Plant growth-promoting abilities of Methylobacterium sp. 2A involve auxin-mediated regulation of the root architecture
Abstract
Methylobacterium sp. 2A, a plant growth-promoting rhizobacteria (PGPR) able to produce indole-3-acetic acid (IAA), significantly promoted the growth of Arabidopsis thaliana plants in vitro. We aimed to understand the determinants of Methylobacterium sp. 2A–A. thaliana interaction, the factors underlying plant growth-promotion and the host range. Methylobacterium sp. 2A displayed chemotaxis to methanol and formaldehyde and was able to utilise 1-aminocyclopropane carboxylate as a nitrogen source. Confocal microscopy confirmed that fluorescent protein-labelled Methylobacterium sp. 2A colonises the apoplast of A. thaliana primary root cells and its inoculation increased jasmonic and salicylic acid in A. thaliana, while IAA levels remained constant. However, inoculation increased DR5 promoter activity in root tips of A. thaliana and tomato plants. Inoculation of this PGPR partially restored the agravitropic response in yucQ mutants and lateral root density was enhanced in iaa19, arf7, and arf19 mutant seedlings. Furthermore, Methylobacterium sp. 2A volatile organic compounds (VOCs) had a dose-dependent effect on the growth of A. thaliana. This PGPR is also able to interact with monocots eliciting positive responses upon inoculation. Methylobacterium sp. 2A plant growth-promoting effects can be achieved through the regulation of plant hormone levels and the emission of VOCs that act either locally or at a distance.
1 INTRODUCTION
Interactions with microbes in the soil are crucial to the health and productivity of plants. The rhizosphere nurtures a diverse microbial community, which includes microorganisms that have the potential to facilitate plant growth. Epiphytes live near or on plant tissue while endophytes reside within plant tissues in leaves, roots, or stems. Endophytes colonise the internal plant tissues without causing any type of harm to the host plant. Obligate endophytes depend on the metabolism of plants for survival, whereas facultative endophytes live outside the host body during a certain stage of their life cycle and are mostly associated with plants from its neighbouring soil environment and atmosphere (Gouda et al., 2016).
Plant growth-promoting rhizobacteria (PGPR) associated with both monocot and dicot roots, augment plant growth through a variety of direct and indirect mechanisms (Vacheron et al., 2013; Vishwakarma et al., 2020). One prominent way in which PGPRs exert their influence is by modifying the architecture of the root system, achieved through the production of phytohormones such as indole-3-acetic acid (IAA). By modulating auxin signalling pathways, PGPR can foster the proliferation of lateral roots (LRs) and the development of root hairs (RHs), which eventually improve nutrient uptake efficiency and enhance overall plant vigour (Grover et al., 2021). Additionally, PGPRs that harbour molecules such as 1-aminocyclopropane carboxylase (ACC) deaminase can limit the production of the plant growth-inhibitory ethylene, increasing the length of roots and aerial structures (Orozco-Mosqueda et al., 2020).
Once a symbiotic relationship is established, PGPRs can exhibit a role as biocontrol agents, effectively withstanding pathogen attacks. This beneficial effect can be achieved through local antagonism as well as the induction of systemic resistance (ISR), which involves the activation of jasmonic acid (JA), ethylene, or salicylic acid (SA) signalling pathways either individually or in combination (Beneduzi et al., 2012). PGPRs have also been shown to produce volatile organic compounds (VOCs) that can stimulate ISR in plants, equipping them with enhanced defence mechanisms against pests and diseases (Zhu et al., 2022). VOCs act as chemical signals that impact on other physiological and biochemical processes in plants, including seed germination, root development, and nutrient uptake. VOCs emitted by PGPR have demonstrated the ability to induce changes in root architecture that positively impact plant biomass (Fincheira et al., 2021).
Methylobacterium species are pink-pigmented bacteria of particular interest within the PGPR group that can colonise a wide range of environments and are among the most abundant bacterial genera associated with the phyllosphere of plants (Knief et al., 2010; Vorholt, 2012). These facultative methylotrophic bacteria can utilise a range of single-carbon compounds such as methanol, methylamine, and formaldehyde as energy and carbon sources (Chistoserdova et al., 2009; Patt et al., 1976). Their prevalence in the phyllosphere can be attributed to their methylotrophic capabilities. Methanol is primarily generated as a byproduct of plant pectin metabolism during cell wall synthesis (Fall & Benson, 1996), but the sloughing of root cells also deposits pectins into the rhizosphere. Active metabolism of methanol has been observed in the rhizosphere (Knief et al., 2012), and 14C-methanol tracing of alive plant material revealed similarly high rates of methanol consumption in the rhizosphere and phyllosphere (Kanukollu et al., 2022). The oxidation of methanol to formaldehyde is catalysed by pyrroloquinoline quinone (PQQ)-dependent methanol dehydrogenases (MDHs), MxaF and XoxF, which utilise calcium (Ca2+) or lanthanide (Ln) as cofactors, respectively (Keltjens et al., 2014; Skovran et al., 2019).
Methylobacterium sp. 2A inoculation in potato and Arabidopsis thaliana plants alleviated salt stress and reduced the size of necrotic lesions caused by Phytophthora infestans infection in potato plants. It produced high amounts of IAA, and genome sequencing revealed metabolic pathways associated with its plant growth-promoting (PGP) capabilities (Grossi et al., 2020). In this study, we sought to further understand A. thaliana–Methylobacterium interaction aiming to elucidate how the bacteria can influence plant growth and root architecture. Moreover, we aim to evaluate if the host range of this PGPR extends to monocots.
