Carboxylation capacity is the main limitation of carbon assimilation in High Arctic shrubs
Abstract
Increases in shrub height, biomass and canopy cover are key whole-plant features of warming-induced vegetation change in tundra. We investigated leaf functional traits underlying photosynthetic capacity of Arctic shrub species, particularly its main limiting processes such as mesophyll conductance. In this nutrient-limited ecosystem, we expect leaf nitrogen concentration to be the main limiting factor for photosynthesis. We measured the net photosynthetic rate at saturated light (Asat) in three Salix species throughout a glacial valley in High-Arctic tundra and used a causal approach to test relationships between leaf stomatal and mesophyll conductances (gsc, gm), carboxylation capacity (Vcmax), nitrogen and phosphorus concentration (Narea, Parea) and leaf mass ratio (LMA). Arctic Salix species showed no difference in Asat compared to a global data set, while being characterized by higher Narea, Parea and LMA. Vcmax, gsc and gm independently increased Asat, with Vcmax as its main limitation. We highlighted a nitrogen-influenced pathway for increasing photosynthesis in the two prostrate mesic habitat species. In contrast, the erect wetland habitat Salix richardsonii mainly increased Asat with increasing gsc. Overall, our study revealed high photosynthetic capacities of Arctic Salix species but contrasting regulatory pathways that may influence shrub ability to respond to environmental changes in High Arctic tundra.
1 INTRODUCTION
The Arctic has warmed at a rate of 0.73°C decade−1 over the last 40 years (Rantanen et al., 2022), leading to major vegetation changes across the Arctic tundra biome. These changes have been characterized by the faster growth, increased height, and northward expansion of shrub species (Forbes et al., 2010; Tape et al., 2006). The ‘shrubification’ phenomenon has been studied extensively in multiple ways (Vowles & Björk, 2019), notably regarding its impacts on species composition and functional diversity of plant communities (Bjorkman et al., 2018), plant-soil interactions (Street et al., 2020), soil properties (Lamarque et al., 2023) and regional climate (Bonfils et al., 2012). Comparatively, few studies have examined the determinants of photosynthetic capacity in Arctic shrubs. Indeed, the latter has only been measured in a handful of species (Betula nana, Salix arctica, Salix glauca, Salix polaris, Salix pulchra) across eight sites to date (Baddeley et al., 1994; Bredahl et al., 2004; Chapin & Shaver, 1989; Fletcher et al., 2012; Johnson & Tieszen, 1976; Muraoka et al., 2002), and we do not yet know the extent to which the underlying diffusional (stomatal and mesophyll) and biochemical (e.g., maximal ribulose-1,5-bisphosphate carboxylation rate, Vcmax) components limit photosynthetic CO2 assimilation in the Arctic. However, a better characterization of CO2 uptake and its limitations among Arctic shrubs would bring new insights on the traits and species strategies that are associated to shrubification. This is important as photosynthetic capacity and particularly, its relationship to leaf nitrogen content are two of the most sensitive parameters of terrestrial biosphere models (Kattge et al., 2009).
Our current understanding of the variation in leaf photosynthetic capacity across species follows the global trend established among leaf functional traits, the so-called leaf economic spectrum (Wright et al., 2004; Diaz et al., 2016), which runs from species with short-lived foliage and fast metabolism to species with slow return on carbon and nutrient investments. The latter functional group is expected to be found in the cold and nutrient-poor environment of low productive Arctic tundra and is notably characterized by species with thick leaves and low nutrient content having low photosynthetic capacity. Although the leaf economic spectrum has been formulated predominantly from data acquired in temperate and tropical environments (Kattge et al., 2011), recent investigations within the tundra biome showed that it tends to hold true in the cold extremes of the planet (Thomas et al., 2020). Yet, Reich & Oleksyn (2004) showed that some Arctic species can reach very high level of leaf nitrogen (N) and phosphorus (P) concentration, while Rogers et al. (2017) demonstrated in seven species that such high N level allowed photosynthetic capacities measured in the tundra to be twofold to fivefold higher than the values used to parameterize current Earth system models. Thus, it is still relatively unclear how well the variation in leaf chemical and biometric traits found among Arctic species reflects their photosynthetic capacity.
Variation in the photosynthetic capacity of leaves versus nutrient content defines their photosynthetic nutrient-use efficiency, which encompasses the nutrient allocation to the photosynthetic apparatus and/or specific activity of photosynthetic enzymes as opposed to investment into structural/protective compounds notably found in cell walls (Onoda et al., 2017). In general, within-species variation of photosynthetic capacity is related to leaf nitrogen content (Hikosaka, 2010 but see Kong et al., 2022). In cold environments, an increase in leaf N both within and across species likely reflects a physiological acclimation (physiological response) and adaptation (evolutionary response) process that compensates for the temperature depression of the Rubisco-catalyzed CO2 carboxylation rate (Baddeley et al., 1994; Körner, 1999). As such, increasing N in leaf tissue is expected to favor the production of N-rich proteins like Rubisco and lead to higher CO2 carboxylation capacity (Hikoska, 2010). In the cold and low nitrogen availability tundra, increasing N availability through temperature and fertilization effectively led to higher photosynthetic capacity in several (but not all) Arctic tundra species (Chapin & Shaver, 1996; Oechel et al., 1994) (path 2, Figure 1). However, leaf N is not necessarily capitalized as functional photosynthetic enzymes, as observed under phosphorus deficiency (Luo, Keenan, et al., 2021; Luo, Peng, et al., 2021; Walker et al., 2014). Nitrogen can be used in the production of photoprotective molecules against daylong light, such as carotenoids (Fernández-Marín et al., 2020) and in cell wall proteins (Onoda et al., 2017). In addition, it has been suggested that tundra productivity is more related with plant sink capacity (ontogenic development) rather than photosynthetic (i.e., source) capacity (Körner, 2015). In such cases, leaf N may not constrain the photosynthetic capacity (Bliss & Wein, 1972; Chapin et al., 1995). Overall, it remains unclear if within Arctic tundra species increasing leaf N content leads to an increase in photosynthetic capacity.
