Caught between two states: The compromise in acclimation of photosynthesis, transpiration and mesophyll conductance to different amplitudes of fluctuating irradiance
Abstract
While dynamic regulation of photosynthesis in fluctuating light is increasingly recognized as an important driver of carbon uptake, acclimation to realistic irradiance fluctuations is still largely unexplored. We subjected Arabidopsis thaliana (L.) wild-type and jac1 mutants to irradiance fluctuations with distinct amplitudes and average irradiance. We examined how irradiance fluctuations affected leaf structure, pigments and physiology. A wider amplitude of fluctuations produced a stronger acclimation response. Large reductions of leaf mass per area under fluctuating irradiance framed our interpretation of changes in photosynthetic capacity and mesophyll conductance as measured by three separate methods, in that photosynthetic investment increased markedly on a mass basis, but only a little on an area basis. Moreover, thermal imagery showed that leaf transpiration was four times higher under fluctuating irradiance. Leaves growing under fluctuating irradiance, although thinner, maintained their photosynthetic capacity, as measured through light- and CO2-response curves; suggesting their photosynthesis may be more cost-efficient than those under steady light, but overall may incur increased maintenance costs. This is especially relevant for plant performance globally because naturally fluctuating irradiance creates conflicting acclimation cues for photosynthesis and transpiration that may hinder progress towards ensuring food security under climate-related extremes of water stress.
1 INTRODUCTION
In natural conditions, steady continuous irradiance is exceedingly rare, even at the top of the canopy (Durand et al., 2022; Kaiser et al., 2018b). The wind, clouds, and diurnal patterns of the sun's position in the sky provide the fundamental impetus for creating fluctuations in the radiation received by plants. Movement in the canopy, altering leaf angle and boundary layer conductance, can be generated by just a gentle breeze; affecting the amount and composition of radiation incident on the leaf (Burgess et al., 2016; Durand & Robson, 2023). In turn, this will determine CO2 assimilation, stomatal opening and photosynthetic enzyme activation in the short and long term (Morales & Kaiser, 2020; Slattery et al., 2018). To prevent a ‘cultural glass wall’ between field and lab researchers (Poorter et al., 2016), it is crucial to consider how the ecophysiology of controlled and field-grown plants differs with regard to light fluctuations. Not only will this enable us to design more-realistic controlled experiments, but it will also ensure that our results are applicable in the field. It is thus not surprising that interest is growing in understanding the mechanisms by which plants respond to light fluctuations. This process may hold potential to improve biomass production (Lawson et al., 2012; Ort et al., 2015) by identifying desirable morphological and physiological traits that can be exploited for targeted selection (Kromdijk et al., 2016; Murchie et al., 2009).
So far, most attention has been focused on the rapid regulation of photosynthetic processes in response to irradiance fluctuations (Durand et al., 2022; Kromdijk et al., 2016; Murchie & Ruban, 2020; Yamori, 2016) or dynamic (i.e. reversible) acclimation responses (Alter et al., 2012; Tikkanen et al., 2010; Yin & Johnson, 2000). Meanwhile, developmental (i.e., irreversible) acclimation response to fluctuating irradiance, here defined as a change in trait value compared to plants grown under steady irradiance, is still largely unexplored. A reduction in LMA of leaves growing under fluctuating irradiance may be the only response that has to date consistently been reported in the literature (Grieco et al., 2012; Kaiser et al., 2018a; Kubásek et al., 2013; Leakey et al., 2002; Vialet-Chabrand et al., 2017; Watling et al., 1997). Still, in their review, Morales and Kaiser (2020) reported that many studies do not compare fluctuating irradiance treatments against the same average steady irradiance as a control treatment (Bellafiore et al., 2005; Caliandro et al., 2013; Kulheim & Jansson, 2005; Suorsa et al., 2012; Tikkanen et al., 2010; Watling et al., 1997; Yin & Johnson, 2000). Moreover, patterns chosen often produce low-frequency fluctuations lasting 3 min or more (Cruz et al., 2016; Kubásek et al., 2013; Leakey et al., 2002), but recent evidence revealed that most light fluctuations are much faster, operating at frequencies shorter than a second (Durand & Robson, 2023; Kaiser et al., 2018b). Other recent studies have used realistic irradiance fluctuations, although their properties were undefined (Matthews et al., 2018; Vialet-Chabrand et al., 2017). Consequently, we are still missing a characterization of how plants develop under specific patterns of irradiance fluctuations, especially since these patterns are diverse, and specific to each canopy depending on its structure (Durand & Robson, 2023).
An increase in irradiance on the leaf also brings a rise in temperature, because part of the energy from the incident photons is converted into heat. Most plants have thin leaves that would severely warm if not for stomata-controlled transpiration allowing for the regulation of leaf temperature (Marchin et al., 2022; Ye et al., 2013). Therefore, changes in temperatures during natural irradiance fluctuations have the potential to dynamically affect transpiration rates, especially in the case of stomatal control lagging behind the pace of high-frequency irradiance fluctuations. Still, whether transpiration is increased due to cumulative radiative loading, or decreased because of intermittent recovery periods, remains to be determined. A change in leaf temperature will also modify the activity of photosynthetic reactions such as RuBP regeneration and CO2 fixation by Rubisco (Bernacchi et al., 2002; Medlyn et al., 2002). Moreover, control of CO2 diffusion into the leaf for photosynthesis operates through stomata, along with mesophyll conductance, itself partly determined by the dynamic positioning of chloroplasts (Tholen et al., 2008). Conductance within the mesophyll and chloroplast movements are known to be affected by both light (Banas et al., 2012; Pang et al., 2023) and temperature (Bernacchi et al., 2002; Łabuz et al., 2015; von Caemmerer & Evans, 2015). It is consequently likely that changes in irradiance and temperature will affect photosynthesis rates, even outside of any dynamic regulation (see e.g., Leakey et al., 2003). This could have substantial repercussions for water-use efficiency (hereafter defined as the ratio of leaf-level CO2 assimilation to transpiration), a metric of importance when considering crop and forest productivity under climate change-induced water stress (Condon et al., 2004).
