sly-miR408b Targets a Plastocyanin-Like Protein to Regulate Mycorrhizal Symbiosis in Tomato
ABSTRACT
Symbiosis between arbuscular mycorrhizal fungi and plants plays a crucial role in nutrient acquisition and stress resistance for terrestrial plants. microRNAs have been reported to participate in the regulation of mycorrhizal symbiosis by controlling the expression of their target genes. Herein, we found that sly-miR408b was significantly downregulated in response to mycorrhizal colonisation. Overexpression of sly-miR408b compromised mycorrhizal colonisation by Rhizophagus irregularis in tomato (Solanum lycopersicum) roots. A basic blue protein gene (SlBBP) was then identified as the new target gene of miR408b in tomato. The expression of membrane-located SlBBP was induced in a copper-dependent manner. Importantly, the loss function of SlBBP decreased the root mycorrhizal colonisation. Overexpression of SlBBP decreased SOD activity, which may interfere with the process of scavenging excessive reactive oxygen species (ROS). Mutation of RBOH1, which encodes ROS-producing enzymes NADPH oxidases, obviously reduced the arbuscule abundance in the mutant roots. Overall, our results provide evidence that sly-miR408b and its target gene SlBBP regulate mycorrhizal symbiosis in tomato through mediating ROS production.
1 Introduction
To better adapt to hostile environments, plants build extensive associations with soil microorganisms (Shi, Wang, and Wang 2023; M. Wang et al. 2024). Arbuscular mycorrhizal symbiosis, a mutually beneficial association between arbuscular mycorrhizal fungi (AMF) and most terrestrial plants including important crops, plays a vital role in plant nutrient acquisition and stress resistance (Smith and Read 2010; Q. Wang et al. 2024). This symbiotic relationship is based on nutrient reciprocity (Jiang et al. 2017).
In response to pathogenic infection, plants have evolved a set of defense mechanisms, known as PTI (pathogen-associated molecular pattern triggered immunity) and ETI (effector triggered immunity), to sense pathogenic invasion and defend against pathogens for further infection (Yuan et al. 2021). In contrast, plants have become more tolerant to symbionts during their biological evolution (Bonfante and Genre 2010). The degree of symbiosis is manipulated by the host plants according to the environment to prevent over-colonisation (Pozo et al. 2015). To promote the harmony of the symbiotic relationship with AMF, plants usually respond at the biological, cellular and molecular levels. Root architectural remodeling is an important characteristic of mycorrhizal symbiosis (Chiu, Choi, and Paszkowski 2018), and the presence of a large number of low-lignified lateral roots is conducive to the contact and penetration of the AMF hyphae (Gutjahr, Casieri, and Paszkowski 2009). Arbuscules are mainly formed in root cortex, where plant cells become more relaxed to wrap the almost entire arbuscule structure, forming the periarbuscular membrane (PAM) interface for nutrients exchange (Choi, Summers, and Paszkowski 2018). Many proteins are embedded in the plant cell membrane and spread out along the PAM interface, such as phosphorus transporters protein PT4 (Harrison, Dewbre, and Liu 2002; Xu et al. 2007) and blue copper protein BCP1 located in PAM (Pumplin and Harrison 2009). Plants undergo transcriptome reprograming during symbiosis (Gutjahr et al. 2015; Shu et al. 2016; Li et al. 2018; An et al. 2018), and it is worth noting that the normal expression of common symbiosis pathway genes (CSPs) is crucial for the establishment and maintenance of the symbiosis (Parniske 2008). Mutation of these genes usually leads to the failure of hyphal infection or incomplete arbuscule development, which severely affects mycorrhizal colonisation in plant roots (Floss et al. 2013; Pimprikar et al. 2016; Floss et al. 2017).
microRNAs (miRNAs) are a class of short-sequence noncoding RNAs that are transcribed from MIR genes (Kim et al. 2011; Summanwar et al. 2020). The transcribed pri-miRNAs undergo a series of splicing processes to produce mature miRNAs (Li and Yu 2021). In plants, mature miRNAs are loaded in AGO proteins to form miRISCs (miRNA-induced silencing complex) to silence expression of their target genes (Mi et al. 2008). Unlike animal miRNAs that mainly play a translational inhibitory role on target genes, plant miRNAs have a high degree of sequence matching with their target genes, and their mode of action on target genes is mainly mRNA splicing (Addo-Quaye et al. 2008; German et al. 2008). miR408 is widely distributed in different plants species, and its family members have been identified in 40 different plants (Gao et al. 2022), it is a 21-nt miRNA that is responsive to copper ion (Leng et al. 2017), light (Jiang et al. 2021) and stress, such as drought (Mishra, Sahu, and Shaw 2022), cold (Ma, Burd, and Lers 2015), and pathogen infection (Djami-Tchatchou and Dubery 2015). The main predicted targets of MiR408 are the genes encoding copper-binding proteins such as laccase proteins and phytocyanin proteins. In Arabidopsis, laccase 13 (LAC13) is the target of MiR408 (Abdel-Ghany and Pilon 2008), while in rice, the target gene of MiR408 is OsUCL8, an uclacyanin (UCL) gene of the phytocyanin family (Zhang et al. 2017). LAC13 competes for copper binding in leaf cells, and ultimately regulates leaf photosynthesis by affecting the amount of copper entering the chloroplast (Zhang et al. 2014). OsUCL8 is associated with the development of rice pollen tubes and thus has an impact on rice yield (Zhang et al. 2017). The functions of MiR408 in rice and Arabidopsis suggest that miRNAs exhibit important roles in plant growth and development.
