Resin acid δ13C and δ18O as indicators of intra-seasonal physiological and environmental variability
Abstract
Understanding the dynamics of δ13C and δ18O in modern resin is crucial for interpreting (sub)fossilized resin records and resin production dynamics. We measured the δ13C and δ18O offsets between resin acids and their precursor molecules in the top-canopy twigs and breast-height stems of mature Pinus sylvestris trees. We also investigated the physiological and environmental signals imprinted in resin δ13C and δ18O at an intra-seasonal scale. Resin δ13C was c. 2‰ lower than sucrose δ13C, in both twigs and stems, likely due to the loss of 13C-enriched C-1 atoms of pyruvate during isoprene formation and kinetic isotope effects during diterpene synthesis. Resin δ18O was c. 20‰ higher than xylem water δ18O and c. 20‰ lower than δ18O of water-soluble carbohydrates, possibly caused by discrimination against 18O during O2-based diterpene oxidation and 35%–50% oxygen atom exchange with water. Resin δ13C and δ18O recorded a strong signal of soil water potential; however, their overall capacity to infer intraseasonal environmental changes was limited by their temporal, within-tree and among-tree variations. Future studies should validate the potential isotope fractionation mechanisms associated with resin synthesis and explore the use of resin δ13C and δ18O as a long-term proxy for physiological and environmental changes.
1 INTRODUCTION
Stable carbon and oxygen isotope compositions (δ13C and δ18O) in tree organic compounds have long been suggested as recording both environmental conditions and tree physiological responses to the environment. While previous studies have explored a diverse array of plant organic molecules for their isotopic variations (Diefendorf et al., 2010; Schmidt, 2003; Siegwolf et al., 2022), relatively little isotopic information is available for nonvolatile resin acids, also known as diterpene acids (Dal Corso et al., 2011, 2017; Murray et al., 1998; Nissenbaum & Yakir, 1995; Nissenbaum et al., 2005; Stern et al., 2008). Resin acids (referred to as ‘resin’ hereafter) constitute major components of pine (Pinus spp.) oleoresin, playing a critical role in defense against herbivores and pathogens (Keeling & Bohlmann, 2006). Resin is also known as an important source of fossil records, and hence can have implications with respect to palaeoenvironmental reconstructions (Tappert et al., 2013). An improved understanding of the natural variations and mechanistic controls of the δ13C and δ18O signals in resin acids is therefore desirable because it cannot only help to interpret (sub)fossilized resin records (Tappert et al., 2013) but also may provide insights into dynamics in resin-based conifer defense mechanisms.
Only a few studies have compared δ13C values between resin and other organic compounds. Dal Corso et al. (2011, 2017) identified a 13C-enrichment in resin of 2.5‰ and 1.7‰ compared to wood and bulk leaf, respectively. In contrast, Gaylord et al. (2013) reported a 0.4‰ depletion of 13C in resin relative to leaf water-soluble carbohydrates (WSCs). However, Tappert et al. (2013) found no consistent δ13C differences between resin and bulk leaf. These variable results are not unexpected, as δ13C in bulk leaf, wood and WSCs can significantly deviate from each other (Bowling et al., 2008; Tang et al., 2023a, 2023b). In this context, a thorough comparison between resin and other organic compounds in trees is essential for a comprehensive overview of δ13C records across diverse plant (sub)fossils (Dal Corso et al., 2017). Among these compounds, sucrose is the main end product of photosynthesis and the dominant transport sugar in trees (Hartmann and Trumbore, 2016), and therefore it is a suitable surrogate for deciphering the 12C/13C fractionation from assimilates to resin. It has been reported that needle sucrose δ13C can better depict the seasonal trends and absolute values of the δ13C of new assimilates for both mature and saplings of P. sylvestris, compared with the aforementioned bulk matter (Rinne-Garmston et al., 2023; Tang et al., 2023b).
Studies on resin δ18O, such as those by Nissenbaum et al. (2005) and Stern et al. (2008), are even more scarce than studies on resin δ13C. Nissenbaum et al. (2005) compared stem resin δ18O in Hymenaea with a compilation of plant cellulose δ18O across a variety of species, suggesting an overall 18O-depletion of 20‰ in resin relative to cellulose. However, it is noteworthy that resin and cellulose undergo different 16O/18O fractionation processes. Cellulose δ18O is mainly determined by source water δ18O, leaf water 18O-enrichment and oxygen isotope exchange between the carbonyl group and water at the site of sucrose production, sucrose transport and cellulose synthesis (Roden et al., 2000; Song et al., 2022). By contrast, the oxygen atoms of resin originate from molecular oxygen (O2) (Hamberger et al., 2011; Meunier et al., 2004) and undergo exchange with surrounding water (Samuel & Silver, 1965; Schmidt et al., 2001) (Figure 1). In other words, resin δ18O is determined by oxidation-related 16O/18O fractionation and oxygen isotope exchange between resin and water. An in-depth understanding of resin δ18O necessitates not only a better grasp of the oxidation-related 16O/18O fractionation, such as its variation with O2 demand, but also a comparison of δ18O between resin and xylem water. However, such analyses are rarely found in existing literature, which motivates the work here.