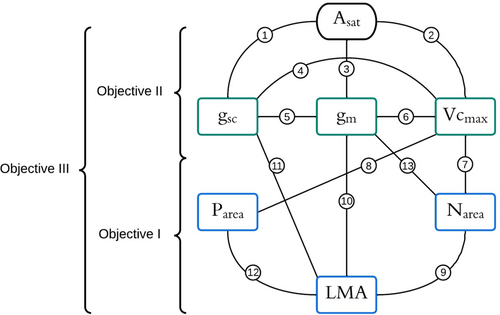
In cold environments, photosynthetic limitation may be dominated by diffusive processes lowering the chloroplastic CO2 concentration (Cc) per unit Rubisco, a condition that may have contributed to the adaptive increase in Rubisco's specificity factor for its CO2 substrate as opposed to O2 in tundra species (Galmés et al., 2016, 2019; Sáez et al., 2017). In Antarctica mesophyll conductance (gm) represented the major photosynthetic limitation for the two known vascular species Deschampsia antarctica and Colobanthus quitensis (Sáez et al., 2018; path 3, Figure 1). Although lower temperature enhances CO2 solubility and favors the liquid phase component of gm, CO2 transfer through cellular membranes may overwhelmingly be reduced under such condition (Evans & von Caemmerer, 2013) (path 3, Figure 1). In the Arctic, the high leaf mass per unit area (LMA) that permits long leaf lifespans (Chapin et al., 1995) is commonly associated with thicker cell walls, greater cell wall mass allocation per unit leaf area, and a thicker and denser leaf mesophyll, all leaf anatomical characteristics that are known to lower gm (Onoda et al., 2017; Théroux-Rancourt & Éthier, 2017) (path 10, Figure 1). Cold environments are also associated with high water viscosity, which can strongly reduce plant hydraulic conductivity and, ultimately, establishes stomatal conductance (gs) as the main limiting factor for photosynthetic capacity (Galmés et al., 2016) (path 1, Figure 1).
- 1.
Determine if Arctic Salix species are particularly characterized by low values of light-saturated photosynthesis rate (Asat), low Narea and Parea despite high LMA, as it would be expected in this biome from the leaf economic spectrum (Wright et al., 2004). From this framework, we also expect that within-species variation of Asat is positively related to Narea and Parea but negatively related to LMA.
- 2.
Disentangle the influence of diffusional and biochemical processes on Asat. While gsc (stomatal conductance to CO2), gm (mesophyll conductance) and photosynthetic capacity (i.e., Vcmax) should be coordinated, we expect the allocation of N towards Vcmax to dominate the limitation of Asat in the low nitrogen availability Arctic tundra.
- 3.
Disentangle the influence of leaf chemical and biometric traits on Asat and diffusive processes among species. While we expect a key role of Narea on Vcmax for the prostrate Salix species, we also expect a different regulatory pathway in S. richardsonii, whose taller stature may constrain the transport of cold and dense water to leaves (Figure 1).
2 MATERIALS AND METHODS
2.1 Site and species selection
The study was conducted in the Qarlikturvik glacial valley of Bylot Island, Nunavut, Canada, in the High Arctic (73°09′ N, 79°57′ W). The High Arctic is a region within the Arctic Circle that experiences extreme cold temperatures, permafrost, limited precipitation, and short growing seasons. This area is typically located north of the tree line and includes parts of northern Canada, Greenland, and the northernmost islands of Norway and Russia. In contrast to the Low Artic, the High-Arctic is devoid of trees; however, woody and shrub species are still present and characterized by small and prostrate stature (Myers-Smith et al. 2011).
The average environmental conditions in the Qarlikturvik valley are as follows: altitude, 20−400 m; growing season solar radiation, ca. 221 W m−2; annual temperature, −14.4°C; growing season temperature, 4.7°C; snow, ~8 months year−1; permanent permafrost; precipitation 77.5 mm during the growing season (Domine et al., 2021). The Qarlikturvik Valley is in the southwestern plain of the island and is crossed at its center by a proglacial river. The valley is about 18 km long, has a terrace about 5 km wide at the bottom and is bordered by plateaus up to 500 m in altitude (Godin & Fortier, 2012). It represents a typical glacial valley geosystem with depositional environments, which includes alluvial, eolian, glaciofluvial, morainic, colluvial and marine sediments (Coulombe et al., 2022). The ground is permanently frozen, with a thaw front depth varying from a few centimeters up to 80 cm (Deschamps et al., 2022). A low-centered polygon landscape characterizes the valley with two baseline vegetation types. Wetlands represent 23% of the valley area and are dominated by sedges (Carex aquatilis, Eriophorum angustifolium, E. scheuzerii), grasses (Dupontia fischeri) and mosses (Calliergon giganteum, Drepanocladus spp.) (Gauthier et al., 1995). Mesic environments occur on polygonal rims, degraded and high centered polygons and hummocky tundra, and support a more diverse group of species composed of Salix spp., Vaccinium uliginosum, Arctagrostis latifolia, Poa arctica and the dominant moss Aulacomnium spp. (Perreault et al., 2016).
S. arctica and S. reticulata are prostrate species whose stature rarely exceeds 15 cm with elliptic to oblong leaves ranging from 1 to 10 cm2 in area. S. arctica leaves are more hypostomatous and pilose, while S. reticulata are more amphistomatous and glabrescent with venation deeply impressed on the adaxial surface. Both species typically dominate the mesic environments of the Qarlikturvik valley. S. richardsonii is the only erect species in the prostrated tundra of Bylot Island, which is located at its northern edge of its distribution range. There, it reaches up to 1 m in height with lanceolate and pilose (and hemiamphistomatous) leaves and is mainly found along small streams and alluvial fans that cross the valley from the surrounding plateaus to the glacial river (Duclos, 2002; Tremblay, 2018).