In rice, MiR164a targets the transcription factor OsNAC60 to mediate rice defense responses to Medicago truncatula infection (Wang et al. 2018). Rhizobia infection induces miR172 expression, which positively regulates nodulation in soybean, lotus, and common beans (Wang et al. 2014; Holt et al. 2015; Nova-Franco et al. 2015). In Medicago sativa, mycorrhizal colonisation induces higher expression of MiR399, leading to repression of its target gene PHOSPHATE2 (PHO2), which in turn reduces PHO2 protein abundance (Branscheid et al. 2010). Due to the PHO2-directed repression, the mycorrhizal symbiosis was sustained, and then the phosphorus uptake was increased (Branscheid et al. 2010). MiR393 targets several auxin receptors in mycorrhizal root of tomato, alfalfa and rice (Etemadi et al. 2014). It serves as a negative regulator of mycorrhization as expression of the precursors of the MiR393 was downregulated during mycorrhization, while overexpression of MiR393 led to undeveloped arbuscules in the roots of the three plants (Etemadi et al. 2014).
In addition to miRNA, the signalling molecules including phytohormones, calcium (Ca2+), reactive oxygen species (ROS) and nitrogen species (RONS) also play pivotal roles in mycorrhizal symbioses. Gibberellins, abscisic acid, ethylene and auxin have been found to be essential for mycorrhizal colonisation (Herrera-Medina et al. 2007; Martín-Rodríguez et al. 2011; Floss et al. 2013; Etemadi et al. 2014; Yu et al. 2014). The activation of sustained Ca2+-dependent signalling in target cells of the host epidermis is crucial for mycorrhizal symbioses (Barker et al. 2017), and a switch in Ca2+ spiking signature is concomitant with mycorrhizal colonisation in M. truncatula (Sieberer et al. 2012). Nitric oxide (NO) is a diffusible and reactive free-radical gaseous molecular that is involved in both pathogenic and mutualistic interactions between plants and microbes (Trapet et al. 2015; Martínez-Medina et al. 2019). Singlet oxygen (1O2), superoxide anion (O2·−), hydrogen peroxide (H2O2), and hydroxyl radical (HO·) are the major forms of ROS in plants (Waszczak, Carmody, and Kangasjärvi 2018). Accumulation of ROS within apoplast, where the RBOH-derived ROS acts as cell-cell signal, contributes to plant immunity of microbe-associated molecular pattern (MAMP) perception and stomatal closure (Mersmann et al. 2010; Macho et al. 2012; Kadota, Shirasu, and Zipfel 2015). In rhizobia–legume symbiosis, ROS plays a central role in infection thread and nodule organogenesis (Fonseca-García et al. 2021). ROS has been regarded as a signal in response to pathogenic infection and root nodule symbiosis, however, the role of ROS in mycorrhizal symbiosis is rarely investigated.
In this study, microRNA sequencing showed that the expression of miR408b was significantly reduced in mycorrhizal tomato plants. Overexpression of miR408b inhibited root mycorrhizal colonisation. The dual luciferase reporter assay identified SlBBP, a plant blue copper protein gene, as the target gene of miR408b. Overexpression of SlBBP decreased SOD activity. Finally, the rboh1 mutant with declined ROS content showed lower mycorrhizal colonisation by Rhizophagus irregularis. Our study presents evidence that miR408b and its target gene SlBBP regulate mycorrhizal symbiosis in tomato through the ROS pathway.
2 Materials and Methods
2.1 Plants Material and AMF Inoculation
The strain of Rhizophagus irregularis was obtained from the Bank of Glomeromycota in China (BGC), Institute of Plant Nutrition, Resources and Environment, Beijing Academy of Agriculture and Forestry Science. Maize (Zea mays L) plants were used for the reproduction of R. irregularis with low-phosphorus nutrient in sandy soil in a greenhouse. After culture for 60 d, maize roots inoculated with R. irregularis were collected together with the sandy soil and used as inoculants. The mixture of corn roots and rhizospheric sand containing approximate 40 AMF propagules per gram was used for mycorrhizal inoculation. The seeds of rboh1 mutant and its corresponding wild type of tomato (Solanum lycopersicum) plants were obtained from Professor Jingquan Yu of Zhejiang University. The variety of tomato used for inoculation experiments and Agrobacterium transformation was Zhongza No. 9 obtained from China Vegetable Seed Industry Technology Co. Ltd (Beijing). After sterilisation with 2% sodium hypochlorite for 10 min, tomato seeds were rinsed with sterile water 8–10 times, placed in a petri dish containing moist filter paper, and incubated at 28°C in the dark for 2 days. Germinated seeds were sown in a seedling tray with a transparent cover and then cultivated in a greenhouse. Four-leaf stage tomato plants were transplanted into a black pot (10 cm upper diameter × 15 cm height × 8 cm bottom diameter) filled with sand that had been autoclaved at 121°C for 2 h. For mycorrhizal treatment, plants were inoculated with mycorrhizal fungus R. irregularis (Rir), a mixture of soil, spores, hyphae and root material, while the control sample received the same amount of autoclaved inoculum. Plants were watered with low-phosphorus (1/4 M) Hoagland's nutrient solution (Hoagland and Arnon 1950). The greenhouse condition was 26°C/22°C, day/night, 14 h/10 h, light/dark), the relative humidity was 70%, the light quantum flux density (PPFD) was 500 μmol·m−2·s−1, and daily total photosynthetically active radiation (DLI) was 15–20 mol·m−2·d−1.