Our previous research highlighted how the pattern of light fluctuation (amplitude, duration, frequency and spectral composition) is governed by the surrounding canopy architecture (Durand & Robson, 2023). Here, we examined how specific differences in the amplitude of fluctuations affect developmental acclimation. We grew Arabidopsis thaliana (L.) plants under irradiance fluctuations of various intensities. Comparing wild-type plants with a mutant deficient in chloroplast movement response allowed us to examine whether potential changes in mesophyll conductance under fluctuating irradiance could be attributed to chloroplast positioning. We also investigated how leaf transpiration is dynamically affected by rapid irradiance fluctuations. We posited the following hypotheses: (1) acclimation to fluctuating irradiance is dependent on the amplitude of fluctuations and it shares some characteristics of both acclimation to high and low irradiances, (2) fluctuating irradiance reduces photosynthetic capacity and will modify transpiration dynamically, (3) mesophyll conductance is affected constitutively in plants growing under a range of irradiance fluctuation patterns.
2 MATERIALS & METHODS
2.1 Plant material and growth conditions
We used the Columbia-0 Arabidopsis thaliana (L.) Heynh. wild type (WT) and the loss-of-function mutant jac1 which lacks the locus AT1G75100 encoding a J-domain protein required for the chloroplast accumulation response, but not for the avoidance response (Hermanowicz et al., 2019; Suetsugu et al., 2005). All seeds were produced in July 2022, by sowing seeds in Jiffy-7 peat pots which were placed in a Percival Scientific breeding chamber (CLF Plant Climatics). The photoperiod was 16 h of light, 8 h of darkness at 21°C for 3 weeks, 60% of humidity. When the flowering bud emerged, the plants were placed in a growing room at 18–24°C, without humidity regulation, with a photoperiod of 16 h of light and 8 h of darkness, light intensity 100–150 μmol m-2 s-1. Before sowing, seeds were hydrated and kept in the dark at 1°C for 24 h. They were sown in 7-by-7-cm square plastic pots filled with a 1:1 mix of limed peat and vermiculite and watered to capacity. The sown seeds were kept in a controlled environment walk-in growth room, in trays with a transparent cover. Light was supplied by fluorescent tubes (L58W/865 Lumilux, Osram; Supporting Information S1: Figure 1) providing 240 µmol m-2 s-1 of photosynthetically active radiation (PAR) (12 h, 7:00–19:00). Relative humidity was maintained at 50/60% and temperature at 23/19°C day/night. The plants were kept well-watered by regular watering. After 7 days, individual plants were transplanted onto a 6-by-6-cm square plastic pot filled in an inverted cone shape (Flexas et al., 2007b) with the same substrate and left to grow in the same conditions until transplantation success was confirmed. Plants were then moved to one of three growth chambers (FitoClima 1200, Aralab) under different light treatments.
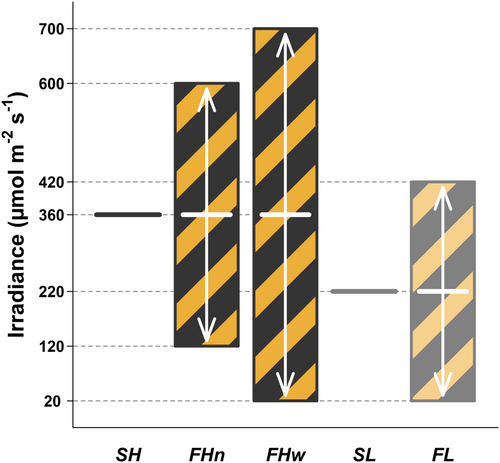
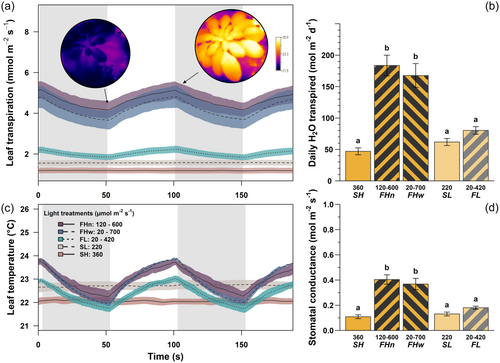
The experiment was repeated in three successive phases (P1-P3), starting with sowing (Day 0) on January 19th, February 20th and March 17th 2023, with transport to the growth chambers on Day 19 (P1), 22 (P2) and 17 (P3). All fluctuating irradiance treatments had a corresponding steady treatment of same average irradiance, and all fluctuations followed a square-wave pattern and lasted 50 s every 100 s (0.01 Hz, 50% duty, that is the light intensity was changed every 50 s). This pattern was conceived to be both rapid enough to echo natural fluctuations and slow enough to have the potential to affect Rubsico, chloroplasts and stomata physiology (Durand et al., 2022). Light treatments were as follows (Figure 1): steady high irradiance (SH: 360 µmol m–2 s–1) and corresponding fluctuating irradiance of either narrow (FHn: 120–600 µmol m–2 s–1) or wide amplitude (FHw: 20–700 µmol m–2 s–1), and steady low irradiance (SL: 220 µmol m–2 s–1) with corresponding fluctuating irradiance (FL: 20–420 µmol m–2 s–1). Light levels were partly chosen based on earlier tests which indicated photosynthesis saturated near 400 µmols m–2 s–1 in these plants. We also wanted to allow comparisons between FHw and FL based on the low part of the fluctuation (20 µmol m–2 s–1). Four replicate plants were grown per treatment combination of light, genotype and phase (i.e., n = 12 biological replicates unless otherwise stated). During each phase, light treatments were changed to another chamber to account for potential chamber effects, and plants were regularly moved around within the chamber to homogenize their light environment. Conditions followed a 11 h/13 h day/night cycle, with relative humidity at 60/70%, and temperature at 22/16°C. These conditions allowed enough time to complete all measurements on mature leaves grown entirely under the chamber conditions, before flowering. Light treatments lasted 24, 18 and 26 days respectively for P1, P2 and P3 counting from their placement in the growth chamber to the end of the measurements; starting on Days 32, 31, and 32 respectively. Leaves were selected visually based on their size, age and ability to fit inside the LI-6800 gas exchange chamber. Light treatments were adjusted by selectively switching off part of the LED panels (Supporting Information S1: Figure 1, Lumitronix) using a pulse generator (YoctoHub-Ethernet, Yoctopuce).
