The B-box transcription factor BnBBX22.A07 enhances salt stress tolerance by indirectly activating BnWRKY33.C03
Abstract
Salt stress has a detrimental impact on both plant growth and global crop yields. B-box proteins have emerged as pivotal players in plant growth and development regulation. Although the precise role of B-box proteins orchestrating salt stress responses in B. napus (Brassica napus) is not well understood in the current literature, further research and molecular explorations are required. Here, we isolated the B-box protein BnBBX22.A07 from B. napus. The overexpression of BnBBX22.A07 significantly improved the salt tolerance of Arabidopsis (Arabidopsis thaliana) and B. napus. Transcriptomic and histological analysis showed that BnBBX22.A07 enhanced the salt tolerance of B. napus by activating the expression of reactive oxygen species (ROS) scavenging-related genes and decreasing salt-induced superoxide anions and hydrogen peroxide. Moreover, BnBBX22.A07 interacted with BnHY5.C09, which specifically bound to and activated the promoter of BnWRKY33.C03. The presence of BnBBX22.A07 enhanced the activation of BnHY5.C09 on BnWRKY33.C03. Overexpression of BnHY5.C09 and BnWRKY33.C03 improved the salt tolerance of Arabidopsis. Functional analyses revealed that BnBBX22.A07-mediated salt tolerance was partly dependent on WRKY33. Taken together, we demonstrate that BnBBX22.A07 functions positively in salt responses not only by activating ROS scavenging-related genes but also by indirectly activating BnWRKY33.C03. Notably, our study offers a promising avenue for the identification of candidate genes that could be harnessed in breeding endeavours to develop salt-resistant transgenic crops.
1 INTRODUCTION
Salt stress negatively impacts plant growth and productivity of crops. It is estimated that approximately 20% of the total cultivable lands and 33% of the irrigated agricultural lands are affected by salinity in the world, and 15% of the irrigated lands are affected by salinity in China (Singh et al., 2020), which has an important impact on agricultural production. To cope with salt stress, plants have developed complex mechanisms at multiple levels, including molecular, cellular, physiological and biochemical processes (Zhao et al., 2020; Zhu, 2016). In plants, two types of proteins, functional proteins and regulatory proteins, are involved in response to salt stress (Lata and Prasad, 2011). As regulatory proteins, transcription factors such as zinc finger proteins play an important role in salt stress responses by regulating downstream stress-responsive genes (Han et al., 2022; Kiełbowicz-Matuk, 2012). Among the zinc finger proteins, the B-box (BBX) family contains one or more B-box domains and sometimes features a CONSTANS, CO-like, and TOC1 domain (Gangappa and Botto, 2014; Talar and Kiełbowicz-Matuk, 2021). The BBX family consists of 32 proteins in Arabidopsis, which can be classified into five subfamilies (groups I-V) on the basis of their phylogenetic relationships and structural features (Khanna et al., 2009). Most of the BBX proteins play a crucial role in seedling photomorphogenesis, photoperiodic regulation of flowering, and shade-avoidance responses (Fan et al., 2012; Gangappa et al., 2013; Valverde, 2011). Some members also function in response to salt stress. For instance, the expression of OsBBX1, OsBBX2, OsBBX8, OsBBX19, and OsBBX24 is markedly induced by salt stress in rice (Shalmani et al., 2019). Heterologous expression of Chrysanthemum CmBBX22, Chimonanthus praecox CpBBX19, and Malus domestica MdBBX1 in Arabidopsis enhanced the tolerance of plants to salt stress (Dai et al., 2022; Gao et al., 2012; Wu et al., 2021). Silencing AhBBX6 from peanut (Arachis hypogaea) compromised the tolerance of peanut plants to salt stress (Tang et al., 2024). In addition, the overexpression of BBX24 in Arabidopsis increases salt tolerance and root growth under salinity (Nagaoka and Takano, 2003). The IbBBX24 regulates salt tolerance by promoting ROS scavenging in sweet potato (Zhang et al., 2022). Although some BBX proteins have been reported in response to salt stress, the molecular mechanisms still remain largely unknown.
Elongated HYPOCOTYL5 (HY5), a member of the bZIP transcription factor family, is the main transcription factor involved in promoting photomorphogenesis (Li et al., 2013). A large number of proteins, including BBX22, play an important role in enhancing HY5-mediated photomorphogenesis in Arabidopsis, and BBX22 acts as a rate-limiting cofactor of HY5 to regulate light signalling (Bursch et al., 2020). In recent years, the role of HY5 in response to salt stress has been under investigation (Gangappa and Botto, 2016). HY5 mediates ABA responses in seed germination and is required for salt and osmotic stresses in seedlings by directly binding to the ABI5 promoter (Chen et al., 2008). COP1 interacts with HY5 to regulate transcription of ABA INSENSITIVE5 (ABI5) to control seed germination under salt stress in Arabidopsis (Yu et al., 2016). In addition, HY5 is required for the expression of P5CS1 and the accumulation of salt stress-induced proline (Feng et al., 2016). However, it is unclear whether HY5 is required in BBX22-regulated salt stress responses.
Salt stress increases the production of ROS (Choudhury et al., 2017). ROS plays a dual role in abiotic stresses in plants, and the steady-state level of ROS depends on the balance between ROS production and ROS scavenging (Mittler et al., 2004). The ROS scavenging mechanisms have been proven to protect plants against salt, drought, or osmotic stresses (Miller et al., 2010). WRKY transcription factors are known to function in response to salt stress (Rushton et al., 2010). For instance, AtWRKY15 (Arabidopsis) and PalWRKY77 (P. alba var. pyramidalis) negatively modulate the growth and salt tolerance of plants (Jiang et al., 2021; Vanderauwera et al., 2012). Overexpression of AtWRKY46 increases the tolerance to salt stress through alleviating H2O2 accumulation in Arabidopsis (Ding et al., 2014). Moreover, WRKY33 is also associated with plant tolerance under salt stress. In Arabidopsis, overexpression of WRKY33 confers tolerance of transgenic plants to NaCl stress (Jiang and Deyholos, 2009). WRKY33 regulates the expression of a cytochrome P450 gene (CYP94B1) and controls apoplastic barrier formation in roots, which enhances salt tolerance in Arabidopsis (Krishnamurthy et al., 2020). However, it remains largely unclear how the WRKY transcription factors respond to salt stress and, in particular, how the upstream regulators of WRKY transcription factors are regulated.
B. napus is a crucial oil crop worldwide that plays a vital role in vegetable oil, biofuel, and livestock feed production (Rokosik et al., 2019). Salt stress significantly restricts the growth and production of B. napus (Machado and Serralheiro, 2017). Therefore, it is urgent to improve the environmental adaptation of B. napus. Mining salt-tolerant genes and understanding their underlying molecular mechanisms under salinity is particularly important for the cultivation of salt-tolerant B. napus. However, little is known about the BBX genes and their function in response to salt stress in B. napus. Here, we isolated the BBX transcription factor BnBBX22.A07 in B. napus and described the biological function of BnBBX22.A07 in response to salt stress. The overexpression of BnBBX22.A07 increased salt stress tolerance by activating the expression of ROS scavenging-related genes. Furthermore, BnBBX22.A07 interacted with BnHY5.C09, thereby enhancing the capacity of BnHY5.C09 to trigger the transcription of BnWRKY33.C03. Overexpression of BnHY5.C09 and BnWRKY33.C03 resulted in a substantial improvement in salt stress tolerance, primarily achieved by scavenging O2.− and H2O2. Collectively, these findings suggested that BnBBX22.A07 can activate not only the expression of genes involved in ROS scavenging, but also BnWRKY33.C03 in response to salt stress.
