DkDTX1/MATE1 mediates the accumulation of proanthocyanidin and affects astringency in persimmon
Abstract
Proanthocyanidins (PAs) is a kind of polyphenols widely distributed in plants, and their astringent properties can protect plants from herbivores and regulate fruit taste. There is a great difference in PA composition between astringent (A)-type and nonastringent (NA)-type persimmon. Here, we studied the potential of DkDTX1/MATE1 in regulating PAs composition through its preferred transport in persimmon fruit. The results of fluorescence microscope showed that the DkDTX1/MATE1 green fluorescence overlapped with the blue light emitted by PA. Overexpression of DkDTX1/MATE1 in persimmon leaves not only significantly increase the concentrations of PA, but also upregulated the expression of PA biosynthesis pathway genes. Further overexpression of DkDTX1/MATE1 in persimmon fruit discs and stable genetic transformation of DkDTX1/MATE1 also led to PA concentrations increased. Molecular docking and transporter assays showed that DkDTX1/MATE1 preferentially transported catechin, epicatechin gallate and epigallocatechin gallate. DkDTX1/MATE1 mainly bound to the PA precursors via serine at position 68. Our findings indicate that DkDTX1/MATE1 play a role in the accumulation of PAs in early stage of fruit development and affects the astringency of persimmon through preferential transport PA precursors, which provided a theoretical basis for the future use of metabolic engineering to regulate the composition of PAs in persimmon.
1 INTRODUCTION
Diet is closely related to immunity, and a nutritious diet can strengthen the immune system and limit infectious diseases such as the recent pandemic of COVID-19 (Verma et al., 2024). Plant products such as grains, fruits and vegetables contain high natural bioactive nutrients and have high nutritional value (Shashirekha et al., 2015). Proanthocyanidins (PAs), also known as condensed tannins, are one of the most important nutrients. They are flavan-3-ols polymers bound to saliva proteins (Dixon & Sarnala, 2020). PAs has a variety of biological activities, not only provides protective functions in many plant (Dixon et al., 2005), but also as an antioxidant, it is considered to have positive effects on human health, including hypolipidemic, cancer-preventive, anti-inflammation, preventing cardiovascular diseases, and improving lipid homeostasis (Qi et al., 2023). PAs can affect the bitterness and astringency of fruits and processed products by changing their composition, thus affecting their taste (Lesschaeve & Noble, 2005). Because the accumulation of PA in plants is toxic, the PA produced in the cytoplasm needs to be stabilised and detoxified by transport to vacuoles, so the accumulation of PA may be a sign of plant stress.
Flavonols, anthocyanins, and PAs belong to flavonoids. The biosynthesis of flavonoids has been deeply understood in many species and is one of the most studied metabolic pathways in plants (Routaboul et al., 2012). There is a common upstream pathway between the biosynthesis of PAs and anthocyanins, and the biosynthesis of PAs is branched out from the anthocyanin biosynthesis pathway at the leucoanthocyanidins level (Dixon et al., 2005). Dihydroflavonol-4-reductase converts dihydroflavonols to flavan-3,4, -diols (leucoanthocyanidins; Shirley et al., 1992), and then leucoanthocyanidins reduced to 2,3-trans-flavan-3-ols (catechin) by leucoanthocyanidin reductase (LAR; Tanner et al., 2003). Anthocyanin synthase (ANS, same as leucocyanidin dioxygenase: LDOX) converts leucoanthocyanidins to anthocyanins (Abrahams et al., 2003), which is then catalysed by anthocyanin reductase (ANR) to produce 2,3-cis- flavan-3-ols (epicatechin [EC]; Xie et al., 2003). Most PAs consist of a mixture of catechin (C) and EC subunits. After these PA precursors and their derivatives are formed, they are transported by transporters and stored in vacuoles (Grotewold, 2004). At present, two mechanisms of PAs transport have been proposed. One is that PA precursors produced in endoplasmic reticulum (ER) cytoplasmic surface transport to vacuoles and polymerise in vacuoles (Kitamura et al., 2010); the other is that PA precursors polymerise into tannosome in chloroplast thylakoids, and enter the vacuole through the invagination of the tonoplast (Brillouet et al., 2013). The first mechanism involves membrane transporter-mediated transport, glutathione S-transferase (GST)-mediated transport, and vesicular transport-mediated transport (Zhao, 2015; Zhao & Dixon, 2010). Among them, detoxification efflux carrier (DTX), also known as the multidrug and toxic compound excretion (MATE) transporter is a kind of transmembrane (TM) transporter (Marinova et al., 2007).
Members of the DTX/MATE family are ubiquitous in various life forms, including eukaryotes and prokaryotes. DTX/MATE transporter is first characterised as multidrug efflux protein in Escherichia coli and Vibrio parahaemolyticus. The length of DTX/MATE protein is generally 400-550 amino acids and usually including 12 TM helices (Kusakizako et al., 2020; Upadhyay et al., 2019). As a model plant, Arabidopsis has 58 DTX/MATE transporters (Upadhyay et al., 2019). Arabidopsis ALF5, as the first DTX/MATE transporter found in plants, is related to the efflux of toxic compounds (Diener et al., 2001). There are many members of the DTX/MATE family, and different members perform different physiological functions (Upadhyay et al., 2019). PA-related DTX/MATE proteins have been found in several plant species, such as Arabidopsis thaliana (TT12, Marinova et al., 2007), Medicago truncatula (MtMATE1, Zhao and Dixon, 2009), Malus ⅹ domestica (apples; MdMATE1 and MdMATE2, Frank et al., 2011), Vitis vinifera (VvMATE1 and VvMATE2, Pérez-Díaz et al., 2014), Gossypium hirsutum (cotton; GhTT12, Gao et al., 2016), Fragaria ⅹ ananassa (strawberries; FaTT12-1, Chen et al., 2018), and Cicer arietinum (CaMATE1, Pal et al., 2023).
