A novel Pik allele confers extended resistance to rice blast
Zhongqiang Qi and Xiuli Meng contributed equally to this work.
Abstract
In the ongoing arms race between rice and Magnaporthe oryzae, the pathogen employs effectors to evade the immune response, while the host develops resistance genes to recognise these effectors and confer resistance. In this study, we identified a novel Pik allele, Pik-W25, from wild rice WR25 through bulked-segregant analysis, creating the Pik-W25 NIL (Near-isogenic Lines) named G9. Pik-W25 conferred resistance to isolates expressing AvrPik-C/D/E alleles. CRISPR-Cas9 editing was used to generate transgenic lines with a loss of function in Pik-W25-1 and Pik-W25-2, resulting in loss of resistance in G9 to isolates expressing the three alleles, confirming that Pik-W25-induced immunity required both Pik-W25-1 and Pik-W25-2. Yeast two-hybrid (Y2H) and split luciferase complementation assays showed interactions between Pik-W25-1 and the three alleles, while Pik-W25-2 could not interact with AvrPik-C, -D, and -E alleles with Y2H assay, indicating Pik-W25-1 acts as an adaptor and Pik-W25-2 transduces the signal to trigger resistance. The Pik-W25 NIL exhibited enhanced field resistance to leaf and panicle blast without significant changes in morphology or development compared to the parent variety CO39, suggesting its potential for resistance breeding. These findings advance our knowledge of rice blast resistance mechanisms and offer valuable resources for effective and sustainable control strategies.
1 INTRODUCTION
Rice (Oryza sativa) is a major staple food crop for over 50% of the world's population, and it particularly dominates Asian food markets (Liu et al., 2019). Rice production is threatened by rice blast caused by the ascomycete fungus Magnaporthe oryzae (Wilson & Talbot, 2009). Incorporation of resistance (R) genes into rice cultivars remains the most effective and eco-friendly method to control rice blast (Wang & Valent, 2017). However, a limitation of this approach is that the majority of R genes do not provide long-term resistance because of the high variability of avirulence (Avr) genes in the pathogen (Cesari et al., 2022; Skamnioti & Gurr, 2009; Xiao et al., 2017). Therefore, it is of great importance to continuously identify new R genes or alleles and analyse the molecular mechanism of their interaction with Avr genes of M. oryzae for controlling rice blast.
Wild rice (Oryza rufipogon) is a major reservoir of genetic diversity, and it can be used to identify valuable genes to improve the quality and quantity of rice (Khush, 1997). For example, Xa21 (Song et al., 1995), an R gene conferring resistance against bacterial blight, and Pid3-A4, Pi9, and Pi54, which confer resistance against rice blast, were cloned from wild rice (Das et al., 2012; Devanna et al., 2014; Lv et al., 2013; Qu et al., 2006). More than 100 R genes conferring resistance against rice blast have been mapped, and more than 30 R genes have been cloned (Wang et al., 2017). Most of cloned R genes encode proteins containing a nucleotide binding site and leucine-rich repeat (NLR protein). Most of the cloned R genes encode nucleotide-binding/leucine-rich repeat (NLR) proteins, which generally contain three conserved domains: a variable N-terminal domain, nucleotide-binding oligomerization domain and leucine-rich repeat (Liu et al., 2010; Skamnioti & Gurr, 2009; Wang et al., 2017). As an intracellular immune receptor, NLR protein is considered to be the switch of plant immune system, after directly or indirectly recognising effector proteins, NLR protein initiates the immune response stimulated by effectors and causes constitutive programmed cell death (Balint-Kurti., 2019). Most R genes have been identified and cloned using a molecular map-based cloning approach, which is time-consuming. An alternative, easier method is bulked-segregant analysis (BSA) combined with next-generation-sequencing (BSA-seq) (Klein et al., 2018; Takagi et al., 2013). Numerous genes in maize, soybean, cotton, sunflower, barley, and rice have been cloned using this approach (Imerovski et al., 2019; Klein et al., 2018; Song et al., 2017; Sun et al., 2018; Zhu et al., 2017).
R genes against rice blast are clustered mostly on chromosomes 6, 11, and 12 (Liu et al., 2010), and some cloned R genes have multiple alleles (Leung et al., 2015; Xiao et al., 2017). For example, 12 alleles at the Pik locus of rice (Pik, Pikp, Pikh, Pikm, Piks, Pike, Pi7, Pik-KA Pi1, Pikg, Pikx and Pikps) have been characterised, and their function is determined by a pair of closely linked CC-NBS-LRR genes (Ashikawa et al., 2008, 2012; Campbell et al., 2004; Chaipanya et al., 2017; Chen et al., 2015; Hua et al., 2012; Kovi et al., 2022; Li et al., 2019a, 2019b; Meng et al., 2021; Yuan et al., 2011; Zhai et al., 2011; Zhai et al., 2014). All the cloned Pik alleles share high amino acid sequence identity, with most variation observed in the region encoding a Heavy-Metal Associated (HMA) domain (De la Concepcion et al., 2018; Kanzaki et al., 2012). Moreover, based on the polymorphisms in the HMA domain, Pik alleles can be divided into two subclasses referred to as K1 and K2 (Zhai et al., 2011). During the recognition of AvrPik by Pik, AvrPik mutated to form multiple alleles due to the selective pressure of Pik alleles. As well known that seven AvrPik alleles (AvrPik-A, -B, -C, -D, -E, -F and Avr-Mgk1) have been cloned, AvrPik-F allele is incapable of activating resistance in rice plants harbouring any of the Pik alleles, and Avr-Mgk1 was unrelated to the AvrPik alleles in sequence and was encoded on a mini-chromosome (Selisana et al., 2017; Sugihara et al., 2023; Yoshida et al., 2009).