2 MATERIALS AND METHODS
2.1 Plant materials, growth conditions and stress treatments
B. napus line 2205, from the Rapeseed Research Laboratory of the College of Agriculture, Northwest Agriculture and Forestry University, were grown in a growth chamber (16 h light/8 h dark, 25/20℃, intensity of 180 umol photons m−2. s−1) with a relative humidity of 60%. In the hydroponic experiments, seeds were germinated on filter papers for 6 days and then transferred to ¼ Hoagland nutrient solution (HS) with a cystosepiment supporter for another 7 days, and finally to ½ HS (Tocquin et al., 2003). The HS was refreshed once a week. Then, the 3-week-old 2205 plants were treated with 200 mM NaCl, and the leaves and roots were harvested at 0, 3, 6, 12, 24 and 48 h for gene expression analysis. Roots, stems, leaves, buds, flowers and horns from the mature line 2205 growing in the field were collected for tissue-specific expression analysis. For phenotypic observation, the overexpression lines at four to five leaf stage were treated with 200 mM NaCl for 10 days in line 2205 background (OE-BnBBX22.A07-2205) under hydroponic conditions. Each biological repeat contained at least six plants per line. In the soil experiments, B. napus seeds were planted in sectors (one plant per sector) of 18 cm × 15 cm (H ×D) round plastic pots for phenotypic observation at various developmental stages. Each biological repeat contained at least three plants per line. The Arabidopsis wrky33 mutant (SALK-006603) was donated by Professor Juan Xu of Zhejiang University. Arabidopsis seeds were sterilised and incubated for 3 days at 4℃ in the dark. Seeds were germinated on half-strength Murashige and Skoog (½ MS) plates for five or 7 days, and then transferred to the soil or ½ MS with supplementation of NaCl for stress treatments. Soil-grown plants were planted in round plastic pots (12 cm × 10 cm/H × D) with a growth medium consisting of a mixture of vermiculite, perlite, and peat moss at a 3:1:1 ratio. For salt tolerance assays on plates, each biological repeat contained at least six plants per line. For salt tolerance assays in soil, each biological repeat contained at least 21 plants per line. All Columbia ecotype (Col-0), wrky33 mutant, transgenic Arabidopsis and Nicotiana benthamiana plants were grown in a growth chamber at 22℃ with a 16 h light/8 h dark photoperiod. For each treatment, three biological replicates were performed.
2.2 Plasmid construction and genetic transformation
The coding sequence of BnBBX22.A07, BnHY5.C09, and BnWRKY33.C03 were amplified and cloned into the modified expression vector pMDC83 for overexpression line construction. The CRISPR/Cas9 construct was generated by designing sgRNA sequences of AtWRKY33 into the pHSN401 vector (Xing et al., 2014). Constructs were introduced into Arabidopsis or B. napus using the Agrobacterium tumefaciens-mediated transformation. The hygromycin-resistant transgenic Arabidopsis plants were generated by a floral dipping method (Clough and Bent, 1998), screened with 25 mg/ml hygromycin and confirmed by PCR amplification. The mutations were confirmed by Sanger sequencing. The transgenic B. napus plants were obtained as described previously (Cardoza and Stewart, 2003). The DNA was extracted from young leaf samples as described previously (Alonso and Stepanova, 2015), and used to verify the presence of the transgene by PCR.
2.3 Protein localisation assay
The coding sequence of BnBBX22.A07 without the termination codon was amplified and cloned into the pCAMBIA1300-GFP vector. The generated construct was transiently transformed into Nicotiana benthamiana by Agrobacterium tumefaciens-mediated transformation. The fluorescence was then observed after infiltration for 2 days with a confocal laser scanning microscope (Olympus, IX83-FV1200). The experiment was independently repeated three times with similar results.
2.4 RNA preparation and gene expression analysis
Total RNA was extracted by the RNA Prep Pure Plant Kit (TIAN GEN). cDNA synthesis was performed using the FastQuant RT Kit (TIAN GEN). Gene expressions were determined by quantitative real-time PCR (RT-qPCR) using the SYBR Green Master Mix (TaKaRa, Japan). Actin2 was used as the internal control gene for normalisation, and the relative expression was calculated by the 2−△△Ct method according to Vandesompele et al. (2002). Each sample was repeated three times and three independent biological experiments were performed. Primers used for RT-qPCR are listed in Table S1.
2.5 RNA sequencing and bioinformatics analysis
Total RNA was isolated from the leaves of three-leaf stage seedlings of BnBBX22.A07-overexpressing plants (OE-B1) and the control plant 2205 (T) under the 200 mM NaCl treatment for 6 h with three biological replicates per sample. The 150-bp paired-end libraries were constructed and sequenced using an Illumina Hiseq. 2500 at Genoseq Company. The raw reads were mapped to the reference genome (http://www.genoscope.cns.fr/brassicanapus/data/) using HISAT2 v2.1.0 (Kim et al., 2015). RSEM v1.2.31 was used to calculate the TPM (Transcripts Per Million) of each gene (Li and Dewey, 2011), and DESeq. 2 v1.10.1 was used to identify the differentially expressed genes (DEGs) (|log2fold change | ≥1; false discovery rate, FDR < 0.05) (Love et al., 2014). The Venn diagram and gene-expression heat map were drawn using OmicShare tools (http://www.omicshare.com/tools). The Kyoto Encyclopaedia of Genes and Genomes (KEGG) enrichment analysis was done using BlastKOALA (Ogata et al., 1999). The MEME FIMO (v5.0.2) tool (p < 1e-5) was used to analyse motifs within the 1000 bp promoter region of differentially expressed genes, and motifs were searched based on the JASPAR cis-element database.
2.6 Physiological measurements
The transgenic seedlings were treated with or without 200 mM NaCl, and then the leaves were harvested and homogenised (1:5 m/v) in an ice-cold mortar using 50 mM sodium phosphate buffer, pH 7.8, containing 1% polyvinylpyrrolidone and 10 mmol/L β-mercaptoethanol. After centrifugation (13 000 g, 15 min, 4℃), the supernatant was used for the determination of the contents of malondialdehyde (MDA), and the activities of superoxide dismutase (SOD) and peroxidase (POD). The content of MDA was measured as described previously (Heath and Packer, 1968). The POD activity was measured according to the method of Chance and Meahly (1955). The SOD activity was assayed according to the method as described previously (Beauchamp and Fridovich, 1971). Three biological repeats were performed, and similar results were obtained. At least three independent plants per biological repeat were tested.