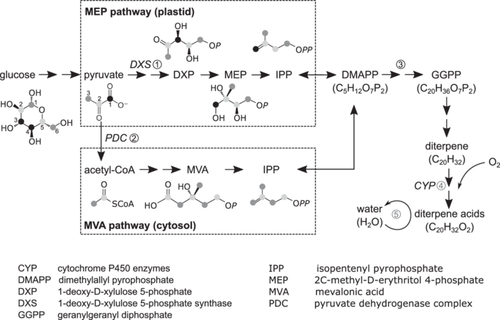
To bridge the understanding from isotopic fractionations associated with resin δ13C and δ18O to their application in environmental studies, it is important to investigate their natural variability and responses to environmental conditions. Stem resin δ13C is known to have low seasonal variability (Gaylord et al., 2013; Stern et al., 2008), which can be attributed to its years-long turnover (Wilson et al., 1963). In contrast, detached shoots and branches often demonstrate a rapid resin turnover within hours (Gershenzon et al., 1993 and reference therein), probably due to smaller resin pools. Yet, it remains unclear whether δ13C and δ18O in twig resin have a larger seasonal variability and thus a better ability to record environmental and physiological changes compared to their counterparts in stem resin. Apart from seasonal variations, it is crucial to consider the isotope variability in resin among individual trees of the same species growing at the same site. Despite similar environmental conditions, tree individuals may still display variations in resin δ13C and δ18O (Stern et al., 2008), due to potential local-scale differences in biotic or abiotic factors. For instance, McKellar et al. (2011) observed higher resin δ13C in bark beetle-infested and water-stressed Pinus contorta compared to control trees, attributing this difference to 13C-enrichment in assimilates mediated by phloem interruption. Rissanen et al. (2021) found a stronger 13C label in stem resin of drought-exposed Pinus sylvestris than that of irrigated trees after 13CO2-pulse labelling. This indicates that drought-affected trees allocate a higher proportion of new assimilates to resin production. While these studies used manipulative approaches to impose treatment effects, which limits their applicability to understanding the natural isotopic variation, they do imply that among-tree variations in resin δ13C can hint at differences in assimilate δ13C and resin production. Building on this, an area that warrants further investigation is how tree tissue growth, known to affect resin production (Redmond et al., 2019), might impact the relationship between isotope compositions in resin and environmental conditions.
- (1)
How do resin δ13C and δ18O relate to assimilate δ13C and xylem water δ18O, respectively?
- (2)
How do the absolute values and seasonal variations of resin δ13C and δ18O differ between twigs and stems, and among trees?
- (3)
How do resin δ13C and δ18O respond to variations in physiological and environmental factors on a seasonal scale?
2 MATERIALS AND METHODS
2.1 Site description
This study was conducted at a 60-year-old boreal forest dominated by Scots pine in southern Finland, Hyytiälä SMEAR II (61°51′ N, 24°17′ E, 170 m a.s.l.). The stand is mixed with Norway spruce (Picea abies (L.) Karst) and birch (Betula pubescens Ehrh. and Betula pendula Roth) in the understory. It had a stand density of 1304 trees per hectare (diameter >5 cm at 1.3 m height) and a basal-area-weighted tree height of 19.9 m in the year 2018 (Kolari et al., 2022). The soil is a haplic podzol on glacial till (FAO–UNESCO, 1990), with an average layer depth of 4, 5, 21 and 34 cm for O, A, B and C horizons, respectively (Kolari et al., 2022). The mean annual temperature was +4.1°C, and the mean temperature between May and September was +12.6°C from 1991 to 2020 (Jokinen et al., 2021). During this period, the mean annual precipitation was 690 mm, with 342 mm falling between May and September (Jokinen et al., 2021).
Environmental data for the study site, including photosynthetically active radiation (PAR), relative humidity (RH), air temperature (T), soil moisture (SM) and SWP, were obtained from the AVAA Smart SMEAR portal (https://smear.avaa.csc.fi/). VPD was calculated from RH and T observations, according to Murray (1967). Daily means of the environmental variables were used in subsequent analysis.
2.2 Sampling of resin, carbohydrates and water
2.2.1 Resin sampling
Resin samples were collected from five mature trees weekly or biweekly, on 17 occasions between May and October 2019, always between 12:00 and 16:00 (UTC + 2). At the top-canopy level, 18 m above ground, resin was collected from sun-exposed 1-year-old twigs, accessed via a 10 m long branch scissor and/or a walk-in scaffolding tower. The resin drops formed at the cut surface were transferred into 2 mL vials or tin and silver cups (IVA Analysentechnik) using a small spatula, which was cleaned with ethanol and dried after each sample. At breast height, 1.3 m above ground, resin was collected from micro-cored holes made with a Trephor tool (Costruzioni Meccaniche Carabin C.) (Rossi et al., 2006). The holes were zigzagged around the tree circumference, with horizontal and vertical distances of 2 and 20 cm between the holes, respectively, to minimize wounding stress-induced resin production. After micro-coring, holes were sealed with PTFE tape, and sampling continued until sufficient resin accumulated within 2 h. Collected samples were put immediately in dry ice and stored at –20°C.
2.2.2 Sampling and extraction of carbohydrates
On each of the 17 resin sampling days, 1-year-old and current-year needles were collected separately for carbohydrate extraction from the same twigs used for twig resin sampling. Additionally, on six specific dates (17 May, 7 June, 28 June, 26 July, 27 August and 23 September), twig bark was also harvested from these twigs. Concurrently, breast-height phloem samples were taken from the five trees used for stem resin sampling, about 10 cm above the micro-cored holes using a 2 cm diameter corer. All needle, twig bark and phloem samples were immediately placed in a cool box with ice bags and, within 2 h of collection, microwaved at 600 W for 1 min to stop enzymatic and metabolic activities (Wanek et al., 2001). These samples were then oven-dried at 60°C for 24 h and stored at room temperature until further processing.