The interaction between Pik alleles and AvrPik alleles is associated with polymorphic residues in the HMA domain and in the effector (De la Concepcion et al., 2018, 2021; Liu et al., 2021; Maqbool et al., 2015; Xiao et al., 2023) and follows an “arm race” model. Kanzaki et al. (2012) reported that the AvrPik-D allele is most likely the ancestral allele and was first recognised by Pikp in rice. AvrPik-E, which escaped recognition by Pikp, appeared after AvrPik-D and then rice evolved Pik to recognise AvrPik-E. AvrPik-A then evolved to escape recognition by Pik, and Pikm evolved in rice to recognise AvrPik-A (Kanzaki et al., 2012). Li et al. (2019b) improved on this model, demonstrating that AvrPik-D, -E, -A, and -B can be recognised by Pikh. Maidment et al. (2023) reported that an engineered Pik allele was capable to recognise AvrPik-C and AvrPik-F. However, until now, no natural Pik allele conferring resistance to AvrPik-F has been reported.
According to our previous monitoring data, the detection frequency of AvrPik alleles were more than 60% of 2206 M. oryzae isolates in Jiangsu province, especially a small number of AvrPik-C haplotype was also found (Data not shown). In addition, isolates carrying AvrPik-C haplotype were also detected in the major rice-growing areas in China (Li et al., 2019b), which leaded to a high risk of resistance loss in varieties carrying the Pik allele. In this study, we explored a novel Pik allele, Pik-W25 from wild rice and clarify the high resistance level of the rice monogenic line containing Pik-W25. Moreover, we analysed the recognition between Pik-W25 and AvrPik alleles. Our findings provide insight into the recognition between effectors and R genes and also provide new material for resistance breeding.
2 MATERIALS AND METHODS
2.1 Plant materials and isolates
Plant materials used in this study were listed in Table S4. Thirty-one species of wild rice (Oryza rufipogon) were obtained from International Rice Genebank Collection (IRGC) of International Rice Research Institute (IRRI). Six monogenic lines harbouring different Pik alleles were maintained at IRRI. Ca89 was collected from Caliraya, Laguna, Philippines. 5008-1, 6030-1, 9482-1-3 and 9149-1a were collected from Ubay, Bohol, Philippines. The six AvrPik alleles overexpressed isolates were received from State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops, Fujian Agriculture and Forestry University (FAFU) in China (Table S5). All strains were cultured on potato dextrose agar (PDA) medium at 28°C.
2.2 Genetic populations construction
For WR25, F2, BC1F1, BC1F2, BC2F1, BC2F2, BC3F1, BC3F2 and BC3F3 populations were generated and CO39 was used as a recurrent parent (Figure S1). Ca89 was used to inoculate the constructed populations to trace the target R gene in WR25. BC1F1 population was inoculated with Ca89 and the resistant lines were transplanted. CO39 was used as female to cross with resistant BC1F1 lines to generate BC2F1. The BC1F2lines was generated by selfing resistant BC1F1 lines. Likewise, BC2F1 population was inoculated with Ca89 and the resistant lines were selected. A cross between resistant BC2F1 lines and CO39 was done to generate BC3F1. Then resistant BC2F2 was also generated by selfing resistant BC2F1 lines. BC3F1 plant population was inoculated with Ca89 and the resistant lines were selected to generate BC3F2. BC3F2 were inoculated and the resistant ones were selected to generate BC3F3 progenies. Then BC3F3 progenies were harvested as single plant. Thirty seeds of every BC3F3 lines were selected and inoculated with Ca89 and only the populations without segregation will be used as monogenic lines of target R gene.
2.3 Construction of sequencing libraries and NGS sequencing
For BSA-Seq, two DNA pools were developed by selecting 100 extreme resistant (R-bulk) and 100 extreme susceptible (S-bulk) individuals and extracting genomic DNAs of two bulks by DNeasy Plant Maxi Kit. Equal amounts of leaves were obtained from two bulks and 10 µg DNA of two bulks were used to construct sequencing libraries. The sequencing was done on an Illumina HiSeqTM 2500 platform by “Novogene: Genome Sequencing Company” (https://en.novogene.com/). We used bwa mem (version 0.7.15) to align (Li & Durbin, 2010) sequencing reads to the Nipponbare reference genome (version RGAP 7). High-confidence single-nucleotide polymorphism (SNP) and insertion-deletion data were obtained by analysing the alignment results using Freebayes software. Samblaster (version 0.1.24) was used to mark duplications (Faust & Hall, 2014) and Samtools (version 1.6) was used to arrange alignment hit (Li & Durbin, 2010). Freebayes (Garrison & Marth, 2012) was used to call variants (SNPs and short InDels). Perl script of Variants Processor (https://github.com/jointgene/VariantsProcessor) was used for filtering out variants with low quality. Only variants which are homozygous in parents and polymorphic in F1 between the parents were remained for further analysis.