2.7 DAB and NBT staining
Hydrogen peroxide (H2O2) and superoxide anions (O2.−) were detected by using 3,3′-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT) staining as previously described (Jabs et al., 1996; Thordal-Christensen et al., 1997), respectively. For DAB staining, the samples were submerged in 1 mg/mL DAB-HCl solution (pH 3.8) for 10 h and then in 95% (v/v) boiling ethanol to decolor. For NBT staining, the samples were submerged in NBT staining solution (AR-0632, DINGGUO) until the dark blue colour appeared, and then the chlorophyll was cleared in 95% (v/v) boiling ethanol solution. The photographs were taken with a fluorescence microscope (Olympus, BX-RFA). The staining quantification was performed with ImageJ. Three biological repeats were performed, and similar results were obtained. At least ten independent plants per biological repeat were tested.
2.8 Yeast one-hybrid and yeast two-hybrid assays
Yeast one-hybrid analysis was performed using the Matchmaker® Gold Yeast One-Hybrid System (Clontech) according to the manufacturer's instructions. The full-length or truncated BnHY5.C09 and BnBBX22.A07 fragments were fused with the pGADT7 vector as the prey. The promoter fragments of different lengths were cloned into the pAbAi vector as the bait. The prey vector was co-transformed with the bait vector into Y1Hgold yeast cells. For yeast two-hybrid analysis, the full-length or truncated BnHY5.C09 and BnBBX22.A07 fragments were respectively cloned into the pGBKT7 vector as the bait and the pGADT7 vector as the prey. The bait and prey constructs were co-transformed into the yeast strain AH109. Medium lacking Leu-Trp-His-Ade was used for selection. Three independent experiments were performed, and similar results were obtained.
2.9 Dual-luciferase transient expression assay
The transcription activity of BnBBX22.A07 and BnHY5.C09 was evaluated through the double luciferase reporter assay system in Nicotiana benthamiana. As an effector, the coding sequences of BnBBX22.A07 and BnHY5.C09 were inserted into the pMDC83 vector. The promoter fragments of WRKY33.C03 were fused into the pGreenII 0800-LUC double-reporter vector. The reporter and effector constructs were separately transformed into A. tumefaciens strain GV3101. The strains carrying both the effector and reporter constructs were co-infiltrated into Nicotiana benthamiana leaves. After infiltration for 48 h, the luciferase activities of LUC and REN were measured using a dual-luciferase reporter assay kit (Promega) and the relative LUC/REN ratios were calculated. Three biological replicates were performed for this analysis, and each biological replicate was tested with three technical repeats.
2.10 Split luciferase (LCI) and bimolecular fluorescence complementation (BiFC) assays
The LCI and BiFC assays were performed in Nicotiana benthamiana leaves using A. tumefaciens-mediated transient expression. For the LCI assay, the coding sequences of BnHY5.C09 and BnBBX22.A07 were fused into the vectors pCAMBIA1300-LUCN and pCAMBIA1300-LUCC, respectively, and then co-infiltrated into the tobacco leaves. After 48 h, the leaves were sprayed with luciferin, and the fluorescence was detected using a multispectral dynamic fluorescence microscopy imaging system (BLT, PlantView100). For the BiFC assay, the coding sequences of BnBBX22.A07 and BnHY5.C09 were fused into the vectors pSPYNE-35S/pUC-SPYNE and pSPYCE-35S/pUC-SPYCE, respectively, and co-expressed in Nicotiana benthamiana leaves. After infiltration for 2 days, the fluorescence was then visualised with a confocal laser scanning microscope (Olympus, IX83-FV1200). Three independent experiments were performed, and similar results were obtained.
2.11 Chromatin immunoprecipitation quantitative PCR (ChIP-qPCR) assay
The ChIP experiments were performed as described previously with slight modifications (Saleh et al., 2008). Briefly, approximately 5 g of 4-week-old BnHY5.C09-overexpressing Arabidopsis seedling (OE-H7) leaves were fixed with 1% (V/V) formaldehyde by vacuum filtration for 30 min. The cross-linking was quenched with the addition of 0.125 M glycine for 5 min. The samples were ground, and the chromatin was extracted and then sonicated into fragments with approximately 200–700 base pair (bp) in length. The chromatin was immunoprecipitated with an anti-GFP antibody. Rabbit Immunoglobulin G (IgG) serum was used as a negative control. The immunoprecipitated DNA fragments were extracted with phenol/chloroform/isoamyl alcohol (25:24:1), precipitated with ethanol, and dissolved in water. The resulting DNA fragments were analysed by qPCR using the SYBR Green Master Mix (TaKaRa) with the real-time PCR detection system (Life Technologies, ABI QuantStudio 7). The enrichment levels were normalised to the input sample and fold enrichment was calculated by normalising against the negative control (IgG). Three biological replicates were performed, and each biological replicate was tested with three technical repeats. The primers are listed in Table S1.
2.12 Electrophoretic mobility shift assay (EMSA)
The coding sequence of BnHY5.C09 was cloned into the pMAL-C5X vector and transformed into Escherichia coli strain BL21 (DE3) to induce the recombinant MBP-BnHY5.C09 protein. The recombinant proteins were purified using amylose resin beads (NEB). The biotin-labelled probes were synthesised by Shanghai Sangon Biotech, and the sequences are listed in Table S1. The EMSA was performed using a LightShift Chemiluminescent EMSA Kit (Thermo Scientific) according to the manufacturer's instructions. The experiment was independently repeated three times with similar results.
2.13 Statistical analyses
Statistical analyses were performed using the SPSS 20.0 software (IBM SPSS Statistics). All charts were obtained using GraphPad Prism 8.0. Statistical significance was computed using a two-tailed Student's t-test. Significance cutoff: *p < 0.05, **p < 0.01, ***p < 0.001.
3 RESULTS
3.1 Salt stress significantly upregulates BnBBX22.A07 transcription
The members of the BBX family in Arabidopsis can be divided into five groups (Khanna et al., 2009). Previous experiments reported that only the members of group IV are induced under salt stress, including AtBBX18, AtBBX22 and AtBBX23 (Figure S1). To better understand the regulatory mechanism of the BBX genes of group IV in B. napus in response to salt stress, we identified the homologues of these genes in B. napus based on BLAST analysis, which were designated as BnBBXN. AY, or BnBBXN. CY, where N represents the homologous group number and Y represents the B. napus chromosome where it is located. The expressions of all homologous alleles were examined by RT-qPCR in the leaves and roots of three-leaf stage seedlings under salt stress (Figure S2A). In leaves, all homologous alleles were upregulated at various points in time with the exception of BnBBX23.A09 (BnaA09g22570D), BnBBX18.C08 (BnaC08g35830D), BnBBX22.A07 (BnaA07g34250D), and BnBBX22.C02 (BnaC02g25060D) expression in leaves was always higher under salt stress compared to the control. In roots, four genes, BnBBX22.A02 (BnaA02g18950D), BnBBX22. Ann (BnaAnng29690D), BnBBX22.A07 (BnaA07g34250D), and BnBBX22.C06.3 (BnaC06g39020D), were induced by salt stress. However, their expression patterns were different. BnBBX22.A02, BnBBX22. Ann, and BnBBX22.C06.3 exhibited an initial upregulation followed by downregulation. In contrast, the expression of BnBBX22.A07 remained consistently higher compared to the control. Taken together, only the expression of BnBBX22.A07 in both leaves and roots was continuously induced by salt stress. These results indicated that BnBBX22.A07 might play an important role in response to salt stress, compared to other homologous alleles. So, BnBBX22.A07 was then selected for further analysis. To further investigate the expression of BnBBX22.A07, we examined the BnBBX22.A07 mRNA levels in various plant tissues using RT-qPCR. The result indicated that BnBBX22.A07 was expressed in all organs examined (Figure S2B).