Persimmon (Diospyros kaki Thunb.) is a kind of fruit crop with a long history of cultivation, and one of its characteristics is that the fruit has strong astringency (Akagi, Ikegami, Tsujimoto, et al., 2009; Matsuo and Ito, 1978). There is a kind of specialised cell, tannin cell in persimmon fruit, and a large amount of PAs in its vacuole is the main cause of astringency (Amorim et al., 2023). PAs interact with salivary proteins to precipitate and aggregate, resulting in a dry and rough uncomfortable taste in the mouth (Wu et al., 2022). Therefore, without artificial treatment to eliminate astringency, these fruits will become inedible (Taira, 1996). Cultivated persimmon varieties can be divided into two types: nonastringent (NA)-type (also termed pollination constant and NA, PCNA) and normal astringent (A)-type (also termed non-PCNA), in which NA-type persimmon is derived by a spontaneous mutation that loses a high level of PAs in fruit (Akagi, Ikegami, Tsujimoto, et al., 2009, 2011; Dong et al., 2022). Compared with A-type, NA-type fruit can lose its astringency in the tree after ripening and can be eaten directly (Akagi, Ikegami, Tsujimoto, et al., 2009). The composition and proportion of PAs in different types of persimmon fruits are different with the development of persimmon fruits (Yonemori et al., 1983). The main PA component in persimmon fruit is epigallocatechin gallate (EGCG), and its content is significantly different in different types of persimmon (Akagi et al., 2010). The PA precursors is synthesised in the ER cytoplasmic surface, then transported into the vacuole, and further accumulated and polymerised in the vacuole to form PAs in persimmon (Chen et al., 2017; Yang et al., 2023). The early biosynthetic gene DkANR, the late polymeric gene DkLAC2 and transcriptional regulatory factors DkMyb4, DkMyb14 have been studied (Akagi, Ikegami, Suzuki, et al., 2009; Akagi, Ikegami, Tsujimoto, et al., 2009; Chen et al., 2021; Zaman et al., 2022), but there are few reports on vacuole transport.
Previously, we found that DkDTX5/MATE5, which affects the formation of different astringency in persimmon, is mainly expressed in mid and late stages of NA-type fruit (Liu et al., 2023). In addition, we isolated DkDTX1/MATE1 gene, which was mainly expressed in the early stage of A-type fruit. Its transient expression in persimmon leaves suggested that it may be a necessary PA precursors membrane transporter (Yang et al., 2016). These results prompted us to explore the PA transport mechanism of DkDTX1/MATE1 and its effect on PA accumulation in the early stage of fruit development. In this study, the overexpression of DkDTX1/MATE1 in persimmon fruit disc proved that DkDTX1/MATE1 was also involved in PA transport in persimmon fruit. We further confirmed that DkDTX1/MATE1 preferred to transport PA precursors C, EC gallate (ECG) and EGCG through molecular docking and E. coli transport experiment. Among them, DkDTX1/MATE1 had a stronger ability to transport EGCG, which is the main component in A-type persimmon. The expression level of DkDTX1/MATE1 in A-type was higher than that in NA-type at the early stage of fruit development. Therefore, the differential expression and preferential transport activity of DkDTX1/MATE1 lead to the difference of PAs between A-type and NA-type persimmon in the early stage of fruit development.
2 MATERIALS AND METHODS
2.1 Plant material and E. coli strains
The persimmon fruits of NA-type ‘Eshi 1’, ‘Youhou’ and A-type ‘Mopanshi’ were collected at 2.5, 5, 10, 15, 20, 25, and 27.5 weeks after blooming (WAB) in the Persimmon Repository of Huazhong Agricultural University, Wuhan, China. The fruit was frozen in liquid nitrogen and stored at −80°C. The tissue-cultured seedlings of ‘Xiaoguo Tianshi’ (NA-type) and ‘Gongcheng Shuishi’ (A-type) were stored in solid half-strength Murashige and Skoog (MS) medium and cultured in photoperiod for 12 h at 24°C. E. coli mutant acrB strain was obtained from the National BioResource Project (National Institute of Genetics), which lacks the ability of drug efflux.
2.2 PA content and composition determination
The PAs content in fruits, transiently transformed fruit discs and leaves were determined by Folin-Ciocalteu method (Oshida et al., 1996). The content of soluble PA was also detected by the printing method (Eaks, 1967). FeCl2 reacted with soluble PA, the darker the colour, the higher the content of soluble PA. The content of PAs in the fruit discs of transiently transformed and transformed persimmon tissue-cultured seedlings leaves was observed by staining with ρ-dimethylaminocinnamaldehyde (DMACA) solution (1% DMACA in methanol-6 M HCl). First, the leaves and fruit discs were soaked in an ethanol solution containing 30% acetic acid for 12 h until the leaves and discs turned white to avoid interference of other pigments with the staining. Then washed 2−3 times with 75% ethanol, and finally stained in 1% DMACA solution for 2 min, observed and photographed for recording. According to the previous description of the method (Tanaka et al., 1994), PAs were cleaved by 2-sulfanylethanol and quantified by high-performance liquid chromatography (HPLC).
2.3 RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Extraction of RNA from flesh and leaves according to the instructions of the spin column plant total RNA purification kit (Sangon Biotech). cDNA synthesis by referring to the instructions of PrimeScriptTM RT reagent Kit with gDNA Eraser (TaKaRa). The RT-qPCR was used to detect the transcription level of DkDTX1/MATE1 and PA biosynthesis-related genes. The primers are shown in Supporting Information S1: Table 1. RT-qPCR was performed on ABI QuantStudio 7 Flex Real-Time PCR system using TB Green® Premix Ex Taq II (TaKaRa). DkActin (GenBank accession no. AB473616) as an internal reference gene, the above cDNA as template, three biological replicates for RT-qPCR. The expression of DkDTX1/MATE1 gene relative to internal reference gene was calculated by CT () method.