2.2 Mycorrhizal Staining Observation and Infection Rate Measurement
The staining method was slightly modified from Jiang et al. (2017): the fresh tomato roots were placed in a centrifuge tube containing 10% KOH and heated at 96°C for 5 min. The following steps were followed: wash roots with sterile water three times to remove the remaining KOH, absorb the water with filter paper and place it in a centrifuge tube containing 2% HCl at 96°C for 5 min. After washing several times with 0.2 M phosphate-buffered saline (PBS) (19 mL 0.2 M NaH2PO4 and 81 mL 0.2 M Na2HPO4 for 100 mL 0.2 M PBS solution), the roots were re-immersed in 0.2 M PBS (pH = 7.4) and placed at room temperature for 3 h. Roots were then transferred to 0.2 M PBS (pH = 7.4) containing 5 µg/mL WGA-488 (wheat germ agglutinin-alexa fluor 488 conjugate, Invitrogen, W11261) and were stained at 4°C overnight. The stained root segments were washed several times with PBS, observed under a Nikon Eclipse Ti2 inverted fluorescence microscope (Shanghai Nikon Instruments Co. Ltd., China), the excitation wavelength was 488 nm and the emission wavelength was 507 nm, and photographed with a monochrome microscope camera (Nikon DS-Ri2). In this study, the weighted method for measurement of root colonisation referred to Biermann and Linderman (1981).
2.3 Total RNA Extraction, Reverse Transcription, and Fluorescence Quantitative Analysis
Based on the TRIZOL method, the total RNA was extracted according to the reagent instructions provided by Invitrogen. The reverse transcription of miRNA was performed by the stem-loop method in accordance with the product instruction manual of Nanjing Vazyme Biotechnology Co. Ltd., and subsequent fluorescence quantitative analysis was performed. The tomato housekeeping gene U6 was used as the reference gene, and the relative expression of each target miRNA was determined by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) using the 2−ΔΔCT method. First-strand cDNA was synthesised according to the product instructions (Promega). The tomato constitutively expressed housekeeping gene Ubi3 was used as a reference gene, and the relative expression of each target gene was determined by qRT-PCR using the 2−ΔΔCT method. Primers for qRT-PCR of mature miR408b and its precursor are listed in Supporting Information: Table S1. The other primers for qRT-PCR are listed in Supporting Information: Table S2.
2.4 Prediction of Target Genes of sly-miR408b
The mature sequence of sly-miR408b was submitted to the psRNATarget website (http://plantgrn.noble.org/psRNATarget), the target genes were then predicted with default parameters (2017 version), and expectation value no more than 3.5 was set to screen out the candidate target genes.
2.5 Dual Luciferase Experiment
The dual luciferase experiment was conducted according to Liu, Wang and Axtell (2014). The primers for dual luciferase assay are listed in Supporting Information: Table S3. The inserted nucleotides sequences for miR408b overexpression and its predicted target genes are listed in Supporting Information: Table S4.
2.6 Copper Treatment
Tomatoes with Rir inoculation (AM) and without inoculation (NM) were treated with copper (added in the form of CuSO4 to the low phosphate Hoagland's nutrient solution) at concentrations of 0, 0.5, 10, and 50 μM, respectively. After 40 days, tomato roots were harvested for total RNA extraction.
2.7 Construction of Composite Plants
A binary vector was constructed for the overexpression and silencing of miR408b and the overexpression of its target gene SlBBP. The binary vector contained the DsRed marker gene for the screening of positively transformed roots. Plasmids were transferred into Agrobacterium MSU440 and used for the induction of tomato hairy roots. The specific transformation steps were described by Ho-Plágaro et al. (2018). The inserted nucleotide sequences for miR408b overexpression and silencing are listed in Supporting Information: Table S5.
2.8 Virus-Induced Gene Silencing (VIGS) of the Target Gene SlBBP
We constructed the pTRV2 plasmid vector containing the target gene SlBBP fragment (400 bp) and transferred it into Agrobacterium GV3101. The pTRV1 strain was constructed at the same time. The tomato VIGS plants were generated according to the method described by H.X. Yan et al. (2012). The inserted nucleotide sequences for target genes are listed in Supporting Information: Table S5.
2.9 Superoxide Anion (O2·−) Observation in Tomato Roots by Nitrogen Blue Tetrazolium (NBT) Staining
Superoxide anion (O2·−) was visualised by staining roots with NBT. NBT (0.125 g) was dissolved in 250 mL of sterile water to prepare a 0.5 mg/mL staining solution (no adjustment of the pH value, placed on ice before use). We washed fresh roots with sterile water and cut them into 2 cm segments immediately. Root segments were placed into a 2 mL sterile centrifuge tube, followed by the addition of 1.8 mL NBT staining solution. Roots were then infiltrated in a vacuum infiltrator (−15 kPa, 4 h). We transferred the root segments into a new 2 mL centrifuge tube, added 95% absolute ethanol, heated the tube in a metal bath (65°C, 20 min), then put the root segments in a new 2 mL centrifuge tube, and added decolorising solution (ethanol: lactic acid: glycerol = 3:1:1) to decolorise the roots for 4 h. The decolorised root segments were observed and photographed by using an optical microscope (Olympus BX51, Olympus, Japan).
2.10 SOD Enzyme Activity Determination
The root SOD activity in overexpressed and silenced plants of SlBBP and sly-miR408b was determined by the NBT method as described by Crapo, McCord and Fridovich (1978).