2 MATERIALS AND METHODS
2.1 Bacterial strains and growth conditions
Methylobacterium sp. 2A wild type (WT) and its transformant were stored at −80°C in R2A broth containing 20% glycerol. Before use, they were streaked on R2A agar (Difco™), LBNS (Luria-Bertani without NaCl), or in Mineral Medium (MM) containing 0.5% methanol (MM methanol) plates and incubated for 2–3 days at 28°C. Single colonies were then transferred to 5 mL of R2A, LBNS, MM methanol, or DF (Dworkin & Foster, 1958) minimal salts medium supplemented or not with ACC and grown with agitation at 180 rpm for the indicated times.
2.2 Chemotaxis assay
Chemotaxis towards methanol and formaldehyde was evaluated using the capillary plug assay technique (Tani et al., 2023). A single colony of Methylobacterium sp. 2A grown overnight on MM methanol at 28°C was suspended in 1 mL of HEPES buffer (20 mM, pH 7.0). After 2-h incubation at room temperature, the activated cell suspension (170 µL) was transferred to a FastWell™ chamber (1 mm thick and 20 mm diameter; Grace Biolabs) on a glass slide. A glass capillary (10–20 µm diameter) containing methanol or formaldehyde (2% w/v) solidified with 1.5% agarose (NuSieve™; Lonza Japan), was immersed in the cell suspension and a cover slip was placed on top. Bacterial cells migrating towards the capillary were observed and recorded with an Olympus microscope (20X). The cell number in the frames was counted using Fiji software (Schindelin et al., 2012).
2.3 Generation of Methylobacterium sp. 2A-mVenus
Methylobacterium sp. 2A was transformed with pAT02m-mVenus plasmid that contains mVenus gene expressed under the methanol-induced mxaF promoter and kanamycin (KAN) resistance gene (Tani et al., 2023). Escherichia coli S17-1 cells were transformed with pAT02m-mVenus and a Bio-Rad Gene Pulser (Bio-Rad) according to the manufacturer instructions. Transformants were selected in LB agar containing KAN (25 mg/L) and colonies fluorescent on UV light were cultured on LB broth with KAN for 24-h. Conjugation with Methylobacterium sp. 2A was conducted in R2A plates. After successive cultures, pink colonies fluorescent on UV light were selected and mVenus production was confirmed with a fluorescence microscopy (BZ-X800; KEYENCE) (Supporting Information: Figure S1A, micrograph). From now onwards, the non-transformed strain will be mentioned as 2A-WT and the transformed one as 2A-mVenus.
To assess the relative burden of the mVenus-expressing plasmid, 2A-WT and 2A-mVenus were grown during the indicated times in a 96-well plate following previously established protocols (Masuda et al., 2018). Each well was filled with 200 µL of MM methanol with or without KAN, or MM supplemented with different carbon sources. Under the conditions tested, the growth rates and viability of both strains were identical. WT cells were not viable in the presence of KAN. Fluorescence of transformed cells was still evident in MM supplemented with methanol and other carbon sources (glucose, glycerol, and succinate) after 26 or 10 days of growth, respectively (Supporting Information: Figure S1).
2.4 Plant material and inoculation with Methylobacterium sp. 2A
In this study, we used various plant species and genotypes: A. thaliana Columbia (Col-0) WT, yucQ (quintuple yucca mutant; Chen et al., 2014), msg2/iaa19 (indole-3-acetic acid inducible 19; Tatematsu et al., 2004), nph4/arf7 and arf19 (auxin-responsive factor 7 and 19; Harper et al., 2000; Wilmoth et al., 2005) mutants, as well as A. thaliana or Solanum lycopersicum L, cv. Micro-tom containing the synthetic auxin-responsive DR5 promoter (Ulmasov et al., 1997) fused to the green fluorescent protein (GFP) and/or β-glucuronidase (GUS) reporter genes (DR5:GFP and DR5:GUS). We also used the following monocot seeds: rye (var. Don Norberto INTA), wheat (cv. Buck Saeta; Buck Semillas S.A., Bs. As., Argentina), rice (cv. Nipponbare), and barley (var. Josefina INTA).
A. thaliana seeds underwent disinfection with 70% and 96% ethanol, while tomato seeds were disinfected with 70% ethanol followed by 10% sodium hypochlorite treatment. Wheat, barley, and rye seeds were disinfected using a 1:40 solution of 10% chlorite, and rice seeds underwent disinfection with 70% ethanol followed by 3% sodium hypochlorite treatment at 80°C.
A. thaliana and tomato seeds were vernalized for 2 days at 4°C and were then allowed to grow under sterile conditions in half-strength MS medium (1/2 MS; Murashige & Skoog, 1962) with 0.8% (w/v) agar. For tomato seeds, 3% (w/v) sucrose was added to the medium. Plants were cultured in a growth chamber under a 16-h light photoperiod at 21–23°C for the indicated times. A root-inoculation approach was used for 1-week-old A. thaliana or tomato plants using a suspension of 2A-WT or 2A-mVenus (0.05 OD600 units in 0.85% NaCl) or sterile saline solution (0.85% NaCl). At the indicated days post-inoculation (dpi), A. thaliana plants were photographed and the number of LR and primary root length were quantified using Fiji software (Grossi et al., 2020). The LR density was calculated as the ratio of the number of LR to the primary root length. The gravitropic response of primary roots of A. thaliana WT and yucQ mutants, whether inoculated with Methylobacterium sp. 2A or not, was stimulated by reorienting the petri dishes by 90° as described in Sun et al. (2008). Pictures were taken 24 h later. Root curvature was measured as the angle of deviation from the initial straight line of the seedling root. Growth length and curvature of roots were measured using ImageJ software. At least 15 seedlings of each condition were used.
