GLR3.6T807I Mutation of Casuarina equisetifolia Is Associated With a Decreased JA Response to Insect Feeding by Lymantria xylina
The first four authors contributed equally to this article.
ABSTRACT
Lymantria xylina is the most important defoliator, damaging the effective coastal windbreak tree species Casuarina equisetifolia. However, the underlying genetic mechanisms through which C. equisetifolia responds to L. xylina attacks remain unknown. Here, we compared the transcriptional, phytohormone and metabolic differences between susceptible (S) and resistant (R) C. equisetifolia cultivars in response to L. xylina feeding. The main L. xylina-induced resistance in C. equisetifolia was a jasmonate (JA) response and JA synthesis was highly induced by L. xylina feeding at both the transcriptional and metabolic levels, thus promoting flavonoid accumulation. The JA response was highly activated by L. xylina feeding on the R but not in the S cultivar, although the JA signalling pathway was intact in both cultivars. We found a single amino acid mutation in the homologues of glutamate receptor-like protein 3.6 (CeGLR3.6T807I) in the S cultivar. Compared with the GLR3.6 homologues in the R cultivar, phosphorylation of CeGLR3.6T807I was not induced by insect feeding, leading to a decreased JA response in the S cultivar. Collectively, this study provides new insights into the function of CeGLR3.6 in regulating the JA response of C. equisetifolia to L. xylina feeding.
1 Introduction
Casuarina equisetifolia is crucial for coastal windbreaks, sand fixation and ecosystem rehabilitation, making it economically and environmentally important species which is widely cultivated in tropical and subtropical areas (Ahmad et al. 2022; Potgieter, Richardson, and Wilson 2014; Sayed 2011; Zhang et al. 2008; Zhong et al. 2010). Because of the extreme conditions persisting in coastal regions, very few plant species can co-inhabit with C. equisetifolia (Xu et al. 2022). Thus, most C. equisetifolia forests have low biodiversity, providing ideal conditions for pest outbreaks (Liu et al. 2014).
Thus far, 155 pest species have been reported in C. equisetifolia forests, and Lymantria xylina (Lepidoptera: Lymantriidae) is the most important defoliator among the pests in the coastal areas of southern China (Wang et al. 2018; Xie et al. 2019). Similar to a closely related species, such as the global quarantine pest Lymantria dispar (Djoumad et al. 2020), L. xylina is a polyphagous herbivore, and the number of recorded host plants for this moth includes and most likely exceeds 424 species of trees and shrubs belonging to 103 families (Zhang 2018). Thus, L. xylina is considered a potential invader of the United States, Canada and Europe in the future (deWaard et al. 2010; Pogue 2007; Wang et al. 2018). Therefore, it is urgent to develop safe and effective control strategies for L. xylina.
Insect herbivory is a major threat to plants which have consequently evolved sophisticated biochemical, genetic and physiological adaptations to stress (Salvador-Recatala 2016). Most woody plants, such as C. equisetifolia, display greater induced resistance to pests in some of their natural cultivars compared to others (Paes et al. 2015). Over the past few decades, resistance has emerged as an effective and ecologically friendly pest control method (Barton, Edwards, and Koricheva 2019; Ribeiro-Barros, Pawlowski, and Ramalho 2022; do Prado Ribeiro et al. 2018; Xu et al. 2022). Therefore, studying the differences in defence responses to L. xylina among C. equisetifolia cultivars is valuable for utilising host plant resistance to control L. xylina.
In higher plants, insect feeding triggers systemic host defence through reactive oxygen species (ROS), electrical signals and cytosolic Ca2+ ([Ca2+]cyt) signals (Choi et al. 2016; Gandhi et al. 2021). The rapid transmission of Ca2+ and electrical signals depends on glutamate receptor-like proteins GLR3.3 and GLR3.6 (Nguyen et al. 2018), which further induce jasmonate (JA) biosynthesis to activate JA responses (Xue et al. 2022). The JA pathway (including both JA biosynthesis and signal transduction) has emerged as the major signalling cascade that integrates information perceived at the plant–insect interface into broad-spectrum defence responses through sophisticated biochemical and genetic mechanisms (Erb, Meldau, and Howe 2012). JA induces secondary metabolite biosynthesis, one of the most important defence against defoliators (Divekar et al. 2022; War et al. 2012; Yang et al. 2023). Thus, GLRs link perception of insect attack to JA responses which further regulate downstream biochemical and genetic processes and activate the plant's chemical defensive traits. Understanding the genetic mechanism by which plants sense insect feeding to activate the JA response is vital for controlling them (Mostafa et al. 2022; Singh, Kaur, and Kariyat 2021). However, research on the response of host plants to L. xylina has focused on the content of C. equisetifolia secondary metabolites, such as compounds terpenoids and phenolics) (Paes et al. 2015; Singh, Kaur, and Kariyat 2021). The underlying genetic mechanisms remain unknown, mainly because of the lack of genome information and an in-vivo system for gene functional analysis (Ren et al. 2022; Ye et al. 2019), which limits the development of new C. equisetifolia cultivars with enhanced defence properties against herbivores.
In our previous work, several C. equisetifolia cultivars with varying levels of susceptibility and resistance to L. xylina were identified from the global germplasm repository from the Chihu State-owned Forest Farm in Huian, China (Huang et al. 2013; Lin et al. 2014). In this study, we explored the transcriptional, phytohormone and metabolic differences between susceptible and resistant cultivars in response to L. xylina feeding. Together with our newly developed in-vivo gene expression system in C. equisetifolia, we identified those genes regulated by L. xylina feeding that affect the accumulation of secondary metabolites in the host plant, thus uncovering L. xylina-induced resistance in C. equisetifolia. This work provides a theoretical basis for developing woody plant cultivars with resistance to insect feeding for sustainable plant production.
2 Materials and Methods
2.1 Sampling Area of Plant Materials
L. xylina-resistant (R; ‘Zhanjiang 3’), -susceptible (S; ‘Zhanjiang 2’) and -neutral (‘Huian 1’) C. equisetifolia cultivars were collected from the C. equisetifolia gene bank in the Chihu state-owned forest farm in Huian, Fujian, China (118° 53′ 24″ E, 24° 53′ 24″ N). The gene bank was established in 2007 by the Chihu State-owned Forest Farm and the Fujian Academy of Forestry Sciences. The area has a subtropical maritime monsoon climate (Huang et al. 2013).