2.4 Protein expression and purification
The extracellular domain of DkDTX1/MATE1 was codon optimised for the synthesis of DkDTX1/MATE1O. DkDTX1/MATE1O was amplified with specific primers containing BamHI and XhoI. DkDTX1/MATE1O and PGEX-6P-1 vector (Amersham Biosciences, Piscataway, NJ) were digested with restriction endonuclease and linked to form PGEX-6P-1-DkDTX1/MATE1O. The recombinant plasmid PGEX-6P-1-DkDTX1/MATE1O was transformed into the expression strain BL21 (DE3), and the single colony was selected and inoculated into the liquid LB medium. When bacterial culture OD600 = 0.6, isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to the final concentration of 1 mM, and shaken at 37°C. The cells were collected by centrifugation after inducing protein expression for 0, 3, 4, 5 and 6 h. After adding 500 μL x1 phosphate-buffered saline (PBS) suspension, ultrasonic crushing the bacterial culture. The supernatant and precipitate were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The bacterial culture was inoculated in 5 L liquid LB medium, and when OD600 = 0.6, IPTG was added to the final concentration of 1 mM. The cells were collected by centrifugation after overnight induction culture at 18°C. 1 x PBS was used to suspend the cell. The supernatant of the broken cell was loaded into GST gel affinity chromatography column for elution to obtain purified target protein.
2.5 Total protein extraction and Western blot analysis
After 5 g ‘Eshi 1’ flesh was ground in liquid nitrogen, phenol extraction buffer (0.7 M sucrose, 0.1 M KCl, 0.5 M Tris-HCl, 50 mM EDTA, pH = 7.5) was added to extract total protein. The upper phenolic phase was transferred to a new centrifuge tube and successively rinsed with methanol and acetone (twice the volume of the phenolic phase for the last collected). The protein precipitates were blown dry under nitrogen. The precipitate was dissolved by adding sodium dodecyl sulfate (SDS) loading buffer, mixed and boiled for 10 min before detection by SDS-PAGE. Polyclonal antibodies raised in rabbits were made using purified DkDTX1/MATE1O protein for immunoblot analysis. The membrane was incubated with anti-DkDTX1O/MATE1O antibody at 1:500, and then incubated with secondary antibody (HRP-Goat anti Rabbit) at 1:10 000. The internal control group was immunoblotted with anti-actin antibody (1:1000 dilution; Sigma-Aldrich). The wild type of Arabidopsis as a negative control.
2.6 Vacuum infiltration and transient gene expression in fruit discs
Primers containing two restriction sites Xba I and Sma I (Supporting Information S1: Table 1) were used to amplify the open reading frame fragment of DkDTX1/MATE1, and then inserted into the overexpression vector pBI121 (Clontech). The silencing expression vector of DkDTX1/MATE1 was constructed by Gateway technique, and the DkDTX1/MATE1 fragment was inserted into pDONR207 vector (Invitrogen) and recombined into the silencing vector pHellsgate8 (Helliwell et al., 2002). The fusion plasmid pBI121-DkDTX1/MATE1 and pHellsgate8-DkDTX1/MATE1 was transformed into Agrobacterium tumefaciens GV3101 and infiltrated into the leaves of ‘Xiaoguo Tianshi’ in vacuum. The vacuum infiltration assay in tissue-cultured seedlings of persimmon was conducted as previously described (Mo et al., 2019). According to the description of Zhang et al. (2022), the transient transformation assay was carried out in Oily persimmon fruit discs.
2.7 Stable transformation
DkDTX1/MATE1 transgenic plants were obtained by A. tumefaciens infecting the leaves of ‘Gongcheng Shuishi’ tissue-cultured seedlings. The leaves were cut into 1.0 cm2 and cultured in the dark on MS medium (1/2 N + 3% sucrose + 0.1 mg/L IAA + 1.0 mg/L ZT). Three days later, the leaves were infected for 15 min with A. tumefaciens and placed on MS medium covered with filter paper. After 7 day of dark culture, the leaves were placed on the callus induction medium (1/2 N + 3% sucrose + 0.1 mg/L IAA + 1.0 mg/L ZT + Cef 400 mg/L) and cultured in the dark at 25°C. The adventitious buds were cultured by light and the transgenic lines were screened on the medium containing kanamycin.
2.8 Observation on autofluorescence of PAs
PA has the characteristic of autofluorescence. The wavelength of excitation is 405 nm and the wavelength range of emission is 505−550 nm (Brillouet et al., 2013). The leaves with transient overexpression of DkDTX1/MATE1 were prepared into sections, 4′,6-diamidino-2-phenylindole staining solution was added after PBS rinsing and incubated for 5 min in the dark. The anti-fluorescence quenching sealer was added, and the fluorescence was observed under a fluorescence microscope (Olympus).
2.9 Molecular docking
Using the most homologous MATE transporter as the template (Protein Data Bank accession code: 3MKU), the structural model of DkDTX1/MATE1 was established by homology modelling. The three-dimensional (3D) structure model of DkDTX1/MATE1 was built using SWISS-MODEL (Waterhouse et al., 2018). The molecular docking protocol was verified by evaluating RMSD between DkDTX1/MATE1 model and 3MKU template. The molecular docking simulation was partly based on the methods of Martiz et al. (2022) and Morales-Quintana et al. (2019). AutoDock has been proved to be a tool that can accurately and quickly predict the binding conformation and binding energy between ligand and receptor (Morris et al., 2009). Using AutoDock 4.0 to remove water molecules, add hydrogen atoms, and calculate the charge of DkDTX1/MATE1, which was stored in PDBQT format as a receptor. We selected five PA precursors (C, EC, ECG, EGC and EGCG) as ligands, which were previously identified as PA components in persimmon (Liu et al., 2023). The 3D structure of the PA precursor was derived from PubChem and modified using Chemdraw18.0. Further add hydrogen atoms, detect root, choose torsions, and save as PDBQT format for docking. Molecular docking using Autodock 4.0 predicted the putative interaction patterns between DkDTX1/MATE1 and different PA precursors. Each precursor was docked three times independently, and 50 conformations were obtained for each docking. The 3D structure of DkDTX1/MATE1 docking with PA precursors was visualised by PyMol. The interaction between DkDTX1/MATE1 and substrate was analysed using LigPlot+. Site-directed mutagenesis of DkDTX1/MATE1 was performed using Swiss-PdbViewer.