After inoculation with 2A-mVenus, A. thaliana WT roots were washed three times with sterile water to remove surface-attached bacteria. The plant cell walls were stained with propidium iodide (PI; 1 mg/mL in water) solution for 2 min. The samples were observed under a ZEISS LSM 880 confocal laser scanning microscope equipped with a C-Apo 40 X NA 1.2 water immersion objective with the 488 nm (mVenus) or the 543 nm (PI) laser excitation. Samples were imaged as Z-stacks and reconstructed with Fiji software. Videos of 3D maximum intensity projections were generated in Zeiss Zen Blue 2.6 software.
A set of the inoculated and non-inoculated roots were homogenised in 1 mL of 0.85% NaCl using a pestle. Another set was surface-sterilised with 70% ethanol, followed by washes with water to remove surface-attached bacteria, and then homogenised in 1 mL of 0.85% NaCl. To ensure complete bacterial removal, 10 µL of the final rinse was plated as a control. Colony forming units (CFU) per gram of root were counted after 4 days of growth on R2A plates.
After rinsing with sterile deionized water, monocot seeds were incubated with a suspension of 2A-WT cells (40 mL, 0.1 OD600 units) or with sterile 0.85% NaCl in an orbital shaker for 30 min. Seeds were dried on Whatman filter paper for 30 min and placed in humid chambers for germination. Following a light pulse, the germination process occurred in darkness. The fresh weight (FW), hypocotyl length, and number of roots were recorded in wheat, barley, and rye seedlings at 4 dpi and in rice seedlings at 7 dpi. Experiments were performed three times using 50 seeds for each treatment. Barley seedlings were transferred to pots containing 250 mL of substrate TS 1 fine + GreenFibre® (Klasmann-Deilmann GmbH) and grown for an additional 15 days. Shoot length was measured every 5 days, and after harvest, root length, dry weight, and FW were determined.
2.5 Plant hormone analysis of A. thaliana plants inoculated with Methylobacterium sp. 2A-WT
We extracted auxins (IAA), abscisic acid (ABA), jasmonic acid (JA), JA-isoleucine (JA-Ile), cytokinins (CK: tZ and iP), and salicylic acid (SA), from 1-week-old A. thaliana WT plants that were inoculated with Methylobacterium sp. 2A. In addition, we evaluated IAA levels in the roots of inoculated WT and yucQ mutant plantlets. In both cases, non-inoculated plants were used as controls. Pictures were taken daily during 5 dpi, and the root length and LR number were counted in each plant using Fiji software. Hormones were purified by solid-phase extraction and quantified using liquid chromatography-electrospray tandem mass spectrometry (LC-ESI-MS/MS) as described in Matsuura et al. (2019). Samples (120 mg) were frozen in liquid nitrogen and ground with zirconium beads in 4 mL of 1% acetic acid in acetonitrile/water (4:1) containing the following stable isotope-labelled compounds: D2-IAA (20 pg/μL, CDN Isotopes Inc.); D6-ABA (20 pg/μL), D2-GA1 (20 pg/μL), D2-GA4 (20 pg/μL), D4-SA (200 pg/μL), D5-tZ (1 pg/μL), D3-DHZ, and D6-iP (1 pg/μL) (Olchemim s.r.o.); D2-JA (20 pg/μL), [13C6]IAA (20 pg/μL), and [13C6]Ja-Ile (1 pg/μL) (Tokyo Chemical Industry Co. Ltd.), and incubated at 4°C for 1-h. After centrifugation (3000g for 10 min at RT), supernatants were collected, and pellets were subjected to re-extraction with 4 mL of the extraction solvent without internal standards and centrifuged. Supernatants were combined, concentrated to 1 mL with the centrifugal concentrator, and applied to an Oasis HLB 1 cc cartridges (30 mg; Waters Corp.) equilibrated with 1% acetic acid. After washing with 1% acetic acid, plant hormones were eluted with acetonitrile/water (4:1) containing 1% acetic acid. The extracts were then applied to Oasis MCX 1 cc cartridge (30 mg; Waters Corp.) pre-equilibrated with 1% acetic acid. After washing with 1% acetic acid, acidic fractions containing IAA, ABA, JA, Ja-Ile, and SA were eluted with 1% acetic acid in acetonitrile/water (4:1). From this eluate, 200 μL were collected, evaporated to dryness, and used for SA quantification. The MCX cartridge was washed further with 5% (v/v) ammonium water. The basic fraction containing tZ and iP was eluted with 5% ammonia in acetonitrile/water (2:3), evaporated to dryness, and used to quantify CKs. The remaining acidic aqueous portion was then applied to a pre-equilibrated Oasis WAX 1 cc cartridge (30 mg; Waters Corp.). After washing, the acidic hormones were eluted with 1% acetic acid in acetonitrile/water (4:1), dried, and used to determine IAA, ABA, JA, and Ja-Ile. Quantitative analysis of analytes was performed using LC-ESI-MS/MS (triple quadrupole mass spectrometer with 1260 high-performance LC, G6410B; Agilent Technologies Inc.) equipped with a Zorbax Eclipse XDB-C18 column (Agilent Technologies Inc.). Mass-to-charge ratio (m/z) transitions of analytes were previously described in a report by Tsukahara et al. (2015). The contents of plant hormones were normalised to the FW of plantlets.
