A Genome-Wide Association Screen for Genes Affecting Leaf Trichome Development and Epidermal Metal Accumulation in Arabidopsis
ABSTRACT
To identify novel genes engaged in plant epidermal development, we characterized the phenotypic variability of rosette leaf epidermis of 310 sequenced Arabidopsis thaliana accessions, focusing on trichome shape and distribution, compositional characteristics of the trichome cell wall, and histologically detectable metal ion distribution. Some of these traits correlated with cLimate parameters of our accession's locations of origin, suggesting environmental selection. A novel metal deposition pattern in stomatal guard cells was observed in some accessions. Subsequent GWAS analysis identified 1546 loci with protein sequence-altering SNPs associated with one or more traits, including 5 genes with previously reported relevant mutant phenotypes and 80 additional genes with known or predicted roles in relevant developmental and cellular processes. Some candidates, including GFS9/TT9, exhibited environmentally correlated allele distribution. Several large gene famiLies, namely DUF674, DUF784, DUF1262, DUF1985, DUF3741, cytochrome P450, receptor-Like kinases, Cys/His-rich C1 domain proteins and formins were overrepresented among the candidates for various traits, suggesting epidermal development-related functions. A possible participation of formins in guard cell metal deposition was supported by observations in available loss of function mutants. Screening of candidate gene lists against the STRING interactome database uncovered several predominantly nuclear protein interaction networks with possible novel roles in epidermal development.
1 Introduction
The plant above-ground organs epidermis is a sophisticated interface between the plant and its environment, providing mecHanical protection, deterring pathogens and predators and allowing for a controlled gas exchange via stomata. Multiple cell types act in a concerted manner to fulfil these functions, and even subtle disruption of their differentiation may cause observable phenotypic alterations (Zuch et al. 2022). Unconspicuous epidermal defects, well tolerated under laboratory conditions, can indicate perturbation of fundamental cell differentiation or cell morphogenesis processes, as documented, for example, by identification of mutants defective in actin nucleation based on a distorted trichome phenotype (see Szymanski 2005).
The most abundant cell type in the leaf epidermis of the model plant Arabidopsis thaliana are the puzzle piece-shaped pavement cells. Their morphogenesis is driven by microtubule rearrangements that constrict cell surface expansion to generate indentations and allow actin-dependent intrusion of neighboring cell's lobes. Coordination of these processes involves cell wall-mediated mecHanical feedback and is orchestrated by a regulatory network including ROP small GTPases, their effectors, locaLized exo- and endocytosis and auxin signalLing (see Sapala et al. 2018; Lin and Yang 2020; Liu et al. 2021; Igisch, Miège, and Jaillais 2022). Leaf epidermis also contains stomatal complexes, hydathodes and trichomes, whose patterning is controlled by complex regulatory networks (see Torii 2021). Differentiation of these specialized cell types involves rearrangement of gene expression patterns, well documented in A. thaliana for both stomatal guard cells (Zhao et al. 2008; Bates et al. 2012; Xia et al. 2022) and trichomes (Jakoby et al. 2008; Huebbers et al. 2022, 2023).
Trichomes contribute to anti-herbivore defense by acting as mecHanical deterrents of herbivore feeding; their ecological relevance is well documented (e.g., Sato et al. 2019; Qu, Bonte, and Vandegehuchte 2022). They also engage in detoxification of heavy metals (e.g., Harada et al. 2010; Yaashikaa et al.2022) and may sense vibrations and mechanical signals (Zhou et al. 2017; Liu et al. 2017). Development of the branched unicellular A. thaliana trichomes is a multi-stage process (Mathur et al. 1999; Han et al. 2022) involving genome endoreduplication, initiation and expansion of trichome branches, and production of secondary cell wall with a characteristic structure and patterns of callose and metal ion deposition (Kulich et al. 2015, 2018). The ablilty to immobilize metal ions in the trichome cell wall may contribute to detoxication of heavy metals such as excessive zinc (Ricachenevsky et al. 2021) or cadmium (Gao et al. 2021, 2022) by sequestration.
Knowledge regarding A. thaliana epidermal development comes mainly from studies on a handful of inbred lineages maintained for decades in the laboratory, such as the widespread Columbia accession. Nevertheless, for some epidermal traits, phenotypic variation among natural Arabidopsis accessions has been attributed to sequence polymorphism in specific genes, such as the GL1, ETC2 and MYC1 transcription regulators affecting trichome density (Hauser, Harr, and Schlötterer 2001; Hilscher, Schlötterer, and Hauser 2009; Symonds, Hatlestad, and Lloyd 2011; see also Hauser 2014) or the STI gene controlling trichome branch number (Ilgenfritz et al. 2003).
With the onset of high throughput sequencing and availabiLity of genetically characterized resources linked to computational tools, genome-wide association studies (GWAS) identifying sequence polymorphisms correlated with distinct phenotypic features became a powerful tool for discovering new gene functions. While the GWAS methodology is well established in Arabidopsis (see, e.g., Brachi, Morris, and Borevitz 2011; Slovak et al. 2015), it found only Limited use in studying epidermal development in this species. A screen for host-side genetic determinants of plant-associated microbiome variability revealed genomic regions enriched in genes participating in cell wall biogenesis, and somewhat surprisingly, also in trichome branching (Horton et al. 2014). A recent report applied GWAS to identify genes responsible for variation in aerial organ trichome density (Arteaga et al. 2022). Epidermal or trichome characteristics have, however, been addressed by GWAS in non-model plant species, including commercially important crops. Recent studies focused on trichome distribution patterns in Brassica napus (Xuan et al. 2020) and Aegilops tauschii (Mahjoob et al. 2022), on cuticular wax deposition in B. napus (Jin et al. 2020; Long et al. 2023) and on cotton trichome development (Li et al. 2020; Wang et al. 2021).
Here we report a GWAS for genes associated with phenotypic variability of selected epidermal traits of rosette leaves in 310 sequenced A. thaliana accessions from the 1001 Genomes collection (Alonso-Blanco et al. 2016), focusing on traits related to trichome development, cell wall composition and epidermal metal deposition. Besides identifying genes known or already suspected to affect these characteristics, our results suggest possible roles in epidermal development for several gene families with hitherto uncharacterized or only partly characterized functions.
2 Results
2.1 Natural Variation of Rosette Leaf Epidermis Traits
We analyzed 310 natural A. thaliana accessions, mostly with available detailed location data and climate parameters of their sites of origin (Supporting Information S2: Table 1) to gain insight into the genetic basis of variability in trichome shape, size and density, epidermal autofluorescence and epidermal metal deposition patterns. We recorded 7 continuous quantitative and 11 categorical semiquantitative (ordinate) or qualitative parameters (Table 1; Bezvoda 2023) that exhibited readily noticeable variabiLity among the accession and were amenable to semi-high-throughput analysis with good interobserver replicability.
| Parameter | Refers to | Variable type | Categorical variable values | Sample preparation |
|---|---|---|---|---|
| Area | Trichomes | Continuous | Callose staining | |
| Circularity | Trichomes | Continuous | ||
| Solidity | Trichomes | Continuous | ||
| Length | Trichomes | Continuous | ||
| Perimeter | Trichomes | Continuous | ||
| Callose content | Trichomes | Continuous | ||
| Stem length | Trichomes | Continuous | Drying (autofluorescence observed under UV light) | |
| Trichome autofluorescence | Trichomes | Categorical ordinate | 0 (none); 0,5; 1; 2; 3 (strongest) | |
| Epidermis autofluorescence | Epidermis outside trichomes | Categorical ordinate | 0 (none); 1; 2; 3; 4; 5 (strongest) | |
| Autofluorescence colour | Epidermis | Categorical qualitative | 0 (none, whitish or colourless); 1 (bluish or brownish hue) | |
| Trichome density | Epidermis | Categorical ordinate | 0 (none); 0.5; 1; 1.5; 2; 2.5; 3; 4 (densest) | |
| Number of branches | Trichomes | Categorical ordinate | Values roughly correspond to typical (or average) trichome branch number | |
| Metal ring | Trichomes | Categorical ordinate | 0 (no rings); 1 (rings in some trichomes); 2 (rings in most or all trichomes) | Metal staining |
| Metal gradient | Trichomes | Categorical ordinate | Scale reflects gradient length - 0 (no gradient); 0.5; 1; 1.5; 2; 2.5; 3 (longest) | |
| Metal base | Trichomes | Categorical ordinate | 0 (no staining of trichome base; 1 (in some trichomes); 2 (in most or all trichomes) | |
| Metal surround | Epidermis around trichome base | Categorical ordinate | 0 (no staining around trichome base; 1 (around some trichomes); 2 (around most or all trichomes) | |
| Metal stomata | Guard cells | Categorical ordinate | 0 (no staining; 1 (in some stomata); 2 (in many stomata); 3 (in most or all stomata) | |
| Shaveproof | Epidermis | Categorical qualitative | 0 (trichomes detachable); 1 (shaveproof) | Detachment of trichomes during callose staining |
The first group of parameters with continuous distribution (Figure 1) describes quantitative aspects of trichome shape, that is, size-related parameters (area, perimeter, overall length and stem length) and descriptors of shape complexity (circularity and solidity). Other morphological features, namely trichome density (Figure 2A,B) and branch number (Figure 2C), were evaluated semiquantitatively.