2.2 Leaf traits and biomass
We measured area-based chlorophyll, and epidermal flavonols and anthocyanins indexes nondestructively (Cerovic et al., 2012), using an optical leaf clip Dualex Scientific+ (Force-A, University Paris-Sud). We measured the adaxial side of one randomly selected mature leaf per plant once per phase, on Day 37. Since some plants showed the first sign of the inflorescence meristem forming, they were harvested immediately after the end of measurements, on Days 42 (P1), 39 (P2) and 41 (P3). On another randomly selected mature leaf per plant, we measured fresh mass and leaf area using ImageJ (Schneider et al., 2012). The sampled leaf and the rest of the whole plant were then placed in a drying oven at 60°C for 3 days, then their dry mass was weighed. Leaf mass per area (LMA) was calculated as dry mass over leaf area. Area-based pigment index was then recalculated as mass-based values using LMA.
2.3 Light- and CO2-response curves
Leaf gas-exchange measurements were done using a portable photosynthesis system (LI-6800, LI-COR, Lincoln) placed inside the growth chamber; measuring three replicate plants from each of the three phases (n = 9) between Days 32 and 42. We selected the first fully expanded leaf that completely grew under growth-chamber conditions. In all cases, leaves were first left to acclimate inside the leaf cuvette, held in the same conditions as those within the growth chamber (CO2 concentration: 400 ppm, block temperature: 22°C, RH: 60% and flow: 500 µmol s–1) and at saturating irradiance (PAR: 1000 µmol m–2 s–1), until stomatal conductance (gs) reached a steady state. Response curves were performed by making the following stepwise changes in irradiance (1800-1400-1000-750-500-400-300-250-150-50-0 µmol m–2 s–1 photons) or CO2 (400-250-150-100-50-0-400-800-1100-1400-1800 ppm). Conditions were equilibrated for 2 min before logging, then a saturating flash was done to obtain chlorophyll fluorescence parameters. A further two CO2-response curves were done at 200 and 50 µmol m–2 s–1 irradiance, after the initial CO2-response curve at 1000 µmol m–2 s–1; waiting for 10 min for gs and the induction state of photosynthesis to reach a steady state. At the end of the measurements, the leaf thermocouple was removed, and the leaf was photographed inside the chamber. In cases when the leaf did not completely fill the cuvette, we corrected the data per leaf area using ImageJ (Schneider et al., 2012). Light-response curves were done after all CO2-response curves were completed. Moreover, all gas-exchange data was corrected for leaks using CO2-response curves taken with an empty chamber.
2.4 Mesophyll conductance
We measured Ci* using the Laisk method (Brooks & Farquhar, 1985; Laisk, 1977), as the CO2 value at the intersection of the three CO2-response curves done at 1000, 200 and 50 µmol m–2 s–1 irradiance. Correspondingly, and by the same method, an estimate of Rd is given as the CO2 assimilation at Ci*.
Since mesophyll conductance (gm) estimation is prone to errors, we cross-calibrated our results using three separate methods of gm estimation. The constant J method described by Harley et al. (1992) to estimate gm assumes that both J and gm are constant when photosynthesis is limited by RuBP regeneration under saturating light. Using those sections of the CO2-response curve for which these conditions occur (which we found to be when atmospheric CO2 concentration was ≥800 ppm, as we determined graphically using fluorescence data), we calculated J by combining Equations 2, 3 and 4, and measured values of Ci* and Rd. The best resulting estimate of gm is the one that minimizes the variance of J.
Finally, the third approach used to estimate gm, along with the photosynthetic capacities Vcmax and Jmax (respectively, the maximum carboxylation rate of Rubisco and the maximum electron transport rate) involved modelling the full biochemical model of photosynthesis from Farquhar, Von Caemmerer & Berry (1980, hereafter the FvCB model) following Ethier and Livingston (2004) to our CO2-response curves (Supporting Information S1: Figure 3). Rubisco kinetic parameters used for the FvCB model followed Bernacchi et al. (2002), temperature-response parameters of Vcmax and Jmax were taken from the widely used ‘plantecophys‘ R package (Duursma, 2015), Rd and Ci* were measured, and subsequently used to calculate Γ* during the fitting using Equation 4. Parameters of the model were fitted using a genetic algorithm, which are stochastic search algorithms inspired by natural selection (Lucasius & Kateman, 1993), and were found to be powerful tools to use the FvCB model with gm (see Su et al., 2009). Sets of parameters (Vcmax, Jmax and gm) were evaluated based on how well they fit the data. At each iteration, the best-fitting sets were kept and crossed over to generate a new population. We used a population size of 500 and 10 000 iterations per curve. A 0.02 mutation chance was applied to control the probability of parameters to be randomly assigned a new value to stimulate diversity and exploration of the parameter space. To constrain gm to lower estimates, the evaluation function of the genetic algorithm defining the best fit was the sum of the root mean square deviation between measured and modelled data using the fitted parameters, and gm itself. This approach was used to prevent the fitting procedure from tending toward biologically impossible values of gm.