2.6 DR5:GUS and DR5:GFP activity
The spatial and temporal patterns of gene expression driven by the DR5 promoter in root samples of A. thaliana or tomato plants were visualised using GUS histochemical activity or GFP fluorescent intensity with a Leica DM 2500 LED microscope (Leica) at 10 dpi. GUS activity was also determined through fluorometric analysis as described in Grossi et al. (2022). Protein quantification was performed with the Bradford method.
2.7 Plant–bacterial dual growth experiments
A 30 μL suspension of 2A-WT cells (1.2 OD600 units in 0.85% NaCl) or a control solution (0.85% NaCl) were spotted at 4 cm from the primary root tip of each 6-day-old A. thaliana WT grown on MS plates. Five days later, detailed root images were captured with a magnifying glass.
VOCs exchange experiments were conducted using two-compartment dishes. In one compartment, six sterilised A. thaliana WT seedlings were placed on 1/2 MS agar, while in the other compartment, a saturated solution (0.8 OD600 units of washed cells in 0.85% NaCl) of 2A-WT or E. coli DH5α was either spotted (10 µL = 1 drop, 30 µL = 3 drops and 90 µL = 9 drops), or spread on R2A, LBNS, or MM methanol agar. Controls were performed using sterile 0.85% NaCl. Each treatment was replicated three times. The plates were sealed with parafilm and incubated in the plant growth chamber for 14 days. Images were captured after 5, 10, and 14 days of growth, and the total rosette diameter and root length were quantified using Fiji software. On Day 14, the plants were harvested, and the FW of each plate was determined.
2.8 Statistical analysis
Statistical analysis was performed by two-way analysis of variance followed by Tukey's HSD test (p < 0.05) or by T-test as indicated in the figures, using GraphPad Prism 8 version 8.4.3 (GraphPad Software).
3 RESULTS
3.1 Methylobacterium sp. 2A genome harbours the genes responsible for methylotrophy and displays chemotaxis towards methanol and formaldehyde
As other members of the genus, Methylobacterium sp. 2A is able to grow in MM methanol (Supporting Information: Figure S1). A search in its genome confirmed the presence of 15 genes responsible for methanol oxidation via Ca2+-dependent MDH in the order mxaWFJGIRSACKLDEHB (Figure 1a, upper panel). mxbDM genes, which are necessary for mxaF expression, were also detected. Additionally, we identified xox cluster encoding Ln-dependent XoxF-type MDH in which xoxF is clustered with cytC, and xoxJ genes (Figure 1a, lower panel).
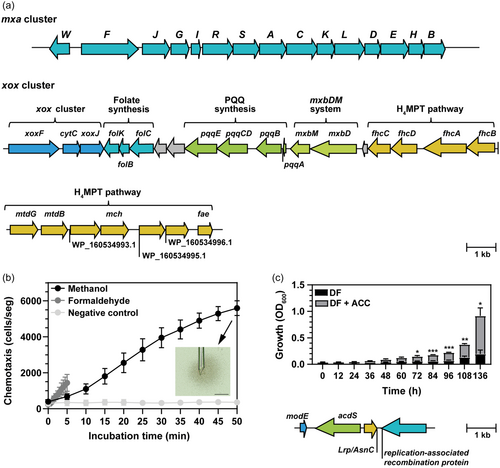
Methylobacterium sp. 2A exhibited chemotactic activity towards methanol (1.08 ± 0.61 cells/s, n = 5) and formaldehyde (4.43 ± 2.43 cells/s, n = 3) (Figure 1b). Both rates were estimated during the first 5 min of the assay; notably, the chemotactic rate towards methanol increased 2.5-fold between 10 and 30 min of exposure to methanol (2.65 ± 0.44 cell/s) showing a biphasic velocity curve. We identified homologues of M. aquaticum 22A MtpB and MtpC genes in Methylobacterium sp. 2A genome with 63.37% and 72.86% identity, respectively. These genes encode the methyl-accepting proteins (MCPs) for methanol-taxis (methylotaxis) and are suggested to be responsible for methylotaxis in the presence and absence of LnCl3, respectively. On the contrary, MtpA gene which is responsible for formaldehyde taxis was not found (Tani et al., 2023). Since plants release methanol, methylotaxis could be critical for plant localisation.
Methylobacterium sp. 2A was able to grow when DF minimal medium was supplemented with ACC as the only nitrogen source indicating that its ACC deaminase encoded by acdS (1011 bp) is active. As in other bacteria, acdS is in opposite orientation to Lrp/AsnC (465 bp) family transcriptional regulator, which is a putative leucine-responsive regulator of the ACC deaminase gene (acdS). Both genes are separated by an intergenic spacer of 226 bp and flanked by modE and a replication-associated recombination protein (Figure 1c). The inoculated A. thaliana plants present a significant increase in the primary root length at 5 dpi compared to controls (Supporting Information: Figure S2) and this could be attributed to ACC deaminase activity.