2.11 Statistical Analysis
The data were processed and plotted using Microsoft Excel 2013 and GraphPad Prism 9 software, and the data were tested for significance by SPSS 19 statistical analysis software. All experiments were conducted in a completely randomised experimental design. Before all statistical analysis, Shapiro–Wilk normality test and Levene's test for homogeneity of variance (p > 0.05) were used to check the normality of the data (p > 0.05). On the premise of satisfying the assumption of normality and homogeneity of variance, the difference between two or more treatments was compared by using student's t-test or analysis of variance (ANOVA) (Tukey's test, p < 0.05). When the assumptions of normality and variance homogeneity were not satisfied, Mann–Whitney U test or the Kruskal–Wallis test was used to analyse the data of two or more groups, and then Fisher's least significant difference (LSD) and Holm correction of adjusted p values were used to calculate the significant difference. Values are means (± SD) of biological replicates.
3 Results
3.1 sly-miR408b Is Downregulated in Tomato Roots Upon Mycorrhizal Colonisation
Mycorrhizal root staining showed that the hyphae were well developed as early as 14 days post R. irregularis (Rir) inoculation, the arbuscule structure could not be observed until 21 days post Rir inoculation, and then finely developed in the root cortex cells with the vesicle formation (Figure 1A). With the elongation of time of Rir inoculation, the mycorrhizal rate was gradually increased (Figure 1B). The expressions of the common symbiotic pathway genes (CSPs) that are critical for symbiosis were induced by mycorrhizal colonisation (Supporting Information: Figure S1). The miRNA sequencing showed that sly-miR408b (with high expression abundance in tomato roots) displayed notably lower expression in mycorrhizal roots than in non-mycorrhizal roots (Supporting Information: Figure S2). Further qRT-PCR analysis showed that the expression of sly-miR408b decreased at later stage of mycorrhizal colonisation (Figure 1C). However, it is worth noting that the mycorrhiza -induced inhibition of sly-miR408b expression occurred 30 days after Rir inoculation (Figure 1C), while PT4, a marker gene for arbuscule formation only expressed in cells with arbuscule structure, was specifically induced 21 days after Rir inoculation (Figure 1D). This suggested that sly-miR408b might not participate in the early stage of mycorrhizal symbiosis and might play a role in the regulation of mycorrhizal symbiosis maintenance. The expression levels of both the sly-miR408b precursor and SPL7, the transcription factor of miR408, miR397, and miR857 involved in copper homoeostasis (Yamasaki et al. 2009), were reduced in mycorrhizal roots compared to non-mycorrhizal roots (Supporting Information: Figure S3B,C), which showed similar expression pattern of sly-miR408b (Supporting Information: Figure S3A). The luciferase assay showed that SPL7 promoted the transcription of sly-miR408b (Supporting Information: Figure S3D), indicating that the reduced expression of sly-miR408b results from mycorrhiza-induced SPL7 downregulation.
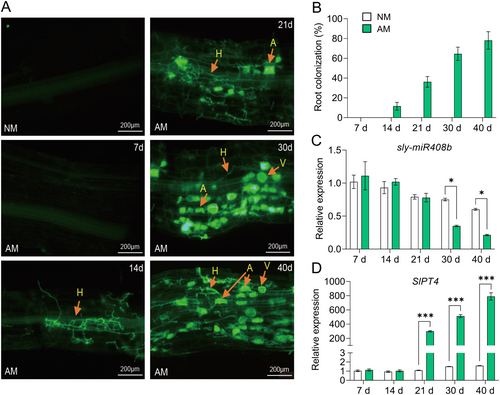
3.2 sly-miR408b Participates in the Regulation of Mycorrhizal Symbiosis
The above results indicate that the expression of sly-miR408b was negatively correlated with mycorrhizal colonisation at later stage. To investigate the function of sly-miR408b, we obtained sly-miR408b overexpression and interference plants by the agrobacterium transformation method (overexpression or interference with sly-miR408b only in the tomato roots, Supporting Information: Figure S4). To obtain the interference plants, short tandem mimic (STTM), a potent approach for silencing miRNAs in plants (J. Yan et al. 2012), was used for root transformation to block the function of sly-miR408b in tomato roots (STTM-sly-miR408b).
The mycorrhizal colonisation rate in CV (tomato plants transformed with the control vector) was 37.5%, while it was 54.1% in sly-miR408b interference plants (STTM-sly-miR408b), which was significantly higher than that in CV plants. However, the mycorrhizal colonisation rate of sly-miR408b-overexpressed roots (OE-sly-miR408b) was only approximately 20%, which was obviously lower than that in the roots of CV and interference plants (Figure 2A). The expression levels of PT4 and BCP1, two marker genes for arbuscule formation, in the transformed plants were significantly lower in OE-sly-miR408b plants compared to those in CV and STTM-sly-miR408b plants (Figure 2B,C). These results suggested that sly-miR408b is a negative regulator of mycorrhizal symbiosis.
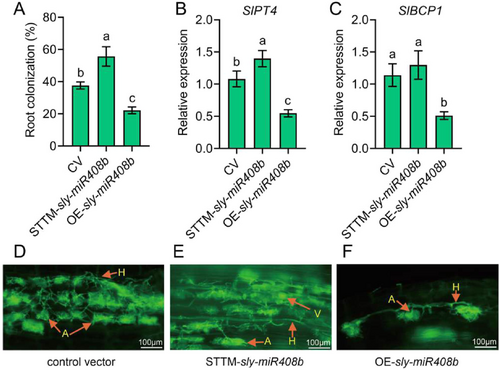
To further elucidate the function of sly-miR408b in arbuscule formation, we observed the arbuscule morphology in three different phenotypes with microscope. The results showed that sly-miR408b overexpression resulted in a lower number of arbuscules but no effect on the arbuscule structure (intact arbuscule structure extend from the hyphae) (Figure 2D–F), suggesting a role of sly-miR408b in the control of mycorrhizal colonisation and arbuscule abundance rather than in the control of arbuscule differentiation.