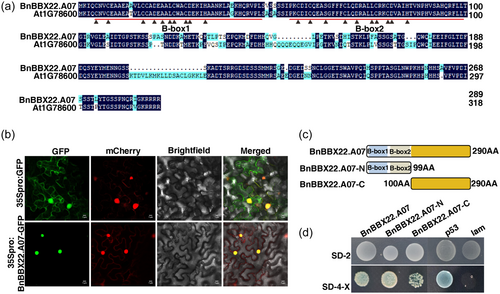
3.2 BnBBX22.A07 encodes a nucleus-localised zinc finger transcription activator
BnBBX22.A07 was amplified in the salt tolerant B. napus 2205 according to the sequence of BnaA07g34250D. The BnBBX22.A07 coding sequence encodes a protein of 290 amino acid including two typical B-box zinc finger domains (amino acids 5–47 and 55-99) in the N terminus with very high homology to Arabidopsis BBX22 (AT1G78600) (Figure 1a). To better understand the biological functions of BnBBX22.A07, the subcellular localisation of BnBBX22.A07 was investigated, and signal was detectable in the nucleus (Figure 1b). We then examined the trans-activity of BnBBX22.A07 and its specific active regions in the yeast system. It was found that the fusion proteins containing the B-box domains exhibited a relatively higher level of transactivation activity than the truncated BnBBX22.A07 (BnBBX22.A07-C) fusion protein at least when expressed in yeast (Figures 1c and d).
3.3 Overexpression of BnBBX22.A07 increases salt tolerance in arabidopsis and B. napus
To investigate the function of BnBBX22.A07 in salt stress responses, we overexpressed BnBBX22.A07 in Arabidopsis and B. napus, and independent BnBBX22.A07-overexpressing (BnBBX22.A07-OE) lines were confirmed by PCR (Figure S3A). Two homozygous BnBBX22.A07-OE lines (OE-B1and OE-B2) with the most increased expression in Arabidopsis and B. napus line 2205 were chosen for subsequent analysis, respectively (Figure S3B,C). We first investigated the phenotypes of 3-week-old WT and BnBBX22.A07-OE Arabidopsis plants under salinity conditions. After ten days of salt treatment with 200 mM NaCl, the BnBBX22.A07-OE plants showed tolerance, compared with the WT plants (Figure 2a). To determine the effects of salt stress on plant growth, 7-day-old BnBBX22.A07-OE and WT seedlings grown on the ½ MS medium were transferred to the plates containing 0 mM or 100 mM NaCl for 1 week. The BnBBX22.A07-OE lines showed longer primary roots compared with the WT under normal condition (Figure 2b,c). Following salt treatment, the root length was reduced by 16.75%-18.79% in BnBBX22.A07-OE lines, but by 37.63% in WT, demonstrating that BnBBX22.A07-OE lines had longer root length compared with WT (Figure 2f).
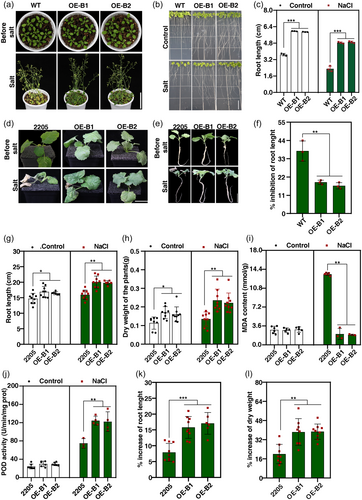
We further investigated the phenotypes of BnBBX22.A07-OE B. napus plants under the background of B. napus line 2205 (OE-BnBBX22.A07-2205). Before salt treatment, the OE-BnBBX22.A07-2205 plants exhibit no obvious differences in phenotype from the control plant 2205. After salt treatment, the leaves of the control plant 2205 turned yellow or wilting. However, the OE-BnBBX22.A07-2205 plants remained green (Figure 2d). Moreover, the root length and dry weight showed significant differences between the OE-BnBBX22.A07-2205 plants and the corresponding control plant 2205 under both normal and salt stress conditions (Figure 2e,g,h). After normalisation to their original root length and dry weight before salt treatment, the relative growth increase of OE-BnBBX22.A07-2205 plants was more than that of control plant 2205 after salt treatment. The increase of root length and dry weight were 15.79%–17.06% and 38.35%–38.68% in OE-BnBBX22.A07-2205 plants and only 7.92% and 19.95% in control plant 2205, respectively (Figure 2k,l). These results are in agreement with the phenotype in Arabidopsis. To further analyse the role of BnBBX22.A07, we measured physiological indicators that characterise oxidation state. The salt-treated OE-BnBBX22.A07-2205 lines exhibited a higher increase in POD activity of 77.14%-78.3% compared to that of the control plant 2205 (62.22%) (Figure 2j). The MDA contents increased 77.52% in the control plant 2205 but exhibited no obvious increased in the salt-treated OE-BnBBX22.A07-2205 lines (Figure 2i). Taken together, these data indicate that BnBBX22.A07 overexpression promotes salt tolerance in Arabidopsis and B. napus.
3.4 Effects of BnBBX22.A07 on plant morphological growth at various developmental stages
To determine whether BnBBX22.A07 overexpression affects the growth of plants, we preliminarily evaluated the phenotypes under normal conditions in the greenhouse. The results showed that the BnBBX22.A07-OE plants exhibited shorter hypocotyl length compared with the control plant 2205 (Figures S4A,B). After 3 months of growth, the leaf numbers showed significant differences between the BnBBX22.A07-OE lines and the control line 2205 at the seedling stage (Figures S4C,E). During the early flowering stage, the BnBBX22.A07-OE lines exhibited multiple main stems (2–3) from the bottom compared with the control line 2205 (Figure S4D). Most importantly, the BnBBX22.A07-OE lines displayed more branch numbers at the mature stage than those of line 2205 (Figure S4F). These results indicate that overexpression of BnBBX22.A07 not only significantly increased tolerance of plant to salt stress, but also affected plant morphological growth under normal growth conditions.