WSCs, comprising sucrose, glucose, fructose, pinitol and myo-inositol, were extracted and purified from the homogenized powder of needles, twig barks and phloem, following the protocols of Wanek et al. (2001) and Rinne et al. (2012). Briefly, c. 60 mg of plant powder was suspended in 1.5 mL deionized water in a 2 mL reaction vial, heated in a water bath at 85°C for 30 min and the supernatant was separated by centrifugation at 10 000 g for 2 min. The supernatant was subsequently purified by three sample treatment cartridges (Dionex OnGuard II H, A & P cartridges, Thermo Fisher Scientific) to remove amino acids, organic acids and phenolic compounds (Rinne et al., 2012). The WSC samples were then stored at –20°C before isotope analysis.
2.2.3 Sampling and extraction of water
Samples of twig water were collected from five mature trees on six occasions (17 May, 7 June, 28 June, 26 July, 27 August and 23 September), each between 12:00 and 16:00. One-year-old twigs were collected, with barks peeled off into separate 12 mL Exetainer glass vials (Labco). All samples were immediately placed in a cool box with ice bags and stored at –20°C before further processing.
Water was extracted from twigs by cryogenic vacuum distillation, at the Swiss Federal Institute for Forest, Snow and Landscape Research (WSL) (Diao et al., 2022). Sample vials were heated in a water bath at 80°C for 2 h under vacuum of <0.05 hPa, and evaporating water was trapped in cooling U-tubes, immersed in liquid nitrogen. Extracted water was filtered through 0.45 µm syringe filters and stored at 4°C before isotope analysis.
2.2.4 Sampling trees
Access limitations to the top canopy and conservation protocols of the SMEAR II station led to a slight variation in our tree selection. Two of the trees for stem resin and phloem sampling (trees 1, 2, 3, 4, 5) were different from those for twig resin, needle and twig bark sampling (trees 1, 2, 3, 6, 7) (Supporting Information S1: Table 1). However, due to insufficient twig resin samples from trees 6 and 7, our analysis of the temporal trends and among-tree and within-tree differences in twig resin δ13C and δ18O was limited to trees 1, 2 and 3. All sampled trees were similar in height, diameter and canopy appearance, and were located within 20 m of each other. Nevertheless, there was some variability in needle water content between trees. For instance, tree 3 had lower needle water content than the other trees (Supporting Information S1: Table 2).
2.3 Isotope analysis
δ13C analysis of resin and WSCs was performed at the Stable Isotope Laboratory of Luke (SILL) (Helsinki, Finland), using an elemental analyzer (EA) (Europa EA-GSL, Sercon Limited) coupled to an isotope ratio mass spectrometer (IRMS) (20-22 IRMS, Sercon Limited). Resin samples, ranging from 0.210 to 0.945 mg, were transferred from 2 mL vials to tin cups (IVA Analysentechnik). Alternatively, any excess resin was removed from the sampling cups. The cups were then placed in an oven at 40°C for 24 h to remove any volatile components. Aliquots of WSCs were pipetted into tin cups and freeze-dried. All cups were wrapped and kept in a desiccator before isotope analysis. The EA-IRMS δ13C values were calibrated by three reference materials: IAEA-CH3 (cellulose, –24.72‰), IAEA-CH7 (polyethylene, –32.15‰) and an in-house sucrose reference (Sigma Aldrich, –12.22‰). The long-term measurement precision of δ13C was 0.1‰ (SD). A total of 105 resin samples were analyzed for δ13C, due to occasional collection challenges and losses during analysis. The δ13C of needle WSCs was calculated as an average from the δ13C series in 1-year-old and current-year needles.
For δ13C analysis of sucrose, aliquots of WSCs from five trees were pooled for each sample type (current-year needles, 1-year-old needles, twig barks and stem phloem) collected on the same sampling day. The analysis was conducted in the Stable Isotope Facility at the University of Vienna (SILVER), using a high-performance liquid chromatography (HPLC) system coupled to an IRMS with a Thermo LC Isolink I interface (Wild et al., 2010). For sugar separation, a Macherey-Nagel Nucleogel Sugar Pb column was used at 80°C and with 0.4 mL min–1 of ultrapure water as eluent. Detected sugars and sugar alcohols, in order, were sucrose, glucose, pinitol, fructose and myo-inositol. To correct the HPLC-IRMS δ13C values (Rinne et al., 2012), interspersed standards containing these sugars and sugar alcohols were injected in six different concentrations (3–100 mg L–1) for each batch of 32–40 samples. Standards were referenced against EA-IRMS (EA 1110, CE Instruments, Milan, Italy, coupled to a Finnigan MAT Delta Plus IRMS, Thermo Fisher Scientific) as pure chemicals. Sucrose δ13C was reported in the current study, as sucrose accurately reflects assimilate δ13C (Tang et al., 2023b) and is the predominant transport sugar in phloem (Rennie & Turgeon, 2009). The measurement precision for sucrose standards was 0.22‰ (SD). Needle sucrose δ13C was calculated as the average δ13C series in 1-year-old and current-year needles.
δ18O analysis of resin and needle WSCs was done at SILL, using high temperature (HT)-EA-IRMS. Resin samples, ranging from 0.365 to 6.24 mg, were weighed in silver cups (IVA Analysentechnik) and oven-dried to remove volatile components. It is noteworthy that resin is hydrophobic (Jagalski et al., 2016) and resin samples contain little water (Sarria-Villa et al., 2021). Therefore, the impact of carbonyl-water oxygen exchange during resin sample preparation is minimal. Aliquots of needle WSCs were pipetted into silver cups and freeze-dried, as suggested by Lehmann et al. (2020) to improve the precision of δ18O analysis. All silver cups were prepared and stored in a desiccator before isotope analysis. δ18O values were calibrated against IAEA-601 (23.14‰), in-house sucrose (36.62‰), and lactose references (Sigma Aldrich, 21.05‰). The long-term precision of δ18O measurements was 0.2‰ (SD). A total of 97 resin samples were analyzed for δ18O. Needle WSC δ18O was calculated as the average of δ18O series from 1-year-old and current-year needles.