3.3 Identification of Target Genes of sly-miR408b
miRNAs function mainly by regulating the expression of their target genes. Therefore, we performed target gene prediction for sly-miR408b. A total of 10 target genes were obtained. The main target genes were plant blue copper protein family genes (Solyc01g104400, Solyc01g090120), laccase (Solyc05g052360, Solyc06g050530) and kinase (Solyc12g007280, Solyc05g056200) (Table 1). LAC12 (Laccase-12), which was confirmed to be targeted by miR408b in Arabidopsis, was also included among the predicted targeted genes. The expressions of all of these target genes were further analysed by qRT-PCR. The results showed that the expression pattern of only two genes, Solyc01g090120 and Solyc01g104400, was negatively correlated with that of sly-miR408b (Supporting Information: Figure S5). Therefore, Solyc01g090120 and Solyc01g104400 were considered to be the potential targets of sly-miR408b.
| Target_gene ID | Description | Expectation |
|---|---|---|
| Solyc07g005740 | Pentatricopeptide repeat-containing protein | 2.5 |
| Solyc05g052360 | Laccase-12 | 2.5 |
| Solyc01g104400 | Basic blue protein-like | 3 |
| Solyc01g090120 | Uclacyanin-3 | 3 |
| Solyc02g091840 | Unknown | 3 |
| Solyc04g071510 | BZIP transcription factor | 3.5 |
| Solyc04g072930 | F-box protein | 3.5 |
| Solyc12g007280 | Protein kinase superfamily protein | 3.5 |
| Solyc06g050530 | Laccase-5-like | 3.5 |
| Solyc05g056200 | LysM domain receptor-like kinase 3 | 3.5 |
The dual-luciferase-based reporter system was used to quantitatively assess the regulation of miRNA and its target genes through agroinfiltration (Liu, Wang, and Axtell 2014). In this experimental system, predicted miRNA target sites were inserted into the 3′-untranslated region (UTR) (3′-UTR sensor) of firefly luciferase (F-LUC) (Figure 3). Relative accumulation of the F-LUC sensor was evaluated at the mRNA level (via qRT-PCR with primers flanking the target site) using Renilla luciferase (R-Luc), expressed from the same T-DNA, as an internal control. To evaluate the efficacy of miRNA to regulate the expression of its target genes, a 21-nucleotide spacer (21-nt spacer) of a random sequence served as a common negative control, perfectly paired sites (perfect match) served as positive controls, and the unmodified 3′-UTR sensor served as a control vector (Figure 3).
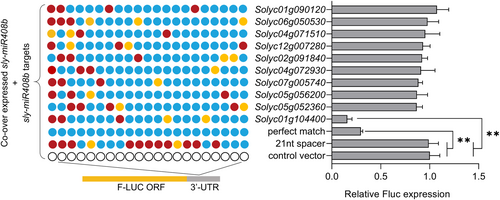
The dual-luciferase reporter assay showed that the luciferase activity in tobacco leaves cotransfected with perfectly matched sequences of sly-miR408b and sly-miR408b was markedly repressed compared to the control vector, indicating the strong regulatory activity of miR408b with respect to its perfectly paired site, while the 21-nt spacer showed no difference from the control vector (Figure 3). With respect to the 10 natural target sites sequences, it was found that only one target site sequence (Solyc01g104400, the natural target sequence) had the same repressed efficacy as the perfect match, indicating that Solyc01g104400 is the target gene of sly-miR408b.
To further verify the inhibitory effects of sly-miR408b on the Solyc01g104400 site sequence, short tandem target mimic miR408b (STTM-sly-miR408b, which can effectively trap the mature sequence of sly-miR408b), was introduced into the dual-luciferase reporter system (Supporting Information: Figure S6A). The results showed that in the presence of STTM-sly-miR408b, the inhibitory effect on the Solyc01g104400 site sequence was prevented compared to the control (STTM-spacer, which cannot absorb the mature sequence of sly-miR408b) (Supporting Information: Figure S6B,C). Mature sly-miR408b is a 21-nucleotide miRNA, and the 21-nucleotide miRNAs are loaded into AGO1 (argonaute protein 1) to form the RISC (RNA-induced silencing complex) with some other proteins in plants (Song et al. 2019). Our RIP (RNA binding protein immunoprecipitation) assay result showed that both mature sly-miR408b and Solyc01g104400 mRNA were detectable in tomato root samples incubated with the AGO1 antibody rather than in the IgG control (Figure S7), suggesting that sly-miR408b might regulate Solyc01g104400 mRNA in vivo. We also determined the expressions of sly-miR408b and Solyc01g104400 in transformed tomato plant roots; decreased expression of Solyc01g104400 in sly-miR408b overexpression plants was seen compared to that in STTM-sly-miR408b (Supporting Information: Figure S8). All of these results together confirm that Solyc01g104400 is one target gene of sly-miR408b in tomato.
3.4 The Target Gene Is Copper Responsive and Located in the Cell Membrane and Cytoplasm
The gene annotation (phytozome 12) showed that Solyc01g104400 is a plastocyanin-like protein (basic blue protein, SlBBP) gene. Plant blue copper proteins consist of three groups including small blue proteins, blue oxidases and coagulation factors. Plastocyanin belongs to small blue proteins and combines only one copper. Phylogenetic homology analysis of target gene SlBBP showed high identity with the basic blue copper gene, Os03g0850900, in rice (Supporting Information: Figure S9A), and two basic blue copper genes, solyc019104390 and solyc019104380, in tomato (Supporting Information: Figure S9B).