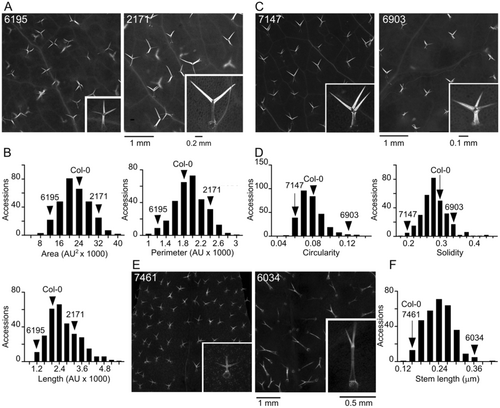
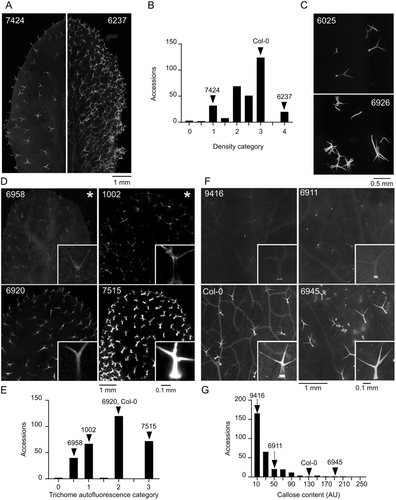
The next group of parameters reflects structural and chemical properties of the trichome cell walls, affecting trichome autofluorescence upon damage, estimated as a semiquantitative categorical variable (Figure 2D,E) and callose content, evaluated semiquantitatively on a continuous scale in a manner capturing also the spatial distribution of callose (Figure 2F,G). Obvious variability in the background epidermal autofluorescence outside trichomes was recorded as a categorical semiquantitative variable (Figure 2D). We introduced another categorical qualitative variable to describe visible differences in the colour of trichome and background fluorescence (Figure 3A).
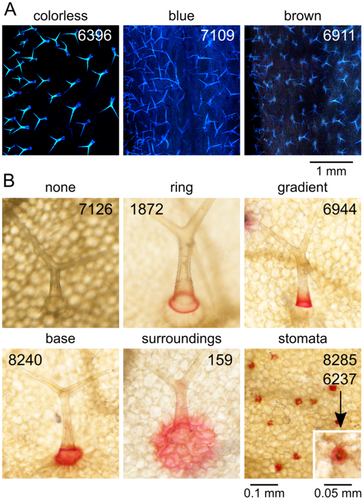
A third group of categorical semiquantitative parameters captures the spatial distribution of metal ion deposits, as detected by dithizone staining, in trichomes and other epidermal cell types (Figure 3B; for description of parameters see Table 1).
Since we noticed that trichomes of some accessions resist mechanical detachment during staining for callose, we recorded this feature as a qualitative parameter ‘shaveproof’.
All seven continuous variable parameters exhibit smooth distribution with a single roughly symmetrical maximum (Figures 1B,D,F, 2G, 4A), except callose content, where most accessions have very Little if any callose in trichomes, resulting in an asymmetric distribution. A roughly symmetrical single maximum distribution, in some cases noisy, was observed also for the semiquantitative categorical variables of trichome autofluorescence, epidermis autofluorescence, trichome density and trichome branch number. The ‘noisy single maximum distribution’ may be explainable by under-representation of visually attributed intermediate values, perhaps reflecting observer uncertainty rather than genuine differences. For several other parameters (metal gradient, metal surround, metal stomata) the distribution was asymmetric with most accessions exhibiting low or no signal. Remaining parameters either varied between only two values (autofluorescence colour, shaveproof) or showed a possibly bimodal distribution, such as in the case of metals at trichome base and metal rings (Supporting Information S1: Figure S1).
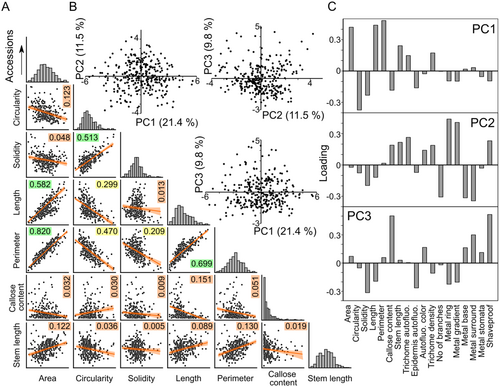
Not surprisingly, some quantitative descriptors of trichome shape were mutually correlated (Figures 4A and Supporting Information S1: Figure 1). Trichome area, perimeter and overall length, which can all be viewed as measures of trichome size, exhibited strong or very strong positive correlation. Weak to moderate positive correlation with these parameters was found also for trichome stem length. Circularity (proportional to the ratio of an object's area and squared perimeter) and soLidity (ratio of the area of the examined object and its convex hull), which both achieve higher values for less intricate shapes, were mutually moderately positively correlated, and negatively correlated with the size-related parameters. While there were some cases of non-negligible correlation among the categorical variables, or between categorical and continuous ones, such correlations were generally weak except of moderate positive correlation between the frequency of metal rings and intensity of metal gradients at the trichome stems, as well as moderate positive correlation between strength of mechanical attachment (the ‘shaveproof’ trait) and trichome callose content, which was also moderately negatively correlated with trichome length. Remarkably, all the strong or moderate correlations were statistically significant (Supporting Information S1: Figure 1).
For a subset of traits including representatives of the mutually correlated trait groups, heritability was estimated from data collected separately for individual plants (Supporting Information S2: Table 2). The broad sense heritability values, ranging between 0.58 and 0.94, indicate a strong genetic contribution to the observed variability (Supporting Information S2: Table 3).
Principal component analysis (PCA) of the 18 recorded parameters did not reveal any obvious clustering of accessions when considering the first three principal components (PCs), which, however, cumulatively explained only less than 43% of total variability (Figure 4B). Analysis of PC loadings indicated that PC1 is positively correlated with the mutually correlated parameters reflecting trichome size, and negatively with circularity and solidity, PC2 correlates either positively or negatively with specific patterns of metal staining, and PC3 mostly reflects callose content and strength of mechanical attachment—that is, two parameters related to cell wall structure (Figure 4C). The PCA results thus support the overall pattern of continuous variability of traits addressed in our study, and also suggest that trichome shape, metal staining and cell wall organization might be determined by distinct sets of genes.
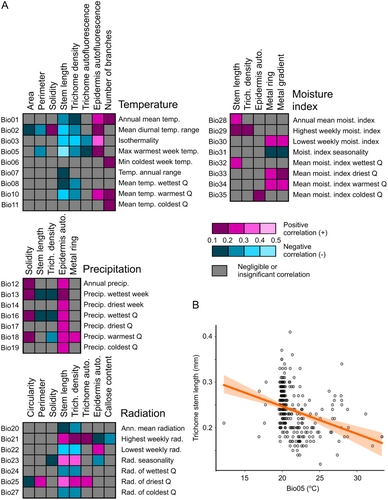
We next examined possible relationships between the 18 phenotypic traits determined in our study and 35 standardized climate variables of the sites of origin of our Arabidopsis accessions, obtained from the CliMond database (Kriticos et al. 2012). Out of the 630 possible trait and cLimatic variable combinations, only 81 exhibited statistically significant non-negligible positive or negative correlation, typically in the weak range (Supporting Information S2: Table 4, Figure 5A). The only exception was a negative correlation between trichome stem length and the maximum temperature of the warmest week (the Bio05 variable), which fell into the moderate range (sensu Schober, Boer, and Schwarte 2018; Figure 5B). The biological interpretation of this observation is not immediately obvious.