Fresh branchlets of C. equisetifolia were cut from trees and placed in Hoagland nutrient solution (LA2061, Solarbio, Beijing, China). After 1 week, the branchlets were treated with a root regeneration-inducing solution, containing 100 ppm indolebutyric acid (I108273, Aladdin, LA, USA) in Hoagland nutrient solution for 24 h to induce root regeneration. Subsequently, the branchlets were subjected to normal Hoagland nutrient solution for root regeneration. The Hoagland nutrient solution was changed daily until an intact plant formed, which took approximately 2 weeks. Rooted branchlet cuttings were used for all experiments, except for the insect-feeding assay (using detached branchlets) and developing transgenic lines (seedlings germinated from seeds of the S cultivar).
2.2 Insect Feeding Assay
L. xylina eggs were collected from a C. equisetifolia forest in Pingtan, Fujian, China (119° 47′ 24″ E, 25° 30′ 36″ N) in May 2020. The eggs were reared in a light chamber (12 h/12 h light/dark cycle, 26°C and 70% humidity). After hatching, the first instar larvae were transferred to Petri dishes (12 cm × 12 cm), and the bottom five larvae were placed on filter paper in each dish (Ma et al. 2021). The branchlets of the neutral cultivar (‘Huian 1’) were fed to the larvae until they reached the third instar stage.
The freshly moulted third instar larvae of L. xylina with similar size and health were transported to cages (42 cm × 42 cm × 70 cm) and reared under similar conditions for further experiments (Ma et al. 2021). The Third instar larvae of L. xylina were fed fresh ~15–20 cm-long branchlets of R and S or transgenic C. equisetifolia in individual cages, with insect feeding assays on R and S cultivars used as inter-controls. Each treatment included three replicates of 15 larvae each. Within 3 weeks, the amount of feeding was measured every 2 days by recording the change in the weights of the branchlets between pre- and post-larval feeding. Every 2 days at the time of measurement, fresh, similarly healthy and sized branchlets kept in water were replaced to measure the change in branchlet weight without insect feeding. Larval and frass weights were measured after 3 weeks of feeding. Additionally, the number of deaths, pupae and pupal weights of the larvae for each individual were recorded. Branchlets that were not fed upon and were measured using the same methods were used as negative controls.
2.3 RNA-Seq and Data Analysis
2.3.1 Sample Preparation
Branchlets (20 g) were collected from the R and S cultivars of C. equisetifolia 1 day before and after feeding with the third instar larvae of L. xylina, respectively. These branchlets were grouped as four separate treatment samples (‘b’ and ‘p’ representing pre- and post-L. xylina feeding, respectively. bR, R cultivar without feeding; pR, R cultivar after feeding; bS, S cultivar without feeding; pS, S cultivar after feeding). Each treatment sample was then crushed in liquid nitrogen. Each treatment included three replicates, with five plants per replicate for each larva feeding on branchlets; a total of 15 plants from each treatment group were used for sequencing. The sample from these 15 plants were divided into three sets, and libraries were independently generated for sequencing.
2.3.2 RNA Extraction and Library Preparation for Transcriptome Sequencing
The total RNA of the four treatment samples was extracted using the Hipure Plant RNA Mini Kit (R4130-02, Magen, CA, USA) according to the manufacturer's protocol for the plant tissues enriched in polysaccharides and polyphenols. RNA degradation and contamination were monitored by 1% agarose gels electrophoresis. RNA was quantified using an Agilent 2100 Bioanalyzer (G2939BA, Agilent Technologies, CA, USA), and the quality and integrity were assessed using a NanoDrop spectrophotometer (840-317400, Thermo Scientific, DE, USA). A total amount of 1.5 μg RNA per sample was used as input material for the RNA sample preparations.
Sequencing libraries were generated using the NEBNext Ultra RNA Library Prep Kit for Illumina (E7770, New England BioLabs Ltd., USA) by Beijing Allwegene Technology Company Limited (Beijing, China). Library quality was assessed using the Agilent Bioanalyzer 2100 system, and sequencing was conducted by Beijing Allwegene Technology Company Limited using Illumina Novaseq6000. RNA sequencing files are available at the National Center for Biotechnology Information (NCBI) under the accession number PRJNA951343 (https://www.ncbi.nlm.nih.gov/).
2.3.3 Data Analysis
Raw data (raw reads) in fastq format were first processed using in-house Perl scripts, with Cutadapt and additional Perl scripts used to define clean data (Wang, Gerstein, and Snyder 2009). All downstream analyses were based on clean, high-quality data. These clean reads were mapped to the assembly and annotation of the C. equisetifolia reference genome (Ye et al. 2019) using STAR (Dobin et al. 2013).
Quantification of transcript abundance (readcount and FPKM) was conducted using HTSeq (v 0.5.4) and differentially expressed genes (DEGs) analysis was performed using the DESeq2 package (R studio, https://bioconductor.org/packages/release/bioc/html/DESeq2.html). p < 0.05 and |log2foldchange| ≥ 2 were set as the threshold for significantly differential expression.
Gene ontology (GO) enrichment analysis of DEGs was implemented using the GOseq R package based on the Wallenius non-central hyper-geometric distribution (Young et al. 2010). The org.Cas.eg.db of GO analysis was built online using eggNOG-mapper (http://eggnog-mapper.embl.de/) with the CCG.rebuild.gff.reorder.noisoforms.pep database (Ye et al. 2019). KOBAS software was used to test the statistical enrichment of DEGs in Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathways (Mao et al. 2005).
2.4 Metabolomics
2.4.1 Metabolite Extraction
Sample preparation (bR, bS, pR and pS) was described in RNA-seq, each treatment included three repeats with five plants per repeat for one larva feeding on branchlets. In total 15 plants for each treatment were used for metabolite analysis. The metabolites from each replicate (20 g) were extracted according to previously described protocol (De Vos et al. 2007).