The SWISS model analysis demonstrated that SlBBP has a copper-binding site for binding one Cu2+ (Supporting Information: Figure S9C,D), suggesting that it is a copper-responsive gene. Consistent with our speculation, the expression of SlBBP was positively regulated by the Cu2+ in a pot experiment (Figure 4B). As for sly-miR408b, Cu2+ as a negative factor inhibited sly-miR408b in tomato roots (Figure 4A). We then measured the copper content of tomato roots and found that mycorrhizal colonisation promoted copper accumulation (Figure 4C). The increased intake of copper might partially explain the downregulated expression of sly-miR408b in mycorrhizal roots.
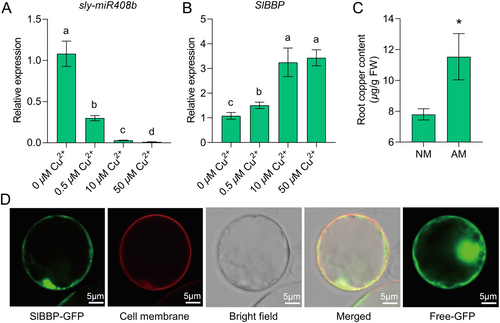
To illustrate the molecular function of SlBBP, the subcellular location of the SlBBP protein was investigated using Arabidopsis thaliana protoplasts transformed with the SlBBP-GFP fusion protein. The results showed that it was located in the cell membrane and cytoplasm (Figure 4D). These results showed that SlBBP is located in the cell membrane and cytoplasm and is responsive to copper.
3.5 Loss of Function of SIBBP Reduces Root Mycorrhizal Colonisation
RT-qPCR analysis showed that the expression of SlBBP exhibited an opposite trend to that of sly-miR408b, and it was significantly induced in mycorrhizal plants 30 days after Rir inoculation (Figure 5A), indicating the relevance between sly-miR408b expression and mycorrhizal colonisation. To further explore the relationship between SlBBP expression and mycorrhizal symbiosis, SlBBP overexpressed (Figure 5B) and silenced (Figure 5D) tomato plants were then inoculated with Rir. Although overexpression of SlBBP did not increase the mycorrhizal colonisation rate (Figure 5C), however, silencing of SlBBP significantly reduced root mycorrhizal colonisation (Figure 5E), indicating that SlBBP has an important effect on tomato mycorrhizal colonisation.

3.6 The ROS Is the Downstream Pathway of sly-miR408b and SlBBP
To determine the functional network of SlBBP in tomato mycorrhizal symbiosis, the string website (https://www.string-db.org) was used to predict and analyse the genes that interact with SlBBP. We found that SlBBP can interact with multiple genes (Supporting Information: Figure S10). Notably, two of these genes encode the superoxide dismutase (SOD) enzyme (Supporting Information: Figure S10, node names: SODCP.2 and SODCC.1), which competitively bind copper with LAC13, a target gene of miR408 in Arabidopsis (Zhang et al. 2014). Therefore, SOD enzyme activity was measured in the transformed tomato roots. Overexpression of SlBBP led to a significant decrease in SOD activity (Figure 6A), indicating that SlBBP had an inhibitory effect on SOD enzyme activity. SOD usually removes excessive ROS in plants. Nitroblue tetrazolium (NBT) histochemical staining showed that overexpression of SlBBP increased the accumulation of superoxide anions (O2·−) in the roots (Figure 6B). In contrast, interference with the expression of SlBBP increased SOD activity (Figure 6C) but reduced the content of superoxide anion (O2·−) in tomato roots (Figure 6D). These results indicated that SlBBP had a negative effect on SOD activity, which affects the removal of O2·−.
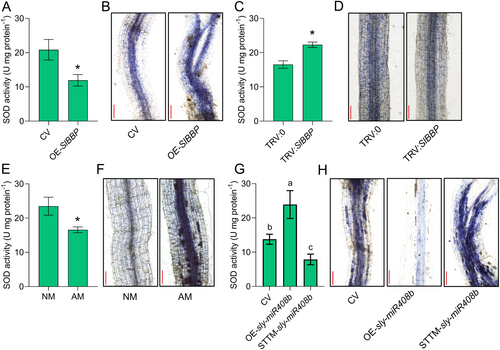
As mentioned earlier, sly-miR408b can regulate the expression of SlBBP. As an upstream factor in this pathway, it is not known whether sly-miR408b also affects the activity of SOD and the accumulation of O2·−. Our results showed that silencing the expression of sly-miR408b also reduced SOD activity (Figure 6G), with increased accumulation of O2·− in the roots (Figure 6H). The opposite trend was observed in OE-sly-miR408b roots (Figure 6G,H). In tomato plants (non-transformed tomato plants), inoculation with Rir reduced SOD activity (Figure 6E) and promoted the accumulation of O2·− in the roots (Figure 6F), indicating the relevance between the ROS pathway and mycorrhizal infection. These results together demonstrated that silencing the expression of sly-miR408b induced by mycorrhizal colonisation may affect the activity of the tomato SOD enzyme through its regulation of SlBBP expression and ultimately lead to the accumulation of O2·− in the roots. Although too much ROS can cause oxidative damage to plants, proper ROS signalling is essential for plant responses (Waszczak, Carmody, and Kangasjärvi 2018). Therefore, we speculated that the accumulation of O2·− in roots may act as a symbiosis signal, which mediates mycorrhizal symbiosis in tomato roots.
