Hydrogen sulphide alleviates root growth inhibition induced by phosphate starvation
Tong Liu and Hao Chen contributed equally to this study.
Abstract
Phosphorus (P) has crucial roles in plant growth and development. Hydrogen sulphide (H2S) has multiple functions in plants, particularly having the ability to promote tolerance to a variety of adversity stresses. However, it is unclear whether H2S has a function when plants suffer Pi-deficiency stress. DES1, encoding L-cysteine desulfhydrase1, is a crucial source of H2S in Arabidopsis thaliana by catalysing the substrate L-cysteine. Under phosphate starvation, the des1 mutant had a significantly shorter primary root length than the wild-type Col-0, and exogenous application of H2S donor NaHS could compensate for the root growth-sensitive phenotype. In contrast, the transgenic lines DES1ox overexpressing DES1 exhibited less sensitivity to phosphate starvation in terms of longer roots compared to the Col-0. These results demonstrate that H2S is involved in the regulation of Arabidopsis root growth under phosphate starvation. Moreover, using quantitative real-time polymerase chain reaction experiments to analyse the changes in genes induced by phosphate starvation in des1 mutant and Col-0, we screened to find that the expression of the Sulfoquinovosyl diacylglycerol 1 (SQD1) gene was significantly downregulated in the des1 mutant. Consistently, exogenous H2S significantly promoted SQD1 expression levels in roots of Col-0. Taken together, we demonstrate that DES1-mediated H2S participates in alleviating root growth inhibition by promoting the expression of SQD1 under Pi starvation.
1 INTRODUCTION
Hydrogen sulphide (H2S) is a signalling molecule that plays a crucial role in regulating and transducing signals in organisms that contain sulphur-containing compounds. (Lisjak et al., 2013; Wang, 2002, 2012). In plants, H2S plays multiple roles in plant growth and development, as well as in resistance to biotic and abiotic stresses (Liu, Wang, et al., 2021; Pandey & Gautam, 2020; Xuan et al., 2020; Zhang et al., 2021). H2S has been widely studied in regulating plant tolerance to drought, heat, chilling, heavy metals, osmotic and saline (Álvarez et al., 2010; Du et al., 2017; Jiang et al., 2019; Jin et al., 2013; Kaya et al., 2020; Li et al., 2013; Lisjak et al., 2011; Scuffi et al., 2014; Yastreb et al., 2020; Ye et al., 2020), and in plant growth and development processes such as seed germination, root development, photosynthesis and photomorphogenesis, cell senescence and programmed cell death and fruit ripening (Álvarez et al., 2012; Baudouin et al., 2016; Chen et al., 2011, 2015; Gotor et al., 2013; Jia et al., 2015; Laureano-Marín et al., 2020; Mukherjee, 2019; Zhang et al., 2017). For example, H2S alleviates cadmium (Cd)-induced root growth inhibition by alleviating Cd-induced oxidative stress and ionic toxicity in Arabidopsis (Arabidopsis thaliana) (Jia et al., 2016; Zhang et al., 2020). Exogenous application of H2S donor NaHS significantly increased the activities of CAT (catalase), APX (ascorbate peroxidase), SOD (superoxide dismutase) and POD (peroxidase) in plants to reduce the toxicity of chromium, aluminium, and hypoxia (Ahmad et al., 2020; Cheng et al., 2013; Dawood et al., 2012; Zhu et al., 2018). H2S in plants is mainly produced by four cysteine desulfhydrase (CDes), including L-cysteine desulfhydrase 1 (DES1), L-cysteine desulfhydrase (LCD), D-cysteine desulfhydrase 1 (DCD) and D-cysteine desulfhydrase 2 (DCD2) that catalysed cysteine (Cys) degradation (Álvarez et al., 2010; Hou et al., 2016; Riemenschneider et al., 2005). Previous research has shown that DES1, working together with O-acetylserine(thiol)lyase (OASTL) in the sulphur metabolism pathway, plays a crucial role in regulating plant tolerance to Cd and oxidative stress by controlling H2S-Cys homoeostasis. The study found that the des1 mutant exhibited significantly improved tolerance to Cd and hydrogen peroxide (H2O2) stress (Álvarez et al., 2010; López-Martín et al., 2008). In addition, a recent study has shown that H2S produced by DES1 controls ABA responses through persulfidation of the transcription factor ABI4 in the ABA signalling cascade, which additionally affects primary root growth (Zhou et al., 2021). Does H2S have a function on plant growth in a nutrient-deficiency condition?