2.4 Allele frequency (AF) and allele frequency difference (AFD) analysis
Every variant has two alleles and the number of alleles from two bulks can form a 2 × 2 list. In our study, the number of allele in S-bulk from CO39 was called “a”. The number of allele in S-bulk from WR25 was called “b”. The number of allele in R-bulk from CO39 was called “c”. The number of allele in R-bulk from WR25 was called “d”. “a”, “b”, “c” and “d” can form a 2 × 2 list. AFCO39 in S-bulk = a/(a + b); AFWR25 in S-bulk = b/(a + b); AFCO39 in R-bulk = c/(c + d); AFWR25 in R-bulk=d/(c + d); AFDCO39in S-bulk and R-bulk = {a/(a + b)} − {c/(c + d)}; AFDWR25 in S-bulk and R-bulk = {b/(a + b)} − {d/(c + d)}. If the SNP has no linkage with candidate gene, the AF of this SNP in two bulks should approach “0.5” and the AFD of two bulks should approach “0”. If one SNP is highly linked with candidate gene, the AF of this SNP in one bulk should approach “1” and in the other bulk, the AF of this SNP should approach “0”, so that the AFD of two bulks should approach “1” or “-1”. A p-value for Cochran-Mantel-Haenszel (CMH) test performed between the S-bulk and R-bulk at each SNP locus was also calculated. The average distributions of the AF and AFD were estimated in a given genomic interval by a sliding window approach with a 3 Mb window size and 10 kb step. Regions in which the average AFD of a locus has a significant peak and windows reflects an average p < 0.05 were considered candidate genomic regions associated with resistance.
2.5 Primer design and sequence analysis
The coding sequence of Pik-W25 was amplified and sequenced. Sequencing was conducted by Biosune (http://www.biosune.com/) in China. BLASTN search programme (https://blast.ncbi.nlm.nih.gov/Blast) and “sequencher 5.4.6” software (http://www.genecodes.com) were used for sequence alignment. RGA4F3/R3 was used to amplify the HMA domain of Pik alleles. For the primers (W25-F/R) for detection of Pik alleles in the rice germplasm resources, we designed the primers with genomic sequence including CC and HMA domains of Pik-W25-1, with a length of 1356 bp.
2.6 Construction of AvrPik alleles overexpressed isolates
For over expression of AvrPik-A/B/C/D/E/F, we amplified a AvrPik-A/B/C/D/E/F gene fragment by PCR with primers RP27-AvrPik-F/R and inserted into the pYF11 (bleomycin resistance) vector with a strong promoter RP27. Then the constructs were used for protoplast transformation of the wild type strain R88002 (Qi et al., 2016). The resulting transformants were first screened by PCR amplification with primers RP27-AvrPik-F/R.
2.7 Y2H analyses
The yeast two-hybrid assay was used to detect protein-protein interactions. Pik-W25-1, Pik-W25-1-HMA and AvrPik-A/B/C/D/E/F complementary DNA were amplified with primers BD-W25-1-F/R, BD-HMA-F/R and AD-AvrPik-F/R, respectively. The amplified products were cloned into the pGBKT7 and pGADT7 vectors (BD Biosciences Clontech). After sequence verification, they were transformed into yeast AH109 strain following the protocol (BD Biosciences Clontech). Yeast transformants grown on synthetic medium minus leucine and tryptophan (SD-Leu-Trp) were transferred to synthetic medium minus leucine, tryptophan, adenine and histidine (SD-Leu-Trp-Ade-His). The interaction between pGBKT7-53 and pGADT7-T was used as the positive control. The interactions between pGBKT7-Lam and pGADT7-T, BD (pGBKT7)-Pik-W25-1 and AD (pGADT7), BD (pGBKT7)-Pik-W25-1-HMA and AD (pGADT7) vectors were used as negative controls (Qi et al., 2012).
2.8 SLC analyses
For SLC assays, the tested coding sequences were cloned into pCAMBIA-35S-nLuc (AvrPik-A/B/C/D/E/F) or pCAMBIA-35S-cLuc (Pik-W25-1 and Pik-W25-1-HMA) with the primers nluc-AvrPik-F/R, Cluc-w25-1-F/R and Cluc-HMA-F/R, and the construct plasmids were transformed into Agrobacterium strain GV3101, cultured overnight in LB medium, collected and suspended in infiltration buffer (10 mM MgCl2, 10 mM methylester sulfnate, 150 μM acetosyringone, pH 5.6), and incubated for 3 h at 30°C before infiltration. The suspensions were then infiltrated into 4-week-old Nicotiana benthamiana leaves in different combinations, after 2 days of growth, luciferase substrate (Promega) was sprayed onto the surface of the leaves and the luciferase signals were imaged using a Tanon (Zhai et al., 2022). All the primers in this study were synthesised by Sangon Biotech Co., Ltd. and were listed in the Table S6.
2.9 Plant infection and disease assessment
For blast fungus inoculation, conidia including R88002 and six AvrPik alleles overexpressed isolates were suspended in equal proportion to a concentration of 5 × 104 spores/mL in a 0.2% (w/v) gelatin solution and 5 mL each was spray on 2-week-old transgenic and wild type rice seedlings. The inoculated plants were kept in the dark for 24 h at 25°C with 100% relative humidity and then moved to the growth room followed by a 16 h:8 h, light:dark cycle. The disease severity was assessed at day 7 after inoculation.
The disease severity of the rice blast infection was evaluated using the standard 0–5 scale, rated on five levels as defined follows: level 0: no evidence of infection; level 1: brown specks smaller than 0.5 mm in diameter, no sporulation; level 2: brown specks about 0.5–1 mm in diameter, no sporulation; level 3: roundish to elliptical lesions about 1–3 mm in diameter with grey centre surrounded by brown margins, lesions capable of sporulation; level 4: typical spindle-shaped blast lesions capable of sporulation, 3 mm or longer with necrotic grey centres and water-soaked or reddish brown margins, little or no coalescence of lesions; level 5: lesions as in 4 but about half of one or two leaf blades killed by coalescence of lesions. Scores of 0–3 were considered resistant reactions, and scores of 4 and 5 were considered susceptible reactions (Qi et al., 2017).