2.4.2 Ultra-High Performance Liquid Chromatography-Mass Spectrometric (UHPLC-MS) Analysis
UHPLC separation was performed using a Dionex Ultimate 3000 RS UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a heated electrospray ionisation source (Thermo Fisher Scientific) metabolic profiles were analysed in both electrospray ion positive and negative ion modes. An ACQUITY UPLC HSS T3 column (1.8 μm, 2.1 × 100 mm, 186009468, Waters, Milford, USA) was employed in both positive and negative modes. Data were obtained from OeBiotech (Shanghai, China). The binary gradient elution system consisted of 0.1% formic acid in water (A) (A117-50, Thermo Fisher Scientific, Waltham, MA, USA) and 0.1% formic acid in acetonitrile (B) (A998-4, Thermo Fisher Scientific, Waltham, MA, USA). Separation was achieved using the following gradient: 0 min, 5% B; 2 min, 5% B; 4 min, 25% B; 8 min, 50% B; 10 min, 80% B; 14 min, 100% B; 15 min, 100% B; 15.1 min 5% B and 16 min, 5%B. The flow rate was 0.35 mL/min, column temperature was set at 45°C, auto-sampler temperature was set at 4°C, and injection volume was 5 μL (Bai et al. 2023); the mass range was 100–1000. The resolution was set to 70 000 for the full MS scans and 17 500 for the HCD MS/MS scans. The collision energies were set to 10, 20 and 40 eV. The mass spectrometer operated under the following conditions: spray voltage, 3800 V (+) and 3000 V (−); sheath gas flow rate, 35 arbitrary units; auxiliary gas flow rate, eight arbitrary units; capillary temperature, 350°C; auxiliary gas heater temperature, 350°C and S-lens RF level, 50.
2.4.3 Data Analysis
Data from different samples were refined to obtain information on insect-induced metabolites. The original LC-MS data were processed using Progenesis QI V2.3 (Nonlinear, Dynamics, Newcastle, UK) for baseline filtering, peak identification, integration, retention time correction, peak alignment and normalisation. Primary parameters of 5 ppm precursor tolerance, 10 ppm product tolerance and a 5% product ion threshold were applied. Compounds were identified on the basis of the precise mass-to-charge ratio (m/z), secondary fragments and isotopic distribution using the Human Metabolome Database, LipidMaps (V2.3), Metlin, EMDB, PMDB and self-built databases for qualitative analysis. The extracted data were further processed by removing any peaks with a missing value (ion intensity = 0) in more than 50% of the groups, replacing the zero value with half of the minimum value and screening according to the qualitative results of the compound. Compounds with scores < 36 (out of 60) were deemed inaccurate and removed. A data matrix was created using the positive and negative ion data. The resulting three-dimensional data, including the peak number, sample name and normalised peak area, were fed into the R package metaX for principal component analysis projections to latent structure discriminant analysis partial least-squares discriminant analysis (OPLS-DA) (Wen et al. 2017). The variable importance in projection (VIP) values obtained from the OPLS-DA model were used to rank the overall contribution of each variable to group discrimination. A two-tailed Student's t test was used to verify whether the metabolite differences between the groups were significant. Differential metabolites were selected based on VIP values greater than 1.0 and p-values less than 0.05. Commercial databases, including KEGG (http://www.kegg.jp) and MetaboAnalyst (https://www.kegg.jp/) were used to search for metabolic pathways (https://www.genome.jp/kegg/pathway.html).
2.5 Jasmonic Acid, Total Flavonoids and Generation Rate of ROS Quantification
Branchlets from the different C. equisetifolia cultivars (bR, pR, bS or pS) and transgenic lines were harvested and immediately frozen in liquid nitrogen. The levels of jasmonic acid and flavonoids and the generation rate of ROS were measured using Plant Jasmonic Acid ELISA Kit (SP29738, Spbio, Shanghai, China), Plant Flavonoid Content Assay Kit (BC1330, Solarbio, Beijing, China) and mitochondrial ROS Generation Rate Assay Kit ELISA Kit (ROS-1-Y, Comin, Shanghai, China), respectively. Each treatment included 15 individual plants that were harvested in three replicates (five individual plants per repeat) for further measurements.
2.6 Jasmonic Acid, L-Glutamic Acid and H2O2 Treatments on Transgenic Lines
Two-week-old seedlings of different C. equisetifolia cultivars (R, S) were used for exogenous jasmonic acid and H2O2 treatments. Seedlings were cultured in Hoagland solution containing jasmonic acid (0, 0.5, 1 and 2 μM; J2500, Sigma, MO, USA), and harvested 0 and 24 h post-treatment for total flavonoid quantification and gene expression analysis. The negative control consisted of seedlings cultured in Hoagland solution with TritonX-100 (0, 0.5, 1 and 2 μM; T8787, Sigma, MO, USA) (Li et al. 2020). Each treatment included 15 individual seedlings that were harvested in triplicate (five individual seedlings per replicate) for further measurement.
Different transgenic seedlings (CeGLR3.6T807-GFP and CeGLR3.6I807-GFP) were treated with 10 mM l-glutamic acid (PHR1107-1G, Sigma, MO, USA) via foliar application, according to Farahmandi's method (Fardus, Hossain, and Fujita 2021) and harvested at 0, 24, 48 and 72 h post-treatment for jasmonic acid and total flavonoid quantification. Different transgenic seedlings treated with ddH2O served as negative controls. Each treatment included 15 individual seedlings that were harvested in triplicate (five individual seedlings per replicate) for further measurement.
Similarly, seedlings were cultured in Hoagland solution supplemented with H2O2 (1 mM; 7722-84-1, Merck, Shanghai, China), and harvested at 0, 6, 12 and 24 h post-treatment for further analysis, including quantification of total jasmonic acid, the generation rate of ROS and gene expression analysis. The negative control consisted of seedlings cultured in Hoagland solution without H2O2. Each treatment included 15 individual seedlings and was harvested as three repeats (five individual seedlings per repeat) for further measurement (Gong et al. 2021).