2.10 Transporter assays in E. coli
To construct vector for E. coli complementary, the full-length coding sequence of DkDTX1/MATE1 was inserted into BamHI and XhoI sites on PGEX-6P-1 vector. The primers are listed in Supporting Information S1: Table 1. The empty and constructed vector were transformed into the E. coli acrB mutant strain which lacked the ability of drug efflux. The single colony was selected and grown in LB liquid medium until the optical density (OD600) was 0.6, and 1 mM IPTG was added to induce DkDTX1/MATE1 expression. The bacterial culture with OD600 of 1.0 was diluted into five continuous decreasing gradients (1.0, 0.8, 0.6, 0.4, 0.2). The equal volume of diluent was spotted onto the medium containing PA precursors and 1 mM IPTG. The concentrations of PA precursors were 0, 50 and 100 μM. Plates were incubated overnight at 37°C.
2.11 Site-directed mutagenesis
Site-directed mutagenesis was performed on the amino acid residues of DkDTX1/MATE1, which interact with the substrate. Gene-specific primers containing mutant sites (Supporting Information S1: Table 1) were used to amplify the full-length coding sequence of DkDTX1/MATE1. Ser 68 was substituted with Ala. The mutation was named dtx1/mate1. The dtx1/mate1 sequence was cloned into the overexpression vector pBI121 for further analysis.
2.12 Statistical analysis
All the experiments involved in this paper contain more than 3 biological repeats. SPSS (IBM SPSS Statistics v22.0) software was used to analyse the significance. The asterisk indicates that a significant difference between the treatment and the control, which was evaluated by analysis of variance, and Fisher's Least Significant Difference test was performed.
3 RESULTS
3.1 The expression level of DkDTX1/MATE1 in A-type is higher than that in NA-type at the early stage of fruit development
To study the changes of PA percentage concentrations during fruit development in A-type and NA-type, fruit at 2.5, 5, 10, 15, 20, 25 and 27.5 WAB were assessed (Figure 1a). The concentrations of soluble PA in persimmon fruit were analysed by imprinting method. With the development of fruit, the colour of filter paper gradually became lighter, and the staining of A-type was deeper than that of NA-type (Figure 1b), indicating that the concentrations of soluble PA in A-type was higher than that of NA-type. This result was further verified by Folin-Ciocalteu method. By analysing the dynamic changes of fruit PA concentrations, it was found that there were significant differences in soluble and insoluble PA percentage concentrations between the two types of persimmon during fruit development (Figure 1c,d). The PA percentage concentrations of A-type and NA-type decreased continuously during fruit development, and the percentage concentrations of soluble and insoluble PA in A-type was higher than that in NA-type as a whole. The difference was that the soluble PA percentage concentrations of NA-type was naturally deastringent (soluble PA concentrations ≤ 0.2%) at 27.5 WAB, reaching the edible level. However, the soluble PA percentage concentrations of A-type remained at about 0.5% at 27.5 WAB, which could not be eaten directly.
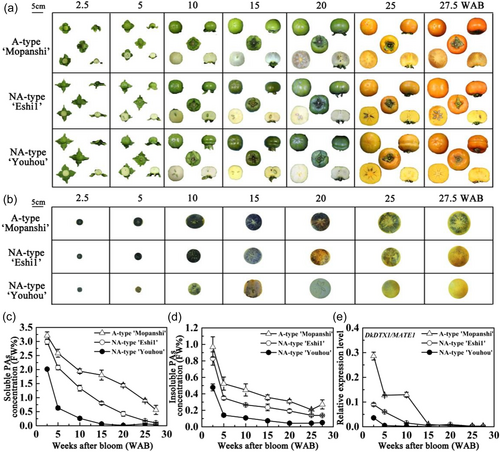
To study the expression pattern of DkDTX1/MATE1, the expression level of fruit in two types of persimmon during fruit development was analysed. With the development of fruit, the expression trend of DkDTX1/MATE1 in the two types was similar, the expression level decreased gradually. The expression level of DkDTX1/MATE1 in A-type was higher than that in NA-type, and its change trend was consistent with the percentage concentrations of PA (Figure 1e).
The protein expression level of DkDTX1/MATE1 was further analysed by western blot. DkDTX1/MATE1 had a complex structure and contained many TM domains, so the extracellular region sequence was selected to synthesise the extracellular gene, named DkDTX1O/MATE1O. DkDTX1O/MATE1O was expressed in E. coli to obtain DkDTX1O/MATE1O recombinant protein, which was further prepared into polyclonal antibody. After DkDTX1O/MATE1O was induced by IPTG at 37°C for 3, 4, 5 and 6 h, a protein band appeared at 40 kD (Supporting Information S1: Figure 1a), which was consistent with the predicted value. Due to the low expression level of DkDTX1O/MATE1O protein in the supernatant, to obtain active DkDTX1O/MATE1O protein, the induction temperature was further reduced to 18°C. After IPTG induction, a large amount of DkDTX1O/MATE1O fusion protein appeared in the supernatant, and the purified target protein was obtained after further elution (Supporting Information S1: Figure 1b). The total flesh protein of NA-type at different fruit development stages was extracted (Supporting Information S1: Figure 2) and incubated with antibody for western blot. The results show that the expression level of DkDTX1/MATE1O protein decreased gradually with fruit development (Supporting Information: Figure 3).
