The bryophyte rhizoid-sphere microbiome responds to water deficit
Abstract
The roots of vascular plants are colonised by a multitude of microbes, which play an important role in plant health and stress resilience. Drought stress in particular is devastating for crop yield and causes major shifts in the rhizosphere microbial communities. However, the microbiome associated to the rhizoids (hereafter termed rhizoid-sphere) of the nonvascular bryophytes remains largely unexplored. Here, we use amplicon sequencing to explore the rhizoid-sphere microbiome of three bryophyte species under drought and well-watered conditions. Comparing rhizoid-sphere microbial communities associated with the two liverworts Marchantia polymorpha and Marchantia paleacea and the moss Physcomitrium patens showed characteristic differences in composition between host species and both conserved and unique changes under drought. At phylum level, these changes were similar to changes in the rhizosphere of angiosperms under drought. Furthermore, we observed strong differences in rhizoid-sphere colonisation between bryophyte species for taxa known for nitrogen fixation and plant growth promotion. Interestingly, M. polymorpha prioritised the growth of belowground organs under osmotic stress, as is the case for angiosperms under drought. Taken together, our results show interesting parallels between bryophytes and angiosperms in the relation with their rhizo(id-)sphere, suggesting evolutionary conservation among land plants in their response to drought stress.
1 INTRODUCTION
Plants colonised land around 500 million years ago, exposing them to numerous new challenges and leading to an immense diversification (Morris et al., 2018). One of the hallmarks of this change from an aquatic to a terrestrial environment was the dramatic change in water availability. Land plant lineages faced the need to develop adaptative strategies to survive temporal fluctuations between wet and dry conditions. These adaptive strategies include changes in plant morphology, metabolism as well as their interaction with their biotic and abiotic environment (Delaux et al., 2012).
The nonvascular bryophyte lineage diverged from the vascular plant lineage shortly after colonisation of land. The bryophytes are thought to be a monophyletic lineage which then split into liverworts, hornworts and mosses (Cox et al., 2014; de Sousa et al., 2019). Comparisons between bryophytes and angiosperms have provided some insight into the evolution of adaptive traits. Studies on the response of model bryophyte species Marchantia polymorpha and Physcomitrium patens to osmotic stress in vitro revealed the use of cellular antioxidants, osmoprotective substances such as proline as well as abscisic acid as a hormonal regulator (Cuming et al., 2007; Erxleben et al., 2012; Ghosh et al., 2021). These adaptations to drought are evolutionarily conserved and shared with vascular plants, such as angiosperms. However, the aforementioned studies typically focused on the aboveground photosynthetic tissue rather than the belowground part. In contrast to vascular plants, the bryophytes have not evolved roots but instead rely on rhizoids for water acquisition and anchorage. These rhizoids are similar to the root hairs of vascular plants, and their genetic regulation shows partial conservation (Jones and Dolan, 2012). They are unicellular in liverworts and multicellular in mosses. Vascular plants modify their root systems in response to drought to improve water acquisition. In particular, they stimulate root growth relative to the shoot, and typically show an elongation of the primary root to reach into deeper parts of the soil (Karlova et al., 2021; Chen et al., 2022). Whether similar plasticity adaptations exist for the rhizoids of bryophytes remains unexplored.
Early land plants were exposed to the microbial communities already inhabiting the land, and therefore evolved various traits to interact with these communities. The relationship between plants and microbes has been shown to be ancient and important in terrestrial plant evolution (Knack et al., 2015; Lambers et al., 2009; Trivedi et al., 2020). Since the land was a harsh and nutrient poor environment, it has been hypothesised that the earliest land plants formed a symbiotic relationship with the present microbes. Mutualistic interactions even seem to have played a pivotal role in facilitating terrestrialization events (Puginier et al., 2022). Of particular interest is the symbiosis between plants and arbuscular mycorrhizal fungi (AMF). Arbuscular mycorrhizal fungi help plants in acquiring nutrients such as phosphorus from the soil, and trade these nutrients for carbon. The ability of plants to engage in symbiosis with AMF is an ancient trait shared by most land plants and with a conserved genetic regulation (Radhakrishnan et al., 2020; Rich et al., 2021). While this trait was retained in most land plants, some lost it, including the bryophyte species Marchantia polymorpha (with the exception of subspecies montivagans) and Physcomitrium patens (Ligrone et al., 2007; Wang and Qiu, 2006). A close relative of M. polymorpha, M. paleacea, has retained the ability to engage in arbuscular mycorrhizal symbiosis and is therefore increasingly used to study of the evolution of this symbiosis (Rich et al., 2021).
Vascular plants harbour and nurture a large variety of microbes inside and around their belowground organs, which are in contact with soil, an environment with an immense microbial diversity (Gams, 2007; Torsvik et al., 2002). By shaping this microbiome, the plants are able to improve their own fitness, which is of particular importance for their survival and nutrient uptake under stress conditions (Berendsen et al., 2012). Among the environmental conditions that plants have to deal with, drought stress is one of the most devastating for crop production, and is increasing in frequency and intensity due to global warming (Dai, 2011; Lesk et al., 2016). Under drought conditions, the root microbiome of plants has been shown to undergo changes (Naylor and Coleman-Derr, 2018). Some of these changes are due to a direct selection of drought-resilient bacteria in the dry soil environment. Notably, monoderm lineages such as Actinobacteria are typically increased in relative abundance, while diderm lineages such as Proteobacteria are reduced (Naylor and Coleman-Derr, 2018). However, additionally to the direct selection based on water availability, drought-induced changes in microbiome are shown also to be driven by the plant, which has an interest in selecting for or against certain microbes to improve its own fitness in the dry soil environment (Xu and Coleman-Derr, 2019). In fact, numerous strains of bacteria have been shown to improve the performance of plants under drought conditions via several different mechanisms (reviewed in Naylor and Coleman-Derr, 2018).