3.2 Methylobacterium sp. 2A colonises A. thaliana roots
Inoculation of A. thaliana WT plantlets with 2A-mVenus promoted root length and increased the number of LRs (Supporting Information: Figure S2), as previously observed with the WT strain (Grossi et al., 2020). At 3 dpi, the bacteria proliferated along the growing roots of A. thaliana; pink-pigmentation due to bacterial colonisation was visible on the primary roots under white light, while bacterial fluorescence was evident under UV light (Figure 2a). The primary root and LRs were surrounded by a matrix containing motile bacterial cells (Figure 2b and Supporting Information: Video S1). Microscopy analysis of surface-sterilised roots revealed that bacteria was attached to the primary root and RHs as single cells or clusters (Figure 2c; Supporting Information: Figure S3). At 7 dpi, confocal analysis revealed that 2A-mVenus colonises the apoplast of cells in the primary root (Figure 2d and Supporting Information: Video S2) and the base of RHs (Supporting Information: Video S3).
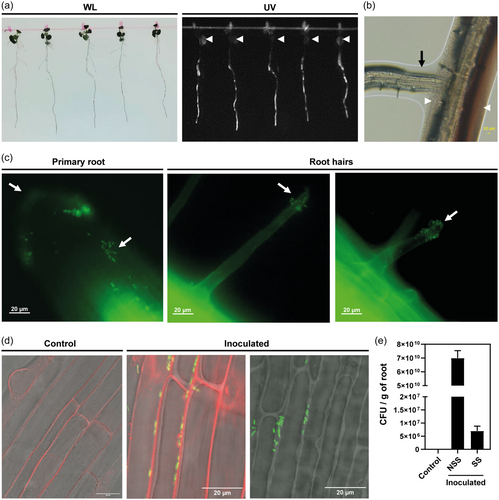
At 7 dpi, 7 × 1010 ± 1.5 × 1010 CFU/g of root were recovered from the macerated root tissue of the inoculated plants. However, only 1 × 107 ± 4.19 × 106 CFU/g were recovered when the roots were previously surface-sterilised. On the other hand, no colonies were recovered from the uninoculated control plants (Figure 2e). The use of fluorescent-tagged bacteria demonstrated that Methylobacterium sp. 2A effectively colonises the roots of in vitro A. thaliana plants. Our results suggest that it is mainly a rhizospheric bacteria, but a small subset of bacterial cells inhabit inner tissues of the primary root, indicating its facultative endophytic nature.
3.3 Inoculation with Methylobacterium sp. 2A modified phytohormone levels in A. thaliana plantlets
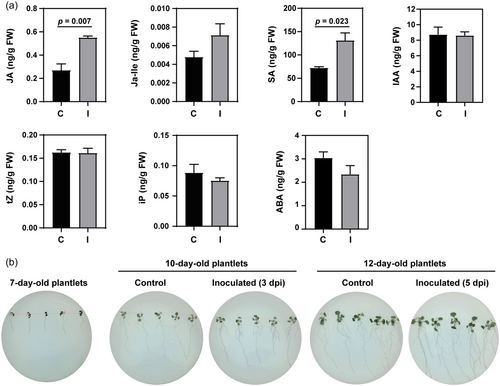
The total content of JA and SA was significantly higher in A. thaliana plantlets inoculated with Methylobacterium sp. 2A compared to controls, and an increase in JA-Ile concentration was also evidenced. Inoculated plants exhibited unaltered CK (tZ and Ip) levels, while ABA concentration decreased (24%) (Figure 3a). The inoculation did not modify plant IAA levels, which was unexpected given that Methylobacterium sp. 2A can synthesise IAA (Grossi et al., 2020) and the inoculated plants exhibited an increase in the number of LRs (Supporting Information: Figure S2) compared with controls.
3.4 IAA secreted by Methylobacterium sp. 2A modulates root architecture
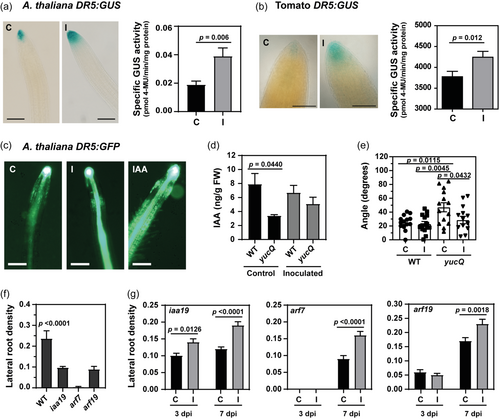
Our previous data using Salkowski's reagent confirmed that Methylobacterium sp. 2A can synthesise IAA (Grossi et al., 2020). Thus, we examined if A. thaliana DR5:GUS and DR5:GFP and the tomato DR5:GUS plants were able to sense it. The DR5 activity pattern mirrors the IAA accumulation pattern (Benková et al., 2003). DR5 activity was detected in the root tips of both species, and the stain was intensified upon inoculation. Fluorometric analysis using whole roots confirmed the increase in GUS activity in A. thaliana and tomato-inoculated plants (Figure 4a,b). This result was further confirmed by comparing control versus inoculated A. thaliana DR5:GFP plants under the microscope. The fluorescence intensity was much higher in the inoculated plants, resembling the levels observed in plants treated with IAA. However, in the latter group, the fluorescent area appeared to be larger (Figure 4c). YucQ mutants have partial auxin deficiency and develop short and agravitropic roots (Chen et al., 2014). In fact, IAA levels measured in the roots of these mutants were significantly lower than in WT roots under control conditions (Figure 4d). However, IAA levels increased when yucQ roots were inoculated (Figure 4d) possibly due to the IAA produced by Methylobacterium sp. 2A. In addition, the inoculation partially restored the gravitropic response in the mutants; curvature measurements indicated that the angle of deviation in inoculated yucQ plants was similar to that of WT plants, and significantly smaller than in non-inoculated yucQ mutants (Figure 4e). The auxin-regulated protein MSG2/IAA19 and the transcriptional activators ARF7 and ARF19 regulate LR formation (Tatematsu et al., 2004; Wilmoth et al., 2005); msg2/iaa19, nph4/arf7, and arf19 mutant plants exhibited defects in LR formation (Figure 4f) but were not entirely insensitive to IAA. At 7 dpi, inoculation with Methylobacterium sp. 2A led to a significant increase in LR density in all mutants (Figure 4g).