The disease severity of the neck blast was evaluated using the standard 0–9 scale, rated on five levels as defined follows: level 0: no disease symptoms; level 1: lesion length ≤5 mm; level 3: lesion length 6–15 mm; level 5: lesion length ≤16 mm and no connection among lesions; level 7: connection among lesions and lesion area accounts for over 50% of the total rice panicle; level 9: connection among lesions and lesion area accounts for over 80% of the total rice panicle (Qi et al., 2023).
3 RESULTS
3.1 Rice blast resistant germplasm WR25 was selected from 27 accessions of O. rufipogon
To screen resistant germplasms to rice blast, 27 accessions of O. rufipogon were selected randomly from IRGC (International Rice Genebank Collection) of IRRI (International Rice Research Institute) and inoculated with four M. oryzae isolates which were preserved in the lab (Table S1). Inoculation results showed that eight accessions (WR4, WR18, WR24, WR25, WR26, WR28, WR29 and WR31) were resistant to the four isolates (Table S1). We chose WR25 for further study. F2 populations derived from crosses between the rice variety CO39 and WR25 were constructed and inoculated with Ca89 (Figure S1), which was commonly used in our lab, and it is also not virulent to WR25. Of the 234 F2 individuals, 188 were resistant and 46 were susceptible (Table 1). The ratio of resistance to susceptibility was found to fit a 3:1 segregation ratio according to the χ2 goodness-of-fit test (χ2 = 3.56, p = 0.06), indicating that the resistance of WR25 against Ca89 is controlled by a single locus/gene (Table 1).
| Isolate | Population | Number of progenies | Chi-square test | ||||
|---|---|---|---|---|---|---|---|
| R | S | Total | Expected ratio (R:S) | χ2 | p | ||
| Ca89 | CO39 × WR25 F2 | 188 | 46 | 234 | 3:1 | 3.56 | 0.059 |
| CO39 × WR25 BC1F2 | 866 | 265 | 1131 | 1.48 | 0.22 | ||
| CO39 × WR25 BC2F2 | 2490 | 956 | 3896 | 0.44 | 0.51 | ||
3.2 The R gene in WR25 was mapped to the telomere of chromosome 11 by BSA-seq
The BSA-seq method was used to map the R gene in WR25 conferring resistance against Ca89, and the BC1F2 population derived from WR25 and CO39 (Figure S1) was inoculated and used for constructing segregating pools (Table 1). In total, 283 071 846 and 350 950 212 clean short reads were generated from the R-pool (average read length of 348.6 bp) and S-pool (average read length of 341 bp), respectively (Table 2). Then, 96.04% of the R-pool reads and 95% of the S-pool reads were aligned to the rice reference genome (http://rice.plantbiology.msu.edu) using bwa mem (version 0.7.15) software (Li & Durbin, 2010). The mean coverage depths for R-pool and S-pool were 98 and 118.73, respectively (Table 2). More than 94.99% of the genome had at least 1× coverage in the two pools, and at least 88.46% had at least 20× coverage (Table 2). High-confidence single-nucleotide polymorphism (SNP) and insertion-deletion data were obtained by analysing the alignment results using Freebayes software (Table S2). Next, the allele frequency (AF) and allele frequency difference (AFD) for each SNP were computed. A candidate region with an average AF > 0.9 for the S-pool and AF < 0.1 for the R-pool was located in the interval 26.9–29 Mb on chromosome 11 (Figure 1a,b). AFD graphs were drawn according to the value calculated for each SNP in the R-pool and S-pool (Figure 1c), and the AFD in the 26.9–29 Mb interval on chromosome 11 was significant according to the Cochran-Mantel-Haenszel test (average p < 0.05) (Figure 1d). The average distributions of the AF and AFD were estimated in a given genomic interval by a sliding window approach with a 3 Mb window size and 10 kb step (Figure 1e), and we focused on the 26.9–29 Mb peak located in the telomere of chromosome 11 (Figure 1f).
| Sample ID | CO39 | R-bulk | S-bulk |
|---|---|---|---|
| Average insert size (bp) | 341.9 | 348.6 | 341 |
| Total reads | 61 291 878 | 283 071 846 | 350 950 212 |
| Mapped reads | 58 469 147 | 271 851 551 | 333 387 344 |
| Mapped rate (%) | 0.9540 | 0.9604 | 0.9500 |
| Mean of coverage depth | 22.61 | 98.00 | 118.73 |
| Coverage at least 1× (%) | 0.9205 | 0.9499 | 0.9512 |
| Coverage at least 7× (%) | 0.8376 | 0.9161 | 0.9191 |
| Coverage at least 10× (%) | 0.7915 | 0.9072 | 0.9108 |
| Coverage at least 20× (%) | 0.5005 | 0.8846 | 0.8904 |
- Note: Mean of coverage depth = mapped reads/size of rice reference genome. Coverage at least 1× (%): the percentage of reference genome had at least 1× coverage in the bulks. R: resistant, S: susceptible.