Phosphorus (P) is the second most important nutrient required for plant growth and reproduction, after nitrogen (Clarkson, 1996; Raghothama, 1999; Schachtman et al., 1998). Phosphorus plays a crucial role in plant metabolism and protein regulation in the form of phosphate (Pi) or phospholipid. The roots of plants absorb Pi from the soil (Clarkson, 1996). Although the soil may contain high levels of total phosphorus, the ability of plants to utilise it is low due to the fixation and precipitation of orthophosphate by other soil ions, as well as the conversion of some orthophosphate into organic phosphorus (Raghothama, 1999; Tinker & Nye, 2000). Pi deficiency inhibits primary root (PR) growth and promotes lateral root (LR) and root hair (RH) growth, resulting in increased total root surface area and making full use of soil phosphorus nutrients (Liu, 2021; Péret et al., 2014). In addition, Low Pi stress can also inhibit above-ground plant growth or reduce Pi consumption by changing plant architecture (Ruan et al., 2018; Sinclair & Sheehy, 1999). Studies have shown that plants can also reduce the damage caused by Pi starvation stress by changing the anabolic mode of critical compounds. Under low Pi stress, the plant cell membrane system phospholipid is replaced by sulfolipid, and the released phosphate can alleviate Pi starvation stress to a certain extent (Nakamura, 2013). When the concentration of Pi in a plant is low enough to cause phosphorus starvation, the plant reacts by initiating an adaptive response to low Pi. This response is known as the Pi starvation rescue system, which helps to increase the efficiency of both Pi acquisition and use efficiency (PUE) (Hasan et al., 2016; Paz-Ares et al., 2022; Shenoy & Kalagudi, 2005). To maintain intracellular Pi homoeostasis, plants activate the whole-body transcriptional response of the MYB transcription factor PHOSPHATE STARVATION RESPONSE 1 (PHR1) under Pi starvation conditions (Bustos et al., 2010; Rubio et al., 2001). As the core positive regulator of Pi starvation signal transduction regulation in plants, PHR1 binds P1BS motifs (GNATATNC) on phosphate starvation-induced (PSI) gene promoters in the form of homolog or heterodimer to activate PSI genes expression (Bustos et al., 2010; Chiou & Lin, 2011; Guo et al., 2015; Rubio et al., 2001; Sun et al., 2016). SQD1 is one of the target genes of PHR1, and its expression is significantly induced in the presence of Pi deprivation (Ha & Tran, 2014; Misson et al., 2005). SQD1 encodes a UDP-sulfoquinovose synthase that catalyses the synthesis of UDP-sulfoquinovose (UDP-SQ) by sulphite and UDP-glucose (UDP-Glc) (Essigmann et al., 1998; Okazaki et al., 2009, 2013; Sanda et al., 2001). UDP-SQ can promote the synthesis of a sulfolipid, sulfolipid 6-sulfo-α-D-quinovosyl diacylglycerol (SQDG), substituting phosphatidylglycerol to maintain the balance of thylakoid membrane charge under Pi restriction (Benning, 1998; Yu et al., 2002). Studies in rice revealed that OsSQD1 affects Pi and S homoeostasis, as well as lipid composition in response to Pi starvation. Under Pi deficiency conditions, primary root growth inhibition is aggravated by OsSQD1 mutation (Sun et al., 2020).
Plants use sulfolipids instead of phospholipids during Pi-deficiency stress to maintain phosphate-sulphate balance in cells. To maintain this balance, PHR1 regulates the transport of sulphate from the soil to the roots by controlling the expression of specific genes like SULTR1;3, SULTR2;1 and SULTR3;4 under conditions of Pi starvation. These genes encode for sulphate transporters that help maintain the balance of phosphate and sulphate in the plant cells (Rouached et al., 2011). It is currently unknown whether H2S, as a sulphide, regulates plant response to Pi deficiency. In this study, we observed a significant increase in both DES1 gene transcript, DES1 protein levels, and DES enzyme activity in roots when subjected to low Pi conditions. DES1-induced H2S production could alleviate the inhibition of primary root growth mediated by Pi starvation stress. During Pi deficiency, the transcription level of SQD1, a PHR1 target gene, is significantly reduced in des1 mutants compared with the wild-type Col-0. Additional results indicate that the SQD1 mutation aggravates primary root growth inhibition under Pi deficiency, which is in line with the rice phenotype. Further study revealed that Pi-starvation stress induced the production of H2S, which further promoted the expression of SQD1. These results suggest that H2S produced by DES1 helps to mitigate root development inhibition under Pi starvation stress by regulation of the expression of SQD1.
2 MATERIALS AND METHODS
2.1 Plant growth conditions and treatment
The Arabidopsis (A. thaliana) seeds were sterilised in 75% (v/v) ethanol for 5 min, followed by 95% (v/v) ethanol for 3 min, and placed on 1/2 Murashige and Skoog (1/2MS) medium containing 1% sucrose and 0.5% agar (Sigma), pH 5.7–5.8. After 3 days of vernalisation at 4°C, Arabidopsis seeds were transferred to grow under the 16 h:8 h, light: dark photoperiod, at 120 μmol m−2s−1 light intensity, and 20°C.
The determination of high or low Pi concentrations is consistent with the previous studies (Li, Li, et al., 2022; Xu et al., 2020). The Pi-repleted medium (+Pi) was made by modifying the 1/2MS medium with an adequate supply of Pi, containing 1 mM KH2PO4. The Pi-limited medium (−Pi) was created by modifying the 1/2MS medium in which Pi (supplied by KH2PO4) was kept at a concentration of 10 μM.