Several findings show the pivotal role of auxin in controlling RH morphogenesis (Vissenberg et al., 2020). In A. thaliana roots inoculated with Methylobacterium sp. 2A, RH growth and number were enhanced (Supporting Information: Figure S4A). Moreover, if the bacteria was spotted at a 4 cm distance from A. thaliana plantlets, when the root grew close to the colony (<1 cm) there was a significant increase in both the length and number of RHs within that specific section of the root. Conversely, the presence of E. coli DH5α did not affect these parameters, which remained comparable to the uninoculated control (Supporting Information: Figure S4B). These results indicated that Methylobacterium sp. 2A, can positively influence root hair growth. Altogether, these findings suggest that Methylobacterium sp. 2A modulates root architecture through the production of IAA.
3.5 VOCs from Methylobacterium sp. 2A impacted A. thaliana development in a dose–responsive manner
Bacterial VOCs play an important signalling role in plant-bacterial interaction; VOCs produced by certain bacterial strains have been shown to result in up to a fivefold increase in plant biomass or in plant death (Bailly & Weisskopf, 2012). The effects of Methylobacterium sp. 2A VOCs were highly dependent on the cultivation medium, the inoculum quantity, and the exposure period. On Day 5, no significant differences in the rosette diameter and root length were observed between the control and bacterial plates (data not shown). On Day 10, a significant increase was observed in the rosette diameter of plants exposed to Methylobacterium sp. 2A VOCs compared with controls regardless of the medium (Figure 5a). On the other hand, a positive effect in root length was only observed in R2A and MM methanol, while a significant decrease occurred in LBNS (Figure 5a). On Day 14, an increase in rosette diameter was detected with a 30 µL inoculum in R2A and with 10 µL and 30 µL inoculum in MM methanol. On the contrary, VOCs negatively impacted A. thaliana development when a high inoculum (90 µL or spread) of Methylobacterium sp. 2A was grown in LBNS; the seedlings ceased to grow and became chlorotic. The VOCs produced by Methylobacterium sp. 2A promoted A. thaliana biomass when cultured in R2A in all concentrations; a 3.8-fold increase in FW was observed with a 30 µL inoculum. However, only a slight increase was evidenced with 10 µL of the cell suspension in LBNS and MM methanol, while greater cell density exerted a negative effect (Figure 5a and Supporting Information: Figure S5) suggesting that Methylobacterium sp. 2A VOCs act in a dose-dependent way. We assessed the growth of Methylobacterium sp. 2A in liquid R2A, LBNS and MM methanol media to evaluate if the differential interaction with plants correlates with any growth defect of the bacterium in these media. However, Methylobacterium sp. 2A strived in all three media (Figure 5b).
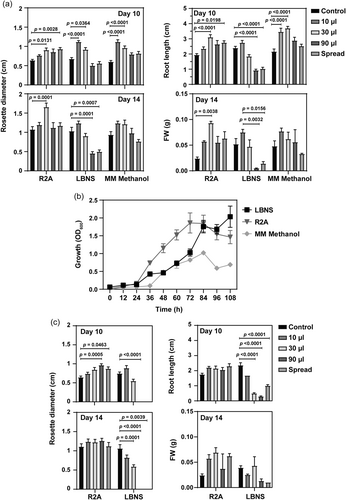
On the other hand, E. coli DH5α VOCs had a positive effect on rosette diameter at Day 10 only when inoculated (90 µL or spread) in R2A medium. VOCs negatively affected rosette diameter or root length in LBNS media (Figure 5c and Supporting Information: Figure S5). The FW determined on Day 14 confirmed these results (Figure 5c).
3.6 Methylobacterium sp. 2A enhanced growth and modulated root architecture in monocot seedlings
Methylobacterium sp. 2A has demonstrated PGP effects in dicots, such as A. thaliana and potato (Grossi et al., 2020). When inoculated in monocot seeds, Methylobacterium sp. 2A slightly increased the germination rate in the four species tested (rye: 89.1% in inoculated (I) vs. 84.2% in control (C) seedlings; barley 83.3% I vs. 79.2% C; wheat: 98.8% I vs. 98.0% C, and rice: 91.1% I vs. 84.5% C). FW and hypocotyl length significantly increased in the inoculated rye and wheat plantlets, hypocotyl length increased in barley, rye and wheat, while the number of roots was enhanced in rice, barley and rye compared to the controls (Figure 6a). Moreover, root length measurements in barley plantlets revealed a significant increase in the inoculated group compared to the controls (Figure 6b). These plantlets were then transferred to pots and grown in the greenhouse. Shoot length was measured at 10, 15, and 20 dpi, showing a significant increase in the inoculated plants compared to the control group that was consistently observed at the three-time points (Figure 6c).