2.2 GWAS Analysis Identifies Loci Associated With Some Epidermal Traits
The phenotypic data were subjected to association analysis using the publicly available GWAPP GWAS platform (Seren et al. 2012) as described in Materials and Methods, aiming first towards identification of all single nucleotide polymorphisms (SNPs) within the transcribed section of genomic DNA, including the CDS, introns and the 5ʹ and 3ʹ UTRs. The numbers of identified significantly associated loci varied among traits on the scale from zero to several hundred, with trichome stem length and guard cell metal accumulation yielding the most candidates (Tables 2 and Supporting Information S2: Table 5). No significant SNPs were found for trichome area, metal ring, metal staining of trichome bases, and resistance towards mechanical detachment, and only one or a few presumably silent polymorphisms (i.e., synonymous mutations or SNPs located in an intron or in untranslated mRNA regions) were identified for overall trichome length, number of branches and the presence of a metal gradient. These traits were therefore not included in subsequent analyses.
| Parameter | Start/stop change | Missense | UTR or intron | Synonymous |
|---|---|---|---|---|
| Area | 0 | 0 | 0 | 0 |
| Circularity | 0 | 44 | 86 | 25 |
| Solidity | 0 | 33 | 66 | 16 |
| Length | 0 | 0 | 1 | 0 |
| Perimeter | 0 | 6 | 15 | 3 |
| Callose content | 0 | 1 | 2 | 1 |
| Stem length | 26 | 657 | 1197 | 574 |
| Trichome autofluorescence | 0 | 21 | 39 | 12 |
| Epidermis autofluorescence | 0 | 11 | 16 | 4 |
| Autofluorescence colour | 1 | 52 | 95 | 35 |
| Trichome density | 2 | 216 | 357 | 182 |
| Number of branches | 0 | 0 | 1 | 0 |
| Metal ring | 0 | 0 | 0 | 0 |
| Metal gradient | 0 | 0 | 2 | 1 |
| Metal base | 0 | 0 | 0 | 0 |
| Metal surround | 0 | 16 | 30 | 17 |
| Metal stomata | 27 | 627 | 970 | 425 |
| Shaveproof | 0 | 0 | 0 | 0 |
The distribution of SNP types for individual traits with multiple SNPs exhibits some differences, although they are not major (Supporting Information S1: Figure 2). In particular, SNPs altering CDS length were found only for traits with overall large SNP numbers, reflecting low probability of polymorphisms affecting start and stop codons. Subsequently, we focused only on loci with SNPs changing the predicted protein product sequence, in most cases due to a missense mutation, and treated all such SNPs as a single category. The full list of genes affected by such SNPs for each trait is provided in Supporting Information S2: Table 6. In total, 1547 different loci exhibited association with at least one of the studied traits, corresponding to ~5.6% of all Arabidopsis genes.
For further analyses, we divided the traits with significantly associated SNPs into four groups: those affecting trichome shape (circularity, soLidity, perimeter and stem length), trichome distribution on the surface of the leaf (i.e., trichome density), wall composition-related parameters (epidermis and trichome autofluorescence, autofluorescence colour, callose content) and metal accumulation (metal staining of stomata and trichome surroundings). While some loci associated with several of the mutually correlated cell shape traits, there was only a very Limited, if any, overlap between candidate lists for traits from the wall composition-related and metal accumulation-related groups. However, polymorphisms in some loci were identified as associating with traits from more than one category (Figure 6A, Supporting Information S2: Table 7).

Assuming that SNPs in transposon-borne genes are unLikely to cause phenotypic differences, we examined what fraction of our candidates corresponds to CDSs from mobile genetic elements to gain insights into the extent of non-specific background. The fraction of transposon-derived loci was significantly depleted compared to whole genome values for the sum of all GWAS candidates, as well as for those associated with shape-related traits or trichome density. However, while depletion of transposon genes was also noticeable for wall-related traits, it was only marginally significant, possibly due to a relatively low number of loci, and practically no depletion was observed for metal-related traits (Table 3). Sets of genes associated with multiple loci usually contained fewer transposon-derived loci than those found on the basis of a single trait (Figure 6B).
| Trait group | All loci | Transposon loci | Fraction from transposons | p value |
|---|---|---|---|---|
| Whole genome (Araport) | 27655 | 3879 | 14% | N.A. |
| All traits | 1547 | 129 | 8% | < 0.0001 |
| Shape traits | 715 | 43 | 6% | < 0.0001 |
| Density | 216 | 5 | 2% | < 0.0001 |
| Wall-related traits | 82 | 6 | 7% | 0.10 |
| Metal-related traits | 654 | 82 | 13% | 0.25 |
- Note: p values indicate significance of the transposon-derived locus depletion compared to the whole genome data set (pairwise χ2 test with Benjamini–Hochberg correction for multiplicity).
- Abbreviation: N.A., not available.
2.3 Multiple GWAS Candidates Encode Proteins With Known or Suspected Epidermal Roles
Next, we examined the traits related to trichome development (i.e., the shape traits, density and the cell wall parameters that largely reflect trichome properties) for enrichment of genes abundantly expressed in trichomes either at the mRNA (Jakoby et al. 2008) or protein (Huebbers et al. 2022) levels. While we did not detect significant enrichment or depletion of these genes in our candidates lists, we identified multiple genes associated with trichome related traits that were abundantly expressed in trichomes, consistent with their participation in trichome development. In some cases, available annotations suggest function in membrane trafficking, biogenesis of cell wall components or turgor generation and maintenance. This might point to possible mechanisms whereby variation in the responsible loci may affect cell growth or cell wall development and generate the observed phenotypic diversity, either by direct modification of trichome morphogenesis or by modulating epidermal cell expansion and hence trichome density (Table 4). Prompted by the finding of several genes engaged in cuticle or epidermal wax biosynthesis, that is, traits often linked to trichome development (see Berhin, Nawrath, and Hachez 2022) among these loci, we examined the rest of our candidates for overlap with a list of 87 putative cuticle and wax biosynthesis genes identified on the basis of their known activities and/or expression patterns (Suh et al. 2005). However, we did not find any additional loci in this manner beyond two genes that were also highly expressed in trichomes—namely AT1G01600/CYP86A4 and AT1G76690/OPR2 (see Table 4).
| Locus (gene name) | Screen | Description | Trait | Allele (effect) |
|---|---|---|---|---|
AT1G01600 (CYP86A4) |
T | Member of the CYP86A subfamily of cytochrome p450 genes. Involved in cutin and wax biogenesis (Camoirano et al. 2020) | Stem length | V341I (up) |
| Density | A476V (down) | |||
| Trichome autofluorescence | A476V (down) | |||
AT1G08920 (ESL1) |
T | ERD (early response to dehydration) six-like 1; ESL1, a transporter for monosaccharides | Stem length | L118F (up) |
| Density | L118F (up) | |||
AT1G12550 (HPR3) |
P | Glyoxylate/hydroxypyruvate reductase HPR3. Transcript restricted to trichomes (Xu et al. 2018) | Stem length | S135A (up) |
AT1G12570 (HTH-like) |
P | Glucose-methanol-choline (GMC) oxidoreductase family protein. Ortholog of maize IPE1 participating in pollen exine development. Involved in cutin and wax biogenesis (Kannangara et al. 2007). | Stem lengtha | K138R (up) N187K (up) |
| Density | D77Gb, S78Ab (up) | |||
AT1G15670 (KFB1, KMD2) |
T | F-box/Kelch-repeat protein. Member of the KISS ME DEADLY (KMD) family, that targets type-B ARR proteins for degradation and is involved in negative regulation of the cytokinin response. Also known as KFB1, a member of a group of Kelch repeat F-box proteins negatively regulating phenylpropanoid biosynthesis. | Stem length | E37A (up) |
AT1G26400 (FOX3, ATBBE5) |
P | Berberine bridge enzyme-like 5. Possible role in defense, related proteins participate in wall modification (Eggers et al. 2021). | Stem length | I16Vb, L39Pb (up) |
AT1G53210 (NCL) |
P | Sodium/calcium exchanger NCL | Stem length | G420D (down) |
AT1G76690 (OPR2) |
P | 12-oxophytodienoic acid reductase with possible role in cutin or wax synthesis (Suh et al. 2005) | Stem length | A90T (down) |
AT1G78920 (AVPL1) |
P | Pyrophosphate-energized membrane proton pump 2. Located at Golgi, strong expression in trichomes confirmed using a GUS reporter (Mitsuda et al. 2001). | Stem length | N16S (up) |
| AT2G20240 (TRM17) | P | GPI-anchored adhesin-like protein, putative (DUF3741) | Stem length | S157P, D576Eb, A671Tb (up) |
AT2G20990 (SYTA, SYT1) |
P | Synaptotagmin A. Regulates endosome recycling at the plasma membrane, but not secretory traffic. Strong expression in trichomes confirmed using a GUS reporter (Schapire et al. 2008). | Stem length | H357Q (up) |
AT2G21410 (VHA-A2) |
P | V-type proton ATPase subunit a2. Required for endocytic and secretory trafficking (Dettmer et al. 2006). | Stem length | I519Vb, I762V (up) |
AT2G21870 (MGP1, PHI1) |
P | Probable ATP synthase 24 kDa subunit, mitochondrial. Essential for pollen formation. | Stem length | K207M (up) |
AT2G35860 (FLA16) |
P | FascicLin-Like arabinogalactan protein. Mutant has reduced cell size in some cell types in the stem (Liu et al. 2020). | Density | K115T (down) |
AT3G28500 (RPP2C, P2X) |
P | 60S acidic ribosomal protein P2-3 | Density | G82A (down) |
| AT3G48000 (ALDH2B4) | P | Aldehyde dehydrogenase family 2 member B4, mitochondrial. May participate in biosynthesis of ferulic acid and sinapic acid, modulating cell wall strength (Brocker et al. 2013). | Stem length | N63D (up) |
AT3G59010 (PME35, PME61) |
P | Pectin methyl esterase, activity controls cell wall mechanic strength; identified in a trichome transcriptome study (Wienkoop et al. 2004) | Density | V291I (down) |
AT4G02930 (TUFA) |
P | Elongation factor Tu, mitochondrial | Stem length | V29I (down) |
AT4G24840 (COG2) |
P | OLigomeric Golgi complex subunit-like protein containing the COG2 domain, part of the membrane trafficking machinery (see Vukašinović and Žárský 2016) | Stem length | L652F (down) |
| AT5G28220 | P | Protein prenylyltransferase (uncharacterized) | Stem length | L190F (down) |
AT5G63910 (FCLY) |
P | Farnesylcysteine lyase (EC 1.8.3.5) involved in a salvage/detoxification of farnesylcysteine residues liberated during the degradation of prenylated proteins (Crowell et al. 2007) | Density | G88S (down) |
- Note: Unless additional references are provided, annotation is derived from Araport 11 or the TAIR description (Reiser et al. 2024). Substitutions and effect directions refer to the Col-0 sequence and phenotype.