2.2 Plant materials
The Arabidopsis wild-type Columbia-0 (Col-0) ecotype was used in this study. All Arabidopsis plants were used in the Col-0 background. The T-DNA insertion lines SALK_205358 (des1), SALK_103855 (des1-1), SALK_058117 (sqd1-1) and SALK_033056 (sqd1-2) mutants have been verified by genotyping. To generate transgenic overexpression plants, the full-length coding sequences of DES1 and SQD1 were cloned into the pEarleyGate101 vector under the control of the cauliflower mosaic virus 35S promoter to generate Pro35S:DES1-YFP and Pro35S:SQD1-YFP. The constructs were transformed into a Col-0 background using the floral dip method (Clough & Bent, 1998). To create transgenic complementation plants, 2000 bp upstream of the DES1 and SQD1 translation start codon and full-length genome sequences of DES1 and SQD1 were cloned into Cut out 35S of pEarleyGate101 vector or pGreenⅡ0179-NOS vector. The constructs were transformed into des1 or sqd1 backgrounds using the floral dip method. For the proSQD1:GUS transgenic plant, 2000 bp upstream of the SQD1 translation start codon was cloned into pGreenⅡ 0179-GUS vector. We hybridised the des1 mutant with the SQD1 overexpression lines to obtain the hybrid lines Pro35S:SQD1-YFP/des1. The primers used are listed in Supporting Information S1: Table S1.
2.3 Confocal microscopy analysis
A confocal laser scanning microscope SP8 (Leica Microsystems) was used for confocal microscopy. The excitation and emission wavelengths of YFP were 514 and 524 nm, respectively, for quantitative analysis of the fluorescence intensity of the YFP. When analysing the quantification of YFP fluorescence intensity, all experiments maintained the same confocal laser scanning microscopy setup parameters. The corresponding confocal data are the relative fluorescence intensities compared to the control, and these values are from untreated ProDES1:DES1-YFP/des1 seedlings.
2.4 RNA isolation and reverse transcription qPCR analysis
Arabidopsis root tissues were collected under +Pi or −Pi conditions and using Trizol reagent (Aidlab) to extract total RNA for qPCR. First-strand cDNA was synthesised from 2 μg of total RNA using the Hifair®III 1st Strand cDNA Synthesis SuperMix (Yeasen). qPCR analysis was performed with 2×SYBR qPCR Mix reagent (Aidlab) in conjunction with a CFX96 detection system (Bio-Rad). The EFα gene was used as an internal control. The primers used for qPCR analysis in this study are provided in Supporting Information S1: Table S1.
2.5 GUS staining and GUS activity
GUS staining is based on the principle that β-glucuronidase can break down 5-bromo-4-chloro-3-indolyl-glucronide into a blue substance (Yuan et al., 2014). The SL7160 GUS staining kit (Coolaber) was used to stain plant materials. According to the kit instructions, the X-Gluc solvent was first dissolved at room temperature, and 1 mL of solvent was added to 1 tube of X-Gluc dry powder to obtain 50×GUS staining concentrate. Subsequently, the concentrate was diluted 50 times with GUS buffer to formulate the GUS staining solution. A stereomicroscope coupled to a DS-Fi1c camera (Nikon) was used to capture GUS images of plants. Image J was used to perform intensity analysis on pictures to determine GUS activity.
2.6 Measurement of H2S content
Measurement of endogenous H2S in root tissues using H2S Content Assay Kit (Solarbio). First, approximately 0.1 g of root tissue was weighed and added with 1 mL Extraction Solution I, homogenised in an ice bath, centrifuged at 12 000 g for 10 min at 4°C; then 0.8 mL of supernatant was taken out, 0.15 mL of Extraction Solution II was added, centrifuged at 12 000 g for 10 min at 4°C and the supernatant was placed on ice for measurement. Second, 50 μL sample, 75 μL Reagent I, and II were added to the test tube. Another 50 μL ddH2O, 75 μL Reagent I, and II were added to the blank tube as a control. Finally, the tubes were mixed thoroughly and kept at room temperature for 10 min. The absorbance of each tube was measured at 665 nm, and the H2S content was calculated using the appropriate formula.
2.7 Expression and purification of DES1 and assay of DES activity
The full-length DES1 CDS was amplified and cloned into the Pet28a vector (HIS). The plasmids were transformed into Escherichia coli strain Rosseta cells. DES1-HIS protein was expressed by induction with 0.5 mM IPTG at 25°C for 6 h and purified through NI-NTA prepacked gravity column (SMART). Unspecific bound proteins were washed with 150 mL buffer A (50 mM Na2HPO4, 0.3 M NaCl, 10 mM imidazole). DES1 protein was eluted by applying a linear gradient to buffer B (50 mM Na2HPO4, 0.3 M NaCl, 40−200 mM imidazole). The obtained DES1 protein was dialysed in buffer C (50 mM TRIS-HCl, 150 mM NaCl, 10% glycerine).