While symbiotic interactions between bryophytes and microbes have been the subject of numerous studies, the microbiome of bryophytes at community level has received less attention. One previous study (Alcaraz et al., 2018) compared the epiphytic bacterial microbiomes associated with M. polymorpha and M. paleacea thalli in their natural environment and in vitro by 16S amplicon sequencing, but not of the rhizoid-sphere. As the main OTUs in the Marchantia microbiome, the genera Methylobacterium, Rhizobium, Paenibacillus, Lysobacter, Pirellula, Steroidobacter and Bryobacter were found. In another study, Knack et al. (2015) surveyed the microbiome associated with early-diverging streptophytes including algae and a liverwort. The authors describe the occurrence of several nitrogen-fixing, methanotrophic and vitamin-producing bacteria and archaea. Marks et al. (2018) showed that the bacterial communities associated with Marchantia inflexa thalli vary between sexes and across habitats. Recently, fungal endophytes were isolated from M. polymorpha and cocultured with M. polymorpha in vitro, revealing effects ranging from aggressively pathogenic to strongly plant growth-promoting (Nelson and Shaw, 2019; Nelson et al., 2018). However, it remains unknown (1) which bacteria and fungi colonise the rhizoid-sphere of bryophytes, (2) how these communities change under drought and (3) how conserved these changes are compared to drought-induced changes in the rhizosphere microbiome of angiosperms.
In this study, we use amplicon sequencing to explore the rhizoid-sphere microbiome of the liverworts M. polymorpha and M. paleacea as well as the moss P. patens grown in the same soil under drought and well-watered conditions. This experimental setup allowed us to directly compare the different rhizoid-sphere communities to determine their commonalities and differences between the different bryophyte species as well as their response to drought stress. We show that drought leads to changes in the composition of these microbial communities and that some of these changes are the same as drought-induced changes in the rhizosphere of angiosperm plants. We also show that certain bacteria and fungi show a strong preference of specificity for certain bryophyte plant hosts. Interestingly, some of the bacteria showing specificity are located within taxa known for nitrogen fixation and beneficial plant-microbe interactions. In addition, we observed that M. polymorpha prioritises rhizoid growth over thallus growth under osmotic stress in vitro, analogous to the prioritisation of root growth over shoot growth in angiosperms under drought. Taken together, comparing the rhizoid-sphere microbiome of bryophytes with the rhizosphere microbiome of angiosperms reveals potentially conserved relations and recruitment strategies and an interesting model to study the evolution of plant-microbe interactions.
2 MATERIALS AND METHODS
2.1 Plant material
Bryophytes used in this study are the two liverwort species Marchantia polymorpha Tak-1 and Marchantia paleacea (Humphreys et al., 2010) as well as the moss Physcomitrium patens, ecotype Gransden. Both Marchantia species were propagated on ½ Gamborg B5 media (pH 5.7) (Duchefa Biochemie) with 1% plant agar (Duchefa Biochemie), in a growth chamber a 22℃ with 16 h light/8 h dark and a light intensity of 80–120 μmol m−2s−1. Moreover, the media for M. polymorpha was supplemented with 29.2 mM sucrose. P. patens protonema were grown on BCDAT medium (1 mM MgSO4, 1.837 mM KH2PO4, [pH 6.5], 10 mM KNO3, 45 µM FeSO4, 0.22 µM CuSO4, 9.93 µM H3BO3, 0.23 µM CoCl2, 0.10 µM Na2MoO4, 0.19 µM ZnSO4, 2 µM MnCl2, 0.17 µM KI, 1 mM CaCl2, 5 mM ammonium tartrate, 0.8% [w/v] Phytoagar), in a growth cabinet at 25℃ under continuous light with an intensity of 60 μmol m−2s−1.
For the angiosperms experiment, we used seeds of Solanum lycopersicum var. Moneyberg and Arabidopsis thaliana Col-0.
2.2 Bryophytes greenhouse experiment
To study the bryophyte rhizoid-sphere under drought conditions, the three bryophyte species were grown in 1 L pots filled with field soil, in a greenhouse. The field soil used in this study, named “MiCRop” soil, was collected from an organic field in Nergena area, Bennekom (coordinates: 51.996250, 5.659375). It was collected in 2014 by excavating to a depth of 80 cm, then stored on a pile outdoors until the experiment in 2022. Before the experiment, the soil was air-dried and sieved with a 5 mm sieve to remove bigger stones and roots. Soil analysis was performed by Eurofins (The Netherlands). Nutrient composition and basic properties of the soil are shown in Supporting Information S1: Table S1. Each 1 L pot was filled with 1000 g of soil, which had a starting soil water content of 2.5% and was watered to a target weight of 1170 g (well-watered condition) before planting. For each bryophyte species, in vitro propagated plant material was transferred to pots with six replicate pots per species and treatment. For M. polymorpha and M. paleacea, we used approximately 10 gemmae per pot. For P. patens, the protonema was transferred to the soil by pressing the plastic circle on which it was growing upside down onto the surface of the soil. The plants were grown in a climate-controlled greenhouse at 22℃ (day)/18℃ (night), with 16 h light/8 h dark and 60% relative humidity. All pots were well-watered for the first 12 days, after which the drought treatment started. The different watering treatments were achieved by re-watering the pots from the top to a certain target weight every 2–3 days, to keep the soil moisture content stable. The target weights for the well-watered, mild drought and severe drought treatments corresponded to gravimetric soil moisture contents of 16.7%, 11.5% and 9.1%, respectively. Sprouting weeds were removed throughout the growing period. After 7 weeks of growth, we photographed the pots, cut off the aboveground part and took rhizoid-sphere samples. Under the severe drought treatment, M. paleacea and P. patens plants almost completely died; only some small patches survived in some of the replicates. Therefore we did not sample rhizoid-sphere for those two species under severe drought. Furthermore, an unplanted soil control was originally included in the experiment for all three treatments, but was later excluded from the analysis due to the growth of mosses naturally present in our field soil.