- Abbreviations: P, proteome; T, transcriptome.
- a The listed minor alleles affecting this parameter are often present together.
- b Not documented in some of the ecotypes carrying the other listed substitution because of sequencing gaps.
Because the loci associated with metal accumulation-related traits mainly reflect stomatal metal deposition, we examined the metal-related candidate list for overlap with a published set of 63 genes upregulated in guard cells (Leonhardt et al. 2004), as well as a list of 1508 proteins repeatedly identified in guard cell proteome (Zhao et al. 2008). We found no overlap between our candidate list and the list of genes highly transcribed in guard cells, but identified 21 loci from the proteome set, all of them associated with metal accumulation in the guard cells and some encoding proteins engaged in ion or other solute transport, membrane trafficking, cell wall biogenesis or gene expression, that is, processes possibly relevant for the observed trait (Table 5). However, there was no significant enrichment of guard cell-expressed genes among our candidates, similar to the trichome case.
| Locus (gene name) | Description | Allele |
|---|---|---|
| AT1G31850 | S-adenosyl-L-methionine-dependent methyltransferases superfamily protein; probable methyltransferase PMT20; found in a Golgi complex proteome study (Parsons et al. 2012) | V113A |
AT1G42550 (PMI1) |
Protein PLASTID MOVEMENT IMPAIRED 1, a plant-specific protein of unknown function conserved among angiosperms | G460A |
AT1G59820 (ALA3) |
PhosphoLipid-transporting ATPase (phosphoLipid translocase) involved in secretory vesicle formation from trans-Golgi | E647K |
AT1G59900 (E1 ALPHA) |
Pyruvate dehydrogenase E1 component subunit alpha-1, mitochondrial | S361P |
AT1G67490 (GCS1, KNF, KNOPF) |
Mannosyl-oLigosaccharide glucosidase GCS1; an alpha-glucosidase I enzyme that catalyzes the first step in N-linked glycan processing. Localized to the endoplasmic reticulum. | D567E |
AT3G10670 (ABCI6, NAP7) |
ABC transporter I family member 6, chloroplastic. Plastidic SufC-Like ATP-binding cassette/ATPase essential for Arabidopsis embryogenesis. Involved in the biogenesis and/or repair of oxidatively damaged Fe–S clusters. | L42V |
AT3G13460 (ECT2) |
Evolutionarily conserved C-terminal region 1; regulates the mRNA levels of the proteasome regulator PTRE1 and of several 20S proteasome subunits, resulting in enhanced 26S proteasome activity | T210P |
AT3G13772 (TMN7) |
Transmembrane 9 superfamily member, localized in the secretory pathway. Overexpression of this protein in yeast alters copper and zinc homeostasis (Hegelund et al. 2010). | N565S |
AT3G48000 (ALDH2B4, ALDH2, ALDH2A) |
Aldehyde dehydrogenase family 2 member B4, mitochondrial | A267T |
| AT4G13360 | 3-hydroxyisobutyryl-CoA hydrolase-Like protein 3, mitochondrial; ATP-dependent caseinolytic (Clp) protease/crotonase family protein | T395A |
AT4G18290 (KAT2) |
Member of the Shaker family potassium ion (K+) channel. Critical to stomatal opening induced by blue light and to circadian rhythm of stomatal opening. Involved in plant development in response to high light intensity. | L170H |
AT4G18670 (LRX5) |
Leucine-rich repeat extensin-like protein 5, involved in cell wall biogenesis and organization. Interacts with several members of the RALF family of ligand peptides. | P507T P813L |
AT4G20400 (JMJ14) |
Histone H3K4 demethylase repressing floral transition | P480L |
| AT4G21180(ERDJ2B) | DnaJ domain protein localized in ER membrane | T67A |
| AT5G35430(NOT10) | Tetratricopeptide repeat (TPR)-like superfamily protein, found also in stress granules | W680C |
| AT5G39040(ABCB27, ALS1, TAP2) | Aluminum sensitive 1, ABC transporter B family member 27; a member of TAP subfamily of ABC transporters that is located in the vacuole. Mutants are hypersensitive to aluminum. May be important for intracellular movement of some substrate, possibly chelated Al, as part of a mechanism of aluminum sequestration. | V266I |
| AT5G43130(TAF4B) | Transcription initiation factor TFIID subunit 4b | Q120K |
| AT5G52470(MED36B, FBR1, FIB1, SKIP7) | Probable mediator of RNA polymerase II transcription subunit 36b; encodes a fibrillarin, a key nucleolar protein in eukaryotes which associates with box C/D small nucleolar RNAs (snoRNAs) directing 2'-O-ribose methylation of the rRNA | L133V |
| AT5G55040(BRD13) | DNA-binding bromodomain-containing protein; interacts with core SWI/SNF complex components. Identified as a specific subunit of BRM-associated SWI/SNF (BAS) complexes. | S256A |
| AT5G58100(IEF7) | Encodes a membrane protein involved in pollen nexine and sexine development | N358H |
| AT5G58140(PHOT2) | Phototropin-2; membrane-bound protein serine/threonine kinase that functions as blue light photoreceptor in redundancy with PHO1. Involved in stomatal opening, chloroplast movement and phototropism. | R898G |
- Note: Unless additional references are provided, annotation is derived from Araport 11 or the TAIR description (Reiser et al. 2024). Substitutions refer to the Col-0 sequence; all Listed alleles exhibited increased guard cell metal deposition compared to Col-0.
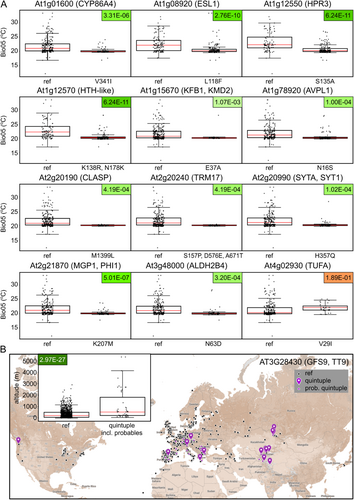
To further verify the performance of our GWAS screen, we inspected the candidate gene lists for genes previously reported to affect the studied traits. While such a search cannot be exhaustive, we found at least five previously characterized relevant genes (Table 6), including loci coding for two cytoskeletal proteins CLASP and SCAR/DIS3, as well as the EXO84B gene, encoding a subunit of the exocyst complex that regulates targeting of membrane vesicles. All three genes exhibit mutant phenotypes involving trichome shape alterations, and all associated with trichome shape traits. In addition, the gene for another exocyst subunit, EXO70H4, documented to participate in the deposition of metals in the trichome cell wall, was picked up as associating with guard cell metal deposits. The fifth gene, GFS9/TT9, is known to affect cell wall autofluorescence and was found in association with trichome density and autofluorescence traits. Notably, five SNPs affecting this gene often occurred together, constituting a quintuple substitution minor allele associated with reduced trichome density and decreased autofluorescence.
| Locus (gene name) | Description | Trait(s) | Allele (effect) |
|---|---|---|---|
AT2G20190 (CLASP) |
CLiP-associated protein; a microtubule-associated protein involved in both cell division and cell expansion. It likely promotes microtubule stability. Loss of function mutants have decreased trichome branch number (Pietra et al. 2013). | Stem length | M1399L (up) |
AT2G38440 (DIS3, ITB1, SCAR2, WAVE4) |
Subunit of the WAVE complex, required for activation of ARP2/3 actin nucleation complex. Mutations cause defects in both the actin and microtubule cytoskeletons that result in aberrant epidermal cell expansion. Loss of function mutants exhibit a distorted trichome phenotype (Basu et al. 2005). | Circularity | I1265V (down) |
| Solidity | I1265V (down) | ||
| Density | I1265V (down) | ||
AT3G09520 (EXO70H4) |
A member of the EXO70 gene family of putative exocyst subunits, conserved in land plants. Involved in trichome cell wall maturation, required for deposition of heavy metals in the cell wall ([Kulich et al. 2015], though guard cell localization was not specifically observed). | Metal stomata | S93N (up) |
AT3G28430 (GFS9, TT9) |
Peripheral membrane protein localized at the Golgi apparatus that is involved in membrane trafficking, vacuole development and flavonoid accumulation. Loss of function mutants exhibit abnormal autofluorescence, though trichomes were not specifically examined (Ichino et al. 2014). | Density | S573A, S626A, Y667Ha, A670Va, D671Ea (down) |
| Trichome autofluorescence. | S573A, S626A, Y667Ha, A670Va, D671Ea (down) | ||
AT5G49830 (EXO84B) |
Exocyst complex component 84B; involved in tethering vesicles to the plasma membrane. Interacts with Exo70H4 that controls trichome cell wall development; loss of function mutant has multiple defects including in trichome morphogenesis (Kulich et al. 2015; Fendrych et al. 2010). | Circularity | E123G (down) |
| Solidity | E123G (down) |
- Note: Substitutions and effect directions refer to the Col-0 sequence and phenotype.