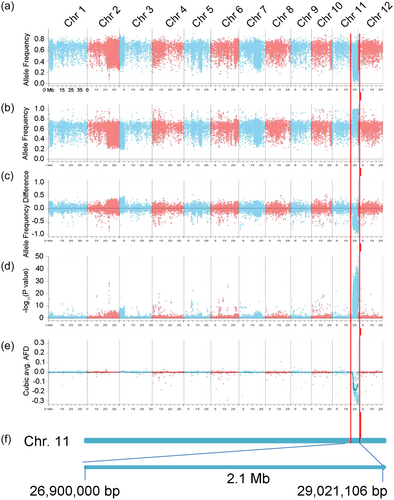
3.3 The R gene in WR25 conferring resistance against Ca89 is tightly linked to the Pik locus
The Pik locus is also located on the telomere of chromosome 11. To determine the relationship between the R gene in WR25 and the Pik locus, linkage analysis was conducted using 900 susceptible lines identified after inoculating the BC2F2 population with Ca89 (Table 1). A dominant marker (RGA4F3/R3) was designed based on the HMA domain in the Pik locus and a 1354 bp fragment was amplified in resistant lines containing Pik alleles (Figure S2). The target band could be amplified from the four resistant lines but not from the 900 susceptible lines, which indicated that the R gene in WR25 conferring resistance against Ca89 is linked to the Pik locus (Figure S2). The new allele was named Pik-W25. The BSA-seq raw data showed that two adjacent genes were resided in the Pik locus. These two genes, called Pik-W25-1 and Pik-W25-2, were transcribed in opposite directions and showed homology with other cloned genes in Pik locus. Three pairs of primers (RGA4F1/R1, RGA4F2/R2 and RGA4F3/R3) were used to amplify Pik-W25-1 and two pairs of primers (RGA5F1/R1 and RGA5F2/R2) were used to amplify Pik-W25-2. The amplicons were sequenced and the sequences were same as the BSA-seq raw data.
3.4 Pik-W25 is a chimera of K1 and K2
Pik-W25-1 consists of three exons with lengths of 564, 566 and 2299 bp, and the deduced gene product encodes a 1142-residue polypeptide (Figure 2a; Figure S3a). The N-terminal region of Pik-W25-1 contains a CC domain, HMA domain (residues 186–263), and NBS-ARC domain, and the C-terminal region contains LRR repeats (Figure 2a). The 3066-bp coding region of Pik-W25-2 is interrupted by a single 164 bp intron, and the deduced gene product encodes a 1021-residue polypeptide (Figure 2a; Figure S3b).
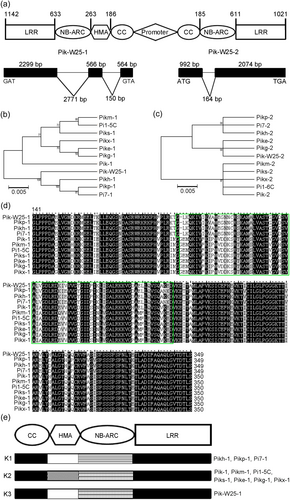
The levels of amino acid sequence identity between Pik-W25-1/Pikh-1, Pik-W25-1/Pi7-1, Pik-W25-1/Pikp-1, Pik-W25-1/Pi1-5C, Pik-W25-1/Pik-1, Pik-W25-1/Pikm-1, Pik-W25-1/Piks-1, Pik-W25-1/Pike-1, Pik-W25-1/Pikg-1 and Pik-W25-1/Pikx-1 are 96.98%, 96.98%, 96.81%, 91.94%, 92.59%, 91.94%, 92.18%, 92.35%, 92.35% and 92.18%, respectively (Figure 2b; Figure S3a). A similar comparison using the Pik-W25-2 amino acid sequence revealed similarity levels of 99.41% (Pikh-2, Pikp-2 and Pi7-2), 99.22% (Pik-2, Piks-2, Pikx-2 and Pikm-2), 99.80% (Pike-2 and Pikg-2) and 99.03% (Pi1-6C) (Figure 2c; Figure S3b). Interestingly, the sequence of HMA domain (residues 186–261) of Pik-W25-1 is consistent with those of Pikh-1, Pikp-1 and Pi7-1, which belong to the K1 subtype, while the sequence of NB-ARC domain of Pik-W25-1 (residues 262–633) is consistent with those of Pik-1, Pikm-1, and Pi1-5C, which belong to the K2 subtype, which indicating that Pik-W25-1 is a chimera of K1 and K2 (Figure 2d,e).
To evaluate the distribution and polymorphism of Pik-W25 in rice varieties, we detected the presence of Pik-W25 with primers (W25-F/R) in 111 cultivated cultivars in Jiangsu province and 901 rice resources collected from all the world. Sequencing results showed that six Pik alleles Pik (50/1012), Piks (65/1012), Pi1 (7/1012), Pikm (6/1012), Pikh (3/1012) and Pikp (3/1012) were detected, but Pik-W25 was not found (Table S3). The result indicated that Pik-W25 was not widely used in rice varieties.
3.5 Pik-W25 conferring resistance against Ca89 specifically recognises AvrPik-C/D/E
To determine whether Pik-W25 conferring resistance against Ca89 is allelic to Pik, we constructed a genetic population of Pik-W25 using rice varieties CO39 (the susceptible variety did not contain any Pik alleles) and WR25 as parents (Figure S1), and we inoculated the BC1F2 individuals with 17 different isolates (Table 3). The BC1F2 population (60 lines) was susceptible to 11 isolates, which do not contain any AvrPik alleles, and three isolates (Ca41, M015-245, and M015-247), which only contain AvrPik-F (Table 3). However, the lines were resistant to IK81-3 and M101-7-2-1-1, which only contain AvrPik-D (Table 3). We also analysed the sequence of Ca89 and found that it contains AvrPik-D and AvrPik-E (Table 3). Based on these results, we speculated that the R gene is likely to be allelic to Pik loci and that it can at least recognise AvrPik-D.