δ18O analysis of water samples was conducted in the Stable Isotope Ecology Laboratory at the University of Basel (Switzerland), using Thermal Conversion (TC)/EA coupled to a Delta V Plus IRMS via a ConFlo IV interface (Thermo Fisher Scientific) (Newberry et al., 2017). Samples were injected more than six times, with the initial measurements omitted to prevent memory effects, and a mean δ18O value was calculated from at least three measurements.
2.4 Modelling needle sugar δ13C and δ18O
Leppä et al. (2022) modelled needle sugar δ13C and δ18O in Scots pine for our study site, and their results were used in this study. The model integrated an advanced version of the classic photosynthetic 13C discrimination model (Farquhar et al., 1982), incorporating a metabolic dissociation between respiratory substrates and fresh assimilates (Wingate et al., 2007). It first simulated δ13C of net CO2 exchange, then predicted needle sugar δ13C, assuming a consistent assimilate mix in the leaves. Regarding δ18O, this model first simulated needle water δ18O, calibrating the Péclet model to non-steady-state conditions (Farquhar & Cernusak, 2005). It then estimated assimilate δ18O, applying a temperature-sensitive fractionation factor (Sternberg & Ellsworth, 2011), and subsequently predicted needle sugar δ18O, assuming a stable needle sugar pool. The parameters and validations of the model are detailed in Supporting Information S1: Note S1 and Leppä et al. (2022). The model was run at half-hourly intervals, and daily averages of needle sugar δ13C and δ18O were used for further analysis.
2.5 Respiration rate
To test if discrimination against 18O during diterpene oxidation varies with tree respiration demand, we examined the correlation between resin δ18O and respiration rate (Rd). To estimate Rd, we measured CO2 fluxes in twig and stem chambers (Supporting Information S1: Note S2) and determined stem and twig Rd based on nighttime CO2 efflux data (Supporting Information S1: Note S2). We installed a transparent twig chamber with a volume of 2.1 L in the top canopy of a tree that was also used for twig resin sampling. Additionally, we used dark stem chambers of about 1 L on two other trees at 8 m height (Supporting Information S1: Table 1). The detailed methodology for our chamber setup and flux calculations is described in Altimir et al. (2002) and Kolari et al. (2012).
2.6 Growth data
To examine whether tree growth periods impact the relationships between isotope compositions in resin and environmental variables, we tracked the seasonal growth of shoots, needles and stems. To measure shoot growth, we tracked the length increment of 16 shoots at the top or the middle of the crown from four mature Scots pine trees (Supporting Information S1: Table 1) (Schiestl-Aalto et al., 2013), three to four times per week from April to June 2019. For needle growth measurement, we monitored the length increase of one needle per measured shoot from May to August 2019. The shoot and needle growth period was defined as the time when 5%–90% of the full length was achieved.
To track stem growth, we collected micro-cores (diameter 2 mm, length 15 mm) at 1.3 m height from the five trees used for stem resin collection (Supporting Information S1: Table 1) on each resin sampling day. We prepared the micro-core sections and analyzed the images of the current-year rings, as described by Tang et al. (2022). We then counted the numbers of total and mature current-year tracheids and fitted the numbers to the Gompertz function (Zeide, 1993) to simulate the growth curves for tracheid production and tracheid maturation, respectively. Based on the growth curves, we defined the tracheid production period and tracheid maturation period as the time when 5%–90% of the total number of tracheids was present and mature, respectively (Jyske et al., 2014). We also defined the growth periods of earlywood and latewood (Supporting Information S1: Figure 1), according to Tang et al. (2023a).
2.7 Data analysis
To answer Question (1), we defined the δ13C offsets between resin and different carbon pools, as well as the δ18O offsets between resin and twig water. To address Question (2), we applied linear mixed effects models using the ‘lme’ function in the R package nlme (Pinheiro et al., 2021) to compare δ13C or δ18O values between different trees or organs, taking the sampling date as a random effect. To answer Question (3), we calculated Spearman's correlation coefficients between resin δ13C and time-series of VPD, T, PAR, SM, SWP and modelled needle sugar δ13C, and between resin δ18O and time-series of VPD, T, PAR, SM, SWP and Rd. We conducted the correlation analysis separately for twig and stem levels, for individual trees, and for the average of all trees and both for the whole growing season and separately for six different tree growth periods (from needle growth period to latewood growth period). Additionally, we considered the temporal integration of recently formed resin by averaging the daily environmental and physiological variables over the period from the current day to the previous n day. We set n at 1, 5, 10, 20, 40 and 60 d. In fact, in a 13C-tracing experiment, the 13C-enrichment in stem resin of mature Scots pine reached its maximum value 2 months after the 13CO2 pulse (Rissanen et al., 2021). Although the varying time window analysis may also shed light on resin formation dynamics, we refrained from overinterpreting the results but encourage future exploration of such possibilities in combination with resin duct production data.
All statistical analyses were performed using R version 4.0.0 (R Core Team, 2020). All data used in this study are provided in Supporting Information S1: Data set S1.