- a Not documented in some of the ecotypes carrying the other listed substitution because of sequencing gaps.
Additional 80 genes with previously reported roles in cytoskeletal organization, membrane trafficking, cell wall biogenesis, tissue patterning or other relevant functions were also found among the candidates, including several genes affecting aspects of epidermal development unrelated to traits addressed in our study (Supporting Information S2: Table 8).
These findings suggest that, despite the lower efficiency of the metal trait-associated gene search, as evident from the lack of transposon gene depletion, the set of candidates found in our screen contains at least a sizeable proportion of genes biologically relevant in the context of leaf epidermis differentiation.
2.4 Some GWAS Candidates Exhibit Environmentally Correlated Allele Distribution
To obtain clues to the mechanisms underlying the observed correlation between trichome stem length and the maximum warmest week temperature of the accession's location of origin (the Bio05 variable), we further examined the set of genes exhibiting SNPs associated with trichome stem length. Since the full List of over 600 stem length-associated candidates was too large for a detailed study, we focused on the 18 genes that were at the same time abundantly expressed in trichomes or encoded proteins with known roles in trichome development (see Tables 4 and 6).
For twelve of these genes, at least 30 studied genotypes (i.e., a tenth of our accessions set) harboured the minor allele. The distribution of Bio05 values for these loci in all but one case exhibited a statistically significant difference between the major and minor alleles, indicating an environmentally correlated bias in allele distribution (Figure 7A), which might suggest natural selection against the minor alleles in hot habitats. Among the affected genes were loci encoding the microtubule-associated protein CLASP, known to have pleiotropic roles in cell expansion and cell division (Ambrose et al. 2007; Pietra et al. 2013), and SYT1, coding for a synaptotagmin engaged in processes related to stress tolerance and immunity towards pathogens (Schapire et al. 2008; Yamazaki et al. 2008; Levy, Zheng, and Lazarowitz 2015; Kim et al. 2016). It this thus conceivable that the selection was operating on some function(s) of these genes unrelated to epidermal morphogenesis.
For all genes with significant environmentally correlated allele distribution, populations harbouring minor alleles originated from multiple sites, i.e. there was no evidence of spatial restriction due to a founder effect. Even variants only found in Scandinavian accessions occured at two or more geographically distant sites (Supporting Information S1: Figure 3). This further supports the relevance of environmental factors and possible natural selection.
Since the GFS9/TT9 gene displayed an unusual co-occurrence of five SNPs, as described above, we also examined the geographic substitution of the Quintuple substitution minor allele. Because the five contributing substitutions were found in mere six accessions of our experimentally characterized collection, we analyzed the whole set of genotypes from the Ensembl database (Yates et al. 2022). Populations carrying the quintuple SNP allele often originated from mountainous regions, and the average altitude of their sites of origin was significantly higher than for the reference variant (Figure 7B). Correspondingly, a significant difference was found in multiple climatic variables from the standard BioClim set, especially in parameters reflecting typical features of high altitude biotops, such as increased temperature seasonality, greater difference among temperature and precipitation extremes, and increased solar radiation (Supporting Information S1: Figure S1). The presence of the quintuple substitution allele across three continents is consistent with its selective advantage under high altitude conditions.
2.5 Overrepresentation of Some Gene Families Among GWAS Candidates Suggests New Gene Functions
We next examined the sets of loci associated with the four trait groups (trichome shape, trichome density, wall-related traits and metal-related traits) for Gene Ontology term association. No enrichment or depletion of any GO categories was found on the p = 0.01 significance level, while on the p = 0.05 level, a 2.73x enrichment of the Transmembrane signal receptor protein class was found among shape-associated loci, and a 12.22x enrichment of the snoRNA-binding molecular function category was observed among metal trait-associated loci. The biological significance of the latter, however, remains unclear in Light of the apparent low reliability of metal-related candidate detection (see above).
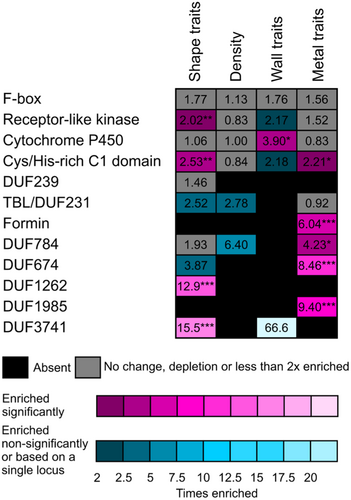
While inspecting our candidate gene Lists, we noticed the presence of multiple members of some gene families, including those encoding evolutionarily conserved domains of unknown function (DUF). We thus examined several such multigene families for possible enrichment among candidates for individual trait groups. Our enrichment analyses included all seven DUF families represented by two or more genes in at least one trait group, as well as five large gene families containing multiple candidates associated with at least one trait group, namely the F-box proteins (568 paralogs in Arabidopsis), receptor-Like protein kinases (307 paralogs), cytochrome P450 isoforms (256 paralogs), cysteine/histidine-rich C1 domain proteins (153 paralogs) and formins (FH2 proteins, 21 paralogs).
For 9 out of these 12 gene families, we found in at least one category of GWAS candidates a reliable, significant at least 2× enrichment compared to whole genome abundance (Figure 8; Supporting Information S2: Table 9). These include receptor-Like kinases (minor but significant enrichment in shape traits association), cytochrome P450 (enrichment in cell wall fluorescence-related traits, compare also Table 4), cysteine/histidine-rich C1 domain proteins (enrichment in shape and metal accumulation-related traits), formins, DUF674, DUF784 and DUF1985 proteins (enrichment in metal accumulation traits), as well as the DUF1261 and DUF3741 families that were found in association with trichome shape traits.
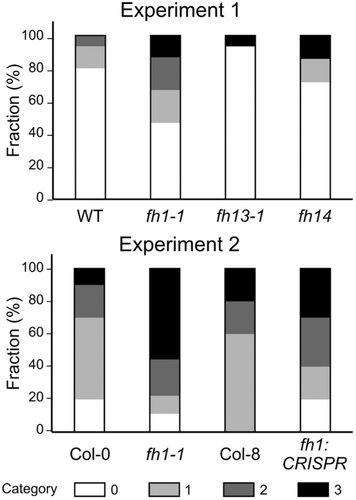
Making use of available previously characterized loss of function mutants affecting all three formin genes associating with the stomatal metal deposition, that is, FH1, FH13 and FH14, we performed single-blinded evaluation of epidermal metal deposition in these mutants and corresponding wild type plants. The results suggest a tendency towards increased stomatal metal accumulation especially in the fh1-1 T-DNA insertion mutant of the formin gene FH1, and possibly also in the CRISPR-generated loss of function allele of the same gene (Figure 9). Although none of the observed between-genotype quantitative differences was statistically significant, this observation is consistent with a possible contribution of formins to epidermal metal deposition that would deserve further attention. It also suggests that also the other observed gene family enrichments may be relevant.
2.6 Associations Among Candidate Genes Indicate Possible Functional Modules
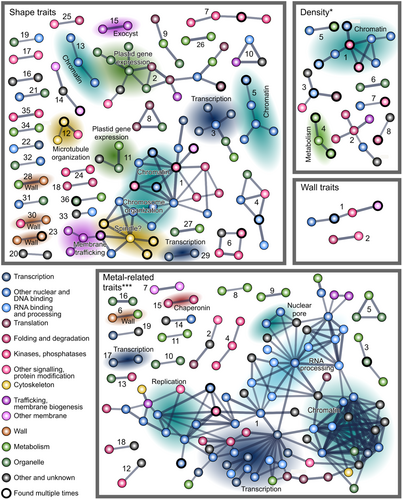
To uncover functional relationships among genes associating with the studied traits, we scanned the candidate Lists for mutual links recorded in the STRING database (Szklarczyk et al. 2023) that registers both experimentally documented and predicted interactions among genes, such as transcriptional co-regulation, clustering on the chromosomes, epistasis, or proteins, such as mutual binding.
Groups of associated genes were found for all four trait groups (Figures 10 and Supporting Information S1: Figure 5, Supporting Information S2: Table 10), but only for trichome density and metal accumulation the numbers of interactions were significantly greater than predicted for a random gene selection (Supporting Information S2: Table 10). Inspection of the found clusters, however, reveals biologically meaningful associations also among candidates Linked to the remaining trait groups. For example, among the shape-associated genes, cluster 15 consists of Exocyst complex subunits. For density and metal deposition, the clusters notably included mainly genes participating in nuclear functions such as chromatin organization, gene expression, RNA processing or nuclear transport.