| Types | Isolates | Rice lines harbouring different Pik alleles | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CO39 | LTH | IRBLk-ka | IRBLkp-K60 | IRBLkh-K3 | IRBL7-M | IRBLkm-Ts | WR25 | ||
| Pik | Pik-p | Pik-h | Pi7 | Pik-m | Pik-W25 | ||||
| I | Ca89 | S | S | R | R | R | R | R | R |
| II | IK81-3 | S | S | R | R | R | R | R | R |
| M101-7-2-1-1 | S | S | R | R | R | R | R | R | |
| III | Ca41 | S | S | S | S | S | S | S | S |
| M015-245 | S | S | S | S | S | S | S | S | |
| M015-247 | S | S | S | S | S | S | S | S | |
| Ⅳ | IK81-25 | S | S | S | S | S | S | S | S |
| M015-9 | S | S | S | S | S | S | S | S | |
| M015-13 | S | S | S | S | S | S | S | S | |
| M015-45 | S | S | S | S | S | S | S | S | |
| M015-52 | S | S | S | S | S | S | S | S | |
| M015-119 | S | S | S | S | S | S | S | S | |
| M015-120 | S | S | S | S | S | S | S | S | |
| M015-144 | S | S | S | S | S | S | S | S | |
| M015-145 | S | S | S | S | S | S | S | S | |
| M015-171 | S | S | S | S | S | S | S | S | |
| M015-249 | S | S | S | S | S | S | S | S | |
- Note: Type I: isolates containing AvrPik-D and AvrPik-E. Type II: isolates only containing AvrPik-D. Type III: isolates only containing AvrPik-F. Type Ⅳ: isolates without AvrPik haplotypes. R: resistant, S: susceptible.
To test this hypothesis, a near-isogenic line (NIL, BC3F3 population G9) containing the R gene from WR25 in the CO39 background was constructed (Figure S1; Table 4) and inoculated with the parent M. oryzae isolate R88002 (without any AvrPik alleles) and six isolates overexpressing AvrPik-A, -B, -C, -D, -E, or -F. CO39 was susceptible to R88002 and all six transgenic isolates (Figure 3a). The NILs were susceptible to R88002 and transgenic isolates overexpressing AvrPik-A, -B, or -F (Figure 3a). However, the NILs were resistant to transgenic isolates overexpressing AvrPik-C, -D, or -E (Figure 3a). This result indicated that the resistance conferred against Ca89 by the R gene in WR25 is controlled by a Pik allele that can recognise AvrPik-C, -D, and -E.
| Isolate | Population | Number of progenies | Chi-square test | ||||
|---|---|---|---|---|---|---|---|
| R | S | Total | Expected ratio (R:S) | χ2 | p | ||
| Ca89 | CO39 × WR25 F1 | 65 | 0 | 65 | – | ||
| CO39 × WR25 BC1F1 | 17 | 14 | 31 | 1:1 | 0.29 | 0.59 | |
| CO39 × WR25 BC2F1 | 12 | 11 | 23 | 1:1 | 0.043 | 0.83 | |
| CO39 × WR25 BC3F1 | 13 | 12 | 25 | 1:1 | 0.04 | 0.84 | |
| CO39 × WR25 BC3F2 | 215 | 67 | 282 | 3:1 | 0.23 | 0.63 | |
| CO39 × WR25 BC3F3 | 33 | 0 | |||||
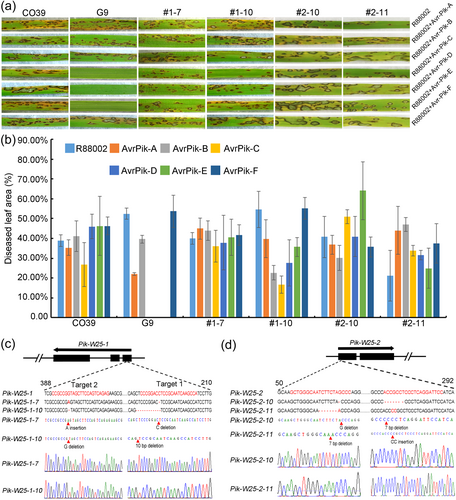
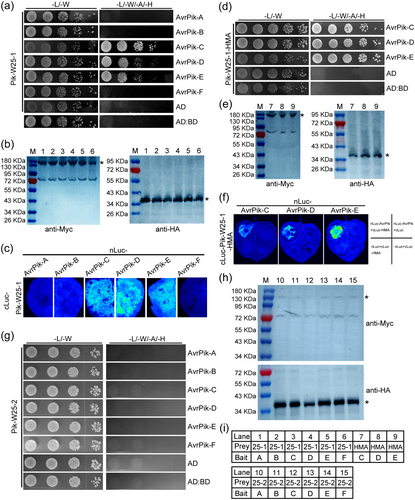
To investigate whether Pik-W25 recognises AvrPik-C, -D, and -E, we generated CRISPR/Cas9-edited transgenic rice lines with loss of function mutations in Pik-W25-1 and Pik-W25-2 in the G9 background. CRISPR/Cas9-edited lines #1–7, #1–10 and #2–10, #2–11 were homozygous characterised by PCR and DNA sequencing (Figure 3c). We tested the resistance of these transgenic rice lines to R88002 and the six transgenic isolates overexpressing AvrPik-A, -B, -C, -D, -E, or -F. The mutations introduced into the four transgenic rice lines restored susceptibility to the M. oryzae isolates overexpressing AvrPik-C/D/E (Figure 3a,b), which indicated that Pik-W25-1 and Pik-W25-2 were involved in the resistance to isolates overexpressing AvrPik-C/D/E. To further confirm the interaction between Pik-W25-1 and the AvrPik alleles, a yeast two-hybrid assay was performed (Figure 4a,b). Yeast cells transformed with both Pik-W25-1 and AvrPik-C, -D or -E grew on selective media. In contrast, yeast expressing Pik-W25-1 or AvrPik-A, -B, or -F alone failed to grow on the selective medium (Figure 4a,b), which suggested that Pik-W25-1 interacted with AvrPik-C/D/E. In addition, we obtained the same result with a split luciferase complementation (SLC) assay in N. benthamiana (Figure 4c). Similarly, Y2H and SLC demonstrated that Pik-W25-1-HMA can also interact with AvrPik-C/D/E (Figure 4d–f). These results indicated that Pik-W25 could interact with AvrPik-C, -D, and -E alleles. Moreover, we found that the Pik-W25-2 could not interact with AvrPik-C, -D, and -E alleles (Figure 4g,h). These results confirmed that Pik-W25 induced immunity required the involvement of both Pik-W25-1 and Pik-W25-2, and Pik-W25-1 act as an adaptor of AvrPik-C, -D or -E, Pik-W25-2 transduced the signal to trigger resistance.