Two hundred milligrams of roots of Col-0 grown on +Pi or −Pi medium for 5 days were taken and powdered in liquid nitrogen for extraction of proteins, respectively. The proteins were extracted at 4°C for 30 min with frequent shaking in 0.5 mL of extraction buffer (100 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 10% SDS and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). The buffer was centrifuged at 10 000 g for 10 min at 4°C, and the supernatant was collected. 500 μL DES-HIS protein was added to the plant protein extract and co-incubated at 4°C for 4 h to obtain DES1-HIS protein under +Pi and −Pi conditions. An assay of DES activity was performed as described previously (Shen et al., 2020). The DES activity was measured by monitoring the release of sulphide from L-cysteine in a total volume of 1 mL containing 0.8 mM L-cysteine, 100 mM Tris-HCl at pH 9.0, and 100 mL of purified DES1 protein. The reaction was initiated by adding L-cysteine. After incubation at 37°C for 15 min, the reaction was terminated by the addition of 100 μL FeCl3 (30 mM) and 100 μL N,N-dimethyl-P-phenylenediamine dihydrochloride (20 mM). The formation of methylene blue was determined at 670 nm in a spectrophotometer, and the relative DES enzyme activity was calculated using the OD values.
2.8 Measurement of Pi content
The Pi content in plant tissues was measured using the Tissue Inorganic Phosphorus Content Assay Kit (Sangon). Approximately 0.1 g of tissue was weighed, 1.0 mL of Reagent I was added on ice, homogenised thoroughly, centrifuged at 4°C, 10 000 rpm for 10 min, and the supernatant was collected and measured. Fifty microliters of sample supernatant, 450 μL water, and 500 μL Reagent III were added to the test tube. To obtain the standard curve, 50 μL standard solution, 450 μL water and 500 μL Reagent III were added to the standard tube. Five hundred microliters of water and 500 µL Reagent III were added to the blank tube as a control. The absorbance was measured at 660 nm after 10 min of incubation in a water bath at 40°C. The absorbance of each tube was measured at a wavelength of 660 nm, and the Pi content was calculated using the appropriate formula.
2.9 Data analysis and image processing
All statistical analyses in this study were performed using GraphPad PRISM software.
Adobe Illustrator was used to typeset the pictures.
3 RESULTS
3.1 DES1 functions in Pi starvation-induced root growth inhibition
Several studies have shown that phosphorus deficiency affects sulphur signalling and metabolism (Allahham et al., 2020; Rouached et al., 2011). However, the specific interactions between sulphur and phosphorus signalling pathways have yet to be well studied. To further explore the effects of phosphorus deficiency on plant sulphur homoeostasis, we constructed T-DNA insertion mutants of the OASTL gene family that function in the Arabidopsis sulphur signalling pathway (Álvarez et al., 2010). We first examined whether the primary root growth of these mutants was affected under Pi-limited and Pi-repleted mediums. We found that among these mutants, the des1 and des1-1 mutants were susceptible to primary root inhibition due to Pi starvation (Figure 1a,b; Supporting Information S1: Figure S1a–c). However, other OASTL family gene mutants oas-a1, oas-b, oas-c, cys-d1, cys-d2, cas-c1 and scs demonstrated no significant differences in root length compared to Col-0 under Pi starvation conditions (Supporting Information S1: Figure S2). In addition, there was no significant difference in seed germination between Col-0, des1 and des1-1 under the Pi-limited and Pi-repleted conditions (Supporting Information S1: Figure S3). Based on these results, we speculate that DES1 is involved in the process of primary root growth inhibition induced by Pi starvation in Arabidopsis.
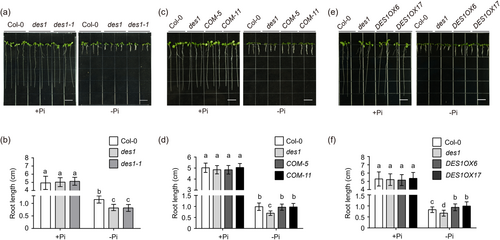
To verify whether DES1 responds to phosphate starvation signalling, we then used the T-DNA insertion mutant des1, the revertant mutations DES1::DES1 des1 and the transgenic overexpressed lines DES1ox for further analysis (Supporting Information S1: Figure S1). We found that there was no significant difference in root length between Col-0 and these transgenic materials grown on a Pi-repleted medium. Moreover, DES1::DES1 des1 lines (COM-5 and COM-11) showed comparable primary root growth with Col-0 under Pi-limitation conditions (Figure 1c,d). In contrast, DES1ox lines were more tolerant to Pi starvation and had longer primary root lengths compared to Col-0 (Figure 1e,f). Thus, these results indicated that DES1 plays a positive role in regulating the growth of primary root under Pi starvation.