2.2 Experimental design and plant sampling
We selected 29 sites in July 2019 to represent the diversity of ecosystems encountered in the Qarlikturvik valley, near the glacier to the ocean and from the wetlands along the river glacier up to the mesic plateau. Across sites, topsoil (0−10 cm) varied from 4.6 to 7.8 in pH, from 9.6 to 74.6% in silt percentage and from 0.3 to 28.2% in soil organic matter content, whereas thaw front depth varied from 20.5 to 71.5 cm (recorded between 10 and 15 August). Microclimate was less variable across sites, ranging from 5.8°C to 6.9°C in mean temperature and from 0.14 to 0.20 kPa in air vapour pressure deficit over the June−July−August growing period. At each site, we sampled the three Salix species, S. arctica, S. reticulata, S. richardsonii, whenever possible (23 of 29 sites presented the three species, with S. reticulata and S. richardsonii absent in one and five sites, respectively). For each species at each site, stems of three different individuals were dug up down to roots then placed in their entirety in an opaque plastic bag to encourage stomatal closure and limit water loss by transpiration during transport to the main camp. There, the individual branches were cut under water and left in the dark for 24 h before beginning the gas exchange measurements.
2.3 Leaf gas exchange measurements
From July 1st to July 20th, we performed instantaneous gas exchange measurements under saturating light on one top-leaf per individual and two individuals per species, and per site (N = 162; n = 58 for S. arctica, n = 56 for S. reticulata, n = 48 for S. richardsonii). Two cross-calibrated portable photosynthesis systems, one LI-6400XT and one LI-6800 (LI-COR Inc.), each fitted with a 2 cm2 window Leaf Chamber Fluorometer to accommodate the small size of Arctic Salix leaves, were used to perform coupled leaf gas exchange and chlorophyll fluorescence measurements. We set the leaf chamber air relative humidity at 60% and leaf temperature (Tleaf) at 10°C. This temperature approximated the average growth condition (mean daily temperature in July = 7.7°C, varying between −1.5 and 22.3°C in the 2013−2019 period (Domine et al., 2021) and prevented condensation issues within the chamber. One hour before measurement, plants were placed under a LED lamp (YGROW S1500 Grow Light) and exposed to a photosynthetic photon flux density (PPFD) of 1000 µmol m–2 s–1. At this level, photosynthesis is considered as light-saturated for Arctic tundra shrub species (Leffler & Welker, 2013; Mbufong et al., 2014; Walther et al., 2018) as circumarctic vegetation is adapted to relatively low light intensities (Mooney & Billings, 1961). After clamping the leaf into the chamber, we waited for steady state conditions (typically 10 min) before recording Asat and gsc as well as steady-state (Fs) and maximal (Fm', determined using a 800 ms rectangular flash of PPFD = 8000 µmol m–2 s–1) chlorophyll fluorescence under ambient (409 ppm) leaf chamber CO2 concentration (Ca), then repeated the measurements under 10 different leaf chamber CO2 concentrations (resulting from setting the instrument's Reference CO2 from 50 to 900 ppm) to generate a CO2 response (A-Ci) curve (see Supporting Information S1: Figure 1). At the end of the A-Ci curve, the leaf chamber CO2 concentration was set back to the ambient atmospheric level (409 ppm) and the chamber incident PPFD reduced to 100 µmol m–2 s–1 to acclimate the leaf to light-limiting conditions before performing light response (A-Q) curve measurements under decreasing PPFD (from 100 to 40 µmol m⁻² s⁻¹), keeping the calculated leaf internal CO2 concentration (Ci) constant throughout to subsequently estimate non-photorespiratory leaf respiration in the light (Rday) using the ‘Kok effect’ method (Kirschbaum & Farquhar, 1987). For comparison, we also recorded the leaf respiration rate in the dark (Rdark) following 10 min stabilization in darkness.
2.4 Estimation of gm, Vcmax and J
The parameters s (an empirical factor relating the chlorophyll fluorescence-based estimation of the photochemical efficiency of photosystem II (ΦPSII = (Fm' – Fs)/Fm') to J (i.e. J = s · ΦPSII · PPFD) by accounting for leaf absorbance, partitioning of the absorbed quanta between photosystems I and II, and nonphotosynthetic electron transport towards alternate sinks) and Γ (leaf CO2 compensation point where Asat = 0) were solved together with gm and Vcmax, with average ( ± SD, n = 162) values of 0.38 (±0.07) and 49 (±16) ppm for s and Γ, respectively, at Tleaf = 10°C. The assumed 10°C values of the Rubisco kinetic constants Γ* (chloroplastic CO2 compensation point), Kc and Ko (Michaelis–Menten constants for RuBP carboxylation and oxygenation, respectively) were taken from Bernacchi et al. (2002). Then, we solved the model for the theoretical case where Ac = Aj, that is, the net photosynthetic rate (Atransition) achieved when Vcmax limitation is in perfect balance with electron transport rate (J) limitation and compared the later with the measured Asat determined at ambient CO2 concentration (Ca = 409 ppm). As Supporting Information S1: Figure 2b shows, Asat correlated tightly with Atransition following essentially a 1:1 relationship, which we took as sufficient ground for assuming a near colimited state for Asat and to carry out our subsequent analyses based on Vcmax limitation (see also Supporting Information S1: Figures 1, 2a and Supporting Information S1: Table 1).
2.5 Biometric and chemical traits
Immediately after the gas exchange measurements, we measured the leaf area using a digital camera and weighed the leaf fresh, then weighted its dry mass after 48 h oven drying at 70°C. The N and P concentration of finely ground dry leaf material was determined using respectively a Thermal Conversion Elemental Analyzer—Isotope Ratio Mass Spectrometer (Agilent technolog) and an Inductively Coupled Plasma Optical Emission Spectroscopy spectrometer (Plasma Model 40, PerkinElmer).
2.6 Statistical analyses
All analyses were performed on R statistical software (v.4.3.1; R Foundation for Statistical Computing, Vienna, Austria).