3.6 The Pik-W25 did not affect the rice agronomic traits
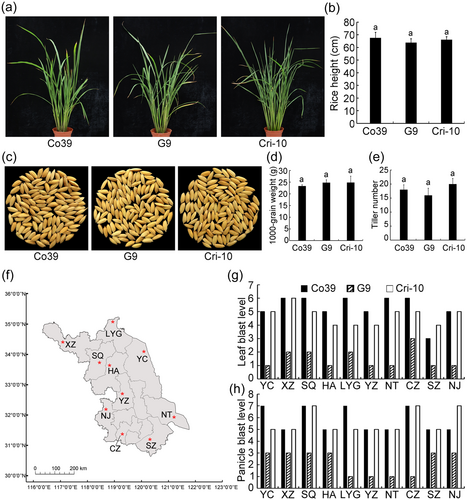
Next, we measured important agronomic traits including rice height, thousand kernel weight and tiller number, and found that there were no significant differences between rice lines CO39, G9, and #10 (Figure 5a–e). To test for differences in resistance against rice blast, we planted all three rice lines in disease nurseries of 10 cities in Jiangsu province (Figure 5f) and found that G9 had higher levels of resistance to leaf and panicle blast than CO39 and #10 (Figure 5g,h). These results indicated that the Pik-W25 improved the resistance to rice blast but had no effects on other agronomic traits. The Pik-W25 had good potential in resistance breeding.
4 DISCUSSION
During domestication of cultivated rice from wild rice, several valuable genes were lost; therefore, wild rice is an important gene bank for exploring new R genes (He et al., 2012; Wambugu et al., 2013), including those controlling resistance to rice blast. In this study, 27 O. rufipogon accessions were selected for exploration of new R genes. Genetic analysis showed that one dominant gene controlled the resistance of WR25 against Ca89 (Table 1).
Conventional gene mapping methods such as map-based cloning are reliable, but they are complicated and time-consuming. Compared with other methods, the most important advantage of BSA-Seq is its simplicity in terms of sample collection and data analysis (Klein et al., 2018). Just as when employing map-based cloning methods, segregating populations such as F2, backcross populations, or RILs are necessary. The population is divided into two pools based on extreme traits such as resistance and susceptibility. In our study, the BC1F2 population derived from a cross and backcross between WR25 and CO39 was used to construct bulk segregated pools (Table 1). To define the smallest mapping interval possible, both the size of the pool and sequencing depth must be considered (Klein et al., 2018). Based on the protocols provided by Novogene company (http://www.novogene.com/tech/service/bsa/qa/), the size of the pool should be at least 20 and the sequencing depth should be at least 20×. In our study, 100 susceptible lines and 100 resistant lines were selected to generate the S-pool and R-pool, respectively, and the sequencing depth was 40×. Using these pools, a new allele of the Pik locus, Pik-W25, was mapped to a 2.1 Mb interval on the telomere of chromosome 11 (Figure 1), indicating that BSA-seq is an effective way to map new R genes.
A total of 12 Pik alleles have been cloned, and all of the encode proteins containing the HMA domain, which is crucial for specificity of recognition between R genes and effectors (Cesari et al., 2013; Maqbool et al., 2015). Genetic analysis indicated that the new allele identified in this study, Pik-W25, was linked with HMA domain (Figure S2). Resistance spectrum analysis indicated that lines containing Pik-W25 were susceptible to non AvrPik isolates and resistant to isolates overexpressing AvrPik-D, which is consistent with the resistance spectrum conferred by other Pik alleles. More importantly, when AvrPik-C, AvrPik-D, and AvrPik-E were transformed into R88002, the transgenic isolates became avirulent on lines containing Pik-W25 (G9), which clearly indicated that Pik-W25 can interact with AvrPik-C, AvrPik-D, and AvrPik-E and trigger resistance. Moreover, we found that the Pik-W25-2 could not interact with AvrPik-C, -D, and -E alleles (Figure 4g,h). These results confirmed that Pik-W25 induced immunity required the involvement of both Pik-W25-1 and Pik-W25-2, and Pik-W25-1 act as an adaptor of AvrPik-C, -D or -E, Pik-W25-2 transduced the signal to trigger resistance. From these data, we conclude that Pik-W25 is a novel Pik allele. Via sequence alignment, we found that the sequence of Pik-W25 is a chimera of the K1 and K2 subtype, which has never been reported before (Figure 2), and we speculate this chimera may affect the recognition of AvrPik alleles by Pik.