2.5 Thermal imagery and dynamic transpiration
2.6 Statistical analyses
Statistics were done using R 4.3.1 (R Core Team, 2023, all data used are available in Supporting Information S1: Table 1). We used a linear mixed model ANOVA with genotype and light treatments as fixed effects using the R packages: ‘car‘ (Fox & Weisberg, 2019), ‘lm4’ (Bates et al., 2015) and ‘lmerTest’ (Kuznetsova et al., 2017). Both the phase (P1-P3) and the chamber were considered as random effects in the model. Residual normality and homoscedasticity were checked graphically. Post hoc pairwise contrast analyses were done to detect differences among factor levels, and P values were adjusted to control for the false discovery rate using ‘emmeans’ (Searle et al., 1980) and ‘multcomp’ (Hothorn et al., 2008). We considered differences significant when p < 0.05 for all tests.
We also performed a principal component analysis (PCA) using the harvest data, pigment index, light and CO2-response parameters, and gm. All data used in the PCA analyses were scaled and mass-based. We fitted a linear model, that included the phase and chamber, to every variable and used the residuals of these models for the PCA so that the main effects (genotype and light treatment) were emphasized.
3 RESULTS
3.1 The effect of irradiance fluctuations on biomass and pigments depends on LMA
We found a large significant decrease in LMA when A. thaliana WT plants were subjected to irradiance fluctuations (by 32%–49% in FHn and FHw, p < 0.001; Table 1), or to lower steady irradiance (by 34% in SL, p <0.001) compared against steady high irradiance (SH). This effect was stronger for a wider amplitude of irradiance fluctuations, that is between FHn and FHw, (p = 0.0007). On the contrary, relative water content (RWC) was highest under irradiance fluctuations (FHn and FHw) or low steady irradiance (SL) than in SH. Similarly, those plants growing under irradiance fluctuations also had higher total leaf area (TLA) by 36% under high (FHn-w compared to SH) and 42% under low irradiance (FL compared to SL), respectively. Because there was only a nonsignificant tendency (FHn compared to SH and FL compared to SL, p > 0.07) for increased leaf number under irradiance fluctuations (except for a significant 36% increase in FHw compared to SH; p = 0.0001), increased TLA was mainly due to larger leaves rather than more numerous leaves. Total dry mass (TDM) was similar among light treatments (except for a 56% decrease in FHw compared to SH; p = 0.007), because increased TLA under irradiance fluctuations counterbalanced lower LMA. Similar results were found in WT and jac1 plants.
| Light treatments | LMA | RWC | TLA | TDM | Number of leaves | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (µmol m–2 s–1) | (mg cm–2) | (%) | (cm2) | (mg) | |||||||
| SH: 360 | WT (Col-0) | 4.76 ± 0.15 | c | 79.9 ± 0.6 | a | 46.3 ± 5.6 | a | 222.1 ± 28.7 | b | 14.3 ± 0.6 | a |
| FHn: 120–600 | 3.04 ± 0.12 | b | 86.4 ± 0.6 | b | 65.7 ± 8.7 | b | 205.9 ± 31.3 | ab | 16.7 ± 1.0 | ab | |
| FHw: 20–700 | 2.41 ± 0.07 | a | 89.0 ± 0.4 | c | 67.4 ± 6.6 | b | 166.6 ± 18.4 | a | 19.5 ± 0.9 | c | |
| SL: 220 | 3.13 ± 0.20 | b | 86.4 ± 0.9 | b | 70.7 ± 8.5 | b | 202.3 ± 21.8 | ab | 17.0 ± 1.5 | abc | |
| FL: 20–420 | 2.11 ± 0.07 | a | 89.7 ± 0.5 | c | 92.6 ± 12.0 | c | 206.2 ± 28.3 | ab | 18.1 ± 0.6 | bc | |
| SH: 360 | jac1 | 4.16 ± 0.13 | c | 82.4 ± 0.5 | a | 41.8 ± 5.5 | a | 181.6 ± 25.7 | bc | 15.2 ± 0.8 | a |
| FHn: 120–600 | 3.30 ± 0.08 | b | 85.9 ± 0.3 | b | 64.0 ± 7.3 | c | 215.0 ± 26.2 | c | 16.6 ± 0.8 | a | |
| FHw: 20–700 | 2.30 ± 0.07 | a | 89.4 ± 0.4 | c | 53.0 ± 6.6 | ab | 124.1 ± 16.9 | a | 17.6 ± 0.7 | a | |
| SL: 220 | 2.93 ± 0.21 | b | 86.4 ± 1.0 | b | 59.9 ± 6.8 | bc | 170.9 ± 20.2 | abc | 16.0 ± 1.0 | a | |
| FL: 20–420 | 2.13 ± 0.09 | a | 89.2 ± 0.6 | c | 77.3 ± 8.7 | d | 157.3 ± 16.1 | ab | 18.2 ± 0.9 | a | |
- Note: The irradiance levels are given in the table. Values are means ± 1 standard error (n = 12). Letters show significant differences (p < 0.05) by post hoc contrast among the five light treatments for each genotype.
Because LMA strongly affected many parameters that can be estimated on a mass or an area basis, we hereafter report analyses of both mass- and area-based data. Figures focus on mass-based data because they account for differences in leaf thickness/density, but classical area-based data are also provided (Table 2 & S2).