3.2 Overexpression or silencing of DkDTX1/MATE1 respectively increases or decreases the PA concentrations in persimmon leaves
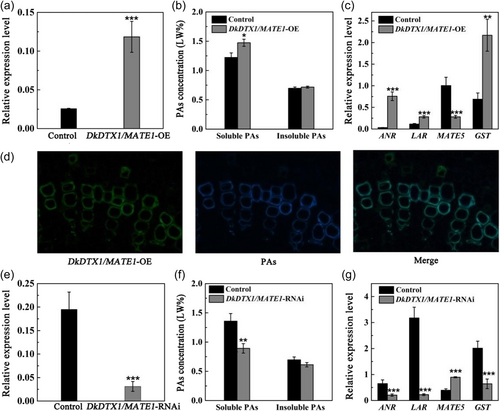
The transient transformation of leaves is unstable by the influence of environment and leaf structure. Therefore, to verify the function of DkDTX1/MATE1, the tissue-cultured seedlings were used for vacuum infiltration. RT-qPCR results showed that after overexpression of DkDTX1/MATE1, the expression level of DkDTX1/MATE1 increased by fivefold (Figure 2a). Compared with the control, the concentrations of soluble PA in DkDTX1/MATE1-overexpressing leaves increased significantly, indicating that DkDTX1/MATE1 was involved in PA accumulation (Figure 2b). The genes related to PAs synthesis were upregulated in DkDTX1/MATE1-overexpressing leaves, in which the expression level of DkANR, DkLAR and DkGST increased by 25-, 2.5- and 3.1-fold, respectively, while the expression of DkDTX5MATE5 decreased by 3.6-fold (Figure 2c). To directly judge the effect of DkDTX1/MATE1 on PAs, the autofluorescence of PAs in the DkDTX1/MATE1-overexpressing leaves was observed. The results showed that the green fluorescence of DkDTX1/MATE1 coincided with the blue fluorescence of PAs (Figure 2d). According to the previous subcellular localisation showed that DkDTX1/MATE1 was located in the tonoplast (Yang et al., 2016), we speculated that both of them may be colocated in the tonoplast, implying that DkDTX1/MATE1 has the ability to transport PAs. After silencing DkDTX1/MATE1, the opposite results were obtained. The expression level of DkDTX1/MATE1, the concentrations of soluble PA, the expression level of DkANR, DkLAR and DkGST decreased significantly (Figure 2e−g). In particular, the expression level of DkLAR decreased the most (14-fold), but the expression level of DkDTX5/MATE5 increased by 2.2-fold, suggesting that the silencing of DkDTX1/MATE1 affects the expression of key genes of PAs synthesis and transport, thus affecting the PA accumulation.
3.3 Overexpression of DkDTX1/MATE1 can promote PAs accumulation in persimmon fruit discs
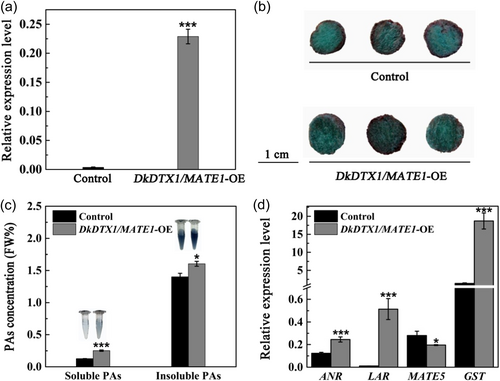
DkDTX1/MATE1 has been shown to be involved in PAs transport in persimmon leaves, while the ultimate goal is to use genetic engineering to regulate the composition and proportion of fruit. Therefore, to study its role in persimmon fruit, pBI121-DkDTX1/MATE1 was transferred into fruit discs. The results of RT-qPCR showed that the expression level of DkDTX1/MATE1 was significantly increased compared with the control (Figure 3a). When PAs are not oxidised, they can react with DMACA to form blue substances. The darker the stained colour, indicating the higher content of PAs. To intuitively judge the accumulation of PAs in fruit discs after overexpression of DkDTX1/MATE1, the fruit discs were stained with DMACA. The results showed that the blue colour of the fruit disc of DkDTX1/MATE1-overexpressing was darker than that of the control (Figure 3b), indicating that there was a high accumulation of PAs after DkDTX1/MATE1 overexpression. The soluble and insoluble concentrations of fruit discs were further extracted by Folin-Ciocalteu method. Observing the colour of the reaction solution, it was found that the colour of the DkDTX1/MATE1-overexpressing was darker than that of the control. Correspondingly, the concentrations of soluble PA in DkDTX1/MATE1-overexpressing fruit discs increased by twofold, and the concentrations of insoluble PA increased significantly (Figure 3c), which was consistent with the results of DkDTX1/MATE1 leaves transformation, and promoted PA accumulation more obviously. The expression levels of PAs synthesis-related genes in fruit discs after DkDTX1/MATE1 overexpression were analysed. It was found that the expression levels of DkANR, DkLAR and DkGST were upregulated in DkDTX1/MATE1-overexpressing fruit discs. The expression of DkLAR increased the most, reaching 47-fold, while DkDTX5MATE5 was downregulated (Figure 3d). It was preliminarily judged that the overexpression of DkDTX1/MATE1 has a great influence on the DkLAR gene in PAs synthesis.
3.4 Overexpression of DkDTX1/MATE1 results in increased PAs levels in transgenic persimmon
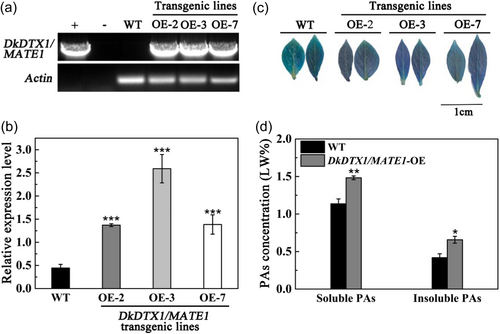
The effect of DkDTX1/MATE1 on promoting PA accumulation has been verified by transient transformation of persimmon leaves and fruit discs. To further study its role in persimmon, a number of DkDTX1/MATE1 overexpression regenerated plants were obtained through stable genetic transformation. Three regenerated plants of DkDTX1/MATE1 OE-2, OE-3 and OE-7 were randomly selected for further detection. The results of PCR detection showed that the three regenerated plants were positive (Figure 4a). Compared with the wild type (WT), the expression level of DkDTX1/MATE1 in DkDTX1/MATE1 OE-2, OE-3 and OE-7 lines increased by 3.1-, 5.8- and 3.1-fold, respectively (Figure 4b). The leaves of WT and DkDTX1/MATE1 regenerated plants stained with DMACA showed that the blue colour of WT leaves was lighter, and the colour of DkDTX1/MATE1 regenerated plants leaves was darker (Figure 4c). The same results were obtained by Folin-Ciocalteu method. Compared with WT, the concentrations of soluble and insoluble PA in leaves of DkDTX1/MATE1 regenerated plants significantly increased (Figure 4d).