2.3 Angiosperms greenhouse experiment
To compare the rhizoid-sphere microbiome of bryophytes with the rhizosphere of angiosperms, a separate greenhouse experiment was conducted. We used the same field soil and greenhouse conditions as described above, with the same method to apply drought treatments, but different soil moisture contents for the treatments. Solanum lycopersicum var. Moneyberg seeds were germinated on wet filter paper for 7 days in the dark. Arabidopsis thaliana Col-0 seeds were cold-stratified for 1 day at 4℃, then germinated for 7 days on the surface of pots filled with 1000 g of soil in the greenhouse. Unplanted soil control pots were also included. In this experiment, the starting soil moisture content was 4.1%. Each pot received 60 mL of ½ Hoagland solution (5.6 mM NH4NO3, 0.4 mM K2HPO4, 0.8 mM MgSO4, 0.8 mM K2SO4, 1.6 mM CaCl2, 0.18 mM FeSO4, 0.18 mM Na2EDTA, 23 µM H3BO3, 4.5 µM MnCl2, 0.3 µM CuSO4, 1.5 µM ZnCl2, 0.1 µM Na2MoO4) and was then watered to a target weight of 1074 g (well-watered condition) before transplanting germinated seedlings. The target weights corresponded to gravimetric soil moisture contents of 13.0%, 10.7% (well-watered), 8.3% (mild drought) and 5.7% (severe drought). The length of the initial well-watered phase for all treatments was 10 days, instead of 12 days for the bryophytes. 33 days after transplanting, photographs of the plants were taken and rhizosphere samples were collected from all treatments except the highest soil moisture content (13.0%) as tomato plants subjected to this treatment grew less than those in the well-watered treatment (10.7%).
2.4 Bryophyte rhizoid-sphere sampling, DNA extraction and amplicon sequencing
For the bryophytes, rhizoid-sphere samples were taken from the top 2 cm of the soil at the site of plant growth, a patch of approximately 50 cm2, with no separation of rhizoids and soil. We used six replicate pots per host species and treatment. The soil samples were immediately stored on ice, then frozen at −20℃. Subsequently, soil samples were freeze-dried and then homogenised per replicate pot. Rhizoid-sphere DNA was isolated from approximately 250 mg of soil per replicate pot, using the DNeasy PowerSoil Pro kit (Qiagen). Two blank extractions were included as a control.
16S and ITS libraries were prepared and sequenced separately by Genome Quebec and BGI, respectively. For 16S amplicons, the V3-V4 region was amplified using the universal primers 341F (CCTACGGGNGGCWGCAG) and 805R (GACTACHVGGGTATCTAATCC), with addition of PNA clamps to block amplification of mitochondrial and plastid DNA (mPNA: GGCAAGTGTTCTTCGGA, pPNA: GGCTCAACCCTGGACAG). For ITS amplicons, the ITS1 region was amplified using the universal primers ITS1f (CTTGGTCATTTAGAGGAAGTAA) and ITS2 (GCTGCGTTCTTCATCGATGC). Samples were multiplexed and paired-end reads of 250 bp were sequenced on a NovaSeq. 6000 (Illumina).
2.5 Angiosperm rhizosphere sampling, DNA extraction and amplicon sequencing
For the angiosperms, plants were taken out of the soil and gently shaken to remove loosely associated soil particles. Plants were then cut at the root-shoot junction. Shoots were weighed fresh, dried for 3 days at 70℃ and weighed again dry. Roots with associated rhizosphere soil were first stored at −20℃. For the unplanted soil control, soil was sampled from the middle of the pot at approximately 5 cm depth. We used seven replicate pots per host species and treatment for rhizosphere samples and two replicates for the unplanted soil control. Later, samples were thawed and rhizosphere or soil DNA was extracted using the DNeasy PowerSoil Pro kit (Qiagen). In the first step, roots with rhizosphere soil or control soil were placed in the PowerBead Pro tube and solution CD1 was added. Samples were shaken in a TissueLyzer for 10 min at 25 Hz, inverting the tubes after 5 min, to dissociate soil particles from the roots and lyse the cells. The roots were then removed from the tube, washed in the tap and weighed. Biomass data was statistically tested for significance across species/treatment combinations using a Kruskal-Wallis test followed by a post-hoc Dunn's test for significance groups, with Benjamini-Hochberg FDR correction and a significance threshold of pFDR < 0.05. Rhizosphere samples were further processed with the DNeasy PowerSoil Pro kit following the manufacturer's protocol.
DNA samples were processed for 16S library preparation and amplicon sequencing by BaseClear (Leiden, The Netherlands). The V3-V4 region was amplified using the universal primers 341F (CCTACGGGNGGCWGCAG) and 806 R (GGACTACHVGGGTATCTAATCC). Samples were multiplexed and paired-end reads of 300 bp were sequenced on a MiSeq (Illumina).
2.6 Amplicon sequence processing and analysis
NovaSeq data from the bryophytes experiment and MiSeq data from the angiosperms experiment were processed with two different pipelines, due to differences between those sequencing platforms.