| Light treatments | Chlorophylls | Flavonols | Anthocyanins | |||||
|---|---|---|---|---|---|---|---|---|
| (µmol m–2 s–1) | ||||||||
| SH: 360 | WT (Col-0) | Area-based | 27.3 ± 1.3 | c | 0.681 ± 0.048 | d | 0.401 ± 0.047 | c |
| FHn: 120-600 | 24.8 ± 0.8 | b | 0.392 ± 0.038 | c | 0.328 ± 0.032 | b | ||
| FHw: 20-700 | 18.5 ± 0.5 | a | 0.316 ± 0.026 | b | 0.333 ± 0.019 | b | ||
| SL: 220 | 26.6 ± 0.9 | c | 0.357 ± 0.033 | bc | 0.278 ± 0.032 | a | ||
| FL: 20-420 | 18.4 ± 0.5 | a | 0.205 ± 0.011 | a | 0.286 ± 0.007 | a | ||
| SH: 360 | jac1 | 28.1 ± 0.8 | c | 0.551 ± 0.043 | d | 0.311 ± 0.032 | b | |
| FHn: 120-600 | 24.2 ± 0.8 | b | 0.412 ± 0.032 | c | 0.319 ± 0.026 | b | ||
| FHw: 20-700 | 17.8 ± 0.4 | a | 0.299 ± 0.022 | b | 0.311 ± 0.013 | ab | ||
| SL: 220 | 24.4 ± 0.9 | b | 0.316 ± 0.021 | b | 0.283 ± 0.026 | ab | ||
| FL: 20-420 | 19.0 ± 0.6 | a | 0.223 ± 0.014 | a | 0.271 ± 0.003 | a | ||
| SH: 360 | WT (Col-0) | Mass-based | 5.85 ± 0.37 | a | 0.140 ± 0.007 | b | 0.080 ± 0.007 | a |
| FHn: 120-600 | 8.45 ± 0.48 | bc | 0.128 ± 0.010 | b | 0.107 ± 0.009 | b | ||
| FHw: 20-700 | 7.73 ± 0.24 | b | 0.132 ± 0.011 | b | 0.140 ± 0.009 | d | ||
| SL: 220 | 9.26 ± 0.65 | c | 0.114 ± 0.007 | a | 0.087 ± 0.005 | a | ||
| FL: 20-420 | 8.48 ± 0.36 | c | 0.097 ± 0.005 | a | 0.129 ± 0.004 | c | ||
| SH: 360 | jac1 | 6.72 ± 0.26 | a | 0.132 ± 0.010 | b | 0.075 ± 0.008 | a | |
| FHn: 120-600 | 7.39 ± 0.27 | ab | 0.127 ± 0.011 | b | 0.098 ± 0.009 | b | ||
| FHw: 20-700 | 7.84 ± 0.19 | b | 0.130 ± 0.008 | b | 0.137 ± 0.005 | c | ||
| SL: 220 | 9.09 ± 0.64 | c | 0.110 ± 0.004 | a | 0.097 ± 0.005 | b | ||
| FL: 20-420 | 9.12 ± 0.34 | c | 0.105 ± 0.004 | a | 0.132 ± 0.005 | c | ||
- Note: The irradiance levels are given in the table. Values are means ± 1 standard error (n = 12) and are given on an area basis (as measured), or on a mass basis calculated by dividing by the leaf mass per area. All values are arbitrary units. Letters show significant differences (p < 0.05) by post hoc contrast among the five light treatments for each genotype Statistical tests were done independently for area- and mass-based data.
Overall, area-based pigment indexes in the leaves for chlorophylls, epidermal flavonols and anthocyanins, were largely driven by differences in LMA (Table 2). On an area basis, both irradiance fluctuations as compared with steady irradiance, and low as compared with high irradiance tended to decrease all these pigment indices; by about 30% for chlorophylls (in all cases p < 0.0007); 45% for flavonols (in all cases p < 0.0001); and 20% for anthocyanins (p < 0.0019, except under low irradiance where p = 0.79). However, calculating mass-based pigment indexes revealed divergent trends. Under irradiance fluctuations compared with steady irradiance, the mass-based chlorophyll index increased by 38% on average under high irradiance (p < 0.0001) but not under low irradiance (p = 0.21). On a mass basis, epidermal flavonols still responded with a small but nonsignificant decrease under fluctuating as compared to steady light, both under high and low irradiance (p > 0.06), consistent with area-based results. Finally, the upper epidermal anthocyanin index increased by 38% under irradiance fluctuations in low irradiance (FL compared to SL), and by 34% or 75% in high irradiance for narrow (FHn) or wide (FHw) amplitudes of fluctuations respectively, as compared to SH. Again, the jac1 plants responded similarly to WT.
3.2 Irradiance fluctuations promote the production of more cost-efficient leaves
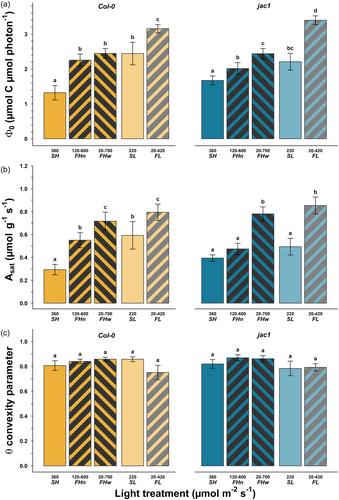
The initial quantum yield of photosynthesis (Φ0) in WT plants on a mass basis increased by 30% under fluctuating irradiance at low irradiance (Figure 2a, FL compared to SL), and by 70% or 85% at high irradiance and respectively narrow (FHn) or wide (FHw) amplitudes of fluctuations compared to SH (in all cases p < 0.0001). On an area basis, only the average irradiance but not fluctuations affected Φ0; increasing it by 22% in SL compared to SH (p = 0.002; Supporting Information S1: Table 2). At saturating irradiance, mass-based CO2 assimilation (Asat) followed a similar pattern under fluctuating irradiance compared to that under steady irradiance, increasing by 1.9 and 2.4 times in narrow and wide fluctuations respectively at high irradiance (Figure 2b, FHn-w compared to SH), and by 1.3 times at low irradiance (FL compared to SL, in all cases p < 0.0007). On an area basis, we only found Asat to be 39% higher under wide (p = 0.0002), but not narrow (p = 0.055) fluctuations, compared with steady irradiance (SH). There was no detectable difference in the convexity parameter θ between light treatments (Figure 2c).