3.5 BnBBX22.A07 regulates the expression of ROS scavenging-related genes and salt-induced O2.− and H2O2 accumulation
To investigate how BnBBX22.A07 contributes to salt tolerance, we evaluated the transcriptomic dynamics between BnBBX22.A07-OE line (OE-B1) and the control plant 2205 (T) under normal conditions and 200 mM NaCl treatment for 6 h by RNA sequencing. In the control plant 2205, 4306 and 5039 genes were upregulated and downregulated, respectively, in response to salt stress (Figure 3a). However, the NaCl treatment led to a less transcriptomic change in the OE-B1 plants, with 2476 induced and 2888 repressed genes, respectively, less than one half of those in the control plant 2205 (Figure 3a). These results indicate that the OE-B1 plants are less sensitive to salt stress compared with the control plants at the transcriptional level. Under normal conditions, the expression of 1326 and 1799 genes in the OE-B1 plants was up- or downregulated, respectively, compared with the control plants (Figure 3b). Under salt treatments, 1545 genes were upregulated, whereas 2064 genes were downregulated in the OE-B1 compared with the control plants (Figure 3b). Following the 6 h NaCl treatment, a total of 9345 differentially expressed genes (DEGs), including 4306 upregulated and 5039 downregulated, were identified in the control plants, which were named as “salt-inducible genes” (Figure 3b). A total of 818 genes (258 up- and 560 downregulated, red + purple), which accounted for 8.75% of the DEGs, were “salt-inducible genes” in OE-B1 compared with the control plants under normal conditions (T-0h vs OE-B1-0h), whereas only 1.80% (92 up- and 77 downregulated, blue + purple) “salt-inducible genes” were found between OE-B1 and control plants under salt conditions (T-6h vs OE-B1-6h) (Figure 3b). These results revealed that the overexpression of BnBBX22.A07 significantly affected the expression of the majority of the “salt-inducible genes”.
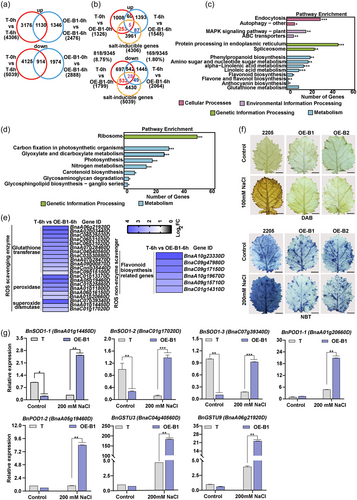
To validate the RNA-seq data, the expression levels of nine randomly selected genes were evaluated using RT-qPCR. As shown in Figure S5, the results were consistent with those of RNA-seq, indicating the validity of the RNA-seq results. To determine which biological functions are affected by the BnBBX22.A07 overexpression under salt stress, we performed KEGG enrichment analysis of DEGs between the salt-treated OE-B1 plants and control plants. The results revealed that 227 upregulated DEGs were significantly enriched in metabolism pathways, genetic information processing, environmental information processing and cellular processes, accounting for 36.56%, 33.92%, 11.45% and 18.06% of the all genes, respectively. The metabolism pathways included linoleic acid metabolism, alpha-linolenic acid metabolism, amino sugar and nucleotide sugar metabolism, flavonoid biosynthesis, flavone and flavonol biosynthesis and phenylpropanoid biosynthesis (Figure 3c), some of which are related to the ROS levels in cells (Dixon et al., 2010; Mittler et al., 2004). Moreover, the downregulated DEGs were mainly found in energy metabolism pathways such as carbon fixation in photosynthetic organisms, glyoxylate and dicarboxylate metabolism, nitrogen metabolism, photosynthesis, carotenoid biosynthesis, glycosaminoglycan degradation, glycosphingolipid biosynthesis-ganglio series and ribosome in salt-treated OE-B1 plants versus control plants (Figure 3d), indicating that BnBBX22.A07 may function in reducing energy loss under salt stress. These results suggested BnBBX22.A07 may play an important role in response to salt stress and ROS homoeostasis. To further explore the influence of BnBBX22.A07 on the transcription levels of ROS-related genes, a total of 27 genes annotated as functioning in ROS scavenging were identified. All of these genes are significantly upregulated in OE-B1 plants compared with the control plants under salt conditions (Figure 3e). Further, some of these genes including superoxide dismutase (SOD), peroxidase (POD), and glutathione S-transferase (GSTU) were selected for expression evaluation using RT-qPCR, which was consistent with those RNA-seq (Figure 3g). These results indicate that BnBBX22.A07 affects the expression of ROS scavenging-related genes at the seedling stage under the NaCl treatment.
To explore the effect of altering the abundance of genes related to ROS scavenging transcripts on the ROS contents, two key ROS, H2O2 and O2.−, were measured in 7-day-old BnBBX22.A07-OE Arabidopsis seedlings pretreated with 100 mM NaCl or 200 mM NaCl. The results showed that the content of H2O2 was almost the same between the BnBBX22.A07-OE and the WT plants after the 200 mM NaCl treatment for 6 h, whereas it was lower in BnBBX22.A07-OE than that in the WT plants after the 100 mM NaCl treatment for 6 h. Meanwhile, the overexpression of BnBBX22.A07 promoted the O2.− accumulation under non-stress conditions in the leaves. The O2.− content was at a lower level in BnBBX22.A07-OE compared with that in the WT plants under the 200 mM NaCl treatment, whereas no difference was observed between the BnBBX22.A07-OE and the WT plants under the 100 mM NaCl treatment (Figure S6A,B). In BnBBX22.A07-OE B. napus lines, consistent results were observed. Overexpression of BnBBX22.A07 increased the O2.− content under normal conditions. Under salt stress, the H2O2 content was reduced at a low NaCl concentration (100 mM NaCl) and the O2.− content was reduced at a high NaCl concentration (200 mM NaCl) (Figure 3f, Figure S6C). Overall, these results implied that BnBBX22.A07 activates the expression of ROS scavenging-related genes and has a role in scavenging O2.− and H2O2 to alleviate salt stress.