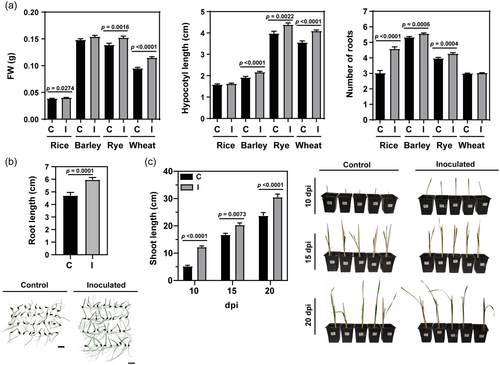
These findings suggested that Methylobacterium sp. 2A has the potential to promote shoot growth and induce modifications in root architecture in monocots as well.
4 DISCUSSION
Methanol consumption occurs both in the phyllosphere and in the rhizosphere (Gouda et al., 2016; Knief et al., 2012). Producing an arsenal of enzymes that enable methylotrophy could confer a significant metabolic advantage for Methylobacterium sp. 2A both within a nutrient-poor environment like the phyllosphere or in a highly competitive one like the rhizosphere (Turner et al., 2013) as was shown for M. extorquens AM1 (Sy et al., 2005). Methylobacterium sp. 2A displayed chemotactic activity to methanol and formaldehyde (Figure 1b). Its genome includes cheY, cheV, the cheAWRB gene cluster, 31 flagellar-related genes, and 52 genes encoding MCPs (Grossi et al., 2020), among which MtpB and MtpC were supposed to be responsible for methylotaxis. However, MtpA, responsible for formaldehyde taxis, was absent in Methylobacterium sp. 2A genome. The high chemotaxis activity for formaldehyde observed may suggest another type of MCP for formaldehyde taxis encoded in its genome. Methylotaxis could be crucial to locate the plants and to migrate toward methanol-available niches, as already shown in M. aquaticum 22A (Tani et al., 2023).
Methanol is a byproduct of plant pectin metabolism during cell wall synthesis (Fall & Benson, 1996) and it was shown that M. extorquens consumes methanol during colonisation (Sy et al., 2005). Interestingly, Methylobacterium sp. 2A-mVenus was able to colonise the apoplast of cells in the primary root (Figure 2d and Supporting Information: Video S2), allowing us to propose the endophytic nature of this strain. Methylobacterium sp. 2A is a facultative endophyte since most of the bacteria was observed to be associated along the primary root and LRs (Figure 2). Motile bacteria was observed to be embedded in a matrix (Supporting Information: Video S1) which is probably rich in root exudates and mucilage as reviewed by Compant et al. (2010). It is established that endophytes can enter through natural breaks in roots or root tips but many endophytic bacteria also express cell-wall-degrading enzymes (Turner et al., 2013). We detected a gene encoding a glycosyl hydrolase from the cellulase family in Methylobacterium sp. 2A genome that could be involved in this process.
Sy et al. (2005) proposed that bacteria can co-metabolise methanol with alternative carbon sources. Another trait that Methylobacterium sp. 2A harbours in its genome is the acdS gene that encodes an active ACC deaminase (Figure 1c). This enzyme cleaves ACC to ammonia and α-ketobutyrate, enabling bacteria to use ACC as a carbon and nitrogen source (Gao et al., 2020). The net result is that the bacterium acts as a sink for ACC. PGPR harbouring ACC deaminase must interact with the root environment to access plant-produced ACC, thus establishing the rhizosphere interaction (Penrose et al., 2001). IAA produced by PGPR can induce the transcription of the plant ACC synthase (ACS) that catalyses the formation of ACC (Glick, 2014). Since Methylobacterium sp. 2A is able to produce substantial amounts of IAA (Grossi et al., 2020), we could speculate that the IAA produced by this strain can induce the expression of ACS resulting in an increase in ACC production. Plants that grow in association with ACC deaminase-containing PGPR generally have longer roots and shoots and are more resistant to growth inhibition by a variety of ethylene-inducing stresses (Glick, 2014). Orozco-Mosqueda et al. (2020) comments that in plants exposed to salt stress, the increased level of ethylene inhibits IAA signal transduction and limits plant growth. As a result of acting as ACC sink, ACC deaminase-containing PGPR can reduce ethylene levels preventing root growth inhibition (Glick et al., 1998, 2007; Glick, 2014). It is tempting to speculate that the significant changes in A. thaliana root length induced by Methylobacterium sp. 2A inoculation, both under control (Supporting Information: Figure S2) and salt stress conditions (Grossi et al., 2020), are the result of the synergistic interaction between IAA and ACC deaminase activity that finely calibrates auxin and ethylene concentration gradients.