3.2 Endogenous H2S responses to phosphate starvation-induced inhibition of primary root growth
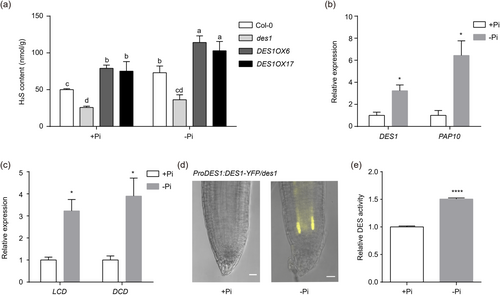
Due to the fact that DES1 is an essential source of endogenous H2S and the above finding that it plays a positive role in the growth of primary roots in plants under Pi starvation, we speculated that DES1-mediated H2S probably contributes to alleviating root growth inhibition induced by Pi starvation. First, we investigated whether endogenous H2S levels were affected for Col-0, des1, DES1OX6 and DES1OX17 grown on Pi-limited and Pi-repleted mediums. The result showed that H2S content in roots of Col-0, DES1OX6 and DES1OX17 was significantly higher under the Pi-limited condition than under the Pi-repleted condition. In contrast, there was no significant change in H2S content in des1 roots (Figure 2a). These results suggested that Pi starvation induces DES1-mediated endogenous H2S generation. We then investigate whether the elevated H2S in Arabidopsis roots is caused by increased DES1 expression. qPCR experiments showed that the transcripts of DES1 in roots were elevated on the Pi starvation condition compared with that on the Pi-repleted condition. The positive control PAP10 showed the same tendency (Figure 2b) (Zhang et al., 2014). In addition, the other hydrogen sulphide synthesis-related genes, LCD and DCD, in roots also showed significantly higher transcript levels under Pi-limited compared to Pi-repleted (Figure 2c). We subsequently determined root length under Pi-repleted and Pi-limited for the T-DNA insertion mutants lcd and dcd. However, we found no significant differences in lcd and dcd under both Pi-repleted and Pi-limited compared to Col-0 (Supporting Information S1: Figure S4a,b). These data suggested that among the H2S synthesis-related genes, DES1 plays a primary role in regulating primary root growth under Pi starvation. To verify whether Pi starvation also promotes DES1 protein expression in Arabidopsis roots, we observed the accumulation of DES1-YFP protein in roots and particularly in root tips under Pi starvation conditions by detecting the fluorescence intensity of ProDES1:DES1-YFP/des1 in complemented lines. The result was consistent with the transcriptional level, with a significantly increased accumulation of DES1-YFP in root tips under Pi starvation (Figure 2d). Moreover, we further examined the DES1 enzyme activity in vitro under Pi-repleted and Pi-limited conditions, and the results showed that the DES1 enzyme activity increased significantly under Pi-limited condition (Figure 2e). These results indicated that DES1 is promoted substantially by Pi starvation both at the transcriptional and protein levels in Arabidopsis roots. DES1-mediated endogenous H2S synthesis is stimulated in the root when challenged with Pi starvation.
3.3 Exogenous H2S alleviates the inhibition of primary root growth under phosphate starvation
To further explore the function of H2S on Arabidopsis primary root growth under Pi limitation, we observed the root growth status of Col-0 under treatment with different concentrations of the H2S donor NaHS in combination with or without Pi limitation. Under low concentrations of NaHS (25, 50, 75 and 100 μM) treatment, Col-0 did not exhibit a significant difference in the primary root growth compared with controls in the Pi-repleted condition (Xiang et al., 2023; Zhang et al., 2017). However, under the Pi-limited growth condition, the inhibition of root growth induced by Pi starvation could be sufficiently alleviated by exogenously applying 25, 50, 75 and 100 μM NaHS, in which 75 μM NaHS has the most significant effect (Figure 3a,b). Next, we determined whether exogenously application of NaHS can alleviate the inhibition of primary root growth in des1 mutant under Pi starvation. The sensitive phenotype of the primary root growth of des1 mutant under Pi starvation was compensated by exogenously applying 75 μM NaHS (Figure 3c,d). Meanwhile, to verify the specificity of NaHS, we imposed the same concentration of Na2SO4 as a control. It was found that the exogenous application of Na2SO4 did not compensate for the primary root growth-sensitive phenotype of des1 under Pi starvation (Supporting Information S1: Figure S5a,b), thus excluding the interference of other sulphide and sodium ions in the experiment. These results suggested that Pi limitation promotes the increase of endogenous H2S content mediated by DES1 in Arabidopsis roots, which was involved in alleviating the inhibition of Arabidopsis primary root growth by Pi starvation.

3.4 SQD1 was depressed in the des1 mutant
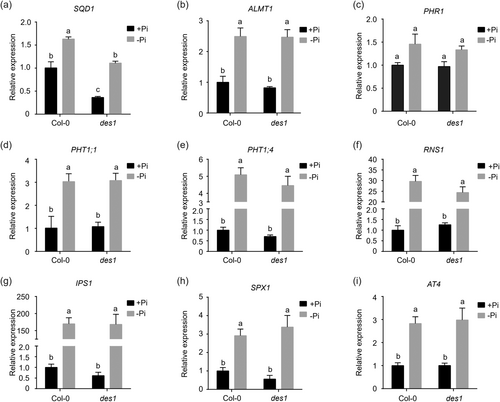