3 RESULTS
3.1 Intra-seasonal resin δ13C
3.1.1 δ13C differences between resin and other carbon pools
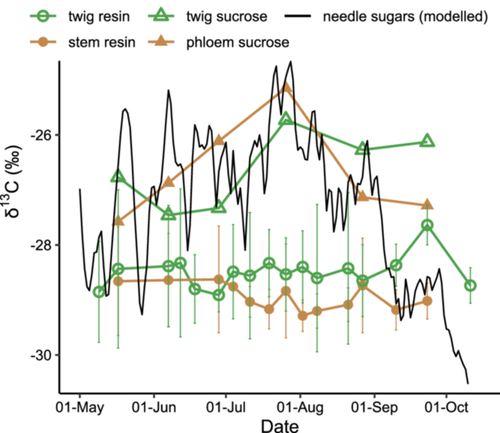
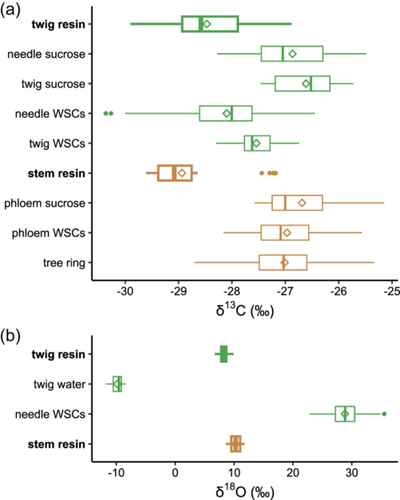
For both top-canopy and breast-height levels, the seasonal variation of resin δ13C did not follow that of sucrose δ13C (Figure 2) or WSC δ13C (Supporting Information S1: Figure 2), both of which peaked at the end of July. Moreover, the amplitude of seasonal variation for resin δ13C was smaller than that of sucrose δ13C at both top-canopy (1.3 vs. 1.7‰) and breast-height (0.7 vs. 2.4‰) levels (Figure 2). Resin was overall 13C-depleted compared with the other carbon pools at the same height (Table 1; Figure 3a). Specifically, twig resin δ13C was 1.8‰ lower than twig sucrose δ13C (p < 0.001), and stem resin δ13C was 2.1‰ lower than phloem sucrose δ13C (p < 0.001) (Table 1; Figures 2 and 3a).
| Tree | Twig resin | Needle WSCs (sucrose) | Twig WSCs (sucrose) | Stem resin | Phloem WSCs (sucrose) | Tree ring |
|---|---|---|---|---|---|---|
| 1 | –28.4 ± 0.3 | –28.2 ± 0.7 | –27.6 ± 0.4 | –28.7 ± 0.1 | –27.1 ± 0.3 | –27.3 ± 0.3 |
| 2 | –27.8 ± 0.7 | –27.8 ± 0.8 | –27.3 ± 0.3 | –29.2 ± 0.2 | –27.2 ± 0.5 | –28.0 ± 0.4 |
| 3 | –29.3 ± 0.6 | –28.3 ± 0.9 | –27.6 ± 0.4 | –29.1 ± 0.1 | –26.9 ± 0.4 | –26.7 ± 0.3 |
| 4 | –29.4 ± 0.1 | –27.6 ± 0.5 | ||||
| 5 | –27.3 ± 0.1 | –26.0 ± 0.4 | –26.1 ± 0.4 | |||
| 6 | –28.3 ± 0.4 | –28.5 ± 0.5 | –27.7 ± 0.4 | |||
| 7 | –27.8 ± 0.2 | –27.9 ± 0.7 | –27.5 ± 0.4 | |||
| 8 | –27.0 ± 0.4 | |||||
| Mean | –28.5 ± 0.3 | –28.2 ± 0.7 (–26.9 ± 0.9) | –27.5 ± 0.3 (–26.6 ± 0.7) | –28.9 ± 0.2 | –27.0 ± 0.3 (–26.7 ± 0.9) | –27.0 ± 0.7 |
- Note: Current-year tree-ring δ13C data are from Tang et al. (2023a).
- Abbreviations: SD, standard deviation; WSCs, water-soluble carbohydrates.
3.1.2 δ13C differences among trees
Twig resin δ13C had different seasonal patterns and absolute values (p < 0.007 for all pairwise comparisons) between different trees (Table 1, Supporting Information S1: S2; Supporting Information S1: Figure 2a). In comparison, stem resin δ13C showed similar seasonal trends for trees 2, 4 and 5 (Supporting Information S1: Table 3; Supporting Information S1: Figure 2b), but had significantly different absolute values among all the trees (p < 0.03 for all pairwise comparisons; Table 1). For example, the stem resin δ13C of tree 5 was 1.9‰ higher than that of the other trees (p < 0.001; Table 1; Supporting Information S1: Figure 2b). Interestingly, tree 5 also had 1.2‰ higher δ13C values in phloem WSCs compared with the other trees (p < 0.001; Table 1; Supporting Information S1: Figure 2d). Additionally, there were positive correlations between tree-paired resin δ13C and WSC δ13C, both averaged annually, in stems (Pearson r = 0.94, p = 0.02), albeit not significant in twigs (r = 0.55, p = 0.33).