For 16S and ITS NovaSeq data from the bryophytes experiment, demultiplexed sequences were processed using a modified DADA2 pipeline optimised for NovaSeq data (https://github.com/ErnakovichLab/dada2_ernakovichlab). This pipeline is different from the traditional DADA2 pipeline in the step of learning the error rates, as NovaSeq data only has four quality score levels. Small changes were made in the settings of this pipeline. Notably, instead of assigning taxonomy using DADA2's assign taxonomy function, we used the function classify-sklearn in Qiime2 version 2022.2 (Caporaso et al., 2010). For 16S amplicons, we used a custom-made classifier based on the Silva database, version 138.1 (Quast et al., 2013), filtered and trimmed to our V3-V4 primers with Rescript in Qiime2. For ITS amplicons, we used a custom-made classifier based on the Unite database, version 8.3 (Kõljalg et al., 2013), which was not filtered or trimmed. All further processing was performed in R, version 4.2.1, and all plots were generated using the ggplot2 package. For 16S data, we filtered out those amplicon sequence variants (ASVs) which were less than 380 bp in length, were not assigned taxonomically to the bacterial kingdom or were assigned as plastids or mitochondria. For ITS data, we filtered out those ASVs which were not assigned taxonomically to the fungal kingdom. Phylogenetic trees of bacterial and fungal ASVs were computed using the align-to-tree-mafft-fasttree function in Qiime2. Alpha-diversity measures were calculated using the phyloseq package and statistically tested with a type II ANOVA, due to the unbalanced design (severe drought treatment was only included for M. polymorpha), using the car package. Tukey's HSD post-hoc test was performed for significance groups with a significance threshold of p < 0.05. For further analysis apart from alpha-diversity, bacterial and fungal ASVs with less than 20 counts in the entire data set or that were present in fewer than three samples were excluded. This resulted in 7672 bacterial and 2660 fungal ASVs. For phylum and ASV level plots, relative abundance was calculated by normalising counts by library size. ASV relative abundance was tested for significance across species/treatment combinations using a Kruskal-Wallis test followed by a post-hoc Dunn's test for significance groups, with Benjamini-Hochberg FDR correction and a significance threshold of pFDR < 0.05. Differential abundance analysis at phylum level was performed on the relative abundances using a Mann-Whitney test and FDR-corrected using the Benjamini-Hochberg procedure (significance threshold: pFDR < 0.05). Differential abundance analysis at ASV level for heatmaps was performed on the unnormalized filtered data set using DESeq. 2 with a likelihood ratio test and FDR-corrected using the Benjamini-Hochberg procedure for the 200 (bacteria) and 100 (fungi) most abundant ASVs, for heatmaps. Differential abundance analysis for drought-induced changes was also performed with all ASVs using DESeq. 2 with a likelihood ratio test, results are reported in Supporting Information S2: Data set 1. For beta-diversity analyses, the filtered data set was normalised by cumulative sum scaling using the metagenomeSeq package. For the UMAP ordinations, Bray-Curtis distances were calculated using the vegan package and the ordination was performed on Bray-Curtis distances using the umap package. For statistical testing of beta-diversity, Bray-Curtis distances were calculated using the phyloseq package and PERMANOVA was performed using the adonis2 function of the vegan package.
For 16S MiSeq data from the angiosperms experiment, demultiplexed sequences were processed using the standard DADA2 workflow in Qiime2. ASVs were then assigned to taxonomy and filtered as described above for 16S NovaSeq data, with the only difference of filtering out ASVs with fewer than 8 counts for the MiSeq data, instead of 20 counts for NovaSeq data, due to the lower sequencing depth. This resulted in 3253 bacterial ASVs. Composition at phylum level was plotted and differential abundance was analysed as described above for the bryophytes data set.
2.7 Rhizoid and thallus growth of M. polymorpha under osmotic stress in vitro
Gemmae of M. polymorpha were grown on ½ Gamborg B5 + vitamins under standard growth conditions previously described. 10 days after transferring gemmae to fresh plates, gemmaelings (immature thalli developed from gemmae) were transferred to treatment plates. We used four gemmaelings per plate and three plates per treatment. Treatment plates consisted of ½ Gamborg B5 supplemented with 100 mM sorbitol and 150 mM sorbitol for osmotic stress, or without sorbitol addition as a control. Plates were kept at a 70° angle in a growth chamber at 22℃ with 16 h light/8 h dark and a light intensity of 100–150 μmol m−2s−1. Rhizoid and thallus growth were assessed at 14 days after start of the treatment by photographing plants from the ventral side using an Epson Perfection V800 Photo scanner at a resolution of 400 dpi. These images were analysed using FIJI running in ImageJ 1.53q software. Thallus surface area was measured through manual outlining and rhizoid surface area was measured using the thresholding function. Statistical testing was performed using a Kruskal-Wallis test followed by a post-hoc Dunn's test for significance groups, with Benjamini-Hochberg FDR correction and a significance threshold of pFDR < 0.05.
3 RESULTS
3.1 Rhizoid-sphere communities of bryophyte species change under drought conditions
To study and compare the rhizoid-sphere microbiome of bryophytes under drought stress, we performed a greenhouse experiment with the two liverworts Marchantia polymorpha and M. paleacea as well as the moss Physcomitrium patens. M. polymorpha showed no apparent growth reduction when subjected to a mild drought treatment, but did grow much less under severe drought. On the other hand, M. paleacea and P. patens showed a clear growth reduction under mild drought, while under severe drought, only very small patches survived in some of the replicate pots (Figure 1a). The severe drought treatment was therefore excluded from the microbiome analysis for the latter two species.
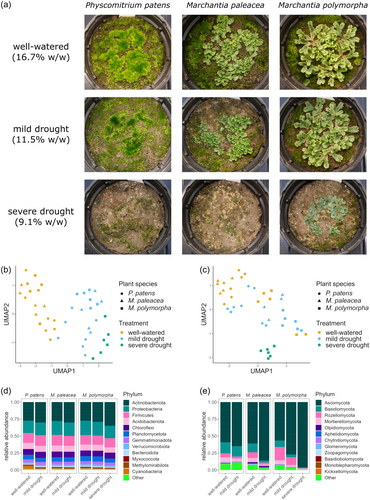
We extracted DNA from the top 2 cm of soil at the site of plant growth and studied the rhizoid-sphere microbiome by 16S and ITS amplicon sequencing, allowing taxonomic profiling of the bacterial and fungal communities, respectively. The composition of both the bacterial and fungal communities were significantly affected by the host plant species and the treatment (Figure 1b,c, Supporting Information S1: Table S2). The effects of the treatment and host species on the fungal rhizoid-sphere were slightly stronger than on the bacterial rhizoid-sphere. For the fungal communities, 15% of the variance was explained by the treatment, 10% by the host species and 7% by their interaction, while for the bacteria the explained variance was 12% for treatment, 8% for host species and 5% for the interaction term (Supporting Information S1: Table S2). The composition of the bacterial and fungal microbiomes were significantly different between all three species pairs, under both well-watered and mild drought treatments (Supporting Information S1: Table S3).