Several Pik alleles recognise specific AvrPik alleles. Previous study had showed that the Pik allele Pikp can recognise AvrPik-D and that AvrPik-DHis46 is essential for this recognition (Maqbool et al., 2015). The Pik allele Pikm recognised the AvrPik-A/D/E alleles, and specific regions within the Pikm-HMA and AvrPik-A/D/E interface are critical for effector recognition by Pikp and in the extended response of Pikm to AvrPik-E and -A (De la Concepcion et al., 2018). Moreover, Pikh also recognises AvrPik-A/D/E, and Pikh-HMAAsn261 and Pikh-HMALys262 play an important role in recognition (De la Concepcion et al., 2021). In this study, using genetic and biochemical techniques, we demonstrated that the novel Pik allele Pik-W25 can recognise AvrPik-C/D/E (Figures 3 and 4), which was different from the recognition patterns of Pikp, Pikh and Pikm. In particular, AvrPik-C is only be recognised by Pik-W25 of the cloned Pik alleles. Moreover, the interaction between Pik-W25-1-HMA and AvrPik-C/D/E were proved by Y2H and SLC tests (Figure 4), which further demonstrated the importance of HMA domain in the recognition of AvrPik by Pik. Alignment of the Pik-W25 and Pikp amino acid sequences showed that there were differences in three sites in the HMA region and 13 sites in the NB-ARC region (Figure 2; Figure S3). We speculate that the HMA domain is not the only element that can determine the specific recognition between effectors and R genes and that the new essential sites need to be explored in the future.
There is often a contradiction between plant yield and resistance, plant growth is slowed by an aggressive immune response, resulting in less production of crops as they fight pathogens (Nelson et al., 2017), but various genes were found in rice that balance growth and resistance (Deng et al., 2017; Li et al., 2017; Xu et al., 2017; Zhou et al., 2018). In this study, we also found that Pik-W25 did not affect rice height, thousand kernel weight and tiller number, but enhanced the resistance level to leaf and panicle blast (Figure 5), which indicate that Pik-W25 can also achieve the win-win effect of yield and resistance. While some genes that can both enhance resistance level and increase yield have been found in recent years (Hu et al., 2023; Sha et al., 2023; Wang et al., 2018), but the number is still very small, and it is still a very important task to explore new genes for increasing yield and broad-spectrum resistance in the future.
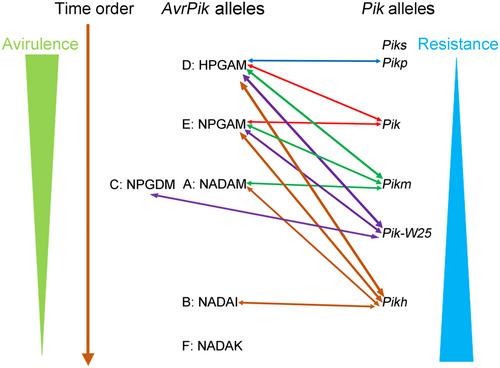
The evolution of M. oryzae AvrPik and rice Pik genes is driven by their physical interactions (Kanzaki et al., 2012). AvrPik-D was derived from an ancestral M. oryzae gene (Kanzaki et al., 2012). The Pik allele, Piks, cannot recognise AvrPik-D; thus, another Pik allele, Pikp, evolved that can recognise AvrPik-D. AvrPik-E was then derived from AvrPik-D via a nucleotide substitution (from His to Asn at position 46), which enabled it to avoid recognition by Pikp (Figure 6). Another Pik allele, Pik, evolved that can recognise AvrPik-D and AvrPik-E. Then, another two alleles evolved from AvrPik-E via nucleotide substitutions: AvrPik-C (from Ala to Asp at position 67) and AvrPik-A (from Pro to Ala at position 47 and from Gly to Asp at position 48), which cannot be recognised by Pikp and Pik. AvrPik-B was derived from AvrPik-A via a change from Met to Ile at position 78t and it cannot be recognised by Pikp, Pik, or Pikm. Next, the rice R gene Pikh arose, recognises AvrPik-D, AvrPik-E, AvrPik-A, and AvrPik-B. Then, another AvrPik allele, namely, AvrPik-F, evolved that cannot be recognised by any of the five Pik alleles (Figure 6). The stepwise evolution of AvrPik and Pik suggests that the evolution of AvrPik loci in M. oryzae to avoid detection by Pik loci in rice drove their interaction and coevolution in nature. In our study, we found that Pik-W25 can recognise AvrPik-C/D/E (Figures 3 and 4). We hypothesise that AvrPik-C is also derived from AvrPik-E because there is only one amino acid difference between these two alleles (Figure 6), and Pik-W25 seems to have evolved to recognise AvrPik-C (Figure 6). The Asian cultivated rice was domesticated from ancestors of the wild rice (Huang et al., 2012), in the process, nucleotide polymorphisms stem from spontaneous mutations, recombination and fixation of beneficial alleles resulting in the genetic based morphological and physiological modifications and ecological adaptation in wild rice populations (Molina et al., 2011; Purugganan & Fuller, 2009; Tan et al., 2008). From the above data, the amino acid structure of Pik-W25 belongs to the chimera of K1 and K2, and we cannot find the presence of Pik-W25 in 1012 rice varieties, which indicated that Pik-W25 was not widely used in rice varieties. While, these results also provide a hypothesis which could be influenced by the frequency of different AvrPik alleles in the global and local pathogen populations. In the process of evolution from wild rice to cultivated rice, the isolates containing AvrPik-D or -E were dominant, almost few isolates containing AvrPik-C allele existed (Li et al., 2019b), so the type K1 or K2 Pik alleles that could recognise AvrPik-D or -E were retained in the cultivated rice and no allele could recognise AvrPik-C. While the chimeric type Pik-W25 was evolved, possibly due to genome recombination and can specifically recognise AvrPik-C, which make Pik-W25 an irreplaceable Pik allele that can be used in rice breeding to control rice blast.
ACKNOWLEDGEMENTS
This work was supported by The Revitalization Foundation of Seed Industry of Jiangsu (No. JBGS(2021)005), the Jiangsu Agriculture Science and technology Innovation Foundation Grant (No. CX19(1008)) and the funding from National Natural Science Foundation of China grants (No. 31861143011) to Y.L.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data are within the manuscript and its Supporting Information files.