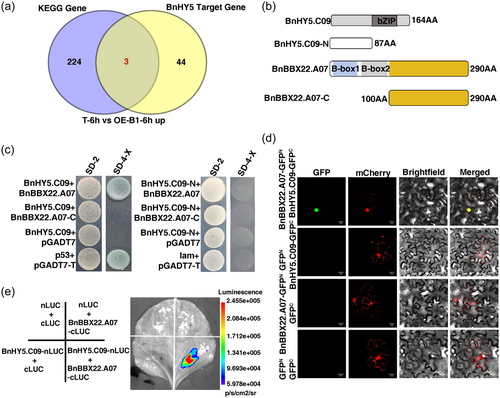
3.6 BnBBX22.A07 interacts with BnHY5.C09
To gain further insights into how BnBBX22.A07 modulates salt tolerance in plants, we next determined the motifs along the 1000 bp promoter region of the 227 upregulated genes between the salt-treated BnBBX22.A07-OE (OE-B1) and the control plants. The results show that no genes were directly regulated by BnBBX22.A07, while 47 genes were detected that might be the target genes of BnHY5 (Figure 4a). Previous studies have shown that BBX22 physically interacts with HY5 which is required for transcriptional regulation in light signalling in Arabidopsis (Datta et al., 2008). Therefore, we speculated BnHY5 might also be required for salt-induced gene expression mediated by BnBBX22.A07. The homologous alleles of BnHY5 on C09 chromosome was selected for further study. To confirm the BnHY5.C09-BnBBX22.A07 interaction, we first examined the trans-activity of BnHY5.C09 in the yeast system. The full-length BnHY5.C09 (BnHY5.C09) and truncated BnHY5.C09 proteins with the basic zipper domain deleted (BnHY5.C09-N) were fused into the pGBKT7 vector (Figure 4b). As shown in Figure 5c, BnHY5.C09 and BnHY5.C09-N did not display trans-activity, at least when expressed in yeast, which is similar to the previous result in Arabidopsis (Ang et al., 1998). Only BnBBX22.A07 containing two B-boxes could interact with BnHY5.C09. BiFC assay also indicated that the interaction between BnBBX22.A07 and BnHY5.C09 occurred specifically in the nucleus (Figure 4d). Furthermore, the LCI assay showed that BnBBX22.A07 could interact with BnHY5.C09 (Figure 4e). Taken together, these observations demonstrated that BnBBX22.A07 interacted with BnHY5.C09 in the nucleus and the basic zipper domain in BnHY5.C09 and the B-boxes in BnBBX22.A07 are essential for BnHY5.C09-BnBBX22.A07 interaction.
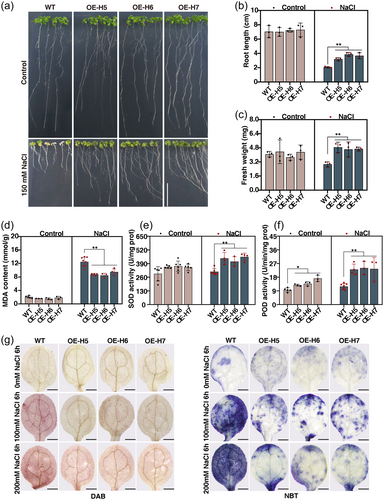
3.7 Overexpression of BnHY5.C09 significantly improves salt tolerance in transgenic arabidopsis by reducing O2.− and H2O2 contents
To investigate whether BnHY5.C09 is involved in plant salt tolerance in Arabidopsis; we overexpressed BnHY5.C09 in Arabidopsis, and independent BnHY5.C09-overexpressing lines (designated as BnHY5.C09-OE) were confirmed by PCR (Figure S7A). Three homozygous BnHY5.C09-OE lines (OE-H5, OE-H6, and OE-H7) with higher BnHY5.C09 transcript abundances were selected for salt tolerance assays (Figure S7B). In addition, BnHY5.C09 was induced by salt stress in both WT and BnHY5.C09-OE plants, with a significantly more pronounced upregulation observed in the BnHY5.C09-OE plants compared to WT plants (Figure S7B). This observation strongly suggests a potential role for BnHY5.C09 in mediating plant responses to salt stress. When 5-day-old seedlings were transferred to ½ MS medium containing 150 mM NaCl for ten days, the BnHY5.C09-OE seedlings exhibited notably superior growth performance, characterised by higher fresh weight and longer root length, in comparison to the WT seedlings under salt stress conditions. However, no discernible disparities in growth were observed between the WT and BnHY5.C09-OE seedlings under control conditions (Figure 5a–c). Consistent with these results, soil-grown BnHY5.C09-OE plants exhibited less growth inhibition with lower MDA contents, higher SOD and POD activity compared to the WT plants upon 200 mM NaCl treatment for 21 days (Figure 5d–f, Figure S8A). Intriguingly, even under control conditions, the POD activity displayed an increase in the BnHY5.C09-OE plants (Figure 5f). These results demonstrate that the overexpression of BnHY5.C09 significantly enhances salt tolerance of Arabidopsis.
To further investigate whether the salt tolerance conferred by the overexpression of BnHY5.C09 is linked to its impact on ROS, the O2.− and H2O2 contents were measured in 5-day-old seedlings pretreated with 100 mM or 200 mM NaCl for 6 h. The results revealed that the BnHY5.C09-OE plants exhibited reduced accumulation of O2.− and H2O2 contents when subjected to salt stress, in contrast to the WT plants. While there were no significant differences between the BnHY5.C09-OE and WT plants when grown under control conditions (Figure 5g, Figure S8B). Collectively, these results indicate that the overexpressing BnHY5.C09 enhances the salt tolerance of Arabidopsis by reducing O2.− and H2O2 contents.
3.8 Interaction between BnBBX22.A07 and BnHY5.C09 enhances the activation of BnHY5.C09 on BnWRKY3.C03
To further identify potential downstream targets of the BnHY5 transcription factor in the regulation of salt tolerance, we performed KEGG pathway analysis, by which 227 upregulated DEGs were identified when comparing the salt-treated BnBBX22.A07-OE plants to the control plants (Figure 4a). Among these genes, three genes were particularly enriched in pathways related to flavonoid biosynthesis and MAPK signalling. Within this subset, we pinpointed two homologous BnWRKY33 genes, namely BnaA03g17820D and BnaC03g21360D, as putative targets of BnHY5 (Figure S9A). To validate this connection, we tested the expression level of WRKY33 in WT and BnHY5.C09-OE Arabidopsis seedlings upon salt exposure. The expression level of WRKY33 in WT seedlings significantly increased under salt stress, while NaCl had a much stronger effect on WRKY33 expression in BnHY5.C09-OE seedlings than in WT seedlings (Figure 6a). The above results indicated that HY5 positively regulated the transcriptional levels of WRKY33 in response to salt stress.
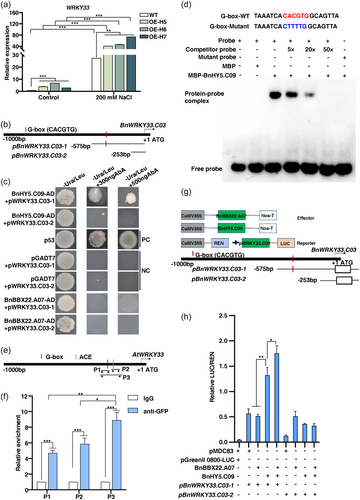
Previous studies have revealed that HY5 regulates the expression of its target genes by binding to G-box or ACGT-containing element (ACE) in the promoter of the target genes (Song et al., 2008). One of the BnWRKY33 genes, BnaC03g21360D (BnWRKY33.C03) with relatively high expression, was selected for subsequent analysis (Figure S9B,C). Promoter analysis revealed the presence of a G-box in the 1000 bp promoter region of BnWRKY33.C03. We performed a yeast one-hybrid (Y1H) assay to investigate whether BnHY5.C09 binds to the BnWRKY33.C03 promoter region, and generated two distinct fragments from the BnWRKY33.C03 promoters (namely pBnWRKY33.C03-1 and -2, Figure 6b). As shown in Figure 6c, only yeast cells co-transformed with pBnWRKY33.C03-1 and AD-BnHY5.C09 displayed normal growth on the SD/-Ura-Leu medium with 300/500 ng/mL AbA. Notably, yeast cells co-transformed with both fragments of the BnWRKY33.C03 promoters and AD-BnBBX22.A07 failed to grow. These outcomes suggest that BnHY5.C09, rather than BnBBX22.A07, specifically interacted with the BnWRKY33.C03 promoter fragment within the yeast system. An electrophoretic mobility shift assay (EMSA) was conducted, which showed that MBP-BnHY5.C09 rather than MBP alone could bind to BnWRKY33.C03 probes with the G-box. However, MBP-BnHY5.C09 could not bind to the mutated probes (Figure 6d). Furthermore, we performed chromatin immunoprecipitation quantitative PCR (ChIP-qPCR) analyses using BnHY5.C09-GFP transgenic Arabidopsis plants. Specific primers were designed for the two core regions (G-box and ACE element) of the AtWRKY33 promoter (P1, P2 and P3, Figure 6e). The results showed that HY5 was significantly enriched on the promoter regions of WRKY33, and the P3 region showed significantly enrichment fold compared to the P1 and P2 regions (Figure 6f). These results revealed that BnHY5.C09 can directly bind to the G-box present in the BnWRKY33.C03 promoter.