The alpha-diversity of the bacterial rhizoid-sphere communities, measured by the Shannon index, was not significantly affected by the treatment or host plant species (Supporting Information S1: Figure S1 and Table S4). On the other hand, for the fungal communities it was significantly affected by both factors. Under drought conditions, the alpha-diversity of fungal communities was lower than under well-watered conditions. Under mild drought, the rhizoid-sphere of P. patens had a higher alpha-diversity than the two Marchantia species (Supporting Information S1: Figure S1).
At phylum level, the bacterial rhizoid-sphere communities were dominated by Actinobacteria, Proteobacteria and Firmicutes (Figure 1d). Actinobacteria relative abundance was increased under drought for all three bryophyte species, but this was only statistically significant for M. polymorpha under severe drought (Figure 4b). Proteobacteria were drought-depleted for both Marchantia species, but this was again only significant for M. polymorpha under severe drought. In contrast, the P. patens rhizoid-sphere was slightly enriched in Proteobacteria under drought, albeit not significantly.
The fungal rhizoid-sphere communities were strongly dominated by the phylum Ascomycota, followed by Basidiomycota and Rozellomycota (Figure 1e). Ascomycota showed a strong increase in relative abundance under drought, especially in the rhizoid-sphere of both Marchantia species, at the expense of most other fungal phyla (Supp. Figure 2).
3.2 Microbiome differences between mild drought and well-watered conditions in the bryophytes rhizoid-sphere
To identify differences in the bryophytes rhizoid-sphere under normal and drought conditions we compared the changes in more detail among the 200 most abundant bacterial ASVs and 100 most abundant fungal ASVs (Supporting Information S1: Figures S3, S4). The three bacterial ASVs with the strongest drought-enrichment were ASV_34, a Pseudonocardia (Actinobacteria), ASV_132, an Allo-/Neo-/Para-/Rhizobium (Proteobacteria) and ASV_149, a Sphingopyxis (Proteobacteria). These three ASVs were consistently drought-enriched across all three bryophyte species, though not always significantly for all species after FDR correction (Supporting Information S1: Figure S3). Some ASVs showed contrasting patterns between the different species. For example, two ASVs assigned to the genus “WD2101 soil group” were drought-enriched only in the rhizoid-sphere of M. polymorpha and three ASVs assigned as Bacillus were drought-enriched only in the rhizoid-sphere of P. patens. On the other hand, we observed a drought-depletion of ASVs belonging to the genera Nocardioides and Terrabacter in the rhizoid-sphere of Marchantia species, but not of P. patens. Generally, drought-induced changes at ASV level were more similar between M. polymorpha and M. paleacea than between Marchantia species and P. patens (Supporting Information S1: Figure S3).
Comparisons of the 100 most abundant fungal ASVs revealed that the strongest changes under mild drought were in one of the two main branches of the order Hypocreales (phylum Ascomycota). In this lineage, six highly abundant ASVs showed a strong enrichment under mild drought, which was consistent in the rhizoid-sphere of all three bryophyte species (Supporting Information S1: Figure S4). Three of these six ASVs belong to the genera Emericellopsis, Acremonium and Gliomastix, the other three were not taxonomically assigned at genus level. These six ASVs strongly dominated the rhizoid-sphere of bryophytes under drought stress, summing up to 58%, 67% and 32% of reads under mild drought for M. polymorpha, M. paleacea and P. patens respectively, compared to 12%, 16% and 3% under well-watered conditions. Interestingly, in M. polymorpha under severe drought, the summed relative abundance of these six ASVs increased to 74%.
3.3 Host species specificity of microbial taxa in the rhizoid-sphere of bryophytes
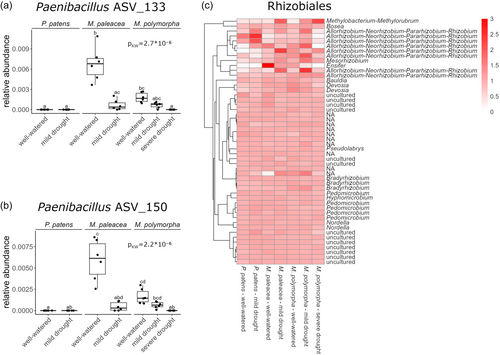
We further investigated which of the most abundant ASVs showed specificity for certain bryophyte species, under well-watered and mild drought conditions (Supporting Information S1: Figure S5). Among the 200 most abundant bacterial ASVs, certain lineages showed stronger differences in relative abundance between species than the rest of the phylogeny. First, two ASVs belonging to the genus Paenibacillus were detected in the rhizoid-sphere of both Marchantia species, but not in that of P. patens. Between the two Marchantia species, these two Paenibacillus ASVs were more abundant in the rhizoid-sphere of M. paleacea in well-watered conditions, but not in mild drought (Figure 2a,b). The Paenibacillus genus has been shown to contain nitrogen-fixing and plant growth-promoting bacteria (Grady et al., 2016). Second, a cluster of ASVs belonging to the order Rhizobiales showed stronger differences between species than the rest of the phylogeny. This is of particular interest because the Rhizobiales order contains rhizobia, a paraphyletic group of nitrogen-fixing bacteria able to engage in root nodule symbiosis with nodulating plants. We therefore further investigated the host species and treatment preferences of the 50 most abundant Rhizobiales ASVs (Figure 2c). While a large part of the Rhizobiales phylogeny shows no specific pattern, strong differences between host species were observed in the closely related genera Allo-/Neo-/Para-/Rhizobium, Ensifer and Methylobacterium/Methylorubrum. Most of these ASVs were more strongly associated with Marchantia rhizoid-spheres. However, two closely related ASVs assigned as Allo-/Neo-/Para-/Rhizobium showed a stronger association with P. patens. One ASV assigned as Methylobacterium/Methylorubrum showed a preference for Marchantia rhizoid-spheres under drought conditions. Bacteria of the genus Methylobacterium were previously reported to live as epiphytes on Marchantia thalli and promote gemmae growth in vitro (Kutschera and Koopmann, 2005; Schauer et al., 2011). Taken together, these data show that several bacteria belonging to genera with nitrogen-fixing or plant growth-promoting potential show clear preferences for certain bryophyte host species.