3.5 Ser-68 of DkDTX1/MATE1 binds to PA precursors
To explore the transport mechanism of DkDTX1/MATE1, that is, the binding with PA precursors, the 3D structure model of DkDTX1/MATE1 was simulated by homology modelling. The RMSD value between the DkDTX1/MATE1 model and the template was 0.46 Å. Based on the analysis of the 3D structure model of DkDTX1/MATE1, it was found that DkDTX1/MATE1 had 12 TM helices, which were arranged into two bundles, each with six TM helices. These TM helices formed a large open cavity that allowed PA precursors to pass through (Figure 5a). The binding free energy (ΔG) of DkDTX1/MATE1 with C, EC, ECG, epigallocatechin (EGC) and EGCG were all negative (Table 1), indicating that these precursors may bind to DkDTX1/MATE1. The ΔG of DkDTX1/MATE1 to C, ECG and EGCG was smaller than that of EC and EGC, indicating that the affinity of DkDTX1/MATE1 to C, ECG and EGCG was higher than that of EC and EGC. Among them, DkDTX1/MATE1 had the highest binding affinity with EGCG. The results of molecular docking showed that the amino acid sites of DkDTX1/MATE1 binding to C were Asn 64, Ser 68, Ile 88, Gln 94, Gln 166 and Ala 228 (Figure 5b). The amino acid sites of DkDTX1/MATE1 interacting with ECG were Ser 68, Ile 88, Gly 92, Tyr 98, Ala 228 and Asn 314 (Figure 5c). Tyr 65, Ser 68, Gln 94, Gly 95, Ile 286, Cys 312 and Asn 314 of DkDTX1/MATE1 could interact with EGCG (Figure 5d). In these amino acid sites of DkDTX1/MATE1, Ser-68 could interact with C, ECG and EGCG, Asn 314 could bind to ECG and EGCG, Ile 88 and Ala 228 could interact with C and ECG.

| Ligand | ΔG (Kcal/mol) |
|---|---|
| DkDTX1/MATE1 | |
| Catechin | −4.45 |
| Epicatechin | −3.75 |
| Epicatechin gallate | −4.75 |
| Epigallocatechin | −3.81 |
| Epigallocatechin gallate | −5.03 |
- Note: The data show the mean binding free energy of the top 10 protein-ligand conformations of three independent dockings.
To verify the role of Ser-68, Ile 88, Ala 228 and Asn 314, we mutated these amino acid residues in silico. In the first group, Ser 68 was substituted with Ala. In the second group, Ile 88 was substituted with Ala. In the third group, Ala 228 was substituted with Leu. In the fourth group, Asn 314 was substituted with Ala. These four groups of mutations were named as dtx1/mate1, dtx1/mate1-1, dtx1/mate1-2 and dtx1/mate1-3, respectively. The results of molecular docking showed that compared with DkDTX1/MATE1, dtx1/mate1-1 only had significant reduced in binding with C. There was no significant difference in the binding of dtx1/mate1-2 to the three precursors. The binding of dtx1/mate1-3 to ECG was significantly reduced (Table 2). Among these mutants, dtx1/mate1 not only significantly reduced the interaction with the three precursors, but also had the largest change in ΔG. In particular, EGCG with the highest binding affinity to DkDTX1/MATE1 was only weakened when interacting with Ser-68 mutants, and no significant difference were observed in other mutants. These results suggest that Ser-68 is one of the key amino acid sites of DkDTX1/MATE1 responsible for PA precursor transport in persimmon.
| Ligand | ΔG (Kcal/mol) | ||||
|---|---|---|---|---|---|
| DkDTX1/MATE1 | dtx1/mate1 | dtx1/mate1-1 | dtx1/mate1-2 | dtx1/mate1-3 | |
| Catechin | −4.51a | −4.13b | −4.31b | −4.50a | −4.52a |
| Epicatechin gallate | −4.64a | −4.11b | −4.61a | −4.71a | −4.32b |
| Epigallocatechin gallate | −4.93a | −4.45b | −4.80a | −4.75a | −4.87a |
- Note: The data show the mean binding free energy of the top 10 protein-ligand conformations of three independent dockings. Different letters indicate that the binding free energy between DkDTX1/MATE1 and mutants are significantly different when bound to the same ligand (p < 0.05).
Furthermore, we transiently overexpressed DkDTX1/MATE1 and dtx1/mate1 in persimmon tissue-cultured seedlings. RT-qPCR showed that the DkDTX1/MATE1 expression levels in overexpressed leaves were significantly elevated compared with that of the control (Figure 5e). The detection of PA concentrations in leaves revealed that compared with the control, the soluble PA concentrations in DkDTX1/MATE1-overexpressing leaves increased significantly, while the PA concentrations in dtx1/mate1-overexpressing leaves had no significant change (Figure 5f), indicating that dtx1/mate1 lost PA transport activity, and DkDTX1/MATE1 bound to PA precursor may be through Ser-68.
3.6 DkDTX1/MATE1 preferentially transports C, ECG, and EGCG
To further confirm the PA precursors transported by DkDTX1/MATE1, the PAs composition in DkDTX1/MATE1-overexpressing leaves were analysed by HPLC. Due to the high degree of polymerisation of PAs in persimmon, to study its composition, PAs were extracted with acetone and further detected with 2-sulfanylethanol thiolysis PAs. C as the starting unit had no cleavage product, so four cleavage products EC-S, ECG-S, EGC-S and EGCG-S were detected. The peaks of C, ECG-S and EGCG-S in DkDTX1/MATE1-overexpressing leaves were strong increased compared with the control (Figure 6a,b). There was no significant difference in EC-S and EGC-S between DkDTX1/MATE1-overexpressing and control leaves. These results indicate that the increase of PAs concentrations caused by DkDTX1/MATE1 overexpression was mainly owing to the elevation of C, ECG and EGCG content.