2.6.1 Assessing where Arctic Salix species stand along the leaf economics spectrum (objective 1)
Leaf trait values measured in Salix species were compared to the global trait distributions from the Globamax database (Maire et al., 2015) in which we only selected C3 photosynthesis type (n = 2409). First, we tested whether Arctic Salix displayed a more conservative strategy compared to species from other biomes. We used a Welch t-test, considering that the variance between the two groups (i.e., Salix versus Globamax) were not equalled. Second, we tested whether Asat covaried positively with leaf Narea and Parea and negatively with LMA, using a mixed regression model (lme4 package) with a Gaussian distribution family. We considered site and sampling date as random factors to focus primarily on trait co-variations that were not related to ontogeny (Bredahl et al., 2004; Muraoka et al., 2002) and between-site environmental differences. We tested whether species differed in the response of Asat to economic traits by selecting the model that minimizes the Aikaike criterion (AICC) among the alternative models that considered all species*trait pair interactions.
2.6.2 Disentangling the influence of diffusional and biochemical processes on Asat (objective 2)
We used two different approaches. The first approach used the equations described in Grassi and Magnani (2005) to calculate the relative photosynthetic limitations imposed by the stomatal (Ls), mesophyll (Lm), and biochemical (i.e., carboxylation) (Lb) resistances operating in series. Using a t-test model with equal variances, we tested whether Ls, Lm, and Lb differed among species. However, this approach is not directly based on Asat variation, but on the relative decrease of Asat associated with the successive CO2 drawdowns leading to the final carboxylation of RuBP. In addition, this approach does not consider the potential interdependency of the reaction-diffusion processes making up the overall photosynthetic limitation. To evaluate the potential influence stomatal and mesophyll CO2 diffusion processes may have on biochemical photosynthetic capacity, we used a second approach consisting in a standardized and mixed regression model. For each species, we considered gsc, gm, Vcmax and the three ways interaction as fixed factors, as well as site and sampling date as random factors. We used a Gaussian family to represent Asat distribution. The values of gsc, gm, Vcmax were scaled (scale function) to estimate their respective importance.
2.6.3 Disentangling the influence of leaf chemical and biometric traits (Narea, Parea, LMA) on the photosynthetic capacity (Asat) and processes (Vcmax, gsc, gm) (objective 3)
We used a series of generalized mixed regression models. We additionally tested if the three Salix species differ in their respective leaf trait relationships considering either the additive or interactive effect of species in the model. We used a gamma family distribution for Vcmax and gm and a Gaussian distribution for gsc. Finally, we used a series of mixed regression structured in path analyses (‘lme4’ and ‘piecewiseSEM’ packages) to explore how variation in Asat can best be understood as driven by both direct and indirect effects between photosynthetic processes (gsc, gm, Vcmax) and leaf biometric and chemistry (Narea, Parea, LMA). We considered site and sampling date as random factors. We built our initial path model based on established relationships in the literature (see Figure 1). We selected a common model for the three species that was the least different from the observations (p > 0) and applied it separately to each species. Finally, we tested whether differences in photosynthetic pathway among species was consistent with photosynthetic nitrogen-use efficiency (PNUE = Asat/Narea) and instantaneous water use efficiency (WUE = Asat/[1.6·gsc]). We used generalized mixed regression models considering site and sampling date as random factors.
3 RESULTS
3.1 Position of Arctic Salix species along the leaf economic spectrum
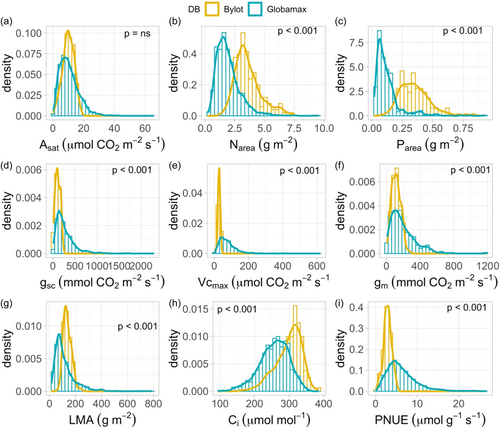
The distribution of Asat values of Arctic Salix species from Bylot Island (median = 10.09, mean = 10.06 ± 0.28 µmol m−2 s−1 at Tleaf of 10°C) was comparable to the one observed at the global scale (median = 9.60, mean = 10.60 ± 0.13 µmol m−2 s−1, Welch-test: p = 0.48 at average Tleaf of 25.5°C), except that it did not include the high value tail (>20 µmol m−2 s−1; Figure 2). Yet, differences arose in trait value distributions between Salix and Globamax for all the other traits measured (Figure 2). LMA and PNUE were higher and lower, respectively, when compared with Globamax values (LMA = 137.2 g m−2, PNUE = 2.89 µmol gN−1 s−1 for Bylot versus LMA = 118.025 g m−2, PNUE = 5.72 µmol gN−1 s−1 for Globamax). Higher values of Narea and Parea as well as leaf intercellular CO2 concentration, Ci, particularly characterized Salix species (Narea = 3.61 g m−2, Parea = 0.353 g m−2, Ci = 350.8 ppm for Bylot versus Narea = 1.94 g m−2, Parea = 0.126 g m−2, Ci = 257.3 ppm for Globamax). Finally, Vcmax, gsc and gm at growth temperature showed significantly lower values for Salix species in comparison with the Globamax distribution (Vcmax = 28.15 µmol m−2 s−1, gsc = 112.63 mmol m−2 s−1 and gm = 116.36 mmol m−2 s−1 for Bylot vs Vcmax = 74.04, gsc = 171.5, gm = 194.36 for Globamax).