Although we knew that Methylobacterium sp. 2A produces IAA, we did not observe an increase in IAA levels in inoculated plants (Figure 3a). PGPR-induced stimulation of root growth is predominantly attributed to their ability to synthesise IAA (reviewed in Kudoyarova et al., 2019). In this study, we confirmed the effect of the secreted IAA using different approaches. In A. thaliana DR5:GUS and DR5:GFP or tomato DR5:GUS roots, inoculation led to an increase in the activity of the synthetic auxin-responsive promoter DR5 (Figure 4a–c). IAA levels were lower in the non-inoculated yucQ mutants than in WT plants but did not differ from WTs after inoculation with Methylobacterium sp. 2A (Figure 4d), suggesting that yucQ roots could uptake the secreted IAA. Moreover, the inoculation of Methylobacterium sp. 2A partially rescued the gravitropic response in yucQ mutants (Figure 4e) and the phenotype of mutant plants with impaired LR formation (Figure 4f). Additionally, the increase in the number and length of RHs observed when A. thaliana roots are in close proximity to the bacteria (Supporting Information: Figure S4), as well as in the number of roots of rice, barley, and rye seedlings, could also be attributed to IAA secretion (Figure 6). The lack of a significant increase in IAA content in inoculated WT plants may be explained by IAA's short half-life and its metabolic and environmental instability since its concentration in plant tissues must be strictly controlled. In fact, at high concentrations, IAA analogues are used as herbicides. Depending on context and concentration, microbially derived auxins can promote plant growth or induce disease (Ludwig-Müller, 2015). IAA is active in submicromolar amounts, and its content in plant tissues is controlled through de novo synthesis, hydrolysis of IAA conjugates, a variety of conjugative and catabolic pathways (Normanly, 1997; Normanly & Bartel, 1999), and the basipetal polar transport system (Estelle, 1998). IAA homoeostasis is a dynamic process that responds to developmental and environmental signals; therefore, it is possible that catabolic processes are activated to ensure that the bacterial IAA does not exert a deleterious effect on the plant. Interestingly, a recent study on root-associated microbiomes had shown that PGPRs belonging to the genus Variovorax contain an auxin-degradation operon that reverses the root growth inhibition effect caused by a wide diversity of IAA-producing bacterial strains (Finkel et al., 2020), suggesting that plant-microbiota interactions coevolve to achieve mutual benefits.
High concentrations of endophytic bacteria can result in the elicitation of a host defence response (Compant et al., 2010; James et al., 2002). It was demonstrated that the inoculation of host plants with PGPR strains can produce a rise in the endogenous level of SA in various parts of plants (Tsukanova et al., 2017). Upon inoculation with Methylobacterium sp. 2A, the total content of JA and SA was significantly increased and a rise in JA-Ile concentration was also evidenced (Figure 3). JA and SA play an important role in the interactions between PGPRs and the host plants. These roles encompass facilitating colonisation, fostering the establishment of the microbial community in the rhizosphere, and contributing to the development of resistance against plant pathogens (Hou & Tsuda, 2022; Paasch & He, 2021). Although the effect was slight, the plants inoculated with Methylobacterium sp. 2A presented reduced ABA levels when compared to controls. It was reported that some PGPR strains can degrade ABA (Belimov et al., 2014) and that others that cannot degrade ABA also decreased ABA levels in host plants (Jiang et al., 2012). In fact, Burkholderia cepacia SE4, Promicromonospora sp. SE188, and Acinetobacter calcoaceticus SE370 counteract salinity and drought stress in cucumber plants, and this was partly achieved by reducing ABA levels (Kang et al., 2014).
VOCs are by-products of microbial metabolism that play important roles in intra- and inter-kingdom interactions. The rhizosphere environment seems favourable for VOCs-mediated communication since the partners are spatially close to each other, and volatiles are more likely to accumulate and reach their activity threshold. The emission of small gaseous signalling molecules from PGPR has emerged as a promising biotechnological tool to promote biomass accumulation in model plants and a few crops, such as tomato, lettuce, and cucumber. However, it is very important to adjust the adequate concentration of the bacterial inoculum. The effect generated by the VOCs emitted by Methylobacterium sp. 2A was dose-dependent; at optimal concentrations, VOCs had a positive effect on plant growth, however, excessive proliferation of the bacteria exerted a negative effect (Figure 5). Bacteria may affect the plants by consuming oxygen; however killing effects of VOCs emitted by Methylobacterium sp. 2A were only observed with LBNS, while growth promotion was obtained on R2A and MM methanol media (Supporting Information:: Figure S5). Methylobacterium sp. 2A grows well in R2A, LBNS and MM methanol, however, we cannot discard the possibility that the media may affect the composition and abundance of these compounds. Approximately 2000 VOCs emitted from almost 1000 bacterial and fungal species were already identified (Lemfack et al., 2018); the specific volatiles emitted by this bacterium remain to be elucidated.
Given the substantial environmental costs associated with contemporary agricultural methods, we need to make farming practices more sustainable. Our findings indicated that Methylobacterium sp. 2A can promote plant growth through the modulation of plant hormone levels and the release of IAA and VOCs. A. thaliana plants responded to these signals by modifying their root architecture which could increase the uptake of water and nutrients, thus improving the overall fitness of the plants. Additionally, monocots positively responded to its inoculation indicating that Methylobacterium sp. 2A is a wide-spectrum PGPR.
ACKNOWLEDGMENTS
The seeds used in this work were kindly provided by Dr. Diego Wengier (tomato Micro-tom DR5:GUS), Dr. Jorge Casal (IFEVA, Facultad de Agronomía, Universidad de Buenos Aires; A. thaliana msg2/iaa19), Dr. José Estevez (Instituto Leloir; A. thaliana DR5:GUS, DR5:GFP, yucQ, nph4/arf7 and, arf19), Cátedra de Química Biológica Vegetal (FFyB, Universidad de Buenos Aires; wheat cv. Buck Saeta). We thank Dr. Pablo Pomata for his helpful advice in confocal microscopy. Cecilia E. M. Grossi is a postdoctoral fellow from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina). Rita M. Ulloa is a member of Carrera de Investigador Científico from CONICET and Associate Professor at Universidad de Buenos Aires (UBA). This work was funded by Proyecto BID PICT 2021 #0440 and supported by the Joint Usage/Research Center, Institute of Plant Science and Resources, Okayama University, and Ohara Foundation.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