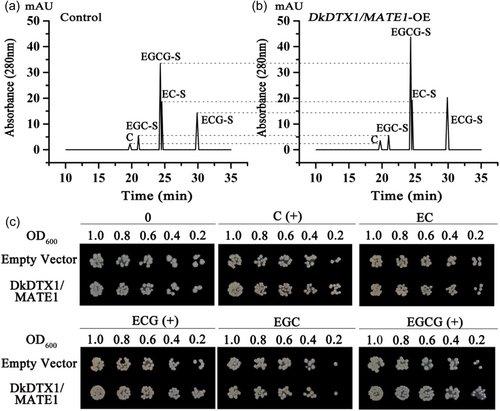
We next investigated the transport activity of DkDTX1/MATE1. We used the acrB mutant strain for complementation analysis, which lost drug efflux capability, and was often used to verify the transport activity of DTX/MATE. First, DkDTX1/MATE1 was expressed in the mutant strain and its growth activity at different concentrations of C was observed (Supporting Information S1: Figure 4). In the absence of C, there was no difference in bacterial growth between the DkDTX1/MATE1 and the empty vector. After adding C, the growth of the mutant strain expressing DkDTX1/MATE1 was better than that of the empty vector. Especially when 100 μM of C was added, the effect was more obvious, so we used 100 μM as the substrate concentration. With this experimental system, we observed the growth status of the mutant strain cultured with different substrates after expressing DkDTX1/MATE1 (Figure 6c). There was no difference in bacterial growth was observed between the DkDTX1/MATE1 transformant strains and the empty vector when cultured without the substrates. Upon supplementation with C, ECG, or EGCG, which was accompanied by decreased bacterial concentration, growth of the mutant strain expressing DkDTX1/MATE1 was superior to that of the strain transformed with the empty vector. This result indicate that DkDTX1/MATE1 expression complemented the lack of drug efflux ability of the E. coli acrB mutant. Furthermore, upon supplementation with other substrates, including EC or EGC, differences in growth were not observed, which indicate that DkDTX1/MATE1 does not transport EC and EGC and preferentially transports C, ECG, and EGCG.
LAR has been shown to produce C-type catechins in several species. The overexpression of DkDTX1/MATE1 also had a significant effect on LAR. Further, we coinjected DkDTX1/MATE1 and DkLAR into ‘Eshi 1’ leaves, and detected the soluble and insoluble PA concentrations in the control and coinjected leaves. We found that the expression levels of DkDTX1/MATE1 and DkLAR in coinjected leaves were significantly increased compared with the control (Supporting Information S1: Figure 5a). Consistent with the previous results, the concentration of soluble PA increased significantly after DkDTX1/MATE1 overexpression. The concentration of soluble and insoluble PA increased more significantly after coexpression of DkDTX1/MATE1 and DkLAR (Supporting Information S1: Figure 5b).
4 DISCUSSION
In recent years, PAs has attracted more and more attention, not only because of its beneficial pharmacological properties, but also due to the special role of PAs in regulating fruit quality (Yu et al., 2020). The content and composition of PAs can be regulated by changing the expression of key genes in the biosynthesis pathway. Improving the transport mechanism of PAs can not only supplement the lack of knowledge of PAs biosynthesis in different species, but also change the content and composition ratio of PAs more quickly and accurately, so as to improve nutritional value and quality of fruit. In this study, it was found that DkDTX1/MATE1 was expressed in the early stage of fruit development, and there was differential expression between A-type and NA-type persimmon. DkDTX1/MATE1 preferred to transport different PA precursors, which provide an idea for regulating the composition of persimmon fruit and cultivating excellent varieties.
A remarkable feature of vacuoles is the secondary metabolites accumulation that enable plants to adapt to environmental stresses (Shitan & Yazaki, 2020). As a secondary metabolite, PA is harmful when it occupies the main part of the cell, so it is necessary to isolate them in the vacuole to avoid harmful effects. In this study, we found that the formation rate of resistant shoots after DkDTX1/MATE1 transformation was very low. In previous reports, only 15 resistant adventitious shoots are produced in 720 infected leaves (Tao et al., 1997). After transformation of DkMyb4, which promotes PA synthesis, the callus turns black and stops growing (Akagi, Ikegami, Tsujimoto, et al., 2009). This may be related to toxicity caused by excessive accumulation of PA. In addition, the oxidation of phenolic compounds can cause plant tissue browning, thus reducing callus growth and regeneration efficiency in vitro. The PAs percentage content of the A-type and NA-type persimmon decreased with the development of fruit (Figure 1c,d). The PAs content of many fruit trees, such as bilberry and blueberry, also decrease gradually during fruit development (Jaakola et al., 2002; Zifkin et al., 2012). The astringency and bitterness caused by high PAs content in young fruits can prevent them from being ingested by herbivores before ripening. It has also been found in sorghum that condensed tannins can prevent sparrows from eating sorghum grains (Wu et al., 2019). These results indicate that PA plays an important role in plant growth and development and resistance to biological stress.
DkANR, DkLAR, and DkGST are crucial structural genes involved in the PA biosynthesis pathway in persimmon. We found that the overexpression of DkDTX1/MATE1 accompanied with upregulation of crucial genes DkANR, DkLAR, and DkGST (Figure 3d). The effect of overexpression and knockout lines on the expression level of biosynthetic structural genes may be due to the feedback mechanism after the interference of transport. This phenomenon was also found in other transporters. The expression level of related structural genes is also decreased after PpGST1 silencing (Zhao et al., 2020). The genetic transformation of NtMATE21 and NtMATE22 affect the expression of structural genes involved in flavonol biosynthesis (Gani et al., 2022). The expression of DcMATE21 was also found to be associated with the increased expression of key genes in anthocyanin biosynthesis (Saad et al., 2023). LAR and ANR are the last step of PAs biosynthesis, in which LAR can produce C. Among these genes, DkDTX1/MATE1 had a greater effect on DkLAR. We speculate that DkDTX1/MATE1 transports C into the vacuole, the content of C in the cytoplasm decreases and feeds back to LAR, thus increasing the expression of LAR. These results suggest that DkDTX1/MATE1 plays an important role in persimmon PA accumulation by influencing the PA biosynthesis pathway genes.