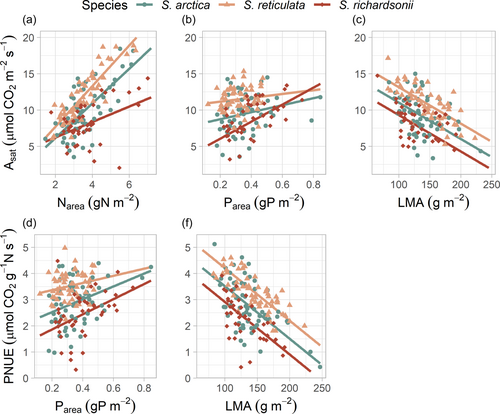
In our study, Asat was positively related to Narea and Parea, and negatively related to LMA (Figure 3a,b,c and Table 1). Across species, S. richardsonii showed a lower increase in Asat for a given increase in Narea compared with S. arctica and S. reticulata ([p(Sp*Narea) < 0.01], Figure 3a). The relationship between Asat and Nmass showed a lower determination coefficient in comparison with an expression on area unit basis (rm2 = 0.12 vs. rm2 = 0.27, respectively; data not shown). Asat and PNUE decreased significantly with increasing LMA, the slope of the relationship being similar across species (Table 1, Figure 3c,e). Parea tended to increase Asat and PNUE, but the effect was not significant.
| Model factors | Asat | PNUE | ||||
|---|---|---|---|---|---|---|
| DF | F Value | p Value | DF | F Value | p Value | |
| Species | 2 | 0.32 | ns | 2 | 5.91 | ** |
| Narea | 1 | 25.81 | *** | — | — | — |
| Parea | 1 | 8.17 | ** | 1 | 10.38 | ** |
| LMA | 1 | 11.42 | *** | 1 | 38.63 | *** |
| Narea* Species | 2 | 7.04 | ** | — | — | — |
| Parea* Species | 2 | 2.13 | ns | 1 | 0.55 | ns |
| Overall | r2m = 0.26; r2c = 0.60; AICC = 753 | r2m = 0.30; r2c = 0.30; AICC = 395 | ||||
- Note: We considered site and sampling date as random factors, and LMA, Narea, Parea and species as fixed factors. For fixed factors, we considered interactions between factor pairs and finally selected the model with the minimum of pairs that minimized the AIC criterion. We used the Gaussian family distribution for both Asat and PNUE (n = 157). We did not consider Narea as an explicative variable in the PNUE model. nsp > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
- Abbreviations: AICC, aikaike criterion; LMA, leaf mass ratio; PNUE, photosynthetic nitrogen-use efficiency.
3.2 Influence of diffusional and biochemical processes (gsc, gm, Vcmax) on Asat
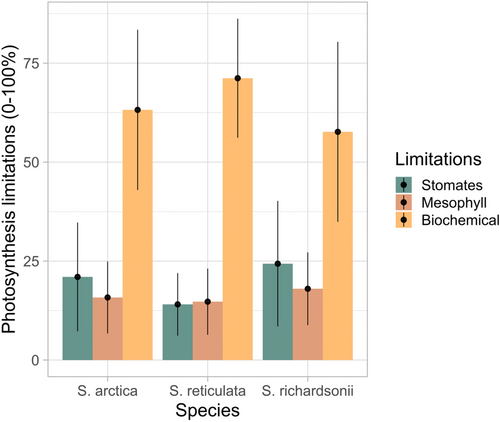
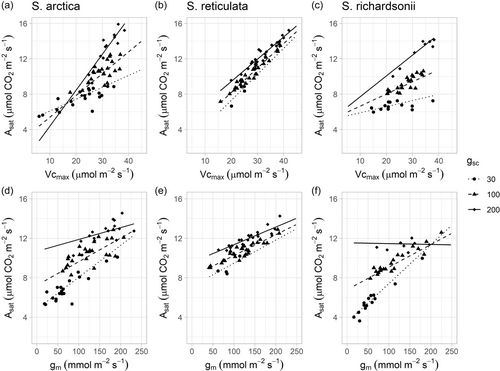
At ambient CO2 concentrations, photosynthesis in the three Salix species was mainly Rubisco limited (Figure 4). There was no significant difference among species and overall gsc, gm, and Vcmax contributed to 21.9%, 19.6%, and 58.5% of Asat limitation, respectively.
The processes, gsc, gm and Vcmax, contributed positively and independently to Asat variation (Table 2, Figure 5). When considering multiple interactions between photosynthetic processes, the positive effect of Vcmax on Asat increased as gsc increased (Figure 5a−c), whereas the positive effect of gm on Asat increased as gsc decreased (p(Vcmax*gsc) < 0.01 and p(gsc*gm) < 0.01, respectively, Table 2, Figure 5). Moreover, the relative importance of photosynthetic processes differed among species, with Vcmax being the main determinant of Asat in S. arctica and S. reticulata, while Asat in S. richardsonii was primarily gsc and gm dependent.
| Variable | Salix arctica | Salix reticulata | Salix richardsonii | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | SE | p Value | Est. | SE | p Value | Est. | SE | p Value | Est. | SE | p Value | |
| Intercept | 9.88 | 0.15 | *** | 11.33 | 0.17 | *** | 9.02 | 0.27 | *** | 10.08 | 0.13 | *** |
| Vcmax | 1.94 | 0.25 | *** | 1.77 | 0.16 | *** | 0.86 | 0.24 | ** | 1.54 | 0.13 | *** |
| gsc | 1.13 | 0.17 | *** | 0.51 | 0.13 | *** | 1.16 | 0.24 | *** | 1.05 | 0.11 | *** |
| gm | 1.31 | 0.21 | *** | 0.94 | 0.18 | *** | 1.38 | 0.25 | *** | 1.28 | 0.12 | *** |
| gsc*gm | −0.38 | 0.24 | ns | −0.09 | 0.14 | ns | −0.63 | 0.32 | * | −0.38 | 0.11 | ** |
| gsc*Vcmax | 0.73 | 0.29 | * | −0.09 | 0.12 | ns | 0.41 | 0.23 | * | 0.31 | 0.12 | *** |
| gm*Vcmax | −0.24 | 0.25 | ns | 0.02 | 0.11 | ns | −0.11 | 0.28 | ns | 0.03 | 0.12 | ns |
| gsc*gm *Vcmax | −0.05 | 0.13 | ns | −0.15 | 0.12 | ns | 0.31 | 0.22 | ns | 0.06 | 0.07 | ns |
| Overall | r2m = 0.98 | r2m = 0.96 | r2m = 0.95 | r2m = 0.98 | ||||||||
- Note: We considered site and sampling date as random factors and used a gamma family distribution. We scaled the values of fixed factors to estimate their relative influence on Asat variations. nsp > 0.1; *p < 0.05; **p < 0.01; ***p < 0.001.