2.7 Gene Expression Quantification Assay
Nine target genes from the four C. equisetifolia samples (bR, bS, pR and pS) were selected from the DEGs identified for functional gene verification. Quantitative real-time PCR (qRT-PCR) was used to quantify gene expression, using Ce-ACT7 as the reference gene (Fan et al. 2017). Primers for the target genes were designed using Primer3 Plus (https://www.primer3plus.com) and are listed in Supporting Information S2: Table S1. RNA extraction was extracted as described previously. For cDNA synthesis, 1 μg total RNA was used with the cDNA Synthesis SuperMix for qPCR Kit (1123ES60, Yeasen, Shanghai, China). qRT-PCR was performed using SYBR-Green PCR Master Mix (11202ES08, Yeasen, Shanghai, China). Three biological replicate and three technical repeats were conducted for each biological, respectively. The relative expression level or fold change of candidate genes was calculated using the comparative CT method () (Chang, Chen, and Yang 2009). Primers synthesis was conducted by BioSune (Shanghai, China).
2.8 Phylogenetic Analysis
The protein sequences of CeGLR3.6 were submitted to the NCBI database for BLAST analysis. Twenty homologous protein sequences were selected for evolutionary analysis, considering sequences those with high alignment values for each species (Supporting Information S2: Table S3). Phylogenetic trees were constructed using the maximum likelihood tree method in TBTools_TRE1.6 and plotted using iTOL (Chen et al. 2023; Zhou et al. 2023).
2.9 In-Vivo Gene Expression Assay in C. equisetifolia
To generate the 35S::CeGLR3.6I807-GFP and 35S::CeGLR3.6T807-GFP constructs, the coding region of CeGLR3.6I807 was directly amplified from the cDNA of C. equisetifolia cultivar S. CeGLR3.6T807 was cloned using the segmented amplification method and two fragments (1–2429 and 2391–2730 bp) were amplified separately from the cDNA of C. equisetifolia cultivar S. PCR conditions using 2 × Profusion PCR Master Mix (HRF0088, Herui, Shanghai, China) were as follows: 95°C for 3 min; 35 cycles of 95°C for 10 s, 55°C for 15 s and 72°C for 50 s and a final extension at 72°C for 5 min. The PCR products were purified using a QIAquick Gel Extraction Kit (28706 × 4, Qiagen, USA). The coding region of CeGLR3.6I807 and the two fragments of CeGLR3.6T807 were cloned into the pFGFP vector in the 3′ end of the double 35S promoter with SpeI and SmaI (R3133L, R0141L, New England BioLabs Ltd., USA) using the In-Fusion system (CU201-02, TransGen, Beijing, China) to generate the 35S::CeGLR3.6I807-GFP and 35S::CeGLR3.6T807-GFP constructs, respectively (Li et al. 2020). All constructed vectors were tested by sequencing and all primers used in this assay are listed in Supporting Information S2: Table S1.
Furthermore, the 35S::CeGLR3.6I807-GFP and 35S::CeGLR3.6T807-GFP constructs were transferred into the agrobacterial strain C58C1 and inoculated with 2-month-old seedlings of S C. equisetifolia S cultivar without true leaf-branches using conventional in-vivo gene expression assay (Supporting Information S1: Figure S1) to develop CeGLR3.6I807-GFP (S) and CeGLR3.6T807-GFP (S) transgenic lines (Supporting Information S1: Figure S2), respectively. The pFGFP (empty vector) served as a negative control. Three months after CeGLR3.6I807-GFP (S) and CeGLR3.6T807-GFP (S) regenerated the roots, the T0 plants of above transgenic lines were fed to the third instar larvae of L. xylina for another 3 weeks, to measure the body weight and feeding amount of each individual insect. At least 30 larvae were used for each transgenic line.
2.10 Subcellular Localisation Assay
For subcellular localisation assay and fluorescence microscopy analyses, the 35S::CeGLR3.6I807-GFP and 35S::CeGLR3.6T807-GFP constructs were transformed into tobacco and C. equisetifolia, respectively. In tobacco (Nicotiana benthamiana), subcellular localisation assays were performed according to Jiang's method (Jiang et al. 2023). The 35S::CeGLR3.6I807-GFP and 35S::CeGLR3.6T807-GFP constructs were co-transformed with the H2B-BFP plasmid from Dr. Jiang into tobacco leaves for 24 h before confocal microscopy. The microscopic images were captured using the Nikon Ti2 confocal microscope equipped with a ‘Plan Apo VC 20× DIC N2’ objective, utilising a DAPI channel (excitation wavelength of 405.0-nm laser and 8.5% laser power) and an EGFP channel (excitation wavelength of 485.8-nm laser and 8.5% laser power).
In C. equisetifolia, 2-week-old seedlings of the ‘Huian 1’ cultivar transformed with the two subcellular localisation plasmids were incubated according to Zhu's method with minor modifications (polypeptide: plasmids = 1:1, volume up to 1.5 mL using 1 × PBS and vacuum treatment for 5 min) (Zhu et al. 2021) to generate CeGLR3.6T807-GFP(H) and CeGLR3.6I807-GFP(H). The seedlings were kept in the dark for 48 h before observing the GFP fluorescence, and images of the radicle were acquired using the Zeiss LSM880 confocal microscope equipped with a ‘Plan-Apochromat 20×/0.8 M27’ objective.
2.11 Protein Phosphorylation Assay
Seedlings (2.5 g) of CeGLR3.6T807-GFP (S) and CeGLR3.6I807-GFP (S) transgenic lines were harvested 24 h after insect feeding and crushed in liquid nitrogen. The sample tissues were dephosphorylated and then suspended in 3 mL lysis buffer without (50 mM Tris-pH = 7.5; 150 mM NaCl; 1 mM EDTA; 1% TritonX-100; 1 mM PMSF; 1 × cocktail; 2 μg/μL Aprotinin; 1 μg/μL pepstain A) with or without 100 U of λ-PPase (P0753S, New England BioLabs Ltd., USA) and lysed for 30 min at 25°C. The crude extract was centrifuged twice at 12 000 rpm for 10 min. The supernatant was subjected to western blot analysis using extraction buffer (0.1 M EDTA, 0.12 M Tris-HCl (pH = 6.8), 4% SDS, 10% β-mercaptoethanol, 5% glycerine, 0.02% bromophenol blue) according to Zuo's method (Zuo et al. 2012).