3.1.3 δ13C differences between twigs and stems
The δ13C offsets between twig resin and stem resin varied significantly from tree to tree (p < 0.02 for all pairwise comparisons), in the order of tree 3 (–0.3 ± 0.5‰) <tree 1 (0.2 ± 0.3‰) <tree 2 (1.7 ± 0.5‰). Correspondingly, the δ13C offsets between twig WSCs and phloem WSCs followed the same order: tree 3 (–0.8 ± 0.3‰) <tree 1 (–0.4 ± 0.5‰) <tree 2 (–0.1 ± 0.6‰), with only a significant difference between tree 3 and tree 2 (p = 0.03). Overall, the sucrose-to-resin δ13C offsets were not significantly different between the top-canopy and breast-height levels (1.8 vs. 2.1‰, p = 0.46; Table 1; Figures 2 and 3a).
3.2 Intra-seasonal resin δ18O
3.2.1 δ18O differences between resin and water pools
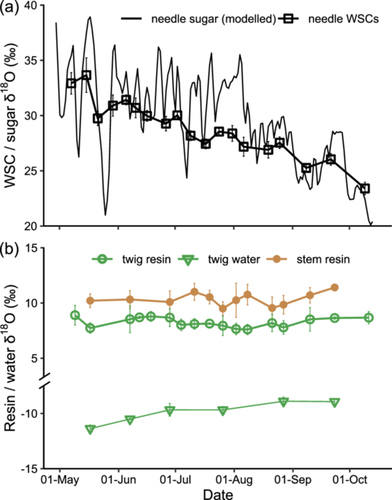
In 2019, mean resin δ18O in both twigs and stems did not follow the increasing trend in twig water δ18O or the decreasing trend in needle WSC δ18O (Figure 4). Mean δ18O values of twig resin, stem resin, twig water and needle WSCs varied by 1.3, 1.9, 2.5 and 10.3‰, respectively (Figure 4). Overall, δ18O values in resin, water and WSC pools were in the following order: twig water (–9.8 ± 0.9‰) <twig resin (8.3 ± 0.4‰) <stem resin (10.3 ± 0.6‰) <needle WSCs (28.8 ± 2.6‰) (p < 0.001; Table 2; Figures 3b and 4).
| Tree | Twig water | Twig resin | Stem resin | Needle WSCs |
|---|---|---|---|---|
| 1 | –9.9 ± 1.0 | 8.5 ± 0.6 | 9.6 ± 0.5 | 28.6 ± 2.5 |
| 2 | –9.8 ± 0.9 | 7.7 ± 0.7 | 9.8 ± 0.8 | 28.9 ± 2.8 |
| 3 | –10.0 ± 1.0 | 8.4 ± 0.7 | 10.6 ± 0.8 | 28.9 ± 2.8 |
| 4 | 11.2 ± 0.4 | |||
| 5 | 9.4 ± 0.7 | |||
| 6 | –9.5 ± 1.0 | 7.8 ± 0.2 | 29.1 ± 2.4 | |
| 7 | –9.9 ± 1.1 | 8.8 ± 0.4 | 28.8 ± 2.7 | |
| Mean | –9.8 ± 0.9 | 8.3 ± 0.4 | 10.3 ± 0.6 | 28.8 ± 2.6 |
- Abbreviations: SD, standard deviation; WSCs, water-soluble carbohydrates.
3.2.2 δ18O differences among trees
Twig resin δ18O was significantly higher in tree 2 than in tree 3 (p = 0.006) and tree 1 (p < 0.001), although there were no significant tree-to-tree differences in twig water δ18O (Table 2; Supporting Information S1: Figure 3a,c). Stem resin δ18O also differed between some of the trees. For instance, trees 3 and 4 had significantly higher δ18O values than the other trees (p < 0.04 for all pairwise comparisons) (Table 2; Supporting Information S1: Figure 3b).
3.2.3 δ18O differences between twigs and stems
Overall, stem resin δ18O was 1.9‰ higher than twig resin δ18O. This difference was significantly lower in tree 1 (1.0 ± 0.9‰) than in tree 2 (2.3 ± 0.6‰; p = 0.005) and tree 3 (2.5 ± 1.2‰; p = 0.02) (Table 2; Supporting Information S1: Figure 3a,b).
3.3 Environmental and physiological signals imprinted in resin δ13C and δ18O
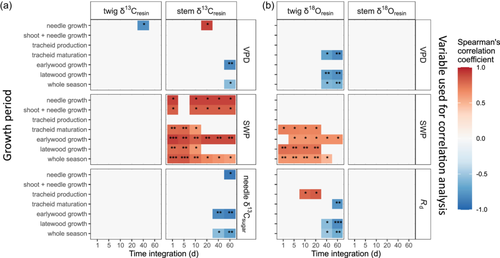
Resin δ13C and δ18O had varying correlations with environmental and physiological variables for different trees and different growth periods (Supporting Information S1: Figures 4 and 5). For instance, during the shoot and needle growth period, twig resin δ13C was positively correlated with needle sugar δ13C for tree 3, but not for the other trees (Supporting Information S1: Figure 4a). Likewise, stem resin δ13C of tree 3 was positively correlated with VPD and needle sugar δ13C during the latewood growth period, but not within the early growing season (Supporting Information S1: Figure 4b). In comparison, trees 2, 4, and 5, which exhibited similar seasonal trends in stem resin δ13C (Supporting Information S1: Table 3), all recorded a SWP signal in stem resin δ13C for the whole growing season (Supporting Information S1: Figure 4b).
Across all trees, environmental and physiological variables correlated better with resin δ13C in stems than in twigs, but better with resin δ18O in twigs than in stems (Figure 5; Supporting Information S1: 4 and 5). Out of all the tested variables, SWP showed the strongest correlations with both resin δ13C and δ18O (Figure 5; Supporting Information S1: 4 and 5).