There are two main types of phosphate starvation (PS) signalling, namely local PS signalling and systemic PS signalling. Specifically, STOP1 and PHR1 (like) transcription factors (TFs) emerge as prominent players in local and systemic signalling, respectively. Although local PS signalling and systemic PS signalling are largely mechanistically independent, there may be some interaction between them (Guo et al., 2022; Paz-Ares et al., 2022). To explore the target genes of H2S in the phosphate starvation signalling pathway, we selected some representative genes known in the phosphate starvation signalling pathway to measure the expression levels in des1 mutant under Pi-starvation condition, among which including ALMT1, a target gene regulated by STOP1, as well as PHR1 and its regulated target PSI genes PHT1;1, PHT1;4, RNS1, IPS1, SQD1, SPX1 and AT4 (Isidra-Arellano et al., 2021). To determine whether these genes participated in the regulation of primary root growth by H2S under Pi limitation, we then measured the expression levels of these genes in the root tissues via a qPCR in des1 mutant and Col-0 growing on Pi-limited and Pi-repleted conditions. The results showed that the expression of the SQD1 gene was significantly reduced in the des1 mutant, either under Pi limitation or Pi-sufficient conditions (Figure 4a). Still, there was no significant difference in the expression of the other PSR genes (Figure 4b–i). It suggested that the mutation in DES1 led to a decrease in the content of endogenous H2S in plants, which might have resulted in downregulated expression of the SQD1 gene. We know that SQD1 generates UDP-sulfoquinovose (UDP-SQ), an intermediate of the sulfolipid SQDG, using UDP-glucose and sulphite as substrates (Sanda et al., 2001). It has been extensively shown that SQDG participates in plant photosynthesis and membrane lipid remodelling during plant phosphate starvation (Nakamura, 2013). In addition, we also measured the amount of inorganic phosphate in the shoots and roots of the des1 mutants and Col-0 under Pi limitation or Pi-sufficient conditions, respectively, and found that there were no significant differences both in the roots and shoots between the des1 mutant and Col-0 (Supporting Information S1: Figure S6a,b). This remained consistent with the qPCR results that there was no significant change in the transcript levels of PHTs, in which PHT1;1 and PHT1;4 played essential roles as high-affinity Pi transporters for Pi uptake, suggesting that the mutation of DES1 did not affect Pi homoeostasis in plants (Chien et al., 2022; Shin et al., 2004). Taken together, these results suggested that DES1 is involved in the regulation of SQD1 gene expression under Pi starvation.
3.5 H2S promotes the expression of SQD1 in Arabidopsis roots
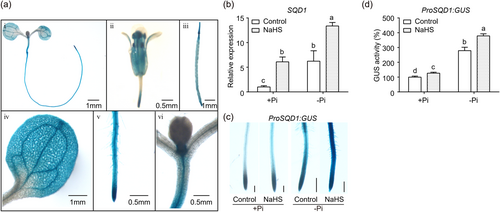
To explore the expression pattern of the Arabidopsis SQD1 gene, we constructed ProSQD1:GUS plants. The GUS staining results showed that SQD1 was expressed in leaves, hypocotyls, flowers, pods and roots of Arabidopsis (Figure 5a). Since the expression of SQD1 was suppressed in the des1 mutant, we then explored whether the exogenous imposition of H2S affects the expression of SQD1. We grew Col-0 on Pi-limited and Pi-repleted medium with or without 75 μM NaHS, respectively, and analysed the expression of SQD1 in roots by qPCR experiments. The results showed a significant increase in the expression of SQD1 after treatment with exogenous NaHS under Pi-limited and Pi-repleted conditions (Figure 5b). Subsequently, we further measured the expression of SQD1 in ProSQD1:GUS plants on Pi-limited and Pi-repleted medium with or without 75 μM NaHS, respectively. The GUS staining of ProSQD1:GUS in roots was increased significantly under Pi-limited or Pi-repleted medium in combination with NaHS treatment compared to Pi-limited or Pi-repleted medium alone (Figure 5c,d). In conclusion, our results demonstrated that H2S promotes the expression of SQD1 in Arabidopsis roots.
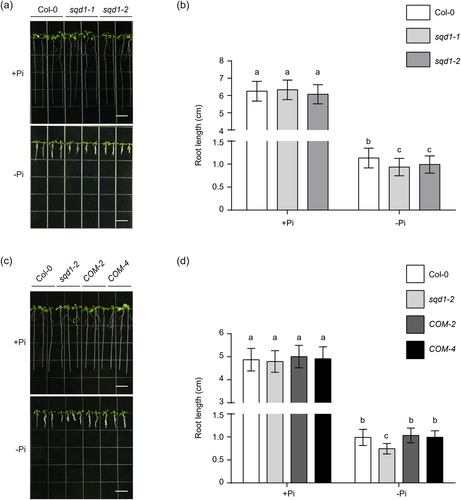
3.6 Arabidopsis sqd1 mutants are hypersensitive to phosphate starvation-induced root growth inhibition
Previous studies have shown that SQD1 is regulated by the TF PHR1 (Ha & Tran, 2014). It has been demonstrated in rice that the mutation of OsSQD1 under Pi limitation results in the inhibition of both primary root, lateral root and adventitious root growth (Sun et al., 2020). To investigate whether the sqd1 mutant is sensitive to primary root growth under Pi starvation in Arabidopsis, we constructed two T-DNA insertion mutants, sqd1-1 and sqd1-2 (Supporting Information S1: Figure S7a–c), to observe their growth status under Pi-limited and Pi-repleted mediums. The data showed that the primary root growth of sqd1-1 and sqd1-2 was shorter than that of Col-0 under the Pi-limited medium (Figure 6a,b). In contrast, there was no difference in primary root growth between the mutants and Col-0 under a Pi-repleted medium, which was consistent with previous studies on rice (Yoshihara et al., 2021). Next, we constructed the revertant mutations SQD1::SQD1 sqd1-2 and the transgenic lines of SQD1OX lines for further analysis (Supporting Information S1: Figure S7d,e). Under a Pi-limited medium, the SQD1::SQD1 sqd1-2 lines compensated for the mutant with primary root growth-sensitive phenotype (Figure 6c,d). The root length of the SQD1OX lines exhibited no significant difference from Col-0 in both the Pi-repleted and Pi-limited mediums (Supporting Information S1: Figure S8a,b). Thus, our results showed that SQD1 is essential for Arabidopsis primary root growth under phosphate starvation.
