generation of a chl- roGFP2-expressing line. Chloroplast targeting was achieved using the 2-Cys peroxiredoxin A (PRXa; Uniprot ID: Q96291) signal peptide. The chl-roGFP2 gene was cloned into the plant cloning vector pART7 using XhoI and HindIII restriction enzymes. The entire construct, including the CaMV 35S promoter and ocs terminator, was cloned into the binary vector pART27 using the restriction enzyme NotI. The pART27 plasmid containing the chl-PRXaTP-roGFP2 construct was transformed into Agrobacterium tumefaciens (GV3101) using heat-shock transformation.
The transformed Agrobacterium were grown on selective plates (LB agar [35 g/1 L dW] + rifampicin [10 ng/mL], gentamicin [30 ng/mL] and spectinomycin [100 ng/mL]), at 28°C for 24–72 h (until visible bacterial coverage). The bacteria were then re-suspended in 50 mL falcons containing 20 mL LB, supplemented with rifampicin (10 ng/mL), gentamicin (30 ng/mL) and spectinomycin (100 ng/mL) and then incubated (28°C, shaken at 200 rpm) until OD = 0.8–1 (measured using a MRC V-1100D spectrophotometer). The tubes were then centrifuged for 5 min (4°C, 4000 rpm) and pellets were re-suspended in 10 mL 1% sucrose in distilled water (dW) to OD = 1, after which, 0.03% 77-L (Agan Adama) was added (referred to below as Transformation Solution). Transformation of A. thaliana was performed by floral dip (Clough and Bent 1998). Transformed lines were selected based on kanamycin resistance and the chl-roGFP2 fluorescence signal.
4.6 Confocal Microscopy
Images were acquired with a Leica TCS SP8 confocal system (Leica Microsystems) using the LAS X Life Science Software and an HC PL APO ×40/1.10 objective. All images were acquired at 4096 × 4096 pixel resolution, with an emission wavelength of 500–520 nm and excitation of 488 nm for chl-roGFP2 fluorescence and emission wavelength of 670 nm and excitation of 488 nm for chlorophyll fluorescence. All images were generated using Fiji (Image J) software.
4.7 Chl-roGFP2 Fluorescence Measurements and Analysis
Whole plant redox imaging was performed on 10 days-old transgenic chl-roGFP2-expressing plants. Fluorescence was detected using an Advanced Molecular Imager HT, and images were taken using the AMIview software. Plants were excited with 405 nm ± 10 or 465 nm ± 10 LED light sources and fluorescence was measured through a 515 nm ± 10 emission filter. For chlorophyll detection, a 405 nm ± 10 LED light source and a 670 nm ± 10 emission filter were used. All images were captured under the following settings: exposure time = 1 s, pixel binning = 2, field view (FOV) = 25 cm, and LED excitation power 40% and 60%, for 405 nm and 465 nm excitations, respectively. Excitation power for chlorophyll detection was 5%. Chlorophyll autofluorescence was measured to generate a chlorophyll mask, which was then used to select pixels that returned a positive chlorophyll fluorescence signal. Only those pixels were subsequently considered for the roGFP analysis. For autofluorescence correction, excitation was performed with a 405 nm ± 10 LED and a 448 nm ± 10 emission filter was used; the excitation power was 60%. Autofluorescence-corrected ratiometric images were generated according to Hipsch et al. (2023).
Quantitative chl-roGFP2 analysis was mainly carried out as per the plate reader method according to (Haber et al. 2021; Lampl et al. 2022) using the Spark Multimode Microplate Reader (Tecan, Switzerland). The following filters were used: excitation: 400/20 nm and 485/20 nm; emission: 520/10 nm. For chlorophyll detection, excitation at 400/20 nm and emission at 670/40 nm. For automatic detection of chl-roGFP2 signals, plants growing in 12-well plates were placed in a Fytoscope FS-SI-4600 (PSI, Czech Republic) chamber and were automatically taken into the fluorometer for fluorescence detection using a KiNEDx KX.01467 robot (paa, UK and USA). A 9-by-9-pixel matrix was formed from each well. Chlorophyll fluorescence was detected at 400 nm/670 nm to create a chlorophyll mask. Only pixels with a positive chlorophyll fluorescence signal were taken into account for the roGFP analysis. Then, the average fluorescence values recorded in nonfluorescent plants (WT) were calculated, and the values were subtracted as background autofluorescence from the values measured in the chl-roGFP2 plants. roGFP2 degree of oxidation (the relative quantity of oxidised roGFP proteins, OxD) was calculated based on the fluorescence signal, as previously described (Meyer et al. 2007).
4.8 Chlorophyll Fluorescence and Gas Exchange Measurements
Chlorophyll fluorescence was measured using a Walz PAM IMAGING PAM M-series IMAG-K7 (MAXI) fluorometer. The effective PSII quantum yield (ΦPSII) was determined by the equation of ΦPSII = (Fm′ − F)/Fm′. Images were analyzed with ImagingWinGigE V2.56p software. A/Ci curves under ambient and 1% O2 in air were conducted using the small plant chamber (6800-17) of the LI-6800 portable infrared gas analyzer (LICOR, Lincoln, NE, USA). To determine the percentage of photorespiration flux out of total gross photosynthesis, the difference between the rate of carbon assimilation recorded at 21% and 1% O2 was divided by the rate recorded in 1% O2.
4.9 Statistics
For 15N enrichment analysis, statistical significance was tested using a two-tail Student's t-test using JMP, and is indicated by asterisks.
Acknowledgements
The work was supported by Israel Science Foundation.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.