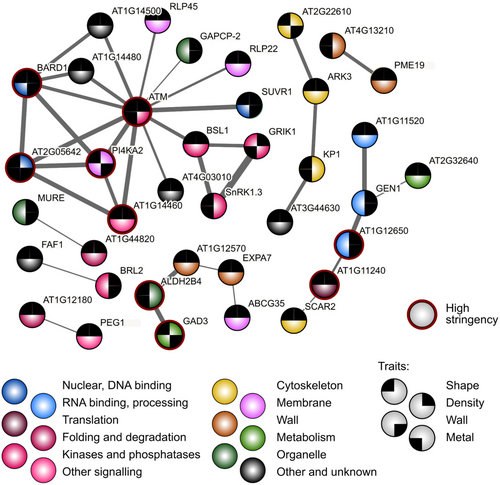
Since we noticed that some genes are shared by clusters found for two or more trait groups, we next performed an analogous search for inter-gene associations among candidates associating with multiple phenotypic trait groups. With somewhat less stringent criteria than those used in the initial interaction searches, this group of genes also exhibited significantly more mutual associations than a random gene selection (Figure 11; Supporting Information S2: Table 10), with one large and two smaller clusters comprising mostly genes associated with shape traits, as well as a small cluster consisting of genes linked to trichome density. We believe that these findings may provide a starting point for exploration of novel regulatory pathways engaged in Arabidopsis epidermal differentiation.
3 Discussion
We employed the GWAS approach to explore the genetic basis of A. thaliana phenotypic variability, focusing on epidermal traits including trichome development, secondary cell wall composition, production of autofluorescent substances, including those deposited in the cell wall, and metal accumulation. To our knowledge, no similar study has been conducted in Arabidopsis so far, except of a recent report (Arteaga et al. 2022) focusing on trichome patterning and trichome density (the latter also included in our study).
We studied 18 quantitative, semiquantitative or qualitative epidermal traits from 310 predominantly Scandinavian A. thaliana accessions from the 1001 Genomes collection (Alonso-Blanco et al. 2016). These traits can be divided into four groups (trichome shape, trichome density, cell wall composition and metal deposition-related). All quantitative parameters, as well as most semiquantitative categorical ones, exhibited continuous variability. As expected, traits reflecting trichome size, as well as those describing trichome shape complexity, were mutually positively correlated. Some of the analyzed traits exhibited also significant, albeit usually weak, correlation with selected climatic variables of the sites of origin of our plant accessions. Notably, epidermis autofluorescence was consistently weakly positively correlated with variables reflecting ambient temperature and amount of precipitation. Autofluorescence of plant tissues is largely due to phenolic compounds that might contribute to the defense of plants against microbial pathogens that thrive in warm, wet conditions (Donaldson 2020; Bhattacharya, Sood, and Citovsky 2010). However, the only observed correlation reaching at least the moderate range (sensu Schober, Boer, and Schwarte 2018) was a negative one between maximum temperature of the warmest week and trichome stem length, where a biological interpretation was not immediately obvious.
Surprisingly, we noticed strong dithizone staining, presumably detecting zinc and other heavy metal ion accumulation (see, e.g., Frederickson 2003; Srivastava et al. 2014; Peco et al. 2020), in stomatal guard cells of some accessions (but not in the common Col-0 Line). A similar pattern was reported for Cd accumulation in cadmium-stressed Col-0 plants (Zeng et al. 2017). In metazoan cells and in yeast, zinc accumulation is associated with increased secretory activity (Yuan 2011), and this also may be the case in the guard cells, which exhibit vigorous membrane turnover.
The outcome of GWAS studies always presents a compromise between specificity and sensitivity (see e.g. Brachi, Morris, and Borevitz 2011). We found statistically significant missense SNPs associated with phenotypic variability for 10 out of the 18 parameters examined, affecting over 5% of all A. thaliana protein-coding genes. Moreover, none of our candidate genes corresponded to any of the 15 trichome density-associated loci found in the recent GWAS report (Arteaga et al. 2022), which was based on phenotyping 191 predominantly Iberian A. thaliana accessions. This raises concerns whether our screen was specific and sensitive enough to be informative. However, genotype collection size in both studies was obviously far below the saturation threshold. For the similarly sized human genome, GWAS saturation was achieved only with population size several orders larger (Yengo et al. 2022). Our List of trichome density-associated genes also did not contain any previously characterized trichome patterning genes from the list compiled by Arteaga et al. (2022), nor did we observe significant enrichment of genes encoding most abundant trichome transcripts (Jakoby et al. 2008) or proteins (Huebbers et al. 2022). In case of the metal accumulation traits, where most candidates reflected the guard cell staining, there was no enrichment of the most abundant guard cell transcripts (Leonhardt et al. 2004), or proteins (Zhao et al. 2008). It is not uncommon that candidate genes from GWAS studies are not enriched in, or even are depleted of, known pathway genes. This would be consistent with the assumption that mutations in large effects genes, which typically tend to be the known genes, might not be beneficial in natural environments (Ristova et al. 2018). Taken together, we beLieve that at least some of our candidates are biologically relevant, because the candidate genes set, both as a whole and for each trait group except metal accumulation, was significantly (or, in one case marginally significantly) depleted of transposon-borne loci, whose SNPs, unLike polymorphisms concerning transposon presence or position (see, e.g., Yan et al. 2022), are rather unlikely to have phenotypic effects. Thus, the sets of trichome shape, density and probably also wall trait-associated GWAS candidates are Likely to contain biologically meaningful genes, while the relevance of candidates found on the basis of metal-related traits remains questionable.
We recovered five loci with well documented mutant phenotypes consistent with the associations identified in our study. First of them is the AT2G20190/CLASP gene coding for a microtubule-associated protein whose loss reduces trichome branch number (Pietra et al. 2013), In this gene, a SNP causing a conserved M1399L substitution in the C-terminal Armadillo-like repeat correlated with longer trichome stems. This substitution occurred more frequently in accessions from locations with higher Bio05 climate variable, reflecting the maximum environmental temperature at the site of the accession's origin. CLASP stabilizes microtubules and consequently affects cell division and cell expansion (Ambrose et al. 2007; Pietra et al. 2013). The stability of Arabidopsis microtubule assemblies can be impaired by high temperatures, resulting in defective meiosis (De Storme and Geelen 2020). Although a direct meiotic role of CLASP has not been documented, it is conceivable that the observed environmentally correlated allele distribution may reflect selection on the basis of a phenotype unrelated to epidermal development. This may be the case also for other loci with environmental bias in allele distribution, such as SYT1, which codes for a synaptotagmin involved in repair of membrane breaks caused by freezing (Yamazaki et al. 2008) and in defense against pathogens (Levy, Zheng, and Lazarowitz 2015; Kim et al. 2016) whose abundance could be linked to environmental conditions.
The second candidate gene with a relevant mutant phenotype is AT2G38440/DIS3, encoding a subunit of the WAVE complex regulating Arp2/3-mediated actin nucleation. Its mutation causes a characteristic distorted trichome shape (Basu et al. 2005). Here, a conserved I1265V substitution in an area immediately preceding the WASP homology motif associated with reduced trichome circularity and solidity, as well as lower trichome density. The third gene, AT5G49830/EXO84B, codes for an exocyst complex subunit with a pleiotropic mutant phenotype that includes trichome deformation (Kulich et al. 2015; Fendrych et al. 2010). A non-conservative E123G substitution at a variable sequence position of this locus (see Cvrčková et al. 2012) associated with decreased trichome circularity and soLidity. A non-conservative S93N substitution in a variable segment of another exocyst subunit, AT3G09520/EXO70H4, involved in metal deposition in the trichome cell wall (Kulich et al. 2015), associated with increased guard cell metal staining.
Last but not least, we found two conservative substitutions (S626A and D671E) associating with a decreae of trichome autofluorescence and also trichome density in AT3G28430/GFS9/TT9, which codes for a Golgi-associated peripheral membrane protein involved in membrane trafficking, vacuole development and flavonoid accumulation whose mutation results in altered autofluorescence (Ichino et al. 2014; Ichino et al. 2020). These mutations are frequently co-occurring with additional three substitutions, constituting a quintuple SNP minor allele, which appears to be present in multiple accessions of mountain origin across the globe. It is tempting to speculate that a variant with reduced cell wall autofluorescence may have altered flavonoid distribution among cell compartments, and perhaps enhanced vacuolar accumulation of anthocyanins that might bring a selective advantage under high altitude conditions characterized by increased UV stress, resulting in the fixation of this minor allele in mountain habitats. The presence of five identical SNPs in geographically disparate populations points towards this allele's unique origin and subsequent elimination in most populations, rather than parallel independent mutations analogous to those found for example in naturally occurring “green revolution dwarf” Arabidopsis mutants (Barboza et al. 2013). The quintuple substitution allele of GFS9/TT9 would clearly deserve a follow-up experimental study.