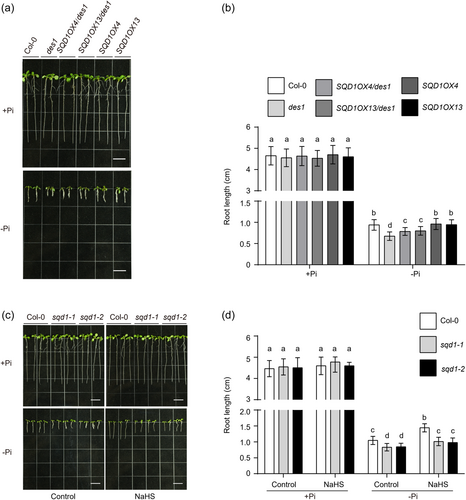
3.7 The function of SQD1 depends on DES1-mediated H2S under phosphate starvation
According to the above results, we conjectured that H2S affects primary root growth under low phosphate conditions by modulating SQD1 in the PS signalling pathway. We then constructed hybrid lines 35S:SQD1-YFP/des1 (Supporting Information S1: Figure S9) to investigate the relationship between DES1 and SQD1 in the regulation of primary root growth under a Pi-limited medium. The result displayed that there was no significant difference in primary root growth among Col-0, des1, SQD1OX and 35S:SQD1-YFP/des1 under Pi-repleted medium, whereas the primary root length of 35S:SQD1-YFP/des1 under Pi-limited condition was intermediate between that of Col-0 and the des1 mutant, that is, shorter than that of Col-0 and longer than that of the des1 mutant (Figure 7a,b). These results indicated that DES1 alleviates inhibition of primary root growth by Pi starvation stress through the regulation of SQD1. To confirm this, we observed the primary root growth of the sqd1 mutants under the application of NaHS and Pi limitation conditions. We found that exogenously applied NaHS did not fully compensate for primary root length to the Col-0 level in the sqd1 mutant under Pi starvation (Figure 7c,d). These results indicated that DES1-mediated H2S regulates primary root growth under Pi starvation through SQD1.
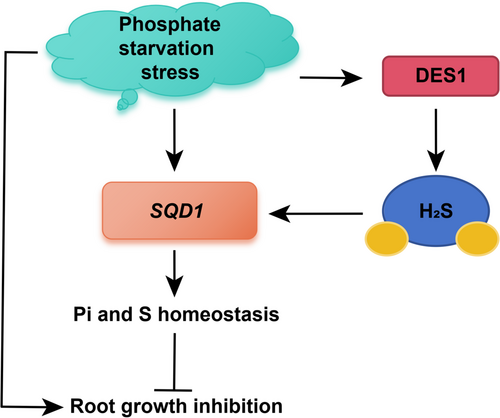
Taken together, our results suggested that DES1-mediated H2S alleviates the inhibition of primary root growth induced by phosphate starvation by promoting the expression of SQD1 (Figure 8). These results elucidated that H2S participates in the regulation of Pi limitation and can alleviate the suppression of primary root growth and development affected by Pi deficiency, which provides a new strategy for plants in alleviating nutrient stresses.
4 DISCUSSION
Phosphorus and sulphur are essential elements for plant growth processes, and they are mainly present in plants as phosphates and sulphates, respectively (Clarkson, 1996; Smith et al., 1995). Phosphate or sulphate deficiency causes inhibition of plant growth, including root development (López-Bucio et al., 2003). Plants replace phospholipids with sulfolipids in case of Pi deficiency and sulfolipids with phospholipids in case of S deficiency (Essigmann et al., 1998; Sugimoto et al., 2007). It has been shown that plants activate PHR1, a core TF that regulates low phosphate signalling, under phosphate starvation and induces the expression of its target gene SQD1 (Ha & Tran, 2014; Misson et al., 2005). SQD1 catalyzes the generation of UDP-SQ from its substrates sulphite and UDP-Glc and promotes the synthesis of sulfolipid-SQDG (Sanda et al., 2001). SQD1 can regulate Pi and S homoeostasis by regulating Pi mobilisation through membrane lipid remodelling, that is, changing the composition of the membrane lipids rich in phospholipids, and its catalytic synthesis of SQDG substitutes phosphatidylglycerol (PG) that is reduced by low Pi in thylakoid membranes, which is one of the general mechanisms of adapting to Pi limitation in plants (Okazaki et al., 2009, 2013). SQD1 deficiency in rice leads to exacerbated effects of Pi deficiency on root development (Sun et al., 2020). In addition, phosphate starvation also activates positive or negative regulation by PHR1 of the sulphate transporter genes SULTR1;3, SULTR2;1 and SULTR3;4, which affects sulphate transport between roots and shoots (Rouached et al., 2011). SQD1 and SULTR maintain plant Pi and S homoeostasis under low Pi conditions by regulating the metabolism or translocation of sulphate, which is reduced and converted to sulphide in plants via the sulphur assimilation pathway (Aarabi et al., 2020). The function of H2S has been extensively studied in plants in response to various abiotic stresses. However, it is unclear whether H2S regulates plant response to phosphate starvation.