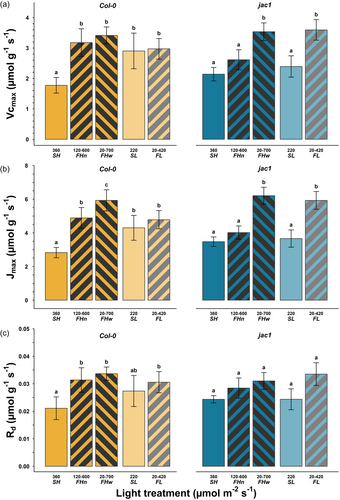
The mass-based maximum carboxylation rate of rubisco (Vcmax) was respectively 79% and 92% higher under narrow and wide irradiance fluctuations compared to steady irradiance (SH, Figure 3a, p < 0.0001) in the WT plants. Similarly, the maximum electron transport rate (Jmax) was 1.7 and 2.1 times as high under high fluctuating than steady light (SH), for narrow and wide fluctuations respectively (p < 0.0001). Daytime respiration (Rd) followed an equivalent pattern of increase under fluctuating irradiance. Under low light, we did not detect significant differences between fluctuating (FL) and steady (SL) light in the WT, but there was an increase in the response of jac1 plants under fluctuating irradiance (FL compared to SL, by 50% for Vcmax, 62% for Jmax, and 38% for Rd). As with the WT, we could not detect significant differences in Vcmax or Jmax between SH and FHn in jac1 plants. There was a tendency for higher Jmax under high compared to low light on an area basis, but differences in Vcmax and Jmax between light treatments on an area basis were generally not statistically significant.
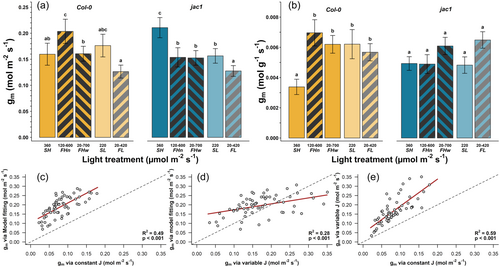
Mesophyll conductance (gm) decreased by 22% under fluctuating irradiance at low irradiance (FL compared to SL, marginally nonsignificant p = 0.08) but not at high irradiance where SHn increased compared with steady light control (p = 0.04), in WT plants on an area basis (Figure 4a). In jac1 plants, gm decreased by 26% under both narrow and wide fluctuations at high irradiance (FHn and FHw compared to SH, p < 0.005), and by 25% at low irradiance (FL compared to SL, p = 0.03). This pattern was reversed when considering gm on a mass basis (Figure 4b), whereby in WT plants gm increased by 88% under irradiance fluctuations compared to SH irradiance (p < 0.0002), but not at low irradiance (p = 0. 84). In jac1 plants, gm tended to increase by about 30% in FHw compared to SH and by 25% in FL compared to SL, but this tendency was not statistically significant (p = 0.08). Overall, all three methods of gm estimation were positively correlated with each other (p < 0.0001, R2 > 0.28; Figure 4c–e, Supporting Information S1: Figure 5).
3.3 Fluctuating irradiance governs dynamics of leaf temperature and transpiration
We found higher transpiration under fluctuating high light (FHn-w) than its steady light control (SH), and a similar nonsignificant tendency between fluctuating and steady under low light (FL compared to SL, Figure 5a,b). Increased transpiration under fluctuating irradiance was the result of both higher leaf temperatures (Figure 5c) and higher gs by as much as four times under high light (FHn-w compared to SH, p < 0.008; Figure 5d), but only by 37% under low light (FL compared to SL, and nonsignificant at p = 0.37). Daily H2O transpired was up to 3.9 times higher under fluctuating than steady light at high irradiance (p < 0.0001), and there was a similar but nonsignificant tendency at low irradiance (FL compared to SL, p = 0.45; Figure 5b).
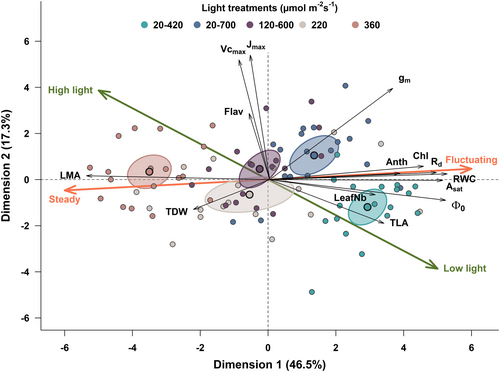
3.4 Principal component analysis reveals distinct axes related to fluctuations versus irradiance
The PCA segregated the data into five groups according to the light treatments (Figure 6). The first and second PC axes accounted for 46.5% and 17.3% of the total variance, respectively. The first four axes accounted for 84% of the total variance. LMA, RWC, chlorophyll index and the light-response parameters Φ0 and Asat contributed most to the first axis, while gm, flavonols and the CO2-response parameters Vcmax and Jmax contributed most to the second axis (Supporting Information S1: Figure 6). Overall, the first axis was mainly related to the differences in response between steady and fluctuating irradiance (red line in Figure 6), while the axis related to trait response to total irradiance formed a diagonal line and was therefore more closely associated with TLA, Vcmax and Jmax. The acute angle between the two axes is equal to 42.2° which implies that some variables contribute to both axes, such as LMA, Φ0 and Asat. On the other hand, mass-based gm appears to be related to the fluctuating/steady axis but not to total irradiance. Using area-based data resulted in similar results, although the axes corresponding to steady/fluctuating irradiance and high/low irradiance were more closely related along the first principal component (Supporting Information S1: Figure 7).
4 DISCUSSION
By subjecting individuals to steady or fluctuating irradiance of various amplitudes with differing average irradiance, we were able to distinguish between acclimation to irradiance fluctuations and to lower irradiance. Framing optical pigment indexes, as well as parameters related to photosynthetic capacity in response to light and CO2, and mesophyll conductance with regard to both leaf area and leaf mass provided a more integrative understanding of the acclimation process at play. Furthermore, our unique examination of the dynamics of leaf temperature under rapid (<1 min) irradiance fluctuations revealed an elevated transpiration rate, drawing attention to the interaction between fluctuating light and water use.