The total proteins were resolved on a 10% SDS-PAGE gradient gel with Acr-Bis (149:1) and transferred to a membrane. The membrane was then incubated with anti-GFP (ab1218, Abcam, UK) or HSP82 (AbM51099-31-PU, Beijing Protein Innovation Co. Ltd, Beijing, China) as the primary antibody, followed by incubation with HRP-linked goat anti-mouse-IgG (ab97051, Abcam, UK) as the secondary antibody. The membrane was incubated with Amersham western blot analysis detection reagent (A38554, Thermo Fisher Scientific, MA, USA) to generate fluorescence of the GFP cassettes. Auto-fluorescence was detected and analysed using the Amersham Image Quant 800 system (29399481, Cytiva, USA).
3 Results
3.1 Feeding on S Cultivar Promotes L. xylina Development
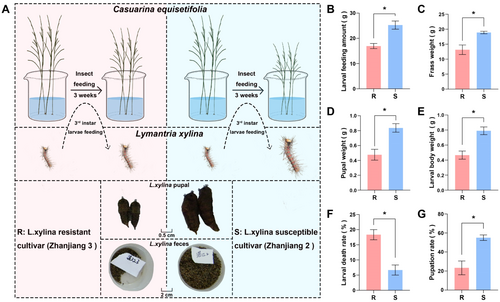
The branchlets of the R and S cultivars were fed to the third instar L. xylina, and their effects on insect development were evaluated after 3 weeks of feeding (Figure 1A). The larval feeding amount, frass weight, body weight, pupal weight and pupation rate were significantly higher in insects fed S than in those fed R (p < 0.05), whereas the larval death rate was significantly lower for the insects fed on S than for those fed on R (p < 0.05). When L. xylina fed on the R and S cultivars, larval feeding amounts of 16.58 ± 1.77 and 25.25 ± 1.62 g (R/S = 0.66), frass weights of 13.15 ± 1.60 and 18.91 ± 0.42 g (R/S = 0.70), body weights of 0.47 ± 0.05 and 0.79 ± 0.07 g (R/S = 0.59), larval death rates of 18.33 ± 1.67% and 6.67 ± 1.67% (R/S = 2.75), pupal weights of 0.48 ± 0.08 and 0.84 ± 0.06 g (R/S = 0.57) and pupation rates of 23.33 ± 7.27% and 55.0 ± 2.89% (R/S = 0.42), respectively, were recorded (Figure 1B–G). This shows that compared to R, feeding on S visibly promoted L. xylina development.
3.2 JA Response Is Different Between the R and S Cultivars
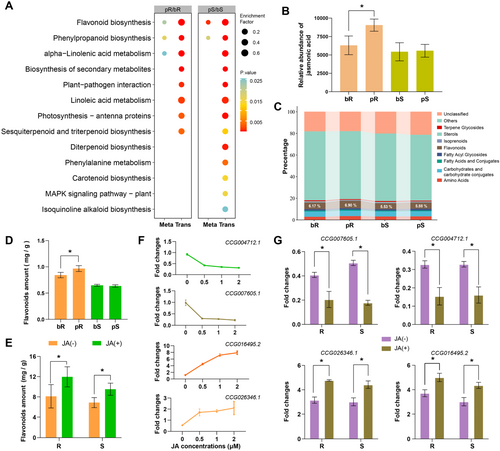
We performed RNA-seq to investigate the differences in transcriptional levels between R and S cultivars in response to L. xylina feeding. In total, 241 534 126 reads were sequenced and analysed, revealing 32 222 coding genes in both the resistant and susceptible cultivars before and after L. xylina feeding (bR, pR, bS and pS) (Supporting Information S1: Figure S3A). Additionally, all metabolites from the different samples (bR, pR, bS and pS) were quantified using LC-MS, resulting in the identification of 19 775 metabolites, of which 12 293 were annotated (Supporting Information S1: Figure S3B, Supporting Information S2: Table S2). We performed a joint analysis of the transcriptome and metabolome and screened three KEGG pathways with differentially expressed transcripts (p < 0.05, Top 13) between pR/bR and pS/bS, which also exhibited similar metabolome differences. These pathways included ‘flavonoid biosynthesis’, ‘phenylpropanoid biosynthesis’ and ‘alpha-linolenic acid metabolism’. Notably, the ‘alpha-linolenic acid metabolism’ pathway overlapped by the transcriptomes of pR/bR and pS/bS, as well as the metabolome of pR/bR, but not by the metabolome of pS/bS (Figure 2A). Given that jasmonic acid, an insect resistance-related hormone, is a major byproduct of linolenic acid metabolism in plants and that both flavonoid and phenylpropanoid biosynthesis can be significantly influenced by JA, we hypothesised that JA plays a critical role in the response to L. xylina feeding across different cultivars. Consequently, we screened the Top 10 GO enrichment pathways in the transcriptome using DEGs from both pR/bR and pS/bS (Supporting Information S1: Figure S5A). This analysis indicated that the JA response in C. equisetifolia might be driven by insect feeding, as differences in JA responses were observed between the R and S cultivars (Supporting Information S1: Figures S4 and S5). Furthermore, we measured JA content in different cultivars using ELISA, which revealed that L. xylina feeding induced high JA content in the R cultivar (pR/bR = 1.44, p < 0.05) but not in the S cultivar (Figure 2B, Supporting Information S2: Table S2). These findings prompted us to further investigate the JA responses across different cultivars to elucidate the varying responses to L. xylina feeding. Because secondary metabolites are crucial substrates regulated by JA in response to insect feeding, we also explored the accumulation patterns of important secondary metabolites in various samples. Results showed that flavonoids were the predominant secondary metabolites identified, with increased synthesis observed in both R and S cultivars after insect feeding. These increases were closely associated with the JA content (Figure 2A,C). Furthermore, we quantified the total flavonoid content of different samples. Consistent with the JA content results, L. xylina feeding induced substantial flavonoid accumulation in the R cultivar (pR/bR = 1.15, p < 0.05) but not in the S cultivar (Figure 2D).