3.3 Direct and indirect effects of leaf biometric and chemistry on photosynthesis
Carboxylation capacity (Vcmax) and gm were positively and significantly related to Narea (Table 3, Supporting Information S1: Figures 3b,c), and negatively and significantly related to LMA (Table 3, Supporting Information S1: Figures 3h,i). In contrast, Parea only had a significant positive influence on Vcmax (Table 3, Supporting Information S1: 3f). Overall, species identity significantly modulated the slope (for gm and Vcmax) and the directionality of the relationships towards Narea (for gsc) (Table 3, Supporting Information S1: Figures 3a–c).
| Model factors | gs | gm | Vcmax | ||||
|---|---|---|---|---|---|---|---|
| DF | F Value | p Value | F Value | p Value | F Value | p Value | |
| Species | 2 | 4.15 | * | 0.75 | ns | 0.21 | ns |
| Narea | 1 | 1.82 | ns | 20.78 | *** | 24.37 | *** |
| Parea | 1 | 0.55 | ns | 2.46 | ns | 9.24 | ** |
| LMA | 1 | 0.55 | ns | 10.83 | ** | 13.24 | *** |
| Narea* Species | 2 | 6.17 | ** | 4.04 | * | 4.63 | * |
| Parea* Species | 2 | 3.12 | ° | 2.07 | ° | 0.23 | ns |
| Overall | r2m = 0.19; r2c = 0.61AICc = 1586 | r2m = 0.18; r2c = 0.50AICc = 1589 | r2m = 0.22; r2c = 0.53 AICc = 988 | ||||
- Note: We considered site and sampling date as random factors, and LMA, Narea, Parea and species as fixed factors. For fixed factors, we considered interactions between factor pairs and finally selected the model with the minimum of pair that minimized the AIC criterion. We used the Gaussian family distribution for all dependent variables and n = 157. nsp > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001.
- Abbreviations: AICC, aikaike criterion; LMA, leaf mass ratio.
Based on a theoretical model, we analysed the direct and indirect influences of leaf biometric and chemistry on photosynthetic processes and capacity. Our initial model assumed that Asat was directly constrained by gsc, gm and Vcmax but indirectly by LMA, Narea and Parea (see Figure 1). We assumed that gsc, gm and Vcmax covaried and were dependent on LMA, Narea and Parea. Finally, Narea and Parea were a function of LMA. This initial model was not rejected by observations for any species (Pmodel = 0.22). The model was improved (Pmodel = 0.41) when we considered a direct path from LMA to Asat, no relationship between Parea−Vcmax and Parea−gm and a causal relationship between Parea → gsc (Figure 6a,b,c).
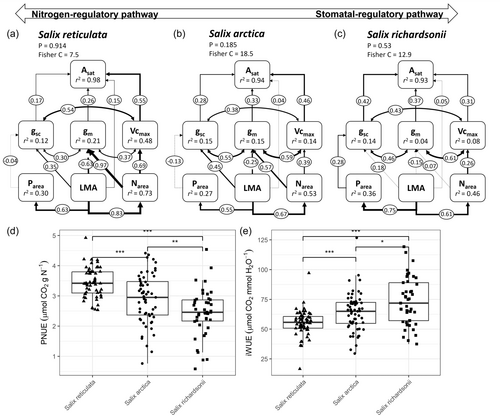
When this model was applied to each species, we showed that the importance of paths to explain Asat variation differed between the three Salix species (Figure 6a,b,c). Vcmax was the most important causal variable among the direct controls to Asat for S. arctica and S. reticulata (ρvcmax = 0.46 vs. ρvcmax = 0.55, respectively), except for S. richardsonii where gsc played a dominant role (ρgsc = 0.42 vs. ρgm = 0.37 and ρvcmax = 0.31). Mesophyll conductance played a direct and substantial role for explaining variation in Asat for the three species (ρgm[S. arc.] = 0.33, ρgm[S. ret.] = 0.26, ρgm[S. ric.] = 0.37). Coordination among photosynthetic processes was a key condition to model stability. Narea increased both Vcmax and gm more substantially for S. reticulata and S. arctica compared to S. richardsonii. LMA influenced positively gsc, but had a substantial negative influence on gm. Finally, there was a weak positive influence of Parea on gsc for S. richardsonii, whereas a negative influence was observed for the two other species.
Path analyses showed contrasting photosynthetic regulatory pathways among species which aligned with species differences in PNUE and iWUE models (r2m[PNUE] = 0.24, r2c[PNUE] = 0.52; r2m[iWUE] = 0.24, r2c[iWUE] = 0.48, Figure 6d,e). The highest PNUE was observed for S. reticulata (S. reticulata = 3.26 ± 0.09 µmol CO2 g−1 N, S. arctica = 2.87 ± 0.14, S. richardsonii 2.43 ± 0.14 µmol CO2 g−1 N, P-values of post-hoc differences among species were all < 0.05), and the highest iWUE for S. richardsonii (S. richardsonii = 74.7 ± 3.48, S. arctica = 65.5 ± 3.20, S. reticulata = 56.4 ± 3.19 µmol CO2 mmol−1 H2O, p Values < 0.05).
4 DISCUSSION
Arctic shrubs are important components of vegetation changes that currently occur across the Arctic tundra biome. This study aimed at exploring the variation of photosynthetic biochemical and diffusive capacities within and among three Salix species co-occurring in the High Arctic. We notably assessed the extent to which variation in leaf biometric and chemistry as well as in the diffusional and biochemical components of photosynthesis constrained Asat. Our observational approach showed that Asat measured under standard leaf micro-environmental conditions and at a standard site and date can strongly vary among individuals (1−18 µmol m−2 s−1) and ~25% of this variation is attributable to Narea, species identity and their interaction within a low nitrogen availability Arctic tundra (Deschamps et al., 2022). When gsc, gm and Vcmax are coordinated, Asat could reach higher values, comparable to other biomes.