4.1 Irradiance fluctuations promote the production of more cost-efficient leaves
Different duration, frequency, amplitude and irradiance level of fluctuations are used in each study investigating irradiance fluctuations, but taken together fluctuating irradiance results in an overall 12% decline in LMA (Morales & Kaiser, 2020). Here, we found that the magnitude of this change depends not only on the average irradiance (Poorter et al., 2019) but also the strength of fluctuations; in that larger fluctuations (i.e., with a wider amplitude) reduced LMA the most. Furthermore, the concurrent increase in RWC with LMA suggests that growth under irradiance fluctuations produces leaves that are both thinner (Vialet-Chabrand et al., 2017) and less dense. Anatomically, these leaves would be less costly to build. The lower cost per leaf was associated with an increase in the TLA of plants growing under irradiance fluctuations, their TDM generally remained unchanged. Increased TLA may be an indication that plants receiving fluctuating irradiance may seek to escape such condition, much like a growth response to escape low irradiance (Poorter, 2002). Although Kaiser et al. (2018a) also found that plant mass did not change with fluctuating irradiance, a decline typically is reported from most studies (Kubásek et al., 2013; Leakey et al., 2002; Vialet-Chabrand et al., 2017). These latter experiments used peak irradiances of three-to-ten times those of the average irradiance. Evidence is scarce, but it is probable that reductions in biomass would be greater for more intense lightflecks; as was found in Lactuca sativa (Bhuiyan & van Iersel, 2021) in agreement with our results comparing SH and FHw (Table 1). The weaker of our irradiance fluctuation treatments did not produce a reduction in biomass, this indicates that the dynamic response of photosynthesis can compensate for the decrease in carbon gain that is inherent to irradiance fluctuations (due to the nonlinear response of photosynthesis to irradiance).
Leaves growing in the shade often have a reduced photosynthetic capacity (Jmax & Vcmax), partly due to their lower LMA (Earles et al., 2017; Liu et al., 2019). In our experiment under fluctuating irradiance, this was not the case. While Asat was higher in FHw than SH, similar to Burgess et al. (2023), this was not a general trend and both Asat and photosynthetic capacity were largely unchanged under fluctuating irradiance, in agreement with Vialet-Chabrand et al. (2017). In fact, we calculated that on a mass basis leaves growing under fluctuating irradiance had much higher photosynthetic capacity, Φ0 and Asat (Figures 2 and 3). This means that these leaves were much more cost-efficient to build, but on the other hand, their maintenance cost per unit mass would be higher due to their preparedness for high photosynthetic activity. The final balance of these costs depends on leaf longevity and the prevailing light environment over the leaf lifespan.
During the high irradiance phase of the irradiance fluctuations, photosynthesis is more likely to be limited by CO2 than insufficient light, especially since 95% light saturation occurred between 200 and 300 µmol m–2 s–1 for our studied plants (Supporting Information S1: Figure 2). gm has been found to respond to light dynamically (Theroux-Rancourt & Gilbert, 2017), and structural changes such as increases in leaf and cell wall thickness additionally modify gm when plants are grown under higher irradiances (Carriquí et al., 2021; Pang et al., 2023). There are some reports that gm may decrease under fluctuating irradiance (Vialet-Chabrand et al., 2017), although gm is notoriously difficult to measure, with many underlying assumptions and small measurement errors can lead to large variations in gm estimates (Pons et al., 2009). Moreover, studies often lack a control treatment with a corresponding irradiance, required if response to fluctuations is to be distinguished from that to average irradiance (e.g. in Huang et al., 2015). Here, we used three separate methods to estimate gm, and showed for the first time that a reduced gm trend under fluctuating versus steady irradiance, when calculated on an area basis, was nevertheless equivalent to an increase in gm on a mass basis when accounting for leaf thickness (Figure 4). The shorter pathlength of CO2 in the thinner leaves growing under irradiance fluctuations is likely to at least partly explain this result. Triose phosphate utilization (TPU) limitation was not accounted for in our study, which might have led to an underestimation of Jmax (Gregory et al., 2021) and of gm by the constant J method (Figure 4). Higher resolution data of the CO2-response near 800 ppm where TPU is not limiting would help reduce the difference between the constant J method and the other two methods. However, recent evidence shows TPU rate is often just higher than the typical CO2 assimilation rate (Fabre et al., 2019; Yang et al., 2016). If this is the case, it is unlikely that a different TPU rate between conditions would have significantly altered our conclusions, which may also contribute to the cross-correlation between the results of our three methods to estimate gm. Still, the TPU rate in dynamic irradiance conditions is still largely unknown, which requires further exploration because its effect has been shown to have importance for dynamic CO2 assimilation (McClain & Sharkey, 2023).
Moreover, jac1 plants differed from WT plants in that they also displayed increased gm under irradiance fluctuations at lower irradiance. In jac1, the accumulation response of chloroplasts is deactivated (Hermanowicz et al., 2019; Suetsugu et al., 2005), which may produce differential chloroplast positioning between plants growing under different patterns of irradiance. For example, a chloroplast avoidance response was found in jac1 mutants even under 1.6 µmol m–2 s–1 of blue light (Hermanowicz et al., 2019). Thus, it is possible that our distinct gm response to high and low fluctuating irradiance in WT and jac1 (Figure 4) may be the result of differences in the position of chloroplasts within the cell, consequently affecting the pathway of CO2 (Pang et al., 2023). Nevertheless, this pattern was not distinct enough for us to make this assertion with confidence. More research is still needed to determine if active acclimation processes related to chloroplast position are involved in the gm response to fluctuating irradiance. Although jac1 plants responded relatively similarly to the WT in our study overall, we know that gm is dynamically regulated and responds to changes in conditions within minutes (Douthe et al., 2012; Flexas et al., 2007a). Measuring gm dynamically in WT and jac1 plants along with nondestructive measurements of chloroplast movements would allow future research to assess the dynamic contribution of chloroplast position to the resistance to CO2 diffusion within the leaf.