The critical role played by H2S in influencing plant growth and development has been widely reported in recent studies, including root development (Li, Chen, et al., 2022). The regulation of root development by H2S has a dual effect, with high concentrations of H2S inhibiting the growth of primary roots by triggering the ROS-NO pathway (Zhang et al., 2017). On the contrary, low concentrations of H2S alleviated the inhibitory effects of abiotic stresses on the root system (Li, Chen, et al., 2022). In this study, we found that H2S could alleviate phosphate starvation-induced inhibition of primary root development. Under a low-Pi medium, exogenous application of H2S partially rescued low Pi-induced inhibition of primary root growth. The main pathway for the synthesis of H2S in plants is an enzymatic pathway from degraded cysteines, a process catalysed by D/L-cysteine desulfhydrase, including DES1, LCD and DCD (Liu, Wang, et al., 2021). des1, lcd and dcd mutants are more sensitive to abiotic and biotic stresses, and the expression levels of stress-induced genes were suppressed in these mutants (Shi et al., 2015). For example, the expressions of DES1 and LCD in roots are induced under Cd stress, resulting in the production of endogenous H2S, which acts as a sulphide catalysed by OASTL to produce Cys and GSH, thereby chelating the Cd-induced ROS burst and alleviating its toxicity to the plant root system. Consistently, the lcd des1 mutant is more sensitive to Cd stress (Jia et al., 2016). In addition, DCD-mediated H2S production alleviated osmotic stress-induced inhibition of root growth (Xiang et al., 2023). In this study, we found that the DES1 expression, DES1 protein, DES enzyme activity, and endogenous H2S content in roots were significantly increased under low Pi conditions. Pi starvation-induced H2S production was impaired in des1 mutants but accumulated in DES1OX lines. This suggests that Pi starvation-induced H2S elevation in the root is actively involved in alleviating inhibition of primary root development under low Pi stress. However, it is still unclear how phosphate starvation induces DES1 expression.
We used qRT-PCR to examine the expression of PHR1 and PHR1-regulated low Pi-responsive target genes under low Pi conditions in des1 mutants and found a significant decrease in SQD1 expression. Moreover, the exogenous application of NaHS significantly increased SQD1 expression. Thus, H2S may alleviate the inhibition of primary root growth by regulating SQD1 due to Pi starvation. Consistent with previous reports of OsSQD1, we found that deletion of SQD1 in Arabidopsis, like the des1 mutant, also leads to plant sensitivity to Pi starvation-induced inhibition of primary root growth. To confirm that H2S mitigates the inhibition of primary root growth by low Pi through modulation of SQD1, we exogenously applied NaHS to sqd1 mutants grown under low Pi conditions. We found that the primary root length was not fully restored in the sqd1 mutant. On the other hand, overexpression of SQD1 in the background of des1 mutants partially restored their primary root growth under a low Pi medium. These results suggest that DES1-mediated H2S regulates primary root growth under Pi starvation through SQD1. In addition, the partial restoration of root length phenotype by exogenous application of NaHS in sqd1 under Pi starvation and the incomplete restoration of root length by overexpression of SQD1 in des1 suggest that H2S may also affect primary root growth through regulation of other low Pi-responsive genes in addition to SQD1 under Pi starvation.
Recent studies have shown that H2S affects protein function by persulfidation-modified cysteine residues of proteins, thereby regulating plant development and response to stress (Aroca et al., 2017, 2021; Jurado-Flores et al., 2023; Liu & Xue, 2021; Siodmak & Hirt, 2021). DES1-mediated H2S persulfidation of SNF1-RELATED PROTEIN KINASE2.6 (SnRK2.6) promotes its enzyme activity and enhances interactions with ABA-responsive element-binding FACTOR2 (ABF2), which promotes ABA signalling and enhances drought tolerance in plants (Chen et al., 2020). Moreover, DES1-mediated H2S persulfidation of the NADPH oxidase RBOHD, which enhances its ability to produce ROS, induces stomatal closure, and thus enhances plant drought tolerance (Shen et al., 2020). ABSCISIC ACID INSENSITIVE 4 (ABI4), a TF in the ABA signalling pathway that integrates ABA and MAPK signalling, is persulfidated by DES1-mediated H2S, which enhances its transactivation activity towards MITOGEN-ACTIVATED PROTEIN KINASE KINASE KINASE 18 (MAPKKK18) (Zhou et al., 2021). In this study, we found that DES1-mediated H2S induced the expression of SQD1 in roots under low Pi, thereby enhancing the tolerance of plant roots to Pi starvation. However, the mechanism by which H2S regulates SQD1 remains to be further explored. Whether DES1-mediated H2S affects the activity of SQD1 by persulfidation and thus influences the homoeostasis of Pi and sulphate in plants under low Pi status and the specific mechanism by which H2S regulates SQD1 remain to be further explored.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (32070214, 31670267).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The authors confirm that all data referred to here are available in the body of this article and its supplementary materials.