3.7 RBOH1 Dependent ROS Production Is Important for Mycorrhizal Symbiosis
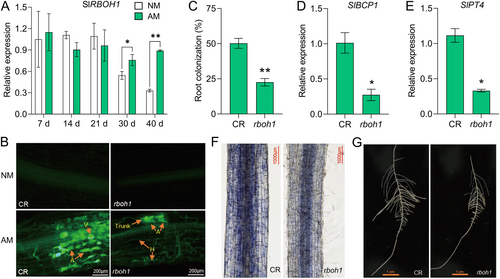
RBOHs are the key genes of ROS metabolic pathways in plants and are important catalytic enzymes for the production of O2·− in plants. In Medicago truncatula, MtRbohE RNAi plant roots showed altered colonisation pattern in the root cortex with fewer arbuscules (Belmondo et al. 2016). While in tomato plants, RBOH1 was the major RBOH gene in response to mycorrhizal symbiosis with the highest expression level among the RBOH genes (Figure 7A and Supporting Information: Figure S11). Mutation of RBOH1 significantly reduces the ROS content in tomato plants (Wang et al. 2019), with lower O2·− in the roots compared to its wild type CR (cv. Condine Red) (Figure 7F). The mycorrhizal colonisation rate of tomato rboh1 mutant plants was significantly lower as compared to CR (Figure 7C), with significantly downregulated PT4 and BCP1 expressions (Figure 7D,E). Interestingly, the reduction in arbuscule number, a phenomenon present in sly-miR408b overexpression roots, was also observed in the roots of the rboh1 mutant (Figure 7B), suggesting that depletion of ROS resulted in low mycorrhizal colonisation. ROS as signalling molecules plays an important role in root development (Tsukagoshi 2016). Declined ROS accumulation in rboh1 mutant led to decreased number and shorter lateral root compared to corresponding wild-type CR (Figure 7G). The above results indicate that the normal content of ROS is an important factor for maintaining well-developed mycorrhiza. The transduction of the ROS signal may play a key role in mycorrhizal symbiosis in tomato.
4 Discussion
It has become evident that small RNAs play essential roles in plant-microbe symbiosis (Hoang, Tóth, and Stacey 2020; Wong-Bajracharya et al. 2022). microRNAs are a class small RNA molecules that are transcribed from MIR genes (Song et al. 2019). Transcriptome data from mycorrhizal roots revealed that some miRNAs were differentially expressed in host plants after mycorrhizal colonisation (Devers et al. 2011), suggesting that these miRNAs are involved in mycorrhizal symbiosis. Hence, the identification of mycorrhizal symbiosis-related miRNAs and their roles is vital for elucidating the symbiosis mechanisms. In the current study, we performed a sequencing analysis of the tomato miRNA transcriptome and identified 47 differentially expressed miRNAs, among which 17 miRNAs were novel miRNAs. These results are consistent with other studies that different host plants have different miRNAs in response to mycorrhizal symbiosis (Gu et al. 2014).
One of the differentially expressed miRNAs, sly-miR408b, which is a conserved miRNA that responds to light, moisture, and temperature in many plants (Jiang et al. 2021; Mishra, Sahu, and Shaw 2022; Sun et al. 2018), was highly expressed in both tomato mycorrhizal roots and non-mycorrhizal roots at the early stage of mycorrhiza formation (Figure 1C). The expression level of sly-miR408b was declined when mycorrhizal symbiosis was well established, which was confirmed by our qRT-PCR and sequencing data in previous research (Wu et al. 2016). Overexpression of sly-miR408b significantly reduced mycorrhizal colonisation rate in tomato roots (Figure 2A), suggesting an important role of sly-miR408b in negative regulation of tomato mycorrhizal symbiosis. However, our microscopic observation showed that arbuscules were well developed in plant cells (Figure 2D–E), which is consistent with the result in MiR171h overexpressed M. truncatula roots (Lauressergues et al. 2012), but different from the result in MiR393 overexpressed roots, in which arbuscules were defect (Etemadi et al. 2014). sly-miR408b overexpression led to decreased number of arbuscules as well as reduced expression of PT4 and BCP1 in tomato roots (Figure 2B,C), two marker genes of AM symbiosis (Harrison, Dewbre, and Liu 2002). It is worth noting that MiR171h was shown to be involved in the lipochito-oligosaccharides (Myc-LCOs) signalling pathway, suggesting its role in fungal perception (pre-contact between plant root and AM fungus); while MiR393 mediated auxin perception that is required for arbuscule development (Etemadi et al. 2014). In our study, the expression of sly-miR408b was not affect by the mycorrhizal symbiosis in the early stage, but was significantly reduced after arbuscules were well developed. These results suggested that sly-miR408b might regulate the range and space of mycorrhizal colonisation within the tomato roots rather than fungal perception and arbuscule development.
miRNAs function mainly by regulating the expression of their target genes. The miRNA-target regulation is usually intertwined with other regulatory networks, which in turn affect plant growth, development and responses to abiotic and biotic stress (Song et al. 2019). Studies have elucidated that in Arabidopsis miR408 targets LAC13 (Laccase 13) (Abdel-Ghany and Pilon 2008), thereby affects photosynthesis by regulating copper flowing into the chloroplast in the leaves (Zhang et al. 2014), while in rice the target gene of miR408 is uclacyanin-like protein 8 (UCL8), which regulates photosynthesis and rice yield via a phytocyanin protein (Zhang et al. 2017). These studies indicate that although the target genes of miR408 are copper-related protein genes, it may differ in different plant species. Our study demonstrated that SlBBP (Solyc01g104400) is the target gene of sly-miR408b (Figure 3 and Supporting Information: Figures S6–7). SlBBP belongs to the plant blue protein family, which is different from the reported miR408 target genes in Arabidopsis and rice. Interestingly, both LAC13 and UCL8 proteins are copper binding proteins for competing copper ions. Our results showed that both sly-miR408b and its target gene SlBBP were copper responsive. The SlBBP expression was increased with copper concentration, while sly-miR408b expression showed opposite pattern (Figure 4). Increasing copper content in mycorrhizal roots may in turn regulates sly-miR408b expression (Figure 4A,C), suggesting possible involvement of sly-miR408b in regulation of copper accumulation in mycorrhizal plants.