To investigate the intricate molecular interplay between BnBBX22.A07 and BnHY5.C09 and its influence on the expression of BnWRKY33.C03, we performed transient dual-luciferase assays in tobacco leaves. The coding sequences of BnBBX22.A07 and BnHY5.C09 driven by the 35S promoter were used as the effectors, and the luciferase (LUC) gene driven by two BnWRKY33.C03 promoter fragments and Renilla luciferase (REN) driven by the 35S promoter were used as the double reporters (Figure 6g). Our results showed that the effector expressing BnHY5.C09 significantly increased LUC activity driven by pBnWRKY33.C03-1, but not pBnWRKY33.C03-2. However, the LUC activity in BnBBX22.A07 reporter construct was not induced by pBnWRKY33.C03. Intriguingly, the introduction of BnBBX22.A07 significantly enhanced the effect of BnHY5.C09 on BnWRKY33.C03 expression (Figure 6h). Together, these results indicate that BnHY5.C09 directly regulates the expression of BnWRKY33.C03, and the presence of BnBBX22.A07 enhances the activation of BnHY5.C09 on BnWRKY33.C03.
3.9 Overexpression of BnWRKY33.C03 enhances salt tolerance in transgenic arabidopsis by reducing O2.− and H2O2 contents
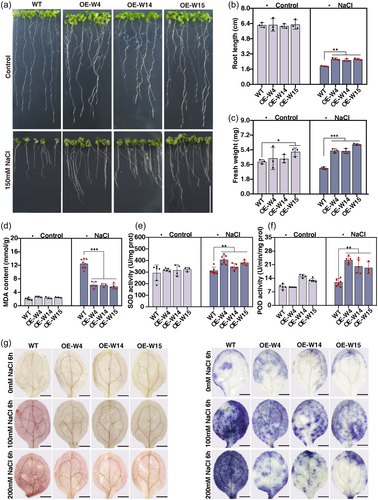
To investigate the role of BnWRKY33.C03 in salt stress tolerance, we overexpressed BnWRKY33.C03 in Arabidopsis, and independent BnWRKY33.C03-overexpressing lines were confirmed by PCR (Figure S10A). Three homozygous BnWRKY33.C03-overexpressing lines (OE-W4, OE-W14, and OE-W15) with higher BnWRKY33.C03 transcript abundances were chosen for salt tolerance assays (Figure S10B). Moreover, BnWRKY33.C03 were strongly upregulated upon salt exposure, and the induction of BnWRKY33.C03 expression was much higher in BnWRKY33.C03-overexpressing (BnWRKY33.C03-OE) plants than that of WT plants (Figure S10B). We first investigated the phenotypes of the WT and BnWRKY33.C03-OE plants under salinity conditions. The BnWRKY33.C03-OE plants exhibited better growth than the WT plants when grown on ½ MS medium containing 150 mM NaCl (Figure 7a). The BnWRKY33.C03-OE plants showed longer root length and higher fresh weight compared with the WT plants under salt stress conditions (Figure 7b,c). We also investigated the phenotypes of soil-grown BnWRKY33.C03-OE plants and WT plants treated with 200 mM NaCl for 21 days. The BnWRKY33.C03-OE plants exhibited less growth inhibition with lower MDA contents, higher SOD and POD activity than the WT plants under salt stress (Figure 7d–f, Figure S11A). We then determined the O2.− and H2O2 contents. DAB and NBT staining revealed that the BnWRKY33.C03-OE plants accumulated less O2.− and H2O2 than the WT plants after salt stress, but not when grown under control conditions (Figure 7g, Figure S11B). Taken together, these results indicate that BnWRKY33.C03 is a positive regulator of tolerance against salt stress by reducing O2.− and H2O2 contents.
3.10 BnBBX22.A07 mediates salt tolerance through WRKY33
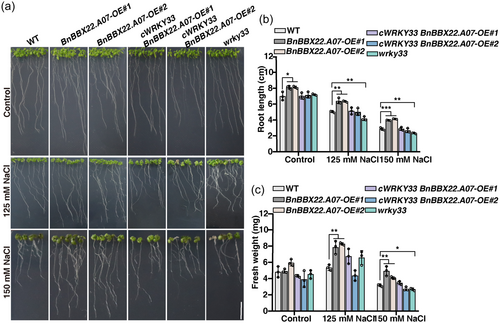
To gain further insights into the regulatory role of WRKY33 in plant salt tolerance mediated by BBX22, we knocked down WRKY33 in BnBBX22.A07-OE Arabidopsis lines using the CRISPR/Cas9 system to create the cWRKY33 BnBBX22.A07-OE lines for subsequent salt sensitivity analyses (Figure S12). The cWRKY33 BnBBX22.A07-OE plants showed a salt-sensitive phenotype comparable to that of WT plants, whereas the salt resistance in the BnBBX22.A07-OE Arabidopsis plants significantly increased compared to the WT plants (Figure 8a). These findings demonstrate that BnBBX22.A07 functions through WRKY33 in the regulation of salt tolerance in plants. The fresh weight and root length were notably increased in the BnBBX22.A07-OE plants compared to the WT plants, but there were no significant differences between the cWRKY33 BnBBX22.A07-OE plants and the WT plants when grown on ½ MS medium containing 125 mM or 150 mM NaCl. Furthermore, the wrky33 mutant seedlings exhibited substantially lower fresh weight and root length values compared with the WT plants under the treatment of 150 mM NaCl. Additionally, the root length of the wrky33 mutant was much lower than that of the WT plants, although there was no significant difference in fresh weight between the WT and the mutant seedlings under the 125 mM NaCl treatment (Figures 8b,c). Taken together, these findings demonstrate that BnBBX22.A07-induced salt tolerance is at least partly dependent on WRKY33.