Interestingly, the expression level of DkDTX5/MATE5 was notably decreased in DkDTX1/MATE1-overexpressing leaves (Figure 2c). Similarly, previous studies also found that DkDTX1/MATE1 was downregulated in DkDTX5/MATE5-overexpressed leaves (Liu et al., 2023). We speculate that on the one hand, the DkDTX5/MATE5 gene may be suppressed by DkDTX1/MATE1. Due to the opposite expression patterns of DkDTX1/MATE1 and DkDTX5/MATE5 during fruit development, when the expression level of DkDTX1/MATE1 is high, the expression level of DkDTX5/MATE5 is lower. Therefore, the increase of the expression level of endogenous DkDTX1/MATE1 may also suppress the expression of endogenous DkDTX5/MATE5. On the other hand, DkDTX5/MATE5 gene may be suppressed by the exogenous DkDTX1/MATE1 transgene. When DkDTX1/MATE1 expression increased, the levels of transported substrates also increased, thereby inhibiting DkDTX5/MATE5 from transporting the substrate, and downregulated its expression level. This phenomenon has also been reported in phloretin glycosyltransferase (PGT). Both PGT2 and PGT3 can catalyse the synthesis of phloridzin. The expression of PGT3 decreased significantly in PGT2-overexpressing lines (Wang et al., 2020). DkDTX1/MATE1 was mainly expressed in the early stage, while DkDTX5/MATE5 was expressed in the middle and late stages of persimmon fruit development. The expression level of DkDTX1/MATE1 in A-type was higher than that in NA-type, but the expression level of DkDTX5/MATE5 was opposite. DkDTX1/MATE1 mainly transports A-type components, and DkDTX5/MATE5 transports NA-type components. These results reveal that although both DkDTX1/MATE1 and DkDTX5/MATE5 are involved in the accumulation of PA, the specific roles are still different.
Different positions of PAs synthesis and storage require effective transport mechanisms to maximise biological effects. GST is a key enzyme in secondary metabolism, which can form GST-PAs complexes (Wei et al., 2021). Recently, some studies have shown that GST transport and membrane protein transport cooperate to transport flavonoid (Zhao, 2015). In Arabidopsis, TT12 and TT19 (GST) are considered to be necessary for PAs vacuolar sequestration (Kitamura et al., 2010). After knocking out TT12 or TT19, PAs can still be detected in the seed coat (Kitamura et al., 2004; Zhao & Dixon, 2009), but the location of PA accumulation changed, suggesting that TT12 and TT19 may cooperate to transport PAs (Zhao, 2015). In this study, we found that overexpression or silencing of DkDTX1/MATE1 caused changes in the expression level of DkGST (Figure 2c,g), indicating that DTX/MATE and GST may also have a cooperative transport mode in persimmon. We speculate that after the PA precursors produced by the ER cytoplasmic surface binds to GST, it is transported to the tonoplast by GST, and then specifically recognises PAs through DTX/MATE and enters the vacuole for storage. In the future, it is necessary to further verify the function of GST and whether there is a cooperative transport of DTX/MATE and GST in persimmon.
Due to the complex structure of PAs, the components of PA in different species are different, and the PAs of different varieties of the same species are also different. The extension unit of PA in blueberry is mainly EC, and the grapevine is C and EC (Yu et al., 2019; Zifkin et al., 2012). Akagi et al. (2010) analysed that the main components of persimmon PA are C, EC, ECG, EGC and EGCG. Similarly, we detected these five components in transformed leaves, and found the highest content of EGCG in DkDTX1/MATE1-overexpressing leaves (Figure 6b). Appropriate concentrations of PAs can improve the quality of fruits and their processed products, which is beneficial to the human body. However, too high concentrations of PA or the proportion of some subunits can cause strong astringency of fruits and seriously affect the edible flavour (Wu et al., 2022). The higher the gallic acid component in the PAs, the stronger the interaction with the protein, and the higher the degree of astringency (de Freitas and Mateus, 2001). Comparing the PA components of different varieties, it was found that the content of EGCG in A-type is higher, while the content in NA-type is lower, with a maximum difference of threefold (Akagi et al., 2010). The high content of EGCG in A-type may be the reason for its high astringency. In our study, we found that DkDTX1/MATE1 had a stronger ability to transport EGCG and the expression level of DkDTX1/MATE1 in A-type was significantly higher than that in NA-type. Therefore, in the early stage of fruit development of A-type, the high expression level of DkDTX1/MATE1 and the preferential transport of EGCG may promote the accumulation of more EGCG in A-type, resulting in a stronger astringency of A-type. Furthermore, our results show that one of the key site of DkDTX1/MATE1 binding to PA precursor was Ser-68. With the publication of the persimmon genome (Li et al., 2023), gene editing technology can be used to edit DkDTX1/MATE1 in the future to reduce the DkDTX1/MATE1 transport activity, thereby reducing astringency of persimmon fruit.
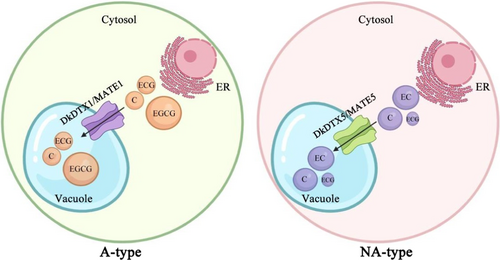
In conclusion, combined with the previous studies of DkDTX5/MATE5, we propose a working model to demonstrate the effect of DkDTX/MATE on the astringency of persimmon (Figure 7). In A-type, DkDTX1/MATE1 preferentially transports the main PA components of A-type, which makes A-type accumulate more EGCG, resulting in stronger astringency of A-type. In NA-type, DkDTX5/MATE5 preferentially transports the main PA components of NA-type, which leads to lower astringency of NA-type. The different expression patterns and substrate transport ability of DkDTX1/MATE1 and DkDTX5/MATE5 improve the PA transport mechanism during fruit development and affect the astringency of different persimmon varieties. PA exists in many fruits, and DTX/MATE has been found to have transport activity in other species. This study analysed the relationship between DTX/MATE and the natural variation of PA accumulation. Our findings not only serve as a basis for future studies aiming to breed new persimmon cultivars with modified PA components in the fruit, but also have potential applications in other fruit species in metabolic engineering, which is of great significance for fruit tree breeding.
ACKNOWLEDGEMENTS
This work was financially funded by the National Key Research and Development Program of China (grant No. 2019YFD1000600).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