3.3 The JA Signalling Pathway Functioned Well in Both the R and S Cultivars
JA responses are mediated by JA synthesis and signalling pathways. In the JA signalling pathway, JA accumulation promotes secondary metabolite biosynthesis through complex genetic regulation (Ge et al. 2015; Ho, Murthy, and Park 2020). Here, we successfully induced flavonoid accumulation in both the R (1.47 times, p < 0.05) and S cultivars (1.17 times, p < 0.05) following exogenous JA treatment (Figure 2E). To explore the genetic regulation of the JA signalling pathway in C. equisetifolia, we also compared the gene expression patterns of the nine differentially expressed JA response genes from the ‘jasmonic acid metabolic process’ GO enrichment pathways (Supporting Information S1: Figure S5A) via JA treatment of the Huian1 cultivar. We found that four of these genes were significantly induced (CCG016495.2 and CCG026346.1) or suppressed (CCG004712.1 and CCG007605.1) after 24 h of treatment with different concentrations of exogenous JA (Figure 2F, Supporting Information S1: Figure S6). Upon quantifying the expression levels of these four JA response genes, we found that exogenous JA treatment significantly affected the expression levels of the four JA response genes in both the R and S cultivars (Figure 2G), indicating that the JA signalling pathway functioned well in both cultivars. Although the JA response differed between the R and S cultivars, the signalling pathways were similar, which this prompted us to explore the differences in JA synthesis between the two cultivars.
3.4 CeGLR3.6 Regulates JA Response by Manipulating JA Synthesis
JA is synthesised using multiple substrates, such as phosphatidylcholine and α-linolenic acid, which is sequentially transformed to 13(S)-HpoTrE, 12,13-EOTrE, 12-OPDA, OPC8, OPC8-CoA and JA-CoA to form (+)-7-iso-JA and finally JA (Wan and Xin 2022). We examined the JA biosynthesis pathway based on the amount of upstream substrates in the pathway and JA, showing that most of the upstream substrates were positively related to JA levels in both R and S cultivars (Figure 3A), indicating that both the cultivars have similar JA biosynthesis pathways. However, JA biosynthesis was not induced by herbivory in S, but was activated in R. These results prompted us to examine the upstream regulators of JA biosynthesis in response to insect feeding on different cultivars. Among the reported upstream regulators of JA biosynthesis, we found that ROS generation rate was significantly induced by insect feeding in both R (1.32 times, p < 0.05) and S cultivars (1.31 times, p < 0.05) (Figure 3B). However, this change failed to upregulate JA biosynthesis) in S but not in R, indicating that the ROS response signalling pathway was different between the R and S cultivars. To resolve this intriguing puzzle, we identified two genes, CCG002650.1 and CCG004668.1 (Figure 3C, Supporting Information S1: Figure S6), which responded both herbivory in both cultivars (overlapping DEGs in pR/bR and pS/bS) in response to ROS (GO terms involved in the ROS response). One of the abovementioned genes, CCG002650.1, was highly induced in both the R (230 times, p < 0.05) and S cultivars (14.5 times, p < 0.05) (Figure 3C), as deduced from the RNA-seq data (triggered in both cultivars by L. xylina feeding). Moreover, through phylogenetic analysis, we found that CCG002650.1 (CeGLR3.6) is the homologue of GLR3.6 in C. equisetifolia (Figure 3D, Supporting Information S2: Table S3), which is capable of manipulating systemic resistance in response to insect herbivores and regulating in vivo JA accumulation (Jakšová et al. 2021; Xue et al. 2022). Thus, we hypothesised that CeGLR3.6 is involved in transmitting the ROS signal to modulate JA biosynthesis.
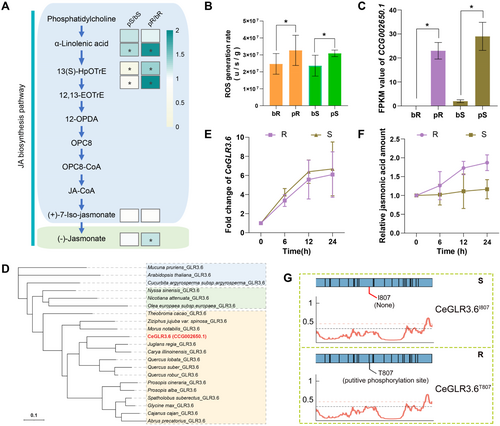
We also tested whether CeGLR3.6 was correlated with ROS generation when treated with H2O2. CeGLR3.6 was upregulated after H2O2 treatment in both R and S cultivars without significant (p > 0.05) differences between the two (Figure 3E). Additionally, JA accumulation in response to H2O2 treatment was detected in the R cultivar, but not in S cultivar (Figure 3F). These findings suggest that the expression of CeGLR3.6 (not the amount of JA) was positively correlated with ROS generation in both the cultivars (Figure 3E, Supporting Information S1: Figure S7); thus, CeGLR3.6 may not be involved in transmitting ROS signals to modulate JA biosynthesis through gene expression regulation. Previous findings suggested that CeGLR3.6 function is mostly regulated at the protein level (especially protein phosphorylation), which prompted us to investigate the differences in protein level regulation. Surprisingly, resequencing of the R and S genomes revealed a C-to-T substitution (2420 bp) in the coding region of CeGLR3.6 in S but not in R, which led to a Thr-to-Ile substitution at position 807 of the CeGLR3.6 protein (CeGLR3.6T807I) and significantly reduced the phosphorylation level of CeGLR3.6 in the S cultivar (Figure 3G, Supporting Information S1: Figure S8, Supporting Information S2: Table S4).