4 DISCUSSION
Most of the BBX proteins are reported to be related to plant growth and development, such as photomorphogenesis (Gangappa and Botto, 2014). Here, we report a novel BBX protein BnBBX22.A07, isolated from B. napus, which enhances salt tolerance in Arabidopsis and B. napus. These findings broadened our understanding of the biological function of the BBX proteins. The root system of dicotyledons comprises an embryonically derived the primary root and postembryonic lateral roots (Bellini et al., 2014). Root system architecture is highly plastic and can be affected by salinity. The primary root length is one of the traits with the most pronounced phenotypic plasticity in response to salt stress in Arabidopsis (Julkowska et al., 2017). The BnBBX22.A07-OE plants exhibited longer primary roots under salt stress (Figure 2b,c,e,g), suggesting that BnBBX22.A07 affected the root length of plants under salt stress. Salt stress can induce the production of ROS, which results in oxidative damage to DNA, proteins and lipids (Choudhury et al., 2017; Miller et al., 2010). MDA is a product of lipid peroxidation and an important indicator of cell membrane damage (Wang et al., 2022). Under long-term salt treatments, the BnBBX22.A07-OE lines had a significantly lower MDA content compared with the control plant 2205 (Figure 2i), indicating that BnBBX22.A07 may enhance salt tolerance of plants by reducing membrane damage caused by cellular oxidation.
Plants have evolved an antioxidant defence system composed of various enzymatic and nonenzymatic components to maintain the balance between ROS production and ROS scavenging (Hasanuzzaman et al., 2020; Mittler et al., 2004). The improvement of stress tolerance is often related to increased expression or activities of antioxidant enzymes. POD is a major ROS-scavenging antioxidant enzyme of plants (Gill and Tuteja, 2010). Our data show that POD activity was higher in the BnBBX22.A07-OE plants than in the control plants under salt stress (Figure 2j), suggesting that the BnBBX22.A07 has a function to mediate ROS levels. In the present study, we further investigated the role of BnBBX22.A07 in plant salt stress by RNA-seq analysis. We found the upregulated DEGs between the BnBBX22.A07-OE plants and the control plants were mainly enriched in metabolism pathways, some of which is involved in facilitating the ROS homoeostasis in plant cells, such as flavonoid biosynthesis, flavone and flavonol biosynthesis and phenylpropanoid biosynthesis (Figure 3c). Therefore, we focused on metabolic pathways associated with ROS. The O2.− and H2O2 are two key ROS, and salt stress induces the production of O2.− and H2O2 (Hasegawa et al., 2000). The O2.− molecules are transformed rapidly into H2O2 by SOD, then H2O2 is converted to hydroxyl radicals by POD (Sagi and Fluhr, 2006). So, we further analysed the expression levels of genes related to ROS scavenging using RT-qPCR, including BnSOD and BnPOD. These genes were significantly upregulated in the BnBBX22.A07-OE plants under salt stress (Figure 3e,g). Additionally, the BnBBX22.A07-OE plants had significantly lower accumulation of O2.− and H2O2 under salt stress, as compared to the control plants (Figure 2j, Figure 3f). The result is consistent with previous studies of soybean under salt stress (Li et al., 2019). In conclusion, our results indicate that overexpression of BnBBX22.A07 protects seedlings development from the deleterious effects of O2.− and H2O2 by regulating expression of ROS scavenging-related genes and enhancing activities of antioxidant enzymes under salt stress.
Interestingly, BnBBX22.A07 contributed to the inhibition of hypocotyl elongation of B. napus during the budding stage (Figures S4A,B). However, heterologous expression of BnBBX22.A07 did not increase the hypocotyl length in Arabidopsis, which is in agreement with the phenotype between the wild-type and bbx22 mutant seedlings in Arabidopsis under long-day conditions (16 h light/8 h dark) (Datta et al., 2008). The mechanism needs to be further investigated. In production practice, this may prevent the occurrence of longer shoot seedlings. Additionally, overexpression of BnBBX22.A07 could significantly increase biomass and leaf numbers of B. napus at the seedling stage (Figure 2h,l, Figure S4C,E), which are critical for improving plant population structure and photosynthetic efficiency, thereby increasing crop yields (Li et al., 2016). Shoot architecture is a complex trait which is crucial for B. napus yield. The branch number is a key factor affecting plant architecture and yield. In our study, overexpression of BnBBX22.A07 significantly increased branch numbers at the mature stage (Figure S4F), indicating that BnBBX22.A07 may be a potential candidate for creating increased-branching B. napus germplasms.
However, the absence of functional WRKY33 led to reduced salt tolerance in BnBBX22.A07-OE Arabidopsis lines (Figure 8). These findings collectively emphasise that the BnBBX22.A07-mediated salt tolerance, at least in part, depends on the presence and functionality of WRKY33. Moreover, it's noteworthy that BnBBX22.A07 alone did not exhibit the capacity to directly bind to the promoter and activate the expression of BnWRKY33.C03 (Figure 6c,h). Rather, BnBBX22.A07 played a pivotal role in enhancing the ability of BnHY5.C09 to activate BnWRKY33.C03 transcription (Figure 6h). Collectively, our results indicate that BBX22 was required in the HY5-mediated expression of the downstream target gene WRKY33.
In conclusion, our results identified a positive regulatory mechanism that confers salt stress tolerance in B. napus by BnBBX22.A07, BnHY5 and BnWRKY33.C03. We found that BnBBX22-07A acts as a negative regulator in the high salinity stress response. In addition, BnHY5, as a transcriptional co-factor of BnBBX22-07A, interacted with BnBBX22-07A and regulated the expression levels of BnWRKY33. However, whether the absence of BnHY5.C09 affects the expression of BnWRKY33.C03 by BnBBX22.A07, thereby affecting the salt tolerance of plants, requires further detailed investigation. Notably, overexpression of BnBBX22-07A affected plant morphological growth of B. napus under normal conditions. Whether BnBBX22.A07 increases or does not affect B. napus yield requires further field experiments. Our findings offer a promising future for breeding salt-resistant transgenic crops.
ACKNOWLEDGEMENTS
We thank Researcher Wei Hua and Researcher Xiaoling Dun (Oil Crops Research Institute, Chinese Academy of Agricultural Sciences) for their guidance on yeast two-hybrid assay and rapeseed transformation, respectively. This work was supported the funding of Biological Breeding-National Science and Technology Major Project (2022ZD04010), Open Project of the Key Laboratory of Biology and Genetic Improvement of Oil Crops, Ministry of Agriculture and Rural Affairs, P. R. China (KF2023004), the Key Research and Development Projects of Yangling Seed-industry Innovation Center (Ylzy-yc2021-01), the Open Project Program (CSBAA202204) of State Key Laboratory of Crop Stress Biology for Arid Areas, NWAFU, Yangling, Shaanxi, China, and the Key Project for “Two Chain” Integrated Crop Breeding of Shaanxi, China (2021LLRH-07).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [the Sequence Read Archive (SRA) database of National Center for Biotechnology Information (NCBI)] at [https://www.ncbi.nlm.nih.gov/bioproject/PRJNA876751/], reference number [PRJNA876751].