SlBBP was predicted to be associated with two copper-binding proteins encoding subunits of the antioxidant enzyme SOD. Our results also showed that SlBBP expression displayed an effect on SOD enzymatic activity, although this association has not been directly experimentally verified. However, there was indirect evidence to illustrate that the enzymatic activity of SOD is positively correlated with the expression of miR408 (Guo, Niu, and Cao 2018), indicating that sly-miR408b may affect SOD enzyme activity by regulating the expression of its target gene SlBBP. The more intuitive evidence is that the expression of miR408 and SOD enzyme activity were both decreased along with increased expression of SlBBP in tomato mycorrhizal roots. These results indicate that the expression levels of miR408 and SlBBP were strongly correlated with SOD activity in the process of tomato mycorrhizal colonisation.
The superoxide anion (O2·−) formed in the apoplast can be dismutated to hydrogen peroxide (H2O2) either spontaneously or by apoplastic SODs (Waszczak, Carmody, and Kangasjärvi 2018). Mutation of tomato RESPIRATORY BURST OXIDASE HOMOLOG 1 (RBOH1) resulted in a dramatic reduction in H2O2 (Wang et al. 2019), suggesting that RBOH1 is critical for tomato O2·− production. The O2·− formed in the apoplast plays an important role in the response of plants to pathogen infection. This may be related to its role as a signalling molecule that can transmit signals between cells and even remote cells (Waszczak, Carmody, and Kangasjärvi 2018), so that cells and tissues far away from the infection site can be prepared for the corresponding immune in advance. For mycorrhizal symbionts, this signalling in plants may prime root cells for symbiotic establishment. In M. truncatula, silencing MtRbohE resulted in decreased number of arbuscules (Belmondo et al. 2016), while opposite result was reported in Phaseolus vulgaris, in which PvRbohB silencing led to Rir over-colonisation in the roots (Arthikala et al. 2013). The possible reason is that different RBOH genes may function differently in different species. In this study, the mycorrhizal colonisation rate of the tomato rboh1 mutant was significantly lower than that of its corresponding wild type (Figure 7B), indicating that the lack of ROS due to the loss of RBOH protein function hinders the development of an intimate symbiosis between tomato plants and mycorrhizal fungus Rir. A further explanation for this should be that the ability of ROS to conduct signal transduction between cells in tomato roots or over long distances is weakened in the mutant rboh1, which is not sufficient to prepare cells adjacent to the colonisation site for symbiosis, and eventually leading to a decreased number of arbuscules. Correspondingly, too much ROS is also unfriendly to mycorrhizal symbiosis, because high accumulation of ROS was observed in the roots of SlBBP-overexpressed tomato (Figure 6B), leading to slightly decreased mycorrhizal colonisation. Therefore, controlling the content of ROS within a certain range is important role for mycorrhizal symbiosis, suggesting the dose effect of ROS in plant-microbe symbiosis. Several studies have demonstrated that ROS signalling is involved in mycorrhizal colonisation in M. truncatula. Zhang et al. showed that mycorrhizal inoculation increased NADPH oxidase activity and H2O2 content in M. truncatula roots, leading to increased antioxidant response under Pb stress (Zhang et al. 2019). Silencing of the Rac1 GTPase MtROP9 in M. truncatula enhances early mycorrhizal colonisations (Kiirika et al. 2012). Our results indicate that sly-miR408b plays a role in maintaining mycorrhizal symbiosis. There exists a positive feedback (symbiosis-copper-sly-miR408b-SlBBP-ROS-symbiosis) in maintenance of AM symbiosis in tomato. Such positive feedback loop helps plants promote mineral nutrient absorption through mycorrhizal pathways and enhance host stress resistance.
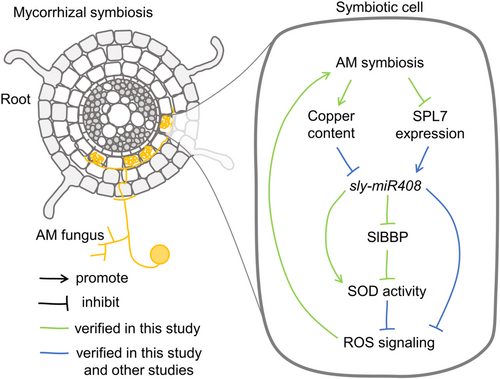
In conclusion, as illustrated in Figure 8, sly-miR408b was identified to be a negative regulator of tomato mycorrhizal symbiosis. sly-miR408b targets a basic blue protein gene (SlBBP) to control the arbuscule abundance in mycorrhizal symbiosis in tomato in a ROS-dependent manner.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. U2005208, 32170350, 32271617, 32371588) to Y.Y.S. and R.S.Z, and Fundamental Research Program of Shanxi Province (20210302122005) to Z.X.S. We thank professor Jingquan Yu of Zhejiang University for providing the seeds of rboh1 mutant and its wild type of tomato.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All the data analysed during this study have been included in this article.