Among the loci associated with trichome shape-related traits and also highly expressed in trichomes were found three genes participating in the biosynthesis of cutin and/or cuticular waxes. AT1G01600/CYP86A4 encodes a cytochrome P450 paralog, associates also with the trichome autofluorescence trait. Its expression is controlled by a transcription network that regulates also trichome branching (Camoirano et al. 2020; see also Berhin, Nawrath, and Hachez 2022). Remarkably, the cytochrome P450 gene family is significantly over-represented among cell wall trait-associated candidates, also consistent with its role in cell wall biogenesis. The second trichome shape-related candidate with high trichome expression, AT1G12570, encodes a glucose-methanol-choLine oxidoreductase related to the maize IPE1 gene participating in pollen exine development, which is co-regulated with CYP86A4 (Kannangara et al. 2007). Polymorphisms in both loci correlate also with variation in trichome density. Remarkably, trichome patterning is altered in some cuticle biogenesis mutants (Berhin, Nawrath, and Hachez 2022), suggesting cuticle contribution to the mechanical cell coupLing and tissue patterning (Reynoud et al. 2021). The third locus implicated in cuticle biogenesis is AT1G76690/OPR2, a 12-oxophytodienoic acid reductase co-regulated with the previous two loci (Suh et al. 2005), which only associated with variation of trichome stem length.
Relevant candidates were also found among guard cell-expressed genes. The loci associated with increased guard cell metal accumulation and highly expressed in guard cells include AT3G13772/TMN7, encoding a membrane protein whose heterologous expression causes yeast to accumulate copper (Hegelund et al. 2010), and AT5G39040/ALS1, coding an ABC transporter whose loss leads to increased aluminum sensitivity (Larsen et al. 2007). Another gene impLicated in stomatal metal accumulation, AT3G13460/ECT2, encodes a mRNA stability-controlLing protein whose mutational loss affects trichome morphogenesis (Scutenaire et al. 2018; Wei et al. 2018). Identification of this locus, which is intensively expressed in guard cells (Zhao et al. 2008) in the context of a guard cell-related phenotype indicates its broader role in epidermal development.
Eighty further candidates with Likely roles in biologically relevant processes such as membrane trafficking, cytoskeletal organization or cell wall biogenesis were recovered. Among them are several members of the trichome birefringence-like (TBL) gene family whose founding member was discovered due to altered trichome cell wall optical properties (Bischoff et al. 2010), an additional exocyst subunit, multiple myosin- or kinesin-related proteins, several extensins and expansins, and about 20 cell wall modifying enzymes.
While these observations can be considered largely confirmatory, our candidate gene Lists are significantly enriched in members from several multigene families not yet reported to participate in relevant aspects of epidermal development. The cysteine/histidine-rich C1 domain proteins, a group of nucleic acid binding proteins with a possible role in plant defense (Hwang et al. 2014) were enriched among trichome shape and metal accumulation-Linked candidates, and receptor-Like kinases (RLKs) associated with trichome shape traits, although no overlaps with a published list of trichome-expressed LRR-RLKs (Wu et al. 2016), representing one of the RLK family branches, were detected. Formins, evolutionarily conserved cytoskeletal organizers that also engage in endomembrane dynamics (see Cvrčková, Ghosh, and Kočová 2024), surprisingly associated with stomatal metal accumulation, including the main housekeeping Class I formin FH1/AT3G25500 (Rosero et al. 2016; Oulehlová et al. 2019; Cifrová et al. 2020). Two candidate conservative amino acid substitutions were found in FH1 (L5F within the membrane insertion signal and D502E in a variable cytoplasmic segment). Observations in two loss of function fh1 mutants suggest a trend towards increased metal accumulation in guard cells. Last but not least, members of five hitherto uncharacterized ‘domain of unknown function’ (DUF) famiLies associated with either trichome shape or metal accumulation traits, including the prolamin-related DUF784 (Zhang 2009), suggesting their participation in relevant developmental pathways.
Although our Gene Ontology term enrichment analyses did not indicate links between the candidates and specific cellular functions, we observed a significant increase in mutual genetic, physical and functional interactions recorded in the STRING database (Szklarczyk et al. 2023) among candidates for the trichome density and metal deposition traits, as well as for candidates associated with multiple traits, compared to a random gene sample. Remarkably, the interacting gene clusters consisted predominantly of genes participating in nuclear functions. A prominent cluster of candidates associated with two or more trait groups comprising mainly signalling and regulatory proteins was centred on a homolog of the mammalian ATM kinase engaged in DNA damage response (Lee and Paull 2021). In plants, ATM homologs participate in DNA repair and UV stress response (Shi and Liu 2021), but also in reaction to exogenous DNA (Vega-Muñoz et al. 2023). Among genes associated with more than one trait category, we also found smaller clusters of shape-associated genes presumably engaged in cytoskeletal and cell wall-related functions, as well as an additional cluster comprising several nuclear proteins and containing genes linked to trichome density.
In summary, our screen identifies several candidate gene families and functional gene clusters that would deserve experimental study to verify their possible roles in epidermal development, possibly leading to uncovering new genetic players in cell morphogenesis and cell differentiation, but also in functions possibly relevant for environmental adaptation under natural conditions.
4 Materials and Methods
4.1 Plant Material
A set of 310 natural Arabidopsis thaliana accessions from the 1001 Genomes collection (Alonso-Blanco et al. 2016), has been included in our study (Supporting Information S2: Table 1). Predominantly, genotypes of North-European origin were chosen to reduce the effect of geographical differences in population structure and related parameters reducing GWAS efficacy (see Gloss et al. 2022). Climate variables data for the locations of origin vere retrieved in the form of standardized BioClim variables from the CliMond database (Kriticos et al. 2012).
Seeds were steriLized with chlorine gas, stratified in tubes at 4°C for 3 days in the dark and sown into standard soil substrate pre-treated with Confidor to prevent blackfly infestation. Plants were grown in growth chambers under controlled long-day conditions (16 h light, 23°C/8 h darkness, 18°C; relative air humidity 60%). Three seedlings per genotype were planted into individual pots at the first true leaf stage. Leaves were collected at the age of 5 weeks (at which point plants of some genotypes were beginning to bolt).
For mutant genotype verification for selected formin-encoding genes, homozygous plant lines carrying previously characterized loss of function mutants fh1-1 (SALK-032981; Rosero, Žárský, and Cvrčková 2013), fh13-1 (SALK_064291C; Kollárová, Baquero Forero, and Cvrčková 2021), and fh14-1 (SALK_058886; Li et al. 2010), available from NASC (RRID: SCR_004576), as well as fh1:CRISPR (generated in our laboratory (see Cifrová et al. 2020), with corresponding congenic wild type plants were used.
4.2 Leaf Sample Processing
Three approximately fingernail-sized mature, non-senescent rosette leaves per plant (i.e., nine leaves per genotype) were harvested, resulting in three pooled samples, each containing one leaf from each individual. Samples were stored for at least 2 days before further processing or observation.
For trichome shape evaluation and for determination of callose content in trichomes, leaves were harvested into a tube containing 1x phosphate-buffered saline (PBS) and 100 mM EGTA. Trichomes were isolated and stained for callose by aniline blue as described previously (Kulich et al. 2015). In genotypes where trichomes failed to detach from the leaves (further referred to as ‘shaveproof’), whole leaves were stained for callose using an analogous procedure (Kulich et al. 2018). This in situ staining was also used for additional documentation, such as the photos shown in Figure 1.
For visualization of autofluorescence and for determination of additional morphological parameters, leaves were pressed adaxial side up between two microscope slides and left to dry up to induce breakage of trichomes resulting in increase of autofluorescence (Kulich et al. 2018).
For visualization of metal content, leaves were collected into a tube containing 3 mL of acetone and stored for at least several days. On the day of observation, they were stained by addition of diphenylthiocarbazone (dithizone) reagent freshly prepared from 1.5 mg of dithizone, 1 mL of distilled water and one to two drops of glacial acetic acid (Seregin and Ivanov 1997). After at least 1 h but not more than 6 h of staining, leaves were briefly washed in distilled water and mounted in water on microscopy slides for observation.
4.3 Microscopy and Imaging
Microscopic images were acquired using a Nikon Eclipse 90i fluorescence microscope equipped with PlanApo 4x/0.2 objective and Nikon DsFi 2 camera as described previously (Kulich et al. 2018). For autofluorescence and callose fluorescence detection, UV-B excitation was employed; in case of callose staining, an additional image was acquired in polarized light for trichome visualization. Leaves stained for metal detection were imaged using bright field settings.
If whole leaves were photographed, tilling images covering at least a half of the leaf lengthwise were automatically stitched from multiple frames, which were generated as maximum projections of three images with 50 μm of Z distance, using the Nikon Imaging Software (NIS Elements AR). Additional image processing was performed using the Fiji platform (Schindelin et al. 2012).
4.4 Epidermal Phenotypes Determination
An overview of phenotypic trauts analyzed in our screen is provided in Table 1.
The first group of parameters was determined from photos of callose stained trichomes. At least 25 interactively selected clearly visible trichomes from three leaves of three plants (detached, or, in shaveproof genotypes, 8–9 in situ trichomes per leaf, selected across its diagonal) were evaluated. Trichome shape parameters (area, circularity, soLidity, length and perimeter) were determined from binary images generated by thresholding polarized light photos using the Yen method as implemented in the Fiji software; the procedure was partially automated using recorded macros to increase throughput, with individual trichome selection being the only manual step. Trichome length was measured manually as the longest trichome dimension. Average parameter values from at least 25 trichomes were recorded for these parameters. Callose content was measured as the fraction of trichome area stained for callose, determined using built-in functions of Fiji from binary images generated by thresholding fluorescence and polarized light photos using the Yen method, again with the aid of recorded macros and manual selection of individual trichomes. The reported value for each accession is the sum of percentage values from 25 trichomes.