4 DISCUSSION
4.1 13C-depletion in resin relative to assimilates
Resin δ13C was on average 1.8‰ and 2.1‰ lower than sucrose δ13C at top-canopy and breast-height levels, respectively (Table 1; Figures 2 and 3a). This offset aligns with the finding that stem resin in mature P. edulis was c. 0.4‰ more 13C-depleted compared to leaf WSCs (Gaylord et al., 2013), which are further depleted in 13C than sucrose due to the presence of 13C-depleted compounds like pinitol (Rinne et al., 2015; Tang et al., 2023b). Likewise, volatile isoprene from leaves, which shares a similar biotic pathway with resin (Hemmerlin et al., 2012), has been reported to be 13C-depleted relative to assimilated CO2, albeit to a larger extent (3‰ to 11‰, Affek & Yakir, 2003; Sharkey et al., 1991).
The relative 13C-depletion of resin compared to sucrose may be partly related to the loss of C-1 atoms of pyruvate during the synthesis of resin precursors, namely isopentenyl pyrophosphate (IPP) (Figure 1). Specifically, in the plastidic methylerythritol phosphate (MEP) pathway (Hemmerlin et al., 2012), only one C-1 atom is preserved for every two pyruvate molecules (Chikaraishi et al., 2004). Alternatively, in the mevalonic acid (MVA) pathway occurring in cytosol (Hemmerlin et al., 2012), no C-1 atoms of pyruvate are retained in IPP (Chikaraishi et al., 2004). Since the C-1 atoms of pyruvate originate from the 13C-enriched C-3 and C-4 positions of the glucose unit (Gilbert et al., 2012), their loss could cause a depletion in 13C of c. 2.2‰ in IPP synthesized via the MVA pathway, and c. 0.9‰ via the MEP pathway, according to the intramolecular 13C-patterns in glucose unit (Gilbert et al., 2012) (Supporting Information S1: Note S3). Additionally, the 13C-depletion of resin may also be attributed to kinetic isotope effects occurring during terpene biosynthesis from IPP, which involves the C–C bond formations and cleavages (Tan et al., 2018).
However, our results are inconsistent with previous studies, which reported 13C-enrichment in resin by c. 1.7‰ and 2.5‰, compared with modern bulk leaf matter (Dal Corso et al., 2017) and fossil wood (Dal Corso et al., 2011), respectively. This inconsistency can be attributed to the following reasons. First, bulk leaf δ13C can substantially deviate from sucrose δ13C. As seen in Scots pine trees at our site, bulk leaf δ13C was up to 3‰ lower than sucrose δ13C (Tang et al., 2023b), due to the presence of 13C-depleted compounds, such as lignin and lipids, in the bulk matter (Bowling et al., 2008). Second, Dal Corso et al. (2011, 2017) analyzed fossilized or solid resin, meaning that the resin, leaves and wood could have been formed at different years with contrasting environmental conditions, thus possibly biasing their δ13C comparisons.
4.2 18O-enrichment in resin relative to plant water
Resin δ18O in twigs and stems was c. 18 and 21‰, respectively, higher than twig water δ18O (Table 2; Figures 3b and 4b). This 18O-enrichment of resin can be attributed to two mechanisms (Figure 1). The first mechanism involves discrimination against 18O during diterpene oxidation, which is catalyzed by Cytochrome P450 enzymes (Hamberger et al., 2011). This process has a fractionation factor of c. 1.020 (Schmidt et al., 2001), and can result in a δ18O value of 3.5‰ for synthesized resin when accounting for the δ18O value of ambient O2 at 23.5‰ (Affek & Yakir, 2014) (Supporting Information S1: Note S3). The second mechanism involves oxygen exchange between the carbonyl group of resin and water, which can cause an 18O-enrichment of c. 27‰ in the carbonyl group above water (Sternberg & DeNiro, 1983; Yakir & DeNiro, 1990). Assuming that twig water δ18O represents xylem water δ18O (Zhao et al., 2016), the oxygen exchange rate for resin would be c. 35% in twigs and c. 50% in stems, based on isotope mass balances (Supporting Information S1: Note S3). However, it should be noted that these values may vary with the 18O-fractionation factor during resin oxidation.
δ18O of twig and stem resin was 20.5 and 18.5‰, respectively, lower than δ18O of needle WSCs (Figures 3b and 4; Table 2), which in turn was c. 2‰ more 18O-enriched than tree-ring cellulose at our site (data not shown). Likewise, Nissenbaum et al. (2005) reported 18O-depletion in resin relative to cellulose by c. 20‰, although it should be noted that their comparison was not based on the same species. These δ18O offsets can be attributed to different isotope fractionation processes associated with the synthesis of WSCs, cellulose, and resin, as well as oxygen atom exchange with surrounding water at their respective locations. Unlike resin δ18O, δ18O of needle WSCs is mainly determined by evaporative enrichment of leaf water and biochemical fractionation associated with oxygen isotope exchange between carbonyl oxygen and leaf water (Gessler et al., 2013), alongside sugar pool size and turnover rate (Song et al., 2014; Leppä et al., 2022). δ18O of tree-ring cellulose is further affected by oxygen exchange with stem water (Gessler et al., 2013; Roden et al., 2000; Song et al., 2022).