For the 100 most abundant fungal ASVs, we observed stronger differences between the three bryophyte species than for the bacteria (Supporting Information S1: Figure S6). While some parts of the fungal phylogeny, such as ASVs belonging to the genus Solicoccozyma, show a similar colonisation in the rhizoid-sphere of the three bryophyte species, many other ASVs show a preference for a specific plant host. Notably, three ASVs assigned to the genus Olpidium showed a much higher abundance in the rhizoid-sphere of M. paleacea compared to M. polymorpha and P. patens, and this trend was confirmed at genus level (Figure 3a). Furthermore, five Fusarium ASVs showed clear preferences for a certain host species and/or treatment (Figure 3b). fASV_22 was specific to P. patens, fASV_59 to M. paleacea under well-watered conditions, fASV_50 to M. paleacea under mild drought, fASV_45 to M. polymorpha under well-watered conditions and fASV_19 to M. polymorpha under severe drought. However, for these Fusarium ASVs, colonisation patterns were not consistent across all replicates, which could be explained by the stochastic nature of fungal community assembly (Supporting Information S1: Figure S7). A similar trend of host species and treatment preference was observed for a large phylogenetically grouped cluster of ASVs that could not be taxonomically assigned (Supporting Information S1: Figure S6).
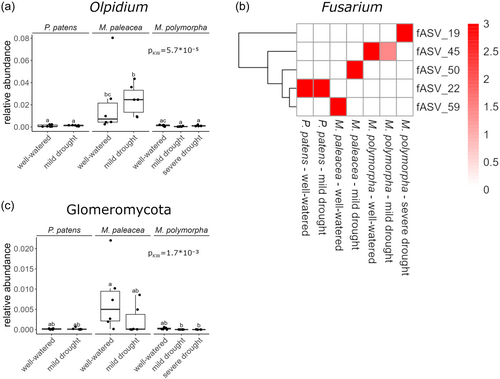
Of particular interest is the difference in fungal colonisation between M. paleacea and M. polymorpha, as the first has retained the ability to engage in symbiosis with arbuscular mycorrhizal fungi, which M. polymorpha has lost. This is reflected by a higher colonisation of the M. paleacea rhizoid-sphere by the phylum Glomeromycota, which contains most described species of arbuscular mycorrhizal fungi (Figure 3c). In our data set, most Glomeromycota ASVs were assigned to the genus Funneliformis and were almost exclusively found in M. paleacea (Supporting Information S1: Figure S8).
3.4 The rhizosphere of angiosperms shows similar changes to that of bryophytes under drought stress
To determine whether the changes that we observed in the rhizoid-sphere of bryophytes under drought are similar to drought-induced changes in the rhizosphere of angiosperm plants, we grew tomato (Solanum lycopersicum) and Arabidopsis thaliana in the same soil as the bryophytes and analysed their rhizosphere microbiomes by 16S amplicon sequencing, for bacteria only. Mild and severe drought treatments led to reduced plant growth compared to the well-watered control (Supporting Information S1: Figures S9, S10). In the angiosperm experiment, the soil moisture contents were kept lower than for the bryophytes, as the latter require a wetter environment to grow optimally. As the soil moisture exerts a direct selection on microbes in the soil, this difference in the treatments hampers the comparison between bryophytes and angiosperms. Nevertheless, many of the changes in relative abundance of the main bacterial phyla under drought compared to the well-watered treatment were conserved between angiosperms (for both mild and severe drought) and M. polymorpha under severe drought: Actinobacteria were enriched, while Proteobacteria, Verrucomicrobiota and Myxococcota were depleted (Figure 4a,b, Supporting Information S1: Figure S11, Figure 1d). On the other hand, Acidobacteriota and Planctomycetota showed contrasting responses to drought; they were depleted in the rhizoid-sphere of M. polymorpha under severe drought and showed almost no change or an enrichment in the rhizosphere of angiosperms under drought. For all three bryophyte species under mild drought, the changes at phylum level were similar, but less pronounced. In unplanted soil controls subjected to the same soil moisture treatments as the two angiosperm species, almost no differences were observed between treatments in the bacterial composition at phylum level under drought (Supporting Information S1: Figure S11). Furthermore, the two Paenibacillus ASVs which were detected in the rhizoid-sphere of the two Marchantia species, but not P. patens, were also completely absent from the rhizosphere of tomato and Arabidopsis. This indicates that these bacteria associate very specifically with Marchantia.
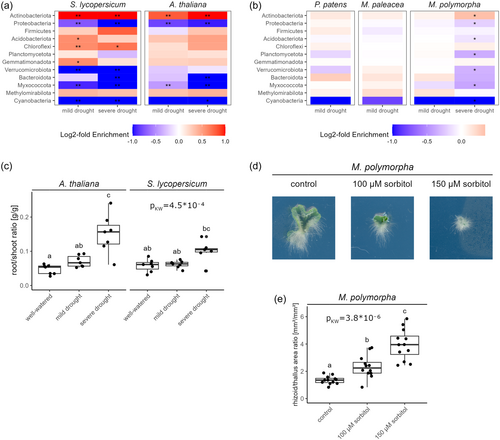
3.5 Marchantia prioritises rhizoid growth under osmotic stress
We further sought to investigate whether another plant adaptation to drought, the prioritisation of belowground growth, is conserved between bryophytes and angiosperms. Angiosperms are known to increase their root growth at the expense of shoot growth to better survive drought (Chen et al., 2022). In line with this, we observed an increase in the root/shoot biomass ratio under drought in A. thaliana and S. lycopersicum (Figure 4c, Supporting Information S1: Figure S10). To test whether a similar strategy is present in bryophytes, we investigated the response of M. polymorpha rhizoids to osmotic stress. Gemmaelings were grown in vitro with and without the addition of sorbitol, and phenotypic traits were measured after 14 days of treatment. Plants grown both in mimicked severe drought (150 mM sorbitol) and mild drought (100 mM sorbitol) showed a dose dependent decrease in their thallus surface area and rhizoid surface area (Figure 4d, Supporting Information S1: Figure S12). However, the reduction in thallus growth was stronger than in rhizoid growth, resulting in an increased rhizoid/thallus ratio under osmotic stress (Figure 4e). This developmental plasticity response to osmotic stress is analogous to the increased root/shoot ratio in angiosperms, suggesting that prioritising the growth of belowground organs could be an evolutionarily conserved plant response to drought stress.