The second group of parameters was determined from fluorescence microscopy images of dried whole leaves, taken in the autofluorescence channel. Trichome stem length was measured using the NIS elements software with manual endpoint identification; average values from at least 25 clearly visible trichomes from 3 leaves, selected 7–9 per leaf across its diagonal, are reported. Additional parameters (trichome autofluorescence, overall leaf epidermis autofluorescence, autofluorescence colour, trichome density and typical number of trichome branches) were visually categorized (see Table 1).
A third group of parameters reflects visually detectable patterns of metal accumulation, categorized as described in Table 1. This group includes the presence of a metal-enriched Ortmannian ring at the bottom part of the trichome stem (Kulich et al. 2015), presence of a visible metal staining gradient in the trichome stem, presence of metal staining at the trichome base, diffuse epidermal staining outside trichomes and staining of stomatal guard cells.
The final categorical parameter, denoted as ‘shaveproof’, reflects the resistance of trichomes against mechanical detachment during staining for callose.
All categorical values were based on the typical appearance of the epidermis of three leaves. Glabrous leaves were attributed the value ‘0’ for all categorical variables. In theexceptional cases where noticeable differences were seen among the three leaves of a given accession, the majority phenotype was recorded.
Phenotype data for a subset of accessions and traits have been independently rescreened by different team members for the purpose of broad sense heritability estimation (Supporting Information S2: Table 2); this also documented good inter-observer replicability of phenotype determination (Supporting Information S1: Figure 6).
4.5 Phenotypic Data Processing and Initial Analyses
Primary phenotypic data were assembled into a structured spreadsheet and deposited in the pubLic AraPheno database (Togninalli et al. 2020) as Study No. 126 (Bezvoda 2023). Broad sense heritabiLity (H2 = VG/VP), that is, the proportion of phenotypic variation (VP) due to genetic variation (VG) estimated from the between- and within-Line phenotypic variance, was calculated from trait values of all individuals in this data set (Alonso-Díaz et al. 2021).
Statistical analysis of phenotypic data and phenotype/environmental parameter correlation analyses were carried out by the Pandas data analysis toolbox (Reback et al. 2021) and pair plots of scatterplots were created by the Seaborn visualization tool (Waskom 2021) v. 0.11.2. Correlation strength is reported using criteria from Schober, Boer and Schwarte (2018).
Principal component analysis (PCA) has been performed using the PAST software (Hammer, Harper, and Ryan 2001) v. 4.11 using the correlation matrix method. All quantitative or semi-quantitative parameters were treated as ordinal variables, the qualitative ‘autofluorescence color’ and ‘shaveproof’ traits were handled as nominal.
4.6 GWAS Analyses and Genomic Data Processing
Phenotype values for each trait were uploaded to the online GWAS application (Seren et al. 2012; Seren 2015) and correlation analyses were run at 5% FDR threshold with all possible combinations of input data transformations and statistical methods available with default settings except the minor allele count (MAC) value that has been lowered to 5. The 1001 Full sequence Data set (TAIR v. 9) was used as the source of genotype information. After initial manual exploration of resulting Manhattan plots, the process of analyzing data was automated.
Raw result data files were downloaded manually and used for subsequent steps. All SNPs identified as significant by at least one method were considered. Additional information about each significant hit was acquired from the GWAS application by web scraping using the Python programming language v. 3.8 (Python Software Foundation 2024) and driver for Google Chrome web browser (Google 2024). Locus annotation was based on TAIR9.0 to maintain compatibility with the 1001 genomes data set. All intergenic hits were discarded from subsequent steps of data processing. Hits were categorized according to the position of the SNP in corresponding gene (INTRON, NON_SYNONYMOUS_CODING, START_LOST, STOP_GAINED, STOP_LOST, SYNONYMOUS_CODING, SYNONYMOUS_STOP, UTR_3_PRIME, UTR_5_PRIME).
Annotation of loci that were subjected to further investigation was individually updated by manual searches of the Araport 11 genome annotation (Cheng et al. 2017) accessed via the BAR Thalemine portal (Pasha et al. 2020).
To determine the direction of phenotypic parameter cHanges for individual minor allele amino acid substitutions, lists of accessions carrying specific substitutions retrieved from the Ensembl resource (Yates et al. 2022) were used to identify subsets of our accessions carrying individual sequence variants. Average values of the phenotypic parameters of question were subsequently determined for each such variant.
4.7 Geographic Mapping of Allele Distribution
Lists of accessions carrying specific alleles of selected loci for the purpose of environmentally correlated allele distribution analyses and for map generation were generated from Ensembl data as described above. Geographic coordinate-based maps were created using Python Folium (Python Visualisation 2024) or Google Maps tools.
4.8 Enrichment and Depletion Analyses of GWAS Candidate Gene Lists
To determine whether lists of candidate genes identified in the GWAS are enriched or depleted for specific gene groups, we used the Arabidopsis genome (Araport 11 version) and compared our data lists after manual annotation with the following gene lists.
As mobile element-derived genes, those with annotations containing the word ‘transposable’ were considered.
As genes with high transcript levels in trichomes, we considered the 164 genes listed as encoding 5% most abundant transcripts in the mature trichome transcriptome (Jakoby et al. 2008).
As genes with guard cell-specific transcription patterns, we considered the list of loci specifically upregulated in the guard cells compared to the mesophyll (Leonhardt et al. 2004).
As genes encoding trichome-abundant proteins, we selected genes from the recently published Arabidopsis trichome proteome study (Huebbers et al. 2022) using the following criteria. A protein had to be significantly enriched in at least two of the four reported proteomic experiments, at least in one case by a factor of two and more, while it was not depleted in any experiment. These criteria produced a list of 455 loci.
For genes encoding guard cell-abundant proteins, we considered the list from a published guard cell proteome study (Zhao et al. 2008).
For gene family enrichment, we included all gene family members as identified by keyword search of candidate gene annotations. Family member counts were based either on Literature, as in the case of F-box proteins (Kuroda et al. 2002), on TAIR (Reiser et al. 2024) Gene FamiLies annotations (as in the case of receptor-like protein kinases and FH2 proteins), or estimated by keyword searches of the Araport 11 annotations (for all DUFs).
Genes from each list were identified among the GWAS candidates and significance of any observed enrichment or depletion was evaluated using pairwise χ2 test (Stagroom 2024) with Benjamini–Hochberg correction for multipLicity performed using an online calculator (Radua, Albajes-Elsagirre, and Fortea 2024).
4.9 Verification of Candidates
Loss-of-function mutants in candidate genes were grown alongside corresponding wild type plants and leaf samples were harvested, processed and imaged as described above for the main ecotype screen. To minimize effects of observer's expectation bias, a single-bLinded experimental design was employed, with one member of the team performing the imaging and others quantitatively evaluating microphotographs with coded labels without knowledge of the plant's genotype. After decoding the identity of the samples, significance of between-genotype differences in the relevant categorical parameters was estimated by the χ2 test (or Fisher's exact test in cases where some categories exhibited zero counts) using online calculators (Stagroom 2024; Vasavada 2016).
4.10 Mapping of Protein–Protein Interactions Among Candidature Gene Products
To gain initial insight into the interactions among candidate genes, the full network STRING protein–protein interaction database v. 11.5 (Szklarczyk et al. 2023) was searched with non-redundant lists of genes associated with the indicated trait groups as queries, using high confidence (score = 0.7) and high stringency (FDR = 1%) settings. Statistical significance of interaction enrichment was obtained during this search. Resulting interaction networks were exported both as tables (further processed in Excel to generate cluster and node lists that were subsequently annotated as described above) and as vector images that were manually edited (to remove unlinked nodes and tidy up the layout), annotated and coloured.
Mapping of interactions among the subset of candidates associated with multiple trait groups was performed analogously except that the confidence threshold was lowered to medium (score = 0.4), that is, to the default settings of the STRING database search tool.
Acknowledgements
We thank Christian Göschl and Samantha Krasnodebski (Gregor Mendel Institute) for help with experiment setup and cultivating plants for the GWAS screen, Marta Čadyová for technical support, and an anonymous reviewer for very inspiring suggestions of additional data analyses.
We acknowledge continuous support from the Charles University Progres Q43 and COOPERATIO programs. The initial stages of this work have been supported by the CSF/GACR/FWF project GF16-34887L (V.Ž., W.B., Y.M.L.-R., F.C.), the formin mutant verification experiments by the CSF/GACR 22-33471S grant (E.K. and F.C.), and the finaLization of this report by the project TowArds Next GENeration Crops, reg. no. CZ.02.01.01/00/22_008/0004581 of the ERDF Programme Johannes Amos Comenius. Open access publishing facilitated by Univerzita Karlova, as part of the Wiley - CzechELib agreement.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Primary data from the phenotype screen are available in the AraPheno database (https://doi.org/10.21958/study:126). Additional data supporting the findings of this study are available in the Supporting Information of this article.