4.1 Rare measurements of Asat on Arctic shrubs
In the Arctic tundra of North−East Canada, we showed that the photosynthetic capacity of three shrub species, S. arctica, S. reticulata and S. richardsonii at Tleaf of 10°C was on average 9.9 ± 0.5, 11.1 ± 0.4 and 8.7 ± 0.5 µmol m−2 s−1, respectively. Such light-saturated photosynthesis rates are also in the range of those reported for deciduous shrub species occurring at similar latitude and measured at saturating light conditions, ambient CO2 concentration, and a Tleaf of ~10°C. In the later studies, Asat varied between 5.9 and 18.0 µmol m−2 s−1 and averaged 11.4 ± 0.9 µmol m−2 s−1 for the following species and sites: S. pulchra in Barrow-Alaska (N71°, Rogers et al., 2017), S. pulchra and S. reticulata in Philip Smith Mountains –Alaska (N68°, Oberbauer et al., 1989), S. arctica in northwest Greenland (N76°, Leffler & Welker, 2013) and in northeast Greenland (N74°, Albert et al., 2011), S. glauca and Betula glandulosa in south Greenland (N61°, Simin et al., 2022), S. glauca and B. nana in northern Sweden (N68°, Fletcher et al., 2012), S. polaris in Svalbard Island (N79°, Muraoka et al., 2002), S. richardsonii, S. glauca and S. kolymensis in Yakutia (N70°, Fan et al., 2018). This range of Asat measured on Arctic species is above the values estimated by terrestrial biophysical models (Asat = 6.75 ± 2.90 µmol m−2 s−1 corrected at 10°C from Rogers et al., 2017), which used temperature functions with parameters approximated from data set in which Arctic shrub species were underrepresented (Rogers et al., 2017). The same observation goes for the estimation of the carboxylation capacity, which averages at Vcmax = 28.2 ± 0.6 µmol m−2 s−1 in our study compared to 12.5 ± 5.6 µmol m−2 s−1 estimated at 10°C by terrestrial biophysical models in Rogers et al. (2017), as well as calculated by Kattge et al. (2009) from Narea−Vcmax equation for deciduous shrubs. Overall, these results emphasize the need to better constrain such key parameters in terrestrial models if we are to improve estimations of growth primary productivity in the Arctic tundra (Euskirchen et al., 2022).
4.2 Salix species are aligned along the leaf economic spectrum for most traits
In agreement with the leaf economic spectrum and earlier observations, Arctic Salix species showed higher LMA and lower PNUE in comparison with the worldwide database, hence highlighting the conservative strategy that Arctic plants use to cope with the cold environment of the tundra (Reich et al., 1992; Thomas et al., 2020; Wright et al., 2004). Among Salix individuals, leaf tissue investment trades off with leaf light-saturated photosynthetic capacity for a given nitrogen unit (Wright et al., 2004). Individuals with a conservative strategy (high LMA, low PNUE) have lower mesophyll conductance (Supporting Information S1: Figure 3h; Parkhurst, 1994; Xie et al., 2020) and lower N partitioning to photosynthetic components (Supporting Information S1: Figure 5; Hikosaka & Hirose, 2000; Onoda et al., 2017; Poorter & Evans, 1998), both of which constrain leaf photosynthesis while ensuring a longer leaf lifespan (Wright et al., 2004).
In contrast to what is expected based on the leaf economic spectrum and the Temperature-Biogeochemical hypothesis (Reich & Oleskyn, 2004), as well as the nutrient limitation usually observed in Arctic tundra (Deschamps et al., 2022; Elser et al., 2007 for Bylot island), the values of Asat, Narea and Parea measured in this study were particularly high, which was highlighted when we compared Salix traits with those from the Globamax data set (Figure 2). As proposed by Reich & Oleskyn (2004), species inhabiting cold environments may exhibit high leaf nutrient concentrations as an adaptation to sustain carboxylation capacity. This is necessary because the biochemical efficiency of nitrogen-rich enzymes and phosphorus-rich RNA diminishes at low temperatures, as discussed by Woods et al. (2003). Rogers et al. (2017) argued that the high Narea of tundra species should allow Asat to be twofold higher than estimated by current Earth system models. Our findings support this assertion and suggest that the models need to be updated with region-specific data to more accurately reflect the photosynthetic capacity of tundra shrub species. In line with these considerations, our study showed that, independently of LMA values, the main contributor to Asat variation was the investment in nitrogen. In addition, we observed higher PNUE with increasing Parea, which spatially extend the previously observed role of P to the tundra biome (Maire et al., 2015). However, this effect was less marked than with increasing Narea, likely because P limitation could be considered weak in this recently weathered valley of artic tundra (6000 cal year BP, Fortier & Allard, 2004). As such, we suggest that the economics of leaf nutrients (Narea-Parea-Asat) should be studied concomitantly with the economics of leaf organic matter (LMA-leaf dry matter content-leaf lifespan) in tundra. Whereas both economic spectra are tightly coupled at large scale (Reich, 2014), having long lifespan at low nutrient concentration for deciduous species may not be a successful strategy when the vegetative period is highly constrained as in High Arctic tundra.