4 DISCUSSION
Bryophytes typically require a high soil moisture content to grow, but have desiccation tolerance strategies in the vegetative tissue to deal with periods of drought (Proctor, 2000). These desiccation tolerance strategies have evolved in a common ancestor of land plants and were crucial for the transition from aquatic environments to land (Marks et al., 2021). In our study M. polymorpha showed a better resilience to drought than M. paleacea and P. patens (Figure 1a). While this may be due to drought adaptation mechanisms of the plant, the growth speed was a potential confounding factor; M. polymorpha grew faster in size than M. paleacea and P. patens and may therefore have established deeper rhizoids, improving its water acquisition under drought. The rhizoid-sphere composition shows changes under drought and differences between bryophyte species. The latter could be explained by species-specific factors such as the differences in metabolism, exudation or rhizoid development. P. patens has single layered multicellular rhizoids while rhizoids of Marchantia are unicellular. This implies different mechanisms related to transport, communication and regulation and likely also mechanisms related to rhizoid-sphere interactions. M. polymorpha is generally a fast growing pioneering species, grown usually in nutrient-rich habitats while M. paleacea acts as a perennial, associated with established and stable habitats (Alcaraz et al., 2018; Ligrone et al., 2007).
In this study we focused on the 200 most abundant bacterial and 100 most abundant fungal ASVs to examine differences in rhizoid-sphere composition due to the drought treatment as well as between bryophyte species. Most ASVs showed similar patterns of enrichment/depletion under drought in the three bryophyte species. The most strongly drought-enriched ASV was taxonomically assigned as Pseudonocardia (phylum Actinobacteria), a genus previously described as persistently drought-enriched and associated with plant growth promotion (Hamedi and Mohammadipanah, 2015; Santos-Medellín et al., 2021). Other ASVs showed contrasting patterns, which could be due to species-specific microbial recruitment strategies under drought. These species-specific recruitment strategies could be due to differences in plant metabolism. For angiosperms, it was shown that root exudates (metabolites secreted into the rhizosphere) can promote or inhibit the presence of specific microbes (Zhalnina et al., 2018). Regarding the fungal rhizoid-sphere communities, we observed a decrease in diversity under drought conditions. The fungal rhizoid-sphere communities showed stronger shifts at phylum level than the bacterial communities (Figure 1d,e). Notably, six ASVs from the order Hypocreales (phylum Ascomycota) completely dominated the drought rhizoid-sphere, summing up to 74% of fungal reads for M. polymorpha under severe drought. Whether these drought-enriched lineages also contribute to their host plant's drought resilience remains to be explored.
When comparing the relative abundance of the 200 most abundant bacterial ASVs between the three bryophyte species, we observed that certain ASVs showed stronger differences in relative abundance compared to the rest of the bacterial phylogeny. Two Paenibacillus ASVs were specific to the two Marchantia species and the Rhizobiaceae family had several members preferentially colonising Marchantia or P. patens (Figure 2). Interestingly, the Paenibacillus genus and as well as the Rhizobiaceae family are both known for nitrogen fixation and plant growth promotion (Grady et al., 2016; Willems, 2006). However, we cannot say with certainty whether the specific bacterial ASVs observed in our data set are able to perform nitrogen fixation or promote plant growth. In angiosperms, both rhizobia and arbuscular mycorrhizal fungi have been reported to alleviate drought stress when added as inoculants (Barquero et al., 2022; Chareesri et al., 2020; Igiehon and Babalola, 2021). Furthermore, the Rhizobiales order, in which the Rhizobiaceae family is embedded, was recently reported as one of the bacterial taxa consistently colonising plant roots, and to also be associated with the chlorophyte algae Chlamydomonas reinhardtii (Durán et al., 2022).
The hypothesised interaction of both bryophytes and angiosperms with bacterial taxa capable of nitrogen fixation and plant growth-promotion raises questions regarding the evolution of these traits. On one hand, these interactions could have evolved in a common ancestor of land plants, as is the case for arbuscular mycorrhizal symbiosis (Radhakrishnan et al., 2020). This mutualistic symbiosis is thought to have played a key role in the colonisation of land by plants, at a time when land was rocky and nutrient availability was low (Puginier et al., 2022). Mutualistic interactions with nitrogen-fixing bacteria could have facilitated this terrestrialization event in a similar way. On the other hand, these interactions could also be the product of co-evolution in several land plant lineages. For the intimate intracellular root nodule symbiosis between plants and nitrogen-fixing rhizobia and Frankia bacteria, it is limited to four orders of land plants and only evolved around 100 million years ago (Griesmann et al., 2018; Soltis et al., 1995). However, free-living nitrogen-fixing bacteria in the rhizosphere can also contribute to plant health and may be recruited in the rhizosphere of plants which are not able to engage in root nodule symbiosis (Van Bouffaud et al., 2016; Van Deynze et al., 2018). The host species specificity patterns of potentially nitrogen-fixing bacteria in the rhizoid-sphere of bryophytes (Figure 2c) suggest that this recruitment may be widespread across the plant kingdom. Understanding the evolution of the less intimate interactions between plants and nitrogen-fixing bacteria in their rhizo(id-)sphere will require more insight into the genetic regulation of these interactions.