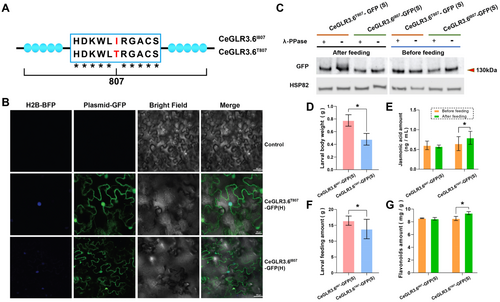
3.5 CeGLR3.6T807I Suppressed the Phosphorylation of CeGLR3.6 and the Downstream JA Response in C. equisetifolia
To investigate the function of CeGLR3.6, we overexpressed CeGLR3.6I807-GFP (CeGLR3.6I807-GFP (H)) and CeGLR3.6T807-GFP (CeGLR3.6T807-GFP (H)) in C. equisetifolia seedlings using a transient expression assay (Figure 4A). GLR3.6 phosphorylation can affect its subcellular localisation, which is crucial for its response to different type of stress (Ahmed et al. 2023; Yong et al. 2021). Thus, we also checked the subcellular localisation of the two CeGLR3.6 variants in the abovementioned overexpression lines to determine whether the phosphorylation of different variants in 807aa could change their subcellular localisation and further affect their functions. The GFP signal was detected in the plasma membrane and condensed inside the cells, indicating that the subcellular localisation of CeGLR3.6 was not affected by the Thr-to-Ile substitution (Figure 4B, Supporting Information S1: Figure S9). Using western blot analysis, a shifted band was detected in CeGLR3.6T807-GFP-overexpressing seedlings of the S cultivar (CeGLR3.6T807-GFP (S)), but not in CeGLR3.6I807-GFP (CeGLR3.6T807-GFP (S)). The shift in CeGLR3.6T807-GFP (S) was suppressed by the dephosphorylation agent PPase, suggesting that CeGLR3.6, but not CeGLR3.6T807I, is phosphorylated (Figure 4C, Supporting Information S1: Figure S10). In addition, the phosphorylation level of CeGLR3.6T807 increased in response to insect feeding, as shown by the band shift of CeGLR3.6T807-GFP (S), which was more pronounced after insect feeding and was suppressed by PPase (Figure 4C, Supporting Information S1: Figure S10). Furthermore, we measured the diet and body weights of third instar L. xylina feeding on different transgenic lines and found that feeding on CeGLR3.6T807-GFP (S) resulted in a reduction in the feeding amount (CeGLR3.6I807-GFP(S) = 16.46 ± 1.458, CeGLR3.6T807-GFP(S) = 13.82 ± 3.072, p < 0.05) and body weight (CeGLR3.6I807-GFP(S) = 0.78 ± 0.09, CeGLR3.6T807-GFP(S) = 0.48 ± 0.09, p < 0.05) of L. xylina compared with those of CeGLR3.6I807-GFP (S) (Figure 4D,E). Moreover, only CeGLR3.6T807-GFP (S) showed an increase in JA (Figure 4F) and flavonoid content (Figure 4G) in response to insect feeding, indicating that CeGLR3.6I807T partially rescued the insect-feeding-insensitive phenotype of the S cultivar. As GLRs have been widely shown to be gated by glutamate in plants (Grenzi et al. 2023), we quantified the amounts of JA and flavonoids in transgenic seedlings treated with exogenous l-glutamate. l-glutamate treatment significantly induced JA and flavonoid accumulation in CeGLR3.6T807-GFP (S) seedlings within 24 h, but not in CeGLR3.6I807-GFP (S) seedlings (Supporting Information S1: Figure S11). In conclusion, our findings suggest that phosphorylation of CeGLR3.6 is crucial for inducing the downstream JA response against insect feeding, and CeGLR3.6T807I suppresses phosphorylation, which diminishes the insect resistance of C. equisetifolia (Supporting Information S1: Figure S13).
4 Discussion
The ability of plants to defend themselves against herbivorous insects is pivotal for adaptive evolution. It is increasingly evident that the JA response is central to the inducible and specific defence responses in plants triggered by leaf-chewing insects (Erb, Meldau, and Howe 2012; Howe and Jander 2008; Mostafa et al. 2022). In the present study, we demonstrated the transcriptional and metabolic differences between S and R cultivars of C. equisetifolia. Biochemical and genetic JA responses were highly induced in the R cultivar in response to L. xylina feeding; however, no obvious changes were observed in the S cultivar. We also found that JA signal transduction and biosynthesis functioned well in the resistant cultivar, whereas JA biosynthesis in the S cultivar was unresponsive to insect feeding, allowing us to uncovered the JA-related biochemical and genetic mechanisms of L. xylina-induced resistance in C. equisetifolia.
In the early stages of leaf damage by insects, ROS, along with electrical and cytosolic Ca2+ ([Ca2+]cyt) signals transfer the damage signal from local to systemic leaves, thus inducing systemic resistance, primarily through GLRs (Choi et al. 2016; Erb and Reymond 2019; Fichman and Mittler 2021; Grenzi et al. 2021; Mousavi et al. 2013; Nguyen et al. 2018; Xue et al. 2022). However, precisely how GLRs are linked to plant resistance to herbivores and whether the induction of ROS by insect feeding affects GLRs remained largely unknown. We observed that ROS generation was highly induced by L. xylina feeding, and the expression of CeGLR3.6 was also induced (Figure 3B,C). In addition, we chose H2O2 as the ROS treatment and found that CeGLR3.6 was upregulated by H2O2 treatment in both the R and S cultivars (Figure 3E); however, JA accumulation in response to H2O2 was detected only in the R cultivar (Figure 3F). Several studies have shown that the transcriptional level of plant GLR3.6 is governed by wounding responses (Mou et al. 2020; Salvador-Recatalà, Tjallingii, and Farmer 2014). However, the level of protein regulation level has not yet been reported, although GLR3.6 phosphorylation has been demonstrated to activate its function during abiotic stress (Silamparasan, Chang, and Jinn 2023). In the present study, we found a single-nucleotide mutation in the coding region of CeGLR3.6 in the susceptible cultivar, leading to a Thr-to-Ile substitution at amino acid 807 (CeGLR3.6T807I), which suppressed the phosphorylation of CeGLR3.6 and inactivated the JA response (Figure 4C,E). The currently confirmed phosphorylation site of GLRs is the Ser861/862 residues using the 14-3-3 protein (Silamparasan, Chang, and Jinn 2023); however, in our results, the Thr807 residue (predicted motif: RLHDKWLTRGACSSE) was not a potential target of the 14-3-3 binding motif (I. F. Chang et al. 2009; Shin et al. 2011; Silamparasan, Chang, and Jinn 2023; Wudick et al. 2018). Therefore, the kinases that phosphorylates CeGLR3.6 requires further investigation. Surprisingly, we found that CeGLR3.6T807I was not phosphorylated in C. equisetifolia seedlings, indicating that GLR3.6 phosphorylation may be specific to different environmental cues. This may provide an important clue for solving the puzzle of insect-specific resistance in plants. GLRs have been detected in various subcellular locations, including the plasma membrane, chloroplasts membranes, mitochondria, ER and vacuoles (Hansen et al. 2021; Silamparasan, Chang, and Jinn 2023; Teardo et al. 2011; Wudick et al. 2018). In addition, protein phosphorylation influences the subcellular localisation of GLRs, affecting their function in both animals and plants. Consequently, subcellular localisation of the two CeGLR3.6 variants is crucial for formulating hypotheses regarding the observed phenotype (Yong et al. 2021) (Ahmed et al. 2023). Therefore, we also examined the subcellular localisation of the two CeGLR3.6 variants in the C. equisetifolia. Regarding the subcellular localisation of CeGLR3.6, we observed that the GFP signal was located in the plasma membrane and condensed inside the cells (Figure 4B, Supporting Information S1: Figure S10). Overall, both Ser861/862 and Thr807 residues are located in the putative cytoplasmic tail of GLRs (Grenzi et al. 2021), indicating that C-terminal GLRs may respond to transfer signals from outside the cell to internal targets, which requires further exploration.