4.3 Carboxylation capacity is the main limitation of Asat in Arctic Salix shrubs
The three analytical approaches we used (c.f. sensitivity analysis, mixed interactive regression model, path analysis) converged toward the same conclusion that carboxylation capacity was the dominant limiting process to Asat for the three Salix species in High Arctic tundra. Such level of leaf biochemical limitation usually occurred in plant species with high Asat, w hereas both gsc and gm are considered major photosynthetic limitations in relatively low Asat species (Gago et al., 2023). This biochemical limitation may occur as low temperatures strongly limit the enzymatic efficiency of RuBP carboxylation and because the nitrogen investment in Rubisco proteins by the three Salix species was relatively low (11.9 ± 0.3%, calculated with Eq. 4 from Niinemets & Tenhunen (1997) and normalized to 25°C) compared with global variation (18.9 ± 6.2%, Onoda et al., 2017). While N could be invested to increase Vcmax (see Figure 6), it may also be required in proteins that protect against the continuous daylight stress of the tundra environment (Huang et al., 2004; Mittler et al., 2022).
This primary limitation by biochemical processes occurred as stomatal and mesophyll conductances of Salix species constrained only weakly the variation of Asat at low temperature (gsc = 112.6 ± 4.5 mmol m−2 s−1; gm = 116.4 ± 4.2 mmol m−2 s−1) when compared with another study at a similar reference temperature of 10°C in Antarctica (gsc = 78 ± 18 mmol m−2 s−1, gm = 50 ± 14 mmol m−2 s−1, Sáez et al., 2017) or with the meta-analysis by Knauer et al. (2022) for deciduous angiosperms when expressed at 10°C using the temperature response of Bernacchi et al. (2002) (gm = 62.3 ± 8.3 mmol m−2 s−1). While gm was not the most limiting process underlying the Asat variation, we found that gm was primarily positively and directly related to leaf chemistry and N concentration, when controlling for LMA in the regression model. N could increase the chloroplast surface area per unit leaf area, which increase CO2 transport through the cell (Gao et al., 2022; Onoda & Wright, 2018). Conversely, LMA significantly decreased gm as observed globally in Onoda & Wright (2018), but this appeared only after considering Narea in the regression model. The negative effect of LMA on gm was partly explained by increases in leaf thickness and leaf dry matter content (see Supporting Information S1: Table S2 and Supporting Information S1: Figures S5). As such, our observations followed the expected trend in the literature (Niinemets et al., 2009), but further leaf anatomical characterisation in Arctic Salix would be required to fully understand the influence of LMA and particularly Narea on gm.
The coordination between the photosynthetic reaction-diffusion processes, gsc, gm and Vcmax was central to the successful execution of the path analysis routine as the model was statistically rejected without considering it. We also observed a tight covariation between the biochemical capacity for RuBP regeneration, Jmax, and Vcmax (Supporting Information S1: Figure 2a, Supporting Information S1: Table 1), which was in line with previous studies (e.g., Walker et al., 2014). The coordination of photosynthetic processes is usually observed and expected to be optimized through natural selection (e.g. Flexas et al., 2013; Maire et al., 2012). Yet, after controlling this coordination, each of the photosynthetic process has played an independent and substantial effect on Asat and interacted together to optimize Asat. As such, the increase of Asat with Vcmax and Asat with gm, i.e. the increase in leaf C return for a given investment in nitrogen (considering that Vcmax and gm increased with Narea), was more rapid whenever stomata are fully open and more prone to lose water. This resulted in a trade-off between the photosynthetic nitrogen use efficiency and the intrinsic water use efficiency (see Supporting Information S1: Figure 5), which has been observed elsewhere in temperate and dry ecosystems (Field et al., 1983; Flexas & Carriqui, 2020; Hikosaka et al., 1998), but never in Arctic tundra to our knowledge.
4.4 Nitrogen-regulated species and stomatal-regulated species
Unlike the limitation analysis, the path analyses distinguished two regulatory pathways for Asat among the co-occurring Salix species. To our knowledge, this is the first study to show these two strategies of photosynthesis for Arctic species, optimizing either nitrogen or water resource. Prostrate S. arctica and S. reticulata, whose dominance typically peaks in mesic Arctic environments (Duclos, 2002), exhibited an N-regulatory pathway. New N units will increase the photosynthetic capacity through both Vcmax and gm processes, resulting in high PNUE for both species (Figure 6d,e). In contrast, erect S. richardsonii, which is adapted to moist to wet habitats and predominates on alluvial fans in the valley (Duclos, 2002), showed Asat regulation mainly through stomatal conductance, resulting in higher iWUE compared to the other two Salix species. Shrubification concerns both nitrogen-regulated and stomatal-regulated species. S. arctica will be the first to colonize glacier retreat areas (Boulanger-Lapointe et al., 2014), while S. richardsonii will increase in height benefiting from climate warming (Buchkowski et al., 2020). Using leaf nitrogen to predict photosynthetic capacity in High Arctic tundra, terrestrial biosphere models have the correct formalism once calibrated for nitrogen-regulated species to simulate growth primary productivity (Rogers et al., 2017). However, neither the formalism nor the calibration appears to be appropriate for stomatal-regulated species, a problem that should be investigated in future research.
5 CONCLUSION
A core result of our study is that key shrub species in the High-Arctic tundra of Bylot island are characterized by higher light-saturated photosynthetic capacity than expected from global databases. Leaf N concentration emerged as the best leaf economic predictors of Asat variation regulating both carboxylation capacity and mesophyll conductance. Such variation of Narea and Asat needs to be better quantified across species and environmental conditions of the Arctic as it can have strong implication for the modelling of Arctic tundra vegetation in Earth system models.
ACKNOWLEDGEMENTS
The authors are thankful to the Inuit community of Pond Inlet and to Parks Canada-Sirmilik National Park, and grateful to D. Bouchard, E. Hardy-Lachance, F. Tanguay, V. Roy-Blais and C. Gignac, for their help with leaves harvests and measurements. This study was supported by the Fonds de recherche du Québec-Nature et technologies (FRQNT-2018-PR-208107), the Natural Sciences and Engineering Research Council of Canada (RGPIN-2023-05596), the Network of Centers of Excellence of Canada ArcticNet and the Northern Scientific Training Program (Indian and Northern Affairs Canada). Logistical support for fieldwork was provided by the Centre d'Études Nordiques (CEN) and Polar Continental Shelf Program (Natural Resources Canada).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