4.2 Thinking about irradiance fluctuations differently
Without accounting for the dynamic response of photosynthesis, the asymptotic shape of the photosynthetic light-response curve implies that almost any fluctuation in light will lead to a reduction in the time-integrated photosynthetic rate. The only exception being at very low irradiances where the curve approaches linearity (Supporting Information S1: Figure S2). The potential loss in carbon assimilation increases exponentially with stronger fluctuations (Bhuiyan & van Iersel, 2021). Such fluctuations in irradiance are typical of plant canopies in natural environments (Chazdon & Pearcy, 1991; Durand et al., 2022; Vierling & Wessman, 2000), and this will likely have an influence on carbon gain at the ecosystem level unless specific acclimation responses are triggered, such as those displayed in this study, to promote the efficient use of light. This means that the corresponding steady average irradiance may not be the most-appropriate control when examining the effect of irradiance fluctuations. As illustrated by our PCA, acclimation to fluctuating irradiance shares some characteristics with acclimation to low irradiance values, but also presents unique features (Figure 6). Using a control treatment that yields the same time-integrated carbon gain may permit acclimation to irradiance fluctuations to be disentangled from acclimation to irradiance level. Although including a control steady treatment of the same average irradiance should always be standard practice (Morales & Kaiser, 2020), perhaps this approach could be a new paradigm through which to investigate the effect of fluctuating irradiance.
Irradiance fluctuations of larger amplitude induced a stronger acclimation response in our experiment, and some traits only responded to the stronger fluctuations. In natural conditions, plants experience a variety of irradiance fluctuations with distinct properties, depending on their location within the canopy (Durand et al., 2022). For example, the intensity of irradiance fluctuations increases with increasing height in the canopy (Durand & Robson, 2023), along with the average incident irradiance. Leaves produced within the upper canopy would be the result of acclimation to both these two drivers, which in some cases would promote antagonistic effects (e.g., LMA), but synergies in others (e.g., anthocyanin index, Asat). In many cases, an increase in irradiance from 220 to 360 µmol m–2 s–1 had a larger impact than fluctuating irradiance from 20 to 420 µmol m–2 s–1. In our experiment, the amplitudes of fluctuations were smaller than the difference between the average irradiance value and darkness. Although in natural conditions, irradiance fluctuations can be stronger and asymmetrical (i.e., the increment increase in irradiance can be larger than the corresponding decrease, as a proportion of the average irradiance). Such an asymmetry may make it more beneficial to acclimate to lower or higher average irradiance, depending on the frequency and duration of fluctuations. The relative importance of irradiance fluctuations and the strength of irradiance in driving leaf development remains to be determined, but our findings reveal that elucidating the mechanisms by which light affects leaf development requires the consideration of both these drivers.
4.3 Large increases in leaf transpiration under fluctuating irradiance
We found a large increase in the amount of water transpired at high irradiances (Figure 5). Moreover, fluctuating irradiance tends to reduce CO2 assimilation potential compared to the corresponding average irradiance, because of the asymptotic shape of the photosynthesis-light response. Indeed, the potential gain from increasing irradiance (during the high part of the fluctuation) is smaller and therefore does not compensate for the potential loss with a corresponding decrease in irradiance. Both the increased transpiration and the potential reduction in photosynthesis that we report (due to the lower assimilation potential), would result in a decline in water-use efficiency at the leaf level. Although such conclusion would require a direct measure of dynamic photosynthesis, which we did perform in this experiment. Still, these two phenomena may create conflicting stimuli whereby an acclimation response to irradiance, which would be advantageous with regard to carbon gains, is detrimental for water use, and conversely. For example, the thinner leaves of plants growing under irradiance fluctuations will have lower specific heat capacity, affecting faster warming with higher radiation loads (Leigh et al., 2012). The effect of fluctuating irradiance on transpiration was larger than we anticipated, and this is potentially important given that the natural amplitude of irradiance fluctuations can be much greater than those we used in our study (Kaiser et al., 2018b). The dynamic response of photosynthesis is considered an important lever by which to potentially improve plant productivity (Long et al., 2022), but our data call for careful attention to be given to the collateral effects on plant water relations. With increasingly frequent and intense droughts under global change, it is unlikely that improvements in photosynthesis via better use of fluctuating light will be sustainable if they come at the cost of reduced water-use efficiency.
4.4 Conclusion
Research into acclimation to and dynamic regulation in fluctuating irradiance is becoming increasingly relevant to improve our understanding of leaf physiology, photosynthesis, and the establishment of forest understorey species (Long et al., 2022; Pearcy & Way, 2012; Way & Pearcy, 2012). From this research, we can now start to unravel specific developmental acclimation responses to fluctuating irradiance from those responses to the average growth irradiance. This study also provides a new approach to creating an appropriate control treatment that provides an alternative perspective on the effects of fluctuating irradiance. The acclimation response to irradiance fluctuations depended both on the amplitude of fluctuations, and on the irradiance levels themselves. The vertical position of leaves will determine the amplitude and duration of light fluctuations they experience (Durand & Robson, 2023), and therefore also drive their acclimation within the canopy. Recent work laid solid foundations upon which to build our understanding of plant response to fluctuating light (Kaiser et al., 2018b; Long et al., 2022; Taylor et al., 2022). Still, more work is needed before we can predict both short-term responses of photosynthesis and long-term acclimation patterns based on the properties of natural fluctuations in light within a canopy. For example, we know that plants have to deal with both long light fluctuations (>8 min; Smith & Berry, 2013) and very short ones (Kaiser et al., 2018b) that are created on top of one another to make a complex pattern reminiscent of fractals. Yet plant regulation and acclimation response to fluctuation of different durations are still misunderstood. This knowledge will open pathways to understanding how ever-present light fluctuations govern ecosystem carbon and water dynamics and play both sides to improve food security and mitigate global changes.
ACKNOWLEDGEMENTS
This experiment was located within Viikki Plant Growth and Experimental Field Facilities (vigour) HiLIFE infrastructure and maintained with the assistance of Daniel Richterich and Leena Grönholm. We would like to thank Pedro J. Aphalo, Justyna Labuz, and David Israel for their technical help. This research was funded by Research Council of Finland decision #351008 and #324555.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article. More information is available upon request.