JA is a plant lipid-derived hormone that regulates herbivore attacks and plant resistance (Li et al. 2022). After L. xylina feeding, multiple response genes were affected, including those involved in wounding, lipid metabolism and JA signalling (Supporting Information S1: Figure S5A). This suggests that JA is vital for the resistance of C. equisetifolia to defoliators. Typically, the JA response can spontaneously induce a vast array of toxic secondary metabolites, and the diversity of these metabolites is essential for effective insect defence because herbivorous insects can detoxify certain groups of plant defence compounds (Han 2017; Heidel-Fischer and Vogel 2015; Li et al. 2022; Xia et al. 2021). However, the diversity of the most significantly altered secondary metabolites in response to L. xylina attacks was relatively low, with most being flavonoids. Furthermore, exogenous JA induced flavonoid accumulation in C. equisetifolia (Figure 2D,E, Supporting Information S2: Table S2). This suggests that the JA response may not be sufficient to directly protect L. xylina larvae, which is supported by the low death rate of larvae feeding on the susceptible or resistant cultivars. A large-area outbreak of L. xylina has only been reported in C. equisetifolia forests, although this pest can damage hundreds of hosts (Shen et al. 2006).
Most reported genes in the JA signalling pathway are regulated at the protein level, primarily through phosphorylation and degradation of the SCFCOI1–JAZ coreceptor complex (Li et al. 2022), which cannot be detected by RNA-seq. However, this regulation was supported by the observation that the majority of DEGs induced by L. xylina were enriched in the protein phosphorylation function groups (Supporting Information S1: Figures S5 and S11), including 231 proteins that function in ‘intracellular signal transduction’, ‘signalling receptor activity’, ‘transmembrane receptor protein kinase activity’, ‘MAP kinase kinase kinase activity’, ‘response to external stimulus’, ‘calmodulin-dependent protein kinase activity’, ‘MAPK cascade’, ‘oxidoreductase activity’, ‘cellular response to hormone stimulus’, ‘response to external biotic stimulus’, ‘hormone-mediated signalling pathway’ and ‘calcium-dependent protein serine/threonine kinase activity’. The phosphorylated proteins identified represent a typical pattern-triggered immunity response (Jakšová et al. 2021; Yu et al. 2024; Yuan et al. 2021). However, pattern-triggered immunity is a general resistance induced by broad-spectrum pests and pathogens, which does not adequately explain the specific resistance to L. xylina feeding in the host plant (Yuan et al. 2021).
OPDA (12-oxophytodienoic acid) is an important substrate in the JA synthesis pathway, and OPDA itself has been widely shown to mediate OPDA-specific responses in plants (Han 2017). In this study, L. xylina feeding significantly induced JA accumulation in the resistant cultivars but not in the susceptible cultivar. Surprisingly, the levels of OPDA and JA differed between the two cultivars, as OPDA was induced by L. xylina feeding in the resistant cultivar but was suppressed in the susceptible cultivar (Figure 2B, Supporting Information S1: Figure S12). The JA content was highly correlated with most JA synthesis substrates besides OPDA. However, the enzyme that promotes OPDA metabolism in the JA synthesis pathway, OPR3 (Han 2017), was highly correlated with JA content at the transcriptional level (Figures 2F and 3A, Supporting Information S1: Figure S12). This suggests that OPDA might serve as an independent L. xylina resistance signal in C. equisetifolia, compensating for the JA response, at least in resistant cultivars.
In conclusion, this study demonstrated that L. xylina feeding strongly induced JA synthesis in C. equisetifolia and promoted the biosynthesis of total flavonoids via the JA signalling pathway. For the first time, we report that the phosphorylation of CeGLR3.6 on the Thr807 residue is crucial to the insect-induced JA response in plants and might provide an important clue for uncovering insect-specific resistance. OPDA may also as a key insect resistance signal in woody plants and warrants further investigation. However, further research is needed to determine the proteins involved in the JA and OPDA signalling pathways, as well as the function of CeGLR3.6 phosphorylation in insect feeding and the JA response.
Acknowledgements
We are immensely grateful to Prof. Chentao Lin of the University of California, Los Angeles (UCLA) for his invaluable suggestions and great help with the experimental techniques employed in this study. Additionally, we are very grateful to the Chihu state-owned forest farm in Huian, Fujian, China, for providing plant materials. This work was supported by the National Natural Science Foundation of China (NSFC) (32071753), the Fujian Forestry Science and Technology Project (ZMGG-0704) and the Forestry Peak Discipline Construction Project of Fujian Agriculture and Forestry University (72202200205).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data are available in the manuscript or as supplemental data. The raw data of the RNA-seq and genome re-sequencing are openly available in the National Center for Biotechnology Information BioProject at https://www.ncbi.nlm.nih.gov/bioproject, reference PRJNA951343, PRJNA1175366. Other data or materials generated in this work are available upon request to the corresponding authors.