In the 100 most abundant fungal ASVs, strong patterns of host species preference were observed. Particularly, the pathogenic genus Olpidium colonised the M. paleacea rhizoid-sphere much more abundantly than that of M. polymorpha and P. patens (Figure 3a). To our knowledge, it is not known whether Olpidium species are able to infect bryophyte plants. However, the specific colonisation of M. paleacea by Olpidium could be related to this plant's ability to engage in arbuscular mycorrhizal symbiosis. In angiosperms, the plants' genetic regulation of arbuscular mycorrhizal symbiosis shows overlap with immunity against pathogens (Jacott et al., 2017; Rey and Jacquet, 2018). This could mean that the ability of M. paleacea to engage in arbuscular mycorrhizal symbiosis comes at the cost of its defence against pathogens. Furthermore, ASVs belonging to the Fusarium genus showed a preferences for a certain host species and treatments (Figure 3b). This could be due to a strong competition between Fusarium fungi, and/or redundancy between those ASVs in their interaction with the host plant. Many strains of Fusarium are plant pathogenic, and it was recently shown that Fusarium oxysporum isolates showing pathogenicity on angiosperms were also able to infect Marchantia polymorpha via a conserved mechanism (Redkar et al., 2022). While the Fusarium genus is known for its plant pathogenicity, many Fusarium species are harmless saprotrophs (Okungbowa and Shittu, 2012).
Compared with the rhizosphere of angiosperms, bryophytes show some similarities in their rhizoid-sphere microbiome under drought stress. Notably, the increase in Actinobacteria and decrease in Proteobacteria, Verrucomicrobiota and Myxococcota under drought were observed in both angiosperms and M. polymorpha (under severe drought; Figure 4a,b). This could be partly due to the direct selection of the dry soil on the microbes. In fact, monoderm bacteria such as the Actinobacteria have a thicker cell wall than diderm bacteria such as Proteobacteria, Verrucomicrobiota and Myxococcota, making monoderm lineages more resilient to desiccation. However, several studies suggest that plants also actively recruit monoderm lineages in their rhizosphere under drought (Santos-Medellín et al., 2021; Xu and Coleman-Derr, 2019). This can contribute to the host plant's drought resilience. For example, a recent study showed that certain Streptomyces were drought-enriched in the endosphere of rice and a corresponding isolate promoted plant growth (Santos-Medellín et al., 2021). Furthermore, many other members of the monoderm phylum Actinobacteria are also described as plant growth-promoting (Hamedi and Mohammadipanah, 2015). To determine whether the similar trends between bryophytes and angiosperms in the response of certain bacterial lineages to drought are caused by conserved recruitment mechanisms, further research is needed.
On the other hand, we also observed differences between the rhizo(id-)sphere microbiomes of bryophytes and angiosperms. For example, two Paenibacillus ASVs were detected in association with the two Marchantia liverwort species but not with the moss P. patens or the angiosperms S. lycopersicum and A. thaliana. The genus Paenibacillus is known to contain bacteria that can promote plant growth through several different mechanisms, including antagonism of root pathogens, phosphate solubilisation, nitrogen fixation as well as production of auxin and siderophores (Grady et al., 2016). This genus was previously shown to be abundant in Marchantia-associated microbiome samples (Alcaraz et al., 2018). Furthermore, the whole genome sequence of a strain isolated from Marchantia polymorpha subsp. ruderalis, named Paenibacillus marchantiae, was published recently (Meierhenrich et al., 2023). In that genome, the authors found gene clusters encoding for the biosynthesis of siderophores, compounds that can extracellularly convert iron ions and potentially help the iron homoeostasis of plants.
Beside comparing the rhizo(id-)sphere microbiomes of bryophytes and angiosperms, we also compared the response of their belowground structures to osmotic stress. We found that M. polymorpha plants preferentially invest in the growth of their rhizoids under osmotic stress, in a similar fashion as angiosperm species preferentially invest in the growth of their root system (Figure 4c–e). This developmental plasticity of belowground organs is an evident advantage for the plants' water acquisition under drought. We therefore hypothesise that this physiological response to drought is widespread across the plant kingdom and that its genetic regulation could be conserved. A conserved regulation could be mediated by the hormone abscisic acid (ABA), which has been shown to regulate responses to water stress in both bryophytes and angiosperms (Takezawa et al., 2011). On the other hand, coevolution of this adaptive trait in the bryophyte and angiosperm lineages cannot be excluded.
In this study, we showed that bryophyte plants are capable of establishing host species-specific rhizoid-sphere communities, which show alterations in response to drought. The drought-induced changes show similarities with drought-induced changes in angiosperm rhizosphere communities and some taxa showing host species specificity are known for their nitrogen-fixing, symbiotic and/or plant growth-promoting potential. Taken together, these results contribute to the hypothesis that plant-microbe interactions in the rhizo(id-)sphere are evolutionarily conserved and evolved early on, contributing to plants' colonisation of land as well as to their resilience to biotic and abiotic stresses.
ACKNOWLEDGEMENTS
We thank Pierre-Marc Delaux for providing M. polymorpha and M. paleacea gemmae as well as for critical reading of the manuscript. We thank Peter van Gisbergen for providing Physcomitrium patens protonema. This work was supported by the Dutch Research Council (NWO/OCW), as part of the MiCRop Consortium Programme, Harnessing the second genome of plants (grant number 024.004.014)
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Raw sequence data can be found under NCBI Bioproject PRJNA1049656 for 16S sequencing of the bryophytes rhizoid-sphere, PRJNA1049759 for ITS sequencing of the bryophytes rhizoid-sphere and PRJNA1049762 for 16S sequencing of the angiosperms rhizosphere. All scripts for microbiome analysis are available at https://github.com/roland-berdaguer/bryophytes-rhizoid-sphere/.