4.3 Implications of among-tree and within-tree differences in resin δ13C and δ18O
Variation in resin δ13C between different trees averaged annually, showed a positive correlation with the variation in WSC δ13C for stems (Pearson r = 0.94; p = 0.02), though not significant for twigs (r = 0.55; p = 0.33). This suggests that differences in resin δ13C among trees are indicative of tree-to-tree differences in assimilate δ13C. It thus supports the notion that resin δ13C can record the physiological responses of stressed trees (McKellar et al., 2011), which have distinct assimilate δ13C values from healthy trees (Churakova (Sidorova) et al., 2018). Building on this, it is important to note that there is some variability among trees in their ability to record environmental variability in resin δ13C. For instance, unlike the other trees, tree 3 displayed a significant correlation between resin δ13C, needle sugar δ13C and VPD (Supporting Information S1: Figure 4). This specificity among trees may imply a higher resin production rate in tree 3, either inherently or triggered by biotic and abiotic stresses (Keeling & Bohlmann, 2006). The observed differences in resin δ18O among trees (Supporting Information S1: Figure 3), unlikely to be due to differences in xylem water δ18O (Table 2), necessitate further investigation of the role of oxidation-related 16O/18O fractionation and carbonyl-water exchange of oxygen.
Twig-to-stem δ13C offsets varied among trees, due to divergent temporal variations in twig resin δ13C and dampened seasonal patterns in stem resin δ13C (Supporting Information S1: Figure 2a, b). This is likely due to the faster turnover rate of resin in twigs than in stems (Gershenzon et al., 1993 and reference therein), which results in more diverse seasonal variability of resin δ13C in twigs. Furthermore, twig-to-stem δ18O offsets likely arise from differences in oxidation-related 16O/18O fractionation and carbonyl-water exchange of oxygen, given the consistent xylem water δ18O signals in stems and twigs (Zhao et al., 2016).
4.4 Are resin δ13C and δ18O good proxies for environmental and physiological studies?
Stem resin δ13C was positively correlated with SWP (Figure 5a). Likewise, McKellar et al. (2011) detected significantly higher resin δ13C values in water-stressed trees compared with control trees. Indeed, soil drought can cause stomatal closure, resulting in higher δ13C values in assimilates and subsequently derived compounds (Galiano et al., 2017; Rinne et al., 2015). However, we did not find significant correlations between resin δ13C and assimilate δ13C (Figure 5a). This discrepancy suggests that there may be other, currently unclear mechanisms mediating the impact of SWP on carbon isotope fractionations during resin synthesis.
Similarly, twig resin δ18O also recorded a SWP signal (Figure 5b). Mild water stress, known to promote resin production (Rissanen et al., 2021), could potentially shorten the turnover time of resin in the resin-producing cells, that is, epithelium, before its secretion into resin ducts (Cabrita, 2018). This accelerated secretion might limit the time available for carbonyl-oxygen exchange with water during resin synthesis, potentially leading to a decrease in resin δ18O. Furthermore, resin δ18O showed positive correlations with Rd in twigs (Figure 5b), suggesting that competition for O2 between resin oxidation and Rd could impact the 16O/18O fractionation for resin oxidation. On the other hand, the lack of significant correlations between resin δ18O and xylem water δ18O in twigs (Spearman p = 0.56) or stems (p = 0.80) can be partly attributed to the temporal integration of resin δ18O, contrasting with the transient nature of xylem water δ18O. Additionally, the correlation can be dampened due to varying oxygen exchange rates between resin and xylem water under different conditions.
There are also limitations in using resin δ13C and δ18O as tracers of seasonal environmental and physiological changes. First, the correlations between isotope compositions in resin and environmental and physiological signals varied not only across different growth periods but also between twigs and stems and among trees (Figure 5; Supporting Information S1: 4 and 5). This indicates that there are temporal, within-tree and among-tree dynamics affecting resin production rates. Second, resin δ13C and δ18O had weaker correlations with environmental variables compared with needle sugar δ13C and δ18O at our study site (Leppä et al., 2022). This can be attributed partly to the dampened seasonal fluctuations in resin δ13C and δ18O caused by the existing resin pool (Figures 2 and 4) (Gaylord et al., 2013), and partly to the high among-tree variability in resin signals. Third, while δ13C remains invariant once the resin hardens (Dal Corso et al., 2017), δ18O in fossilized resin can be further modified due to oxygen exchange with environmental water (Stern et al., 2008), especially when resin is preserved in moist environments such as aquatic sediments. This can complicate the environmental interpretation of fossilized resin δ18O.
4.5 Conclusion
In this study, we quantified the δ13C and δ18O offsets between resin and their potential precursor molecules and investigated the imprints of physiological and environmental signals in resin δ13C and δ18O over a single season. Our results showed that resin was c. 2‰ more depleted in 13C than sucrose and c. 20‰ more enriched in 18O than xylem water. Furthermore, we found a strong SWP signal imprinted in stem resin δ13C and twig resin δ18O at an intraseasonal scale. These results indicate the need for further investigation into the isotopic fractionation mechanisms associated with resin synthesis. Future experiments should aim to validate the potential fractionation mechanisms and assess how they vary under different environmental conditions. This knowledge can help to improve the interpretation of environmental and physiological signals from (sub)fossil resin δ13C and δ18O records.
ACKNOWLEDGEMENTS
Many thanks to Aino Seppänen for water extraction, to Fana Teferra and Marine Manche for carbohydrate extraction, to Natalia Kiuru and Nikol Ilchevska for micro-core preparation, and to Kersti Leppä for providing modelled data of needle sugar δ13C and δ18O. We also greatly thank our reviewers for their constructive comments and suggestions. This study was financially supported by the European Research Council (no. 755865), the Academy of Finland (no. 295319, 341984, 343059, 357902), the Academy of Finland Flagship Program (no. 337549) and the China Postdoctoral Science Foundation (no. 2024M750075).